Abstract
MicroRNAs (miRNAs) have an important role in the regulation of tumor development and metastasis. In this study, we investigated the clinical and prognostic value as well as biological function of miR-466 in colorectal cancer (CRC). Tumor and adjacent healthy tissues were obtained from 100 patients diagnosed with CRC. miR-466 expression was determined by quantitative reverse transcription polymerase chain reaction (qRT-PCR). mRNA and protein levels of cyclin D1, apoptosis regulator BAX (BAX), and matrix metalloproteinase-2 (MMP-2) were analyzed by qRT-PCR and Western blot, respectively, in SW-620 CRC cells transfected with miR-466 mimics or negative control miRNA. Effects of miR-466 on SW-620 cell proliferation, cell cycle and apoptosis, and invasion were investigated using CCK-8 assay, flow cytometry and Transwell assay, respectively. miR-466 expression was significantly downregulated in tumor tissues compared to matched adjacent non-tumor tissues. Low expression of miR-466 was significantly correlated with the tumor size, Tumor Node Metastasis stage, lymph node metastasis, and distant metastasis. The overall survival of CRC patients with low miR-466 expression was significantly shorter compared to high-miR-466 expression group (log-rank test: p = 0.0103). Multivariate analysis revealed that low miR-466 expression was associated with poor prognosis in CRC patients. The ectopic expression of miR-466 suppressed cell proliferation and migration/invasion, as well as induced G0/G1 arrest and apoptosis in SW-620 cells. Moreover, the ectopic expression of miR-466 decreased the expression of cyclin D1 and MMP-2, but increased BAX expression in SW-620 cells. In conclusion, our findings demonstrated that miR-466 functions as a suppressor miRNA in CRC and may be used as a prognostic factor in these patients.
Keywords: miR-466, colorectal cancer, CRC, prognosis, proliferation, migration, invasion
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer-related death in the United States, with estimated 136,830 new cases (52.5% men and 47.5% women) and 50,310 deaths (52.2% men and 47.8% women) in 2014 [1,2]. Despite a decrease in the morbidity and mortality rates, CRC remains a challenge for clinical diagnosis [3]. Age, adenomatous polyps, inflammatory bowel disease, and inherited genetic risk are the main non-modifiable risks factors for CRC, while high-fat diet, obesity, cigarette smoking, and alcohol are the major environmental factors that affect the CRC development [4]. The use of biological markers in diagnosis and prognosis of CRC has been recommended. However, despite improved understanding of the clinicopathological features of CRC as well as the molecular mechanisms of initiation and progression of colorectal tumorigenesis, limited biomarkers have been available up to now.
MicroRNAs (miRNAs) are small non-coding RNAs of 21-22 nucleotides in length, which regulate gene expression through mRNA cleavage or translational repression [5]. It is assumed that miRNAs regulate approximately 30% of protein-coding genes and thus affect various biological processes in eukaryotes [6]. Importantly, a large number of miRNAs are differentially expressed in healthy and cancer tissues, and are associated with disease development and outcome, indicating their potential as biomarkers for cancer [7]. To date, numerous studies have demonstrated the role of miRNAs in regulating the progression of CRC. For example, the down-regulation of miR-193a-3p was associated with worse prognosis of patients with CRC [8]. miR-552 was shown to have a tumor-promoting role in CRC development by targeting Dachshund family transcription factor 1, increasing the rates of CRC cell proliferation and migration [9]. miR-10b expression was positively correlated with advanced Tumor Node Metastasis (TNM) stages, colorectal liver metastasis, lymph node metastasis, venous infiltration, poorer differentiation, and served as an independent prognostic factor of poor overall survival (OS) [10]. A recent study showed that miR-466 was underexpressed in prostate cancer (PC) and that the restoration of miR-466 expression in metastatic PC cell lines led to the impaired oncogenic functions, induced cell cycle arrest, and apoptosis in cancer cells. Moreover, miR-466 exerted the anti-tumor effect through direct targeting of bone-related transcription factor RUNX2 [11]. On the contrary, the occurrence and development of cervical cancer were closely associated with increased expression of miR-466 [12]. To the best of our knowledge, a potential role of miR-466 in CRC has not been investigated up until now.
