Abstract
Ampullary carcinoma or cancer of the ampulla of Vater is a rare malignancy with a high recurrence rate. Although cost-effective biomarkers, such as neutrophil-to-lymphocyte ratio (NLR), have been investigated in other cancers for predicting postoperative prognosis in patients, studies on the role of NLR in ampullary cancer are scarce. Here we aimed to evaluate the prognostic significance of preoperative NLR in patients with operable ampullary carcinoma. We retrospectively reviewed 87 patients who underwent pancreaticoduodenectomy for the treatment of ampullary carcinoma between December 1999 and April 2014. The association between NLR and prognosis (overall survival [OS] and disease-free survival [DFS]) was evaluated. Possible correlations between NLR and clinicopathological features were also assessed. The 5-year DFS and OS rates after surgery in patients with ampullary carcinoma were 51% and 63%, respectively. A high NLR (≥3.0) was found in 40 patients. The NLR was a significant prognostic factor for both OS and DFS. Multivariate analysis revealed a significantly worse OS in patients with positive surgical margins and NLR ≥3 (p = 0.001). Patients with T3-T4 stage (p = 0.029) and NLR ≥3 (p = 0.043) had a lower DFS. Patients with a high NLR had a significantly worse Eastern Cooperative Oncology Group performance score. Preoperative NLR is an independent and significant predictive factor of prognosis in patients with ampullary carcinoma. An elevated pretreatment NLR (e.g. NLR ≥3) may be considered as a biomarker for poor prognosis in patients with ampullary carcinoma.
Keywords: Carcinoma of the ampulla of Vater, neutrophil-to-lymphocyte ratio, prognosis, survival, inflammation, NLR, ampullary carcinoma
INTRODUCTION
Accounting for only 0.2% of all gastrointestinal malignancies, ampullary adenocarcinomas are exceedingly rare. Among periampullary tumors, ampullary carcinoma (also known as cancer of the ampulla of Vater) is the second most common malignancy, following carcinoma of the head of the pancreas [1,2]. Whipple operation is still the treatment of choice [3,4], and compared with periampullary carcinomas, ampullary carcinomas have a relatively better prognosis and higher rate of resection. The 5-year survival rate of patients with resected ampullary carcinoma ranges between 30% and 67.9% in different series [5-8]. Approximately 80% of all ampullary carcinoma cases are resectable at the time of diagnosis, although the cancer recurs in almost half of the patients [9,10]. Considering the high recurrence rate, the important role of prognostic markers is emphasized. Recently, researchers have identified numerous prognostic factors associated with systemic inflammation in various types of malignancies. These factors include serum albumin and C-reactive protein (CRP) levels, as well as the preoperative neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), among others [11-15].
Our study is aimed to determine the prognostic value of preoperative NLR and its association with survival outcomes following resection in patients with ampullary adenocarcinoma.
MATERIALS AND METHODS
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
This retrospective study was initially approved by the Ethical Committee of Ankara Numune Training and Research Hospital, Ankara, Turkey.
Informed consent
Informed consent was waived for individual participants included in the study given the retrospective nature of this work.
Study design
We consecutively included 87 patients with histologically confirmed carcinoma of the ampulla of Vater who underwent curative pancreaticoduodenectomy at our institution, including R0 and R1 resections, from December 1999 to April 2004.
All patients were above the age of 18 years and some received adjuvant therapies. Patients who had metastatic disease at the time of diagnosis or who were inoperable for other reasons, as well as patients who were previously treated for another primary malignancy or who were pregnant, were excluded from the study.
Initially, the association between clinicopathological parameters and the disease-free survival (DFS) or overall survival (OS) rate following pancreaticoduodenectomy was determined using univariate and multivariate analyses. The age, sex, pathologic tumor stage (pT-stage), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), lymph node metastasis (LNM), surgical margin positivity, applied treatments and treatment responses, postoperative Eastern Cooperative Oncology Group (ECOG) performance score, and NLR were investigated.