Here we investigated the expression of miR-466 in CRC and normal adjacent tissues using quantitative reverse transcription polymerase chain reaction (qRT-PCR). We then correlated the miR-466 expression results with the OS, clinicopathological and prognostic parameters in CRC patients. Finally, we explored the biological function of miR-466 in CRC by analyzing its effect on cyclin D1, apoptosis regulator BAX (BAX), and matrix metalloproteinase-2 (MMP-2) expression.
MATERIALS AND METHODS
Patients and tissue specimens
We enrolled 100 patients diagnosed with CRC by pathology and with complete clinicopathological data. The patients underwent complete surgical resection at the Second Affiliated Hospital of Zhejiang University School of Medicine, from March 2015 to October 2016. None of the patients received chemotherapy or radiotherapy before the surgery. Patients were followed up for 5 years and the clinical assessment was performed in each patient at the end of study.
The tumor and adjacent healthy tissues (<3 cm away from tumor) were obtained from the 100 patients. The resected tissues were immediately snap frozen in liquid nitrogen and stored until RNA extraction.
Informed consent was obtained from all patients and the study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (approval number SA23853).
Cell culture and transfection with miRNA mimics
SW-620 cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, US) and cultured in Leibovitz’s L-15 medium (GIBCO, NY, US) containing 10% fetal bovine serum (FBS, GIBCO), penicillin (100 units/mL), and streptomycin (100 units/mL). The cells were maintained in a humidified incubator at 37 °C containing 5% CO2. Before transfection, SW-620 cells (1 × 105 cells/well) were seeded into 6-well plates and incubated overnight. miR-466 mimics and scrambled negative control miRNA (Neg-miR) were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). When the cells reached 70-80% confluence, they were transfected with 100nM miR-466 or Neg-miR using Lipofectamine 2000 (Life Technologies, CA, US), according to the manufacturer’s protocol. The transfected cells were incubated for 48 hours at 37 °C, until further analysis.
qRT-PCR
Total RNA was extracted using TRIzol reagent. Complementary DNA synthesis was performed with 1 µg of the total RNA in 20 µl reaction mixture using the PrimeScript RT reagent kit (TakaRa, Dalian, China). Quantitative expression analysis was performed on 7900HT Fast Real-Time PCR System (Applied Biosystems, CA, US). The following primers were used: MIR466 forward: 5’-CACTAGTGGTTCCGTTTAGTAG-3’, reverse: 5’-TTGTAGTCA CTAGGGCACC-3’; U6 small nuclear RNA (RNU6-1) forward: 5’-CTCGCTTCGGCAGCACA-3’, reverse: 5’-AACGCT TCACGAATTTGCGT-3’; BAX forward: 5’-GATGCGTCCACCAAGAAGCT-3’, reverse: 5’-CGGCCCC AGTTGAAGTTG-3’; MMP2 forward: 5’-TGTACCG CTATGGTTACACTCG-3’, reverse: 5’-GGC AGG GACAGTTGCTTCT-3’; and GAPDH forward: 5’-AA GGTGAAGGTCGGAGTCA-3’, reverse: 5’-GGAAGATG GTGATGGGATTT-3’.
The relative expression level of MIR466, CCND1, BAX and MMP2 was calculated and statistically compared using the 2-ΔΔCt method after normalization with the RNU6-1 or GAPDH expression.
OS analysis using Kaplan–Meier estimator
According to the median MIR466 expression value used as a cutoff, 100 CRC cases were classified into higher-than-median group (n = 51) or lower-than-median group (n = 49). The value of ‘1’ was entered in the event of death and ‘0’ was entered if a patient was alive. The survival was estimated using the Kaplan–Meier method, and the survival distributions in relation to miR-466 expression were compared between the two groups using the log-rank test.
Western blotting
After 48 hours of transfection, SW-620 cells were collected and lysed in lysis solution. Total protein was extracted and quantified by a BCA protein assay kit (Pierce Biotechnology, Rockford, IL, US), according to the manufacturer’s instructions. Approximately 30 µg of proteins was separated on sodium dodecyl sulfate (SDS)-polyacrylamide gel (10%) and then transferred to a polyvinylidene fluoride (PVDF) membrane. Next, the membranes were blocked with 5% non-fat dried milk and incubated with rabbit anti-human Cyclin D1 (1:500, ab2502, Abcam, Cambridge, UK), BAX (1:1000, B8429, Sigma-Aldrich, MO, US), MMP-2 (9662, Cell Signaling Technology, MA, US), and glyceraldehyde 3-phosphate dehydrogenase [GAPDH] (1: 500000, 10494-1-AP, Proteintech, Wuhan, China) overnight at 4°C. We then incubated the membranes with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:5000, SC-2054, Santa Cruz, CA, US) for 2 hours at room temperature. After washing, the blots were visualized with an enhanced chemiluminescence (ECL) system (Santa Cruz, CA, US). GAPDH was used as the internal control.
Cell proliferation assay
The cell proliferation was determined using the CCK-8 assay (Beyotime, China), according to the manufacturer’s instructions. After 48 hours of transfection, SW-620 cells (3 × 103/well) were seeded in a 96-well plate and incubated until they attached to surface. Following the incubation of cells every 24 hours (up to 72 hours), 10 µL of CCK-8 reagent was added to each well and incubated for 2 hours. The absorbance was measured at 450 nm in a microplate reader. All data were calculated from three samples.
Cell cycle and apoptosis assays
Cell cycle and apoptosis were analyzed by flow cytometry in SW-620 cells after transfection. Briefly, the cells were harvested, washed with phosphate-buffered saline (PBS), and fixed with cold methanol overnight. For cell cycle analysis, the cells were immediately stained with propidium iodide (PI) using the BD Cycletest plus DNA reagent kit (BD Biosciences, CA, US). For apoptosis analysis, the cells were incubated with binding buffer supplemented with Annexin V-FITC and PI for 30 minutes in the dark at room temperature, according to the manufacturer’s protocol. The stained cells were immediately analyzed on a BD FACSVerse flow cytometer (BD Biosciences, US).
Transwell migration and invasion assays
For cell migration analysis, transfected SW-620 cells (1 × 105 cells) were plated into 24-well Boyden chambers (Corning, NY, US) using a pore polycarbonate membrane. Then, serum-free medium was seeded in the upper chambers and lower chambers were filled with medium containing 10% FBS, serving as a chemoattractant. After the incubation for 24 hours, the cells that migrated to the lower chambers were fixed in methanol and stained using 0.1% crystal violet. A similar method was used for cell invasion analysis, except the chambers were precoated with 20 µg Matrigel. In both assays, three randomly-selected visual fields were imaged and analyzed using a Nikon TE2000 microscope (Nikon, Tokyo, Japan). The results were averaged over three independent experiments.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0. (IBM Corp., Armonk, NY). All quantified data represent an average of at least three samples. Differences were determined with the analysis of variance (ANOVA), Student’s t-test, or Chi-squared test where appropriate. Data that were statistically significant in the univariate survival analysis (p<0.05) were analyzed in a multivariate survival analysis using Cox’s regression model. A two-tail p value of <0.05 was considered statistically significant.
RESULTS
Association between miR-466 expression and clinicopathological features of CRC
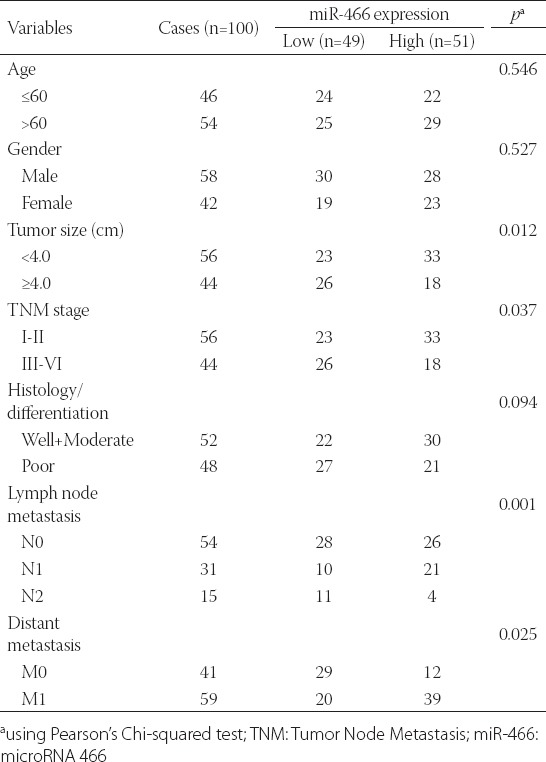
A total of 100 patients (58 males and 42 females) were enrolled in this study. The expression of miR-466 was first determined in pairs of CRC and adjacent non-tumor tissues, normalized to U6 expression. As shown in Figure 1, the miR-466 expression was significantly downregulated in CRC tissues compared with the adjacent normal tissues, in all cases (p < 0.001). The CRC cases were then classified either into high-expression group (n = 51) or low-expression group (n = 49), using the median value of miR-466 expression as a cutoff. As shown in Table 1, low expression of miR-466 in CRC patients was significantly correlated with the tumor size (p = 0.012), TNM stage (p = 0.037), lymph node metastasis (p = 0.001), and distant metastasis (p = 0.025). No significant difference was observed between miR-466 expression and the age, gender, and histological differentiation (p > 0.05).