Routine preoperative blood tests were performed on venous blood from all patients. The NLR was calculated by dividing the ANC by the ALC. Lymphopenia was defined as a lymphocyte count ≤1.5 × 109/L and neutrophilia as a neutrophil count >7 × 109/L according to the reference values used in our laboratory. The NLR cut-off value (<3 or ≥3) was determined in accordance with previous studies [11,12,16].
Recurrence was defined as newly formed local or distant metastatic tumors evident in imaging studies such as ultrasonography, magnetic resonance imaging or computed tomography during follow-up, either with or without an associated increase in serum carcinoembryonic antigen (CEA) or carbohydrate antigen 19-9 (CA 19-9).
Statistical analysis
PASW Statistics for Windows, Version 18.0. (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. A value of p ≤ 0.05 was considered statistically significant. Descriptive statistics are reported as percentages and medians. A survival analysis was performed using the Kaplan-Meier method. Variables with p < 0.1 in the univariate analysis were entered in a Cox regression model with backward selection to determine independent predictors of survival. Among the correlated factors with similar effects on survival, only those with clinical significance were included. DFS was defined as the interval between the 1st day of surgery and the 1st day of recurrence or death due to any cause, whichever occurred first. OS was defined as the interval from the 1st day of surgery to the date of death from any cause or the last follow-up.
RESULTS
Patient characteristics
Eighty-seven patients were included in the study. The demographic features of the patients are summarized in Table 1. The median age was 62 years (range, 37-83 years) and 51 of the patients were male (58.6%). Thirty-eight (43.7%) patients had T1-T2 tumors and 49 (56.3%) had T3-T4 tumors. LNM was detected in 51 (58.6%) patients. Seven patients (8.0%) had positive surgical margins. Fifty-eight (66.7%) patients received adjuvant chemotherapy. The median ANC and ALC were 4.7 × 109/L (range, 0.2 × 109/L-17.5 × 109/L) and 1.66 × 109/L (0.51 × 109/L-3.64 × 109/L), respectively. Lymphocytopenia (ALC <1.5 × 109/L) was evident in 36.8% of patients and neutrophilia (ANC ≥7 × 109/L) in 24.1% of patients.
TABLE 1.
Demographic and clinical characteristics of patients with ampullary carcinoma
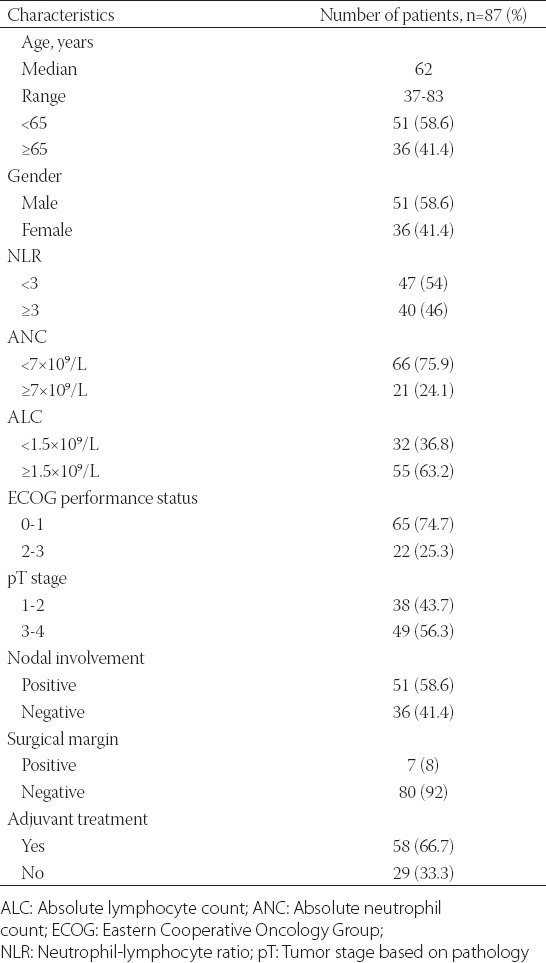
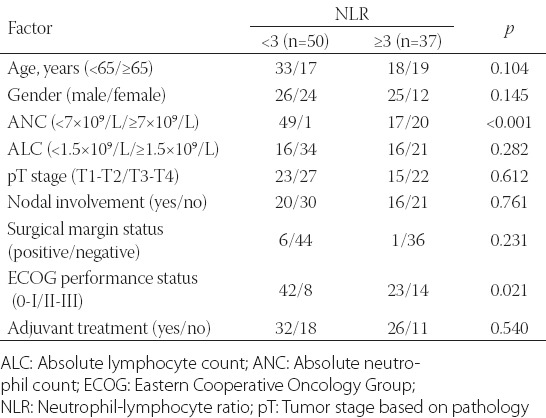
The median NLR was 2.7 (range, 1.1-8.2). The preoperative NLR was >3 in 40 patients (46.0%). When the relationship between the clinicopathological parameters and NLR was evaluated, a high NLR was associated with poor ECOG performance status (p = 0.021) and a higher ANC [p < 0.001] (Table 2).