FIGURE 1.
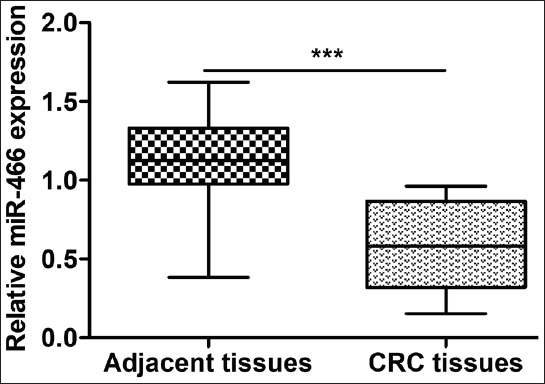
Expression of miR-466 in colorectal cancer (CRC) and adjacent normal tissues. The microRNA 466 (miR-466) expression was significantly downregulated in CRC tissues compared with matched adjacent normal tissues, in all 100 cases; ***p < 0.001.
TABLE 1.
Relationship between miR-466 expression and clinicopathological parameters in colorectal cancer (CRC) patients
Association between miR-466 expression and patient prognosis
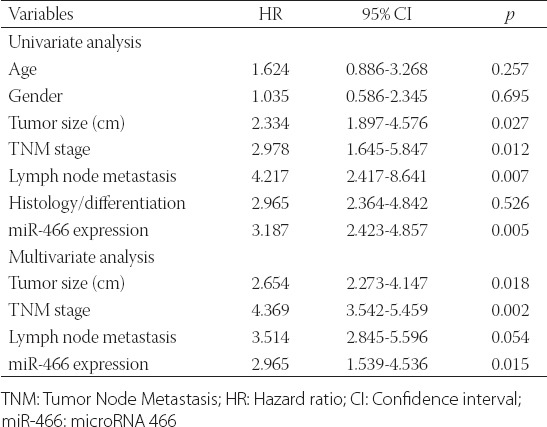
The Kaplan–Meier analysis and log-rank test showed that CRC patients with low miR-466 expression had significantly shorter survival (median OS: 28 months) compared to CRC patients with high miR-466 expression [median OS: 42 months] (Figure 2, p = 0.0103). Furthermore, the univariate analysis indicated that the tumor size (p = 0.027), TNM stage (p = 0.012), lymph node metastasis (p = 0.007), and low miR-466 expression levels (p = 0.005) were significantly correlated with OS. The multivariate Cox regression analysis showed that low miR-466 expression (hazard ratio [HR] = 2.965; 95% confidence interval [CI]: 1.539-4.536; p = 0.015) was an independent prognostic factor for OS in CRC patients (Table 2). Overall, these results indicated that low miR-466 expression in CRC is associated with poorer prognosis.
FIGURE 2.
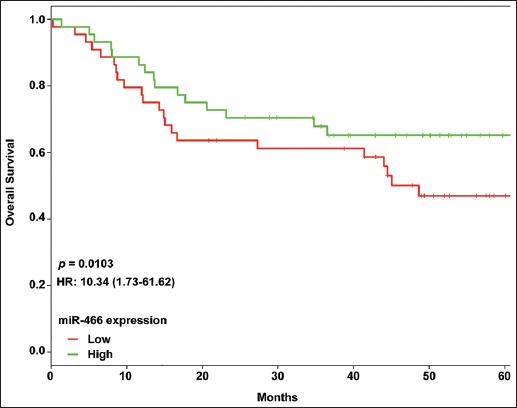
Overall survival (OS) analysis using Kaplan–Meier method and long-rank test in colorectal cancer (CRC) patients with low and high microRNA 466 (miR-466) expression. CRC patients with low miR-466 expression had significantly shorter survival (median OS: 28 months) compared to CRC patients with high miR-466 expression (median OS: 42 months).
TABLE 2.
Univariate and multivariate analyses of prognostic parameters in colorectal cancer (CRC) patients
Ectopic expression of miRNA-466 affected cell proliferation, cell cycle distribution and apoptosis in CRC in vitro
Human SW-620 cells were transfected with miR-466 mimics or non-target scrambled Neg-miR, to assess the functional significance of miR-466 in CRC cells. As shown in Figure 3A, increased levels of miR-466 were confirmed by qRT-PCR in the cells transfected with miR-466 mimics compared to Neg-miR-transfected cells (p < 0.001). The CCK-8 assay revealed that the proliferation rates were significantly decreased in miR-466 mimics-transfected cells at 48 hours and 72 hours (Figure 3B, p < 0.05 and p < 0.01, respectively) compared to the scrambled control.
FIGURE 3.
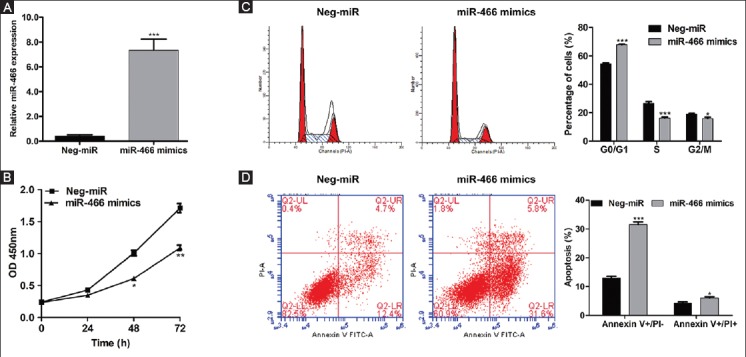
Ectopic expression of microRNA 466 (miRNA-466) inhibits proliferation, induces cell cycle arrest and apoptosis in colorectal cancer (CRC) cells. SW-620 cells were transfected with miR-466 mimics or negative control miRNA (Neg-miR). (A) Increased levels of miR-466 were confirmed by quantitative reverse transcription polymerase chain reaction in the cells transfected with miR-466 mimics compared to Neg-miR-transfected cells (p < 0.001). (B) CCK-8 assay showed significantly decreased proliferation rates in miR-466 mimics-transfected cells at 48 hours and 72 hours (p < 0.05 and p < 0.01, respectively) compared to the control. (C) PI staining showed that the percentage of cells in G0/G1 phase was significantly increased, from 54.45% in Neg-miR-transfected cells to 68.01% in miR-466 mimics-transfected cells (p < 0.001). Moreover, the overexpression of miR-466 remarkably decreased the percentage of SW-620 cells in S and G2/M phase (p < 0.001 and p < 0.05, respectively). (D) Flow cytometry analysis showed that the early apoptosis and late apoptosis rates were significantly increased in SW-620 cells transfected with miR-466 mimics compared to Neg-miR-transfected cells (p < 0.001 and p < 0.05, respectively). Values are expressed as mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs. Neg-miR group.
As illustrated in Figure 3C, the PI staining showed that the percentage of cells in G0/G1 phase was significantly increased, from 54.45% in Neg-miR-transfected cells to 68.01% in miR-466 mimics-transfected cells (p < 0.001). Moreover, the over-expression of miR-466 remarkably decreased the percentage of SW-620 cells in S and G2/M phase (p < 0.001 and p < 0.05, respectively), indicating that the cell cycle was arrested in G0/G1 phase by miR-466 mimics-transfection. In addition, the early apoptosis and late apoptosis rates were significantly increased in SW-620 cells transfected with miR-466 mimics compared to Neg-miR-transfected cells (p < 0.001 and p < 0.05, respectively), as shown by flow cytometry analysis in Figure 3D.