TABLE 2.
Correlation between clinicopathological characteristics and the neutrophil-to-lymphocyte ratio in patients with ampullary carcinoma
OS and DFS analysis
The median follow-up from the initial diagnosis was 40.9 months (range, 1.68-186.15 months). The 5-year DFS and OS rates following pancreaticoduodenectomy were 51% and 63%, respectively.
Prognostic factors
In the univariate analysis, factors which may have potential prognostic significance in terms of DFS and OS were investigated. These parameters included age, sex, ECOG performance status, T-stage, ANC, ALC, lymph node status, surgical margin positivity, history of adjuvant treatment, and NLR.
NLR ≥3 (p = 0.048) and T3-T4 stage (p = 0.038) were associated with a poor DFS (Table 3). In the multivariate analysis, NLR ≥3 (p = 0.041) and T3-T4 stage (p = 0.033) remained significant predictors of recurrence (Figure 1A).
TABLE 3.
Univariate and multivariate analyses of clinicopathological features of patients with ampullary carcinoma in relation to disease-free survival
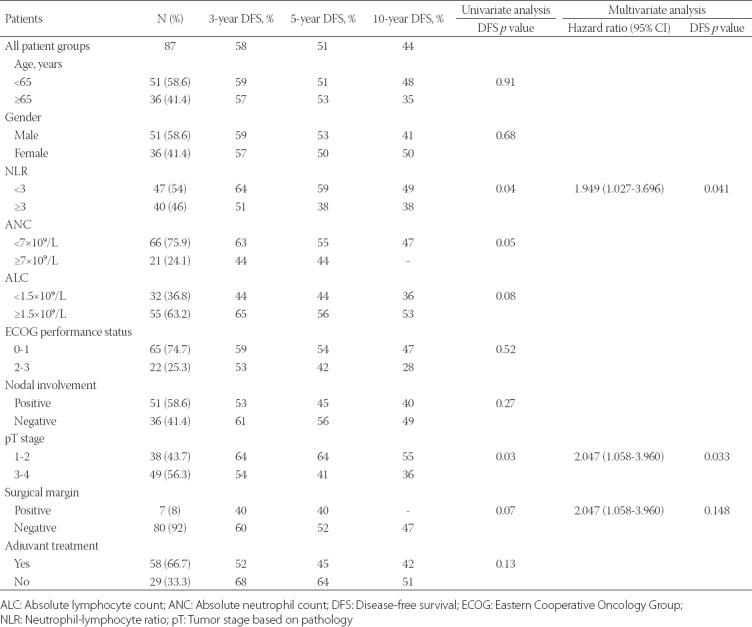
FIGURE 1.
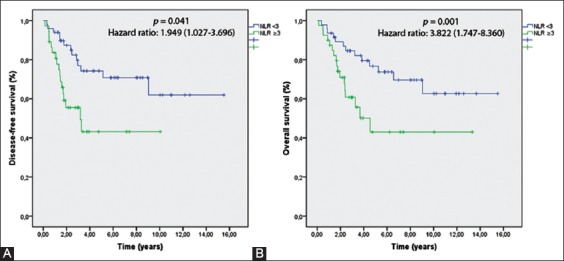
(A) Disease-free survival (DFS) rates according to the neutrophil-to-lymphocyte ratio (NLR) classification. (B) Overall survival (OS) rates according to the NLR classification. NLR ≥3 was significantly associated with poor DFS (p = 0.041) and OS (p = 0.001).
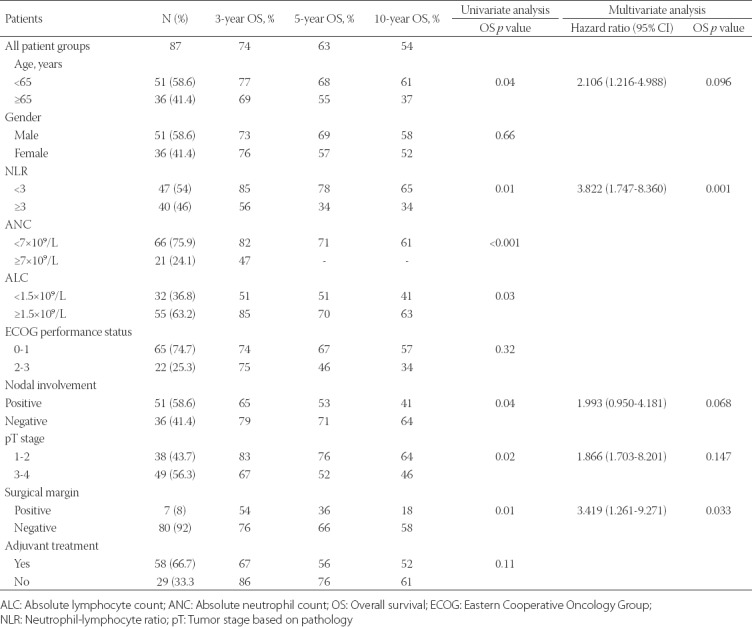
Table 4 summarizes the relationships of the clinical and pathological parameters with the OS rate according to the univariate and multivariate analyses. In the univariate analysis, the median OS was significantly lower in patients with NLR ≥3 (p = 0.012), pT3-T4 stage (p = 0.025), ANC ≥7 × 109/L (p < 0.001), ALC <1.5 × 109/L (p = 0.034), lymph node involvement (p = 0.047), age ≥65 years (p = 0.045), and positive surgical margins (p = 0.015). In the multivariate analysis, positive surgical margins (p = 0.033) and NLR ≥3 (p = 0.001) remained independent and significant prognostic predictors of a poor OS (Figure 1B).
TABLE 4.
Univariate and multivariate analyses of clinicopathological features of patients with ampullary carcinoma in relation to overall survival
DISCUSSION
There has been a growing interest in the field of oncology to identify prognostic markers that are cost-effective, easily accessible, and simple to evaluate. To contribute to these ongoing efforts, we investigated the predictive value of NLR in determining the prognosis of patients with operable ampullary carcinoma. Our multivariate analysis showed that a high NLR was significantly correlated with a poor DFS and OS after the treatment in patients with ampullary carcinoma. To the best of our knowledge, this is the most comprehensive report (i.e. we included the highest number of cases) on the therapeutic outcomes of pancreaticoduodenectomy in ampullary carcinoma.
It is now well accepted that inflammation and immunity have a critical role in tumor development. Immune cells that infiltrate tumors communicate with cancer cells and control the tumor growth. Moreover, immune response affects all stages of tumor development, including initiation, invasion, promotion, malignant conversion, and metastasis [17]. The prognostic significance of inflammatory factors such as albumin, CRC, and PLR has been demonstrated in numerous malignancies. The importance of NLR, as a readily accessible and cost-effective prognostic parameter, was also indicated in different types of cancers, including esophageal, colorectal, bladder, gastric, kidney, breast, lung, pancreatic, and gallbladder cancers, glioblastoma, cholangiocarcinoma, hepatocellular carcinoma, as well as in carcinoma of the ampulla of Vater [11-23]. Other prognostic parameters associated with survival of patients with ampullary carcinoma include T-stage, involvement of lymph nodes, surgical margin positivity, perineural invasion, lymphovascular invasion, ECOG performance score at the time of diagnosis, and tumor differentiation [24-28].