Ectopic expression of miRNA-466 suppressed cell migration and invasion in CRC in vitro
The Transwell assay revealed that the upregulation of miR-466 dramatically reduced the migration (130.0 to 42.5, p < 0.001) and invasion (44.7 to 10.7, p < 0.01) ability in SW-620 cells (Figure 4). Collectively, our data demonstrated that the ectopic expression of miR-466 could suppress the migration and invasion of CRC cells.
FIGURE 4.
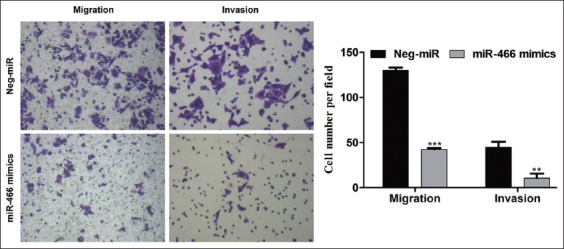
Ectopic expression of microRNA 466 (miRNA-466) impaired migration and invasion of colorectal cancer (CRC) cells. Transwell migration assay (left) and invasion assay (right) of SW-620 cells transfected with miR-466 mimics or negative control miRNA (Neg-miR). The upregulation of miR-466 dramatically reduced the migration (130.0 to 42.5, p < 0.001) and invasion (44.7 to 10.7, p < 0.01) ability in SW-620 cells. Representative images are shown on the left and quantification of three randomly selected fields is shown on the right. Values are expressed as the mean ± SD of three independent experiments. **p < 0.01, ***p < 0.001 vs. Neg-miR group.
Ectopic expression of miRNA-466 altered the expression of Cyclin D1, BAX and MMP-2 in CRC cells
According to qRT-PCR, the ectopic expression of miR-466 mimics significantly reduced the mRNA expression of Cyclin D1 and MMP-2, but elevated BAX mRNA expression (Figure 5A). Consistently, the Western blot analysis showed that the protein expression of Cyclin D1 and MMP-2 was downregulated, while BAX expression was upregulated in miR-466 mimics-transfected cells compared to Neg-miR-transfected cells (Figure 5B).
FIGURE 5.
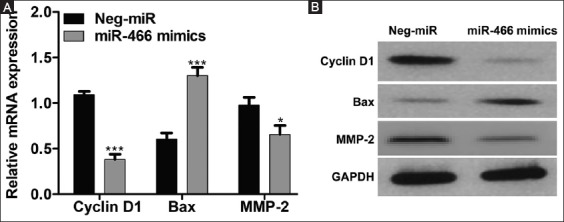
Ectopic expression of microRNA 466 (miRNA-466) altered the expression of cyclin D1, apoptosis regulator BAX (BAX), and matrix metalloproteinase-2 (MMP-2) in colorectal cancer (CRC) cells. (A) Quantitative reverse transcription polymerase chain reaction and (B) Western blot analysis showed that the mRNA and protein expression, respectively, of cyclin D1 and MMP-2 was downregulated, while BAX expression was upregulated in miR-466 mimics-transfected SW-620 cells compared to negative control miRNA (Neg-miR)-transfected cells. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. Values are expressed as the mean ± SD of three independent experiments. *p < 0.05, ***p < 0.001 vs. Neg-miR group.
DISCUSSION
CRC remains a life-threatening disease largely due to the absence of specific clinical symptoms and lack of biomarkers with high specificity and sensitivity [13,14].
Previously, the role of miR-466 in cancer growth and metastasis has been indicated. In this study, we showed that the expression of miR-466 was downregulated in CRC compared to matched adjacent normal tissues, suggesting that miR-466 may be associated with the progression and development of CRC. Similar results were reported on the role of miR-466 in PC [11].
Next, we analyzed the correlation between miR-466 expression, clinicopathological characteristics, and clinical outcome in CRC patients. Most notably, low expression levels of miR-466 were correlated with the tumor size, TNM stage, lymph node metastasis, and distant metastasis, suggesting a role of miR-466 in the tumor growth and metastasis in CRC. Furthermore, the Kaplan–Meier analysis revealed that the patients with low expression of miR-466 had a shorter survival compared to patients with high miR-466 expression. Thus, miR-466 may serve as an independent prognostic factor of OS in CRC patients.