Although previous studies have shown that a high NLR is associated with poor survival in different malignancies, the exact relationship between NLR and patient prognosis remains unclear [18]. It is assumed that patients with elevated NLR have relative lymphocytopenia and decreased leukocyte response, which increases the recurrence rate of a tumor [29]. Another hypothesis is that the high neutrophil count may lead to the accelerated progression of neoplasm through the release of vascular endothelial growth factor [30]. When activated, neutrophils suppress cytotoxic T-cells and natural killer cells while regulating the activation of T cells; therefore, an immunosuppressive environment is established [31]. In addition to the mechanisms described above, it was reported that tumor-associated macrophages (TAMs) affect NLR. TAMs are capable of secreting cytokines, particularly interleukin (IL) 6 and IL-8, which are critical mediators of systemic neutrophilia [13,32].
To the best of our knowledge, only one other study [16] has investigated the role of NLR in patients with ampullary carcinoma. The study included 37 postoperative patients diagnosed with ampullary carcinoma, and revealed that pN2 or pN3 stage (p = 0.025) and NLR ≥3 (p = 0.026) were independent and significant predictors of a poorer OS, according to multivariate analysis [16]. In our study, positive surgical margins (p = 0.033) and NLR ≥3 (p = 0.001) were independent and significant prognostic predictors of a poor OS. Moreover, although not significant, there was a tendency toward poorer outcomes in our patients with lymph node involvement (p = 0.068). The differences between our and the study of Haruki et al. [16] may be due to our study design, i.e. we classified LNM only into two groups (present [N] or absent [N0]), while they grouped the LNM into pN0-N1 and pN2-N3. They showed that DFS was negatively correlated with pN2-N3 stage (p = 0.027). In contrast, we reported that NLR ≥3 (p = 0.043) and pT3-T4 stage (p = 0.029) were independent and significant predictors of a poor DFS. Also, Haruki et al. reported 5-year DFS and OS rates following pancreaticoduodenectomy of 73.4% and 82.1%, respectively, compared to 51% and 63% in our study. This difference may be due to our patient selection since the number of patients with relatively poor prognostic factors was higher in our population. In their study [16], patients with pT3-T4 and NLR ≥3 constituted 38.0% and 27.0% of the total number of patients compared with 56.3% and 46.0%, respectively in our study. A high NLR was associated with an advanced T-stage (p = 0.007) in the study of Haruki et al. and it was associated with poor ECOG performance (p = 0.021) status and a higher ANC (p < 0.001) in our study. Since the tumor microenvironment produces cytokines, patients with a high NLR may have a poorer ECOG performance status.
Our study is limited by its retrospective design. However, the advantage is that we included the highest number of ampullary carcinoma cases described in the literature so far.
CONCLUSION
There is an increasing trend of utilizing easily accessible and cost-effective markers to predict postoperative prognosis in cancer patients. Here, we report that preoperative NLR may predict both DFS and OS rates in patients with ampullary carcinoma. Since it is cost-effective and routinely measured during preoperative blood tests, NLR is easy to evaluate in almost all patients. As an indicator of poor prognosis, NLR may be useful in monitoring and managing patients with ampullary carcinoma.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Rosenberg R, Friederichs J, Schuster T, Gertler R, Maak M, Becker K, et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: A single-center analysis of 3,026 patients over a 25-year time period. Ann Surg. 2008;248(6):968–78. doi: 10.1097/SLA.0b013e318190eddc. https://doi.org/10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi K, Enjoji M. Carcinoma of the ampulla of vater. A clinicopathologic study and pathologic staging of 109 cases of carcinoma and 5 cases of adenoma. Cancer. 1987;59(3):506–15. doi: 10.1002/1097-0142(19870201)59:3<506::aid-cncr2820590326>3.0.co;2-#. https://doi.org/10.1002/1097-0142(19870201)59:3<506: AID-CNCR 2820 590326 >3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: The Mayo clinic experience. Int J Radiat Oncol Biol Phys. 2006;66(2):514–9. doi: 10.1016/j.ijrobp.2006.04.018. https://doi.org/10.1016/j.ijrobp.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Willett CG, Warshaw AL, Convery K, Compton CC. Patterns of failure after pancreaticoduodenectomy for ampullary carcinoma. Surg Gynecol Obstet. 1993;176(1):33–8. [PubMed] [Google Scholar]
- 5.Bettschart V, Rahman MQ, Engelken FJ, Madhavan KK, Parks RW, Garden OJ. Presentation, treatment and outcome in patients with ampullary tumours. Br J Surg. 2004;91(12):1600–7. doi: 10.1002/bjs.4787. https://doi.org/10.1002/bjs.4787. [DOI] [PubMed] [Google Scholar]
- 6.Colussi O, Voron T, Pozet A, Hammel P, Sauvanet A, Bachet JB, et al. Prognostic score for recurrence after whipple’s pancreaticoduodenectomy for ampullary carcinomas; Results of an AGEO retrospective multicenter cohort. Eur J Surg Oncol. 2015;41(4):520–6. doi: 10.1016/j.ejso.2015.01.010. https://doi.org/10.1016/j.ejso.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T, et al. Biliary tract cancer treatment: 5,584 results from the biliary tract cancer statistics registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16(1):1–7. doi: 10.1007/s00534-008-0015-0. https://doi.org/10.1007/s00534-008-0015-0. [DOI] [PubMed] [Google Scholar]
- 8.Morris-Stiff G, Alabraba E, Tan YM, Shapey I, Bhati C, Tanniere P, et al. Assessment of survival advantage in ampullary carcinoma in relation to tumour biology and morphology. Eur J Surg Oncol. 2009;35(7):746–50. doi: 10.1016/j.ejso.2008.10.010. https://doi.org/10.1016/j.ejso.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T, et al. Tumor of the ampulla of vater: Experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134(5):526–32. doi: 10.1001/archsurg.134.5.526. https://doi.org/10.1001/archsurg.134.5.526. [DOI] [PubMed] [Google Scholar]
- 10.Sellner FJ, Riegler FM, Machacek E. Implications of histological grade of tumour for the prognosis of radically resected periampullary adenocarcinoma. Eur J Surg. 1999;165(9):865–70. doi: 10.1080/11024159950189375. https://doi.org/10.1080/11024159950189375. [DOI] [PubMed] [Google Scholar]
- 11.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200(2):197–203. doi: 10.1016/j.amjsurg.2009.08.041. https://doi.org/10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 12.Malietzis G, Giacometti M, Askari A, Nachiappan S, Kennedy RH, Faiz OD, et al. A preoperative neutrophil to lymphocyte ratio of 3 predicts disease-free survival after curative elective colorectal cancer surgery. Ann Surg. 2014;260(2):287–92. doi: 10.1097/SLA.0000000000000216. https://doi.org/10.1097/SLA.0000000000000216. [DOI] [PubMed] [Google Scholar]
- 13.Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: A retrospective analysis. Ann Surg. 2013;258(2):301–5. doi: 10.1097/SLA.0b013e318297ad6b. https://doi.org/10.1097/SLA.0b013e318297ad6b. [DOI] [PubMed] [Google Scholar]
- 14.McMillan DC. An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc. 2008;67(3):257–62. doi: 10.1017/S0029665108007131. https://doi.org/10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- 15.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23(1):31–9. doi: 10.1016/j.suronc.2013.12.001. https://doi.org/10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Haruki K, Shiba H, Horiuchi T, Shirai Y, Iwase R, Fujiwara Y, et al. Neutrophil to lymphocyte ratio predicts therapeutic outcome after pancreaticoduodenectomy for carcinoma of the ampulla of Vater. Anticancer Res. 2016;36(1):403–8. [PubMed] [Google Scholar]
- 17.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(1):883–99. doi: 10.1016/j.cell.2010.01.025. https://doi.org/10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34(1):55–60. doi: 10.1016/j.ejso.2007.02.014. https://doi.org/10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Han S, Liu Y, Li Q, Li Z, Hou H, Wu A, et al. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. doi: 10.1186/s12885-015-1629-7. https://doi.org/10.1186/s12885-015-1629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Na N, Yao J, Cheng C, Huang Z, Hong L, Li H, et al. Meta-analysis of the efficacy of the pretreatment neutrophil-to-lymphocyte ratio as a predictor of prognosis in renal carcinoma patients receiving tyrosine kinase inhibitors. Oncotarget. 2016;7(28):44039–46. doi: 10.18632/oncotarget.9836. https://doi.org/10.18632/oncotarget.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viers BR, Boorjian SA, Frank I, Tarrell RF, Thapa P, Karnes RJ, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with advanced pathologic tumor stage and increased cancer-specific mortality among patients with urothelial carcinoma of the bladder undergoing radical cystectomy. Eur Urol. 2014;66(6):1157–64. doi: 10.1016/j.eururo.2014.02.042. https://doi.org/10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 22.Yao M, Liu Y, Jin H, Liu X, Lv K, Wei H, et al. Prognostic value of preoperative inflammatory markers in Chinese patients with breast cancer. Onco Targets Ther. 2014;7:1743–52. doi: 10.2147/OTT.S69657. https://doi.org/10.2147/OTT.S69657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao QT, Yang Y, Xu S, Zhang XP, Wang HE, Zhang H, et al. Prognostic role of neutrophil to lymphocyte ratio in lung cancers: A meta-analysis including 7,054 patients. OncoTargets Ther. 2015;8:2731–8. doi: 10.2147/OTT.S90875. https://doi.org/10.2147/OTT.S90875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JW, Bhandari M, Astill DS, Wilson TG, Kow L, Brooke-Smith M, et al. Predicting patient survival after pancreaticoduodenectomy for malignancy: Histopathological criteria based on perineural infiltration and lymphovascular invasion. HPB (Oxford) 2010;12(2):101–8. doi: 10.1111/j.1477-2574.2009.00140.x. https://doi.org/10.1111/j.1477-2574.2009.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein F, Jacob D, Bahra M, Pelzer U, Puhl G, Krannich A, et al. Prognostic factors for long-term survival in patients with ampullary carcinoma: The results of a 15-year observation period after pancreaticoduodenectomy. HPB Surg. 2014;2014:970234. doi: 10.1155/2014/970234. https://doi.org/10.1155/2014/970234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowe MC, Coban I, Adsay NV, Sarmiento JM, Chu CK, Staley CA, et al. Important prognostic factors in adenocarcinoma of the ampulla of Vater. Am Surg. 2009;75:754–60. [PubMed] [Google Scholar]
- 27.Pomianowska E, Westgaard A, Mathisen Ø, Clausen OP, Gladhaug IP. Prognostic relevance of number and ratio of metastatic lymph nodes in resected pancreatic, ampullary, and distal bile duct carcinomas. Ann Surg Oncol. 2013;20(1):233–41. doi: 10.1245/s10434-012-2592-z. https://doi.org/10.1245/s10434-012-2592-z. [DOI] [PubMed] [Google Scholar]
- 28.Qiao QL, Zhao YG, Ye ML, Yang YM, Zhao JX, Huang YT, et al. Carcinoma of the ampulla of Vater: Factors influencing long-term survival of 127 patients with resection. World J Surg. 2007;31(1):137–43. doi: 10.1007/s00268-006-0213-3. https://doi.org/10.1007/s00268-006-0213-3. [DOI] [PubMed] [Google Scholar]
- 29.Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39(6):585–9. doi: 10.1136/jcp.39.6.585. https://doi.org/10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6(4):283–7. doi: 10.1023/B:AGEN.0000029415.62384.ba. https://doi.org/10.1023/B: AGEN.0000029415.62384.ba. [DOI] [PubMed] [Google Scholar]
- 31.Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol. 2012;19(1):217–24. doi: 10.1245/s10434-011-1814-0. https://doi.org/10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 32.Maniecki MB, Etzerodt A, Ulhoi BP, Steiniche T, Borre M, Dyrskjot L, et al. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012;131(10):2320–31. doi: 10.1002/ijc.27506. https://doi.org/10.1002/ijc.27506. [DOI] [PubMed] [Google Scholar]