The uncontrolled growth is a typical feature of CRC. We investigated the effect of miR-466 on several cellular processes in CRC. Our results showed that the increased expression of miR-466 suppressed the proliferation, migration and invasion as well as induced apoptosis in CRC cells. Similar results were also reported in highly metastatic PC cells [11]. Overall, our results indicated that the overexpression of miR-466 impaired the oncogenic function in CRC cells, which may also explain the positive association between miR-466 downregulation and the tumor size, TNM stage, metastasis, and poor outcome. All this suggest that miR-466 may be useful as a biomarker in CRC diagnosis and treatment.
The control of cell cycle progression plays a central role in cell proliferation, which is accomplished by the temporal activation of cyclin/cyclin-dependent kinase (CDK) complexes [15]. Cyclin D1, D2, D3, and E are the key regulators of the G1-to-S phase transition [16]. In this study, miR-466 mimics-treated SW-620 cells showed a significantly decreased cyclin D1 expression, implying that the overexpression of miR-466 could prevent the G1-S transition, leading to the accumulation of cells in G0/G1 phase and attenuation of cell proliferation. We also observed a higher expression of proapoptotic protein BAX in miR-466 mimics-treated SW-620 cells compared to negative control. The mitochondrial and death receptor pathways are the two main apoptosis signaling pathways in mammalians [17]. BAX has been shown to play a key role in the induction of cytochrome c release from mitochondria, in response to diverse apoptotic stimuli, such as growth factors and cytokines [18]. Impaired release of cytochrome c leads to the loss of activation of caspase-3, the executor of apoptosis [19]. In addition to its role in the mitochondrial apoptosis pathway, BAX is involved in the regulation of apoptosis in cancer cells, mediated by the death receptor signaling [20]. LeBlanc et al. [20] showed that human CRC cells deficient in BAX were resistant to death-receptor ligands, while the sister clones with active BAX expression were sensitive to this pathway. In this study, the overexpression of miR-466 induced early and late apoptosis, suggesting that miR-466 might be involved in the mitochondrial and/or death receptor signaling pathways.
The spread of cancer cells to the surrounding tissue is considered the first step in cancer metastasis [21]. Accumulating evidence confirmed that the remodeling of extracellular matrix (ECM) is required for the passage of invasive tumor cells through the tissue barrier [22]. The proteolytic degradation of various ECM components is controlled by matrix metalloproteinases (MMPs), especially MMP-2 and MMP-9 [23]. Oku et al. [24] showed that tight junction protein claudin-1 increased the invasion capability of oral squamous cell carcinoma cells through the activation of MMP-2 and regulation of membrane-type MMP-1 (MT1-MMP) expression. Moreover, claudin-expressing ovarian epithelial cells had increased MMP-2 activity, indicating a possible role of MMP proteins in claudin-mediated invasion [25]. In the current study, we observed a significant decrease in the mRNA and protein expression of MMP-2 in SW-620 cells transfected with miR-466-mimics, compared to control. Our results suggest that the inhibition of invasion and migration of CRC cells by upregulation of miR-466 might be associated with the inactivation of MMP-2.
Future studies in this area may help gain a deeper understanding of the relationship between miR-466, clinicopathological features and poor outcome in CRC.
CONCLUSION
We confirmed that miR-466 is underexpressed in CRC tissues and that the low expression of miR-466 is associated with the tumor size, TNM stage, lymph node and distant metastasis, as well as poor survival. Therefore, miR-466 may be suitable as a biomarker for CRC diagnosis and prognosis. Furthermore, miR-466 exerts its tumor-suppressing effects in CRC partially through the regulation of cyclin D1, BAX, and MMP-2. These findings provide important clues on the role of miR-466 in developing new therapeutic strategies for CRC.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Giantonio BJ, Catalano PJ, Meropol NJ, O’Dwyer PJ, Mitchell EP, Alberts SR, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;20-25(12):1539–44. doi: 10.1200/JCO.2006.09.6305. https://doi.org/10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):104–17. doi: 10.3322/caac.21220. https://doi.org/10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. https://doi.org/10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(4):191–7. doi: 10.1055/s-0029-1242458. https://doi.org/10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, et al. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer. 2010;126(5):1166–76. doi: 10.1002/ijc.24827. DOI: 10.1002/ijc.24827. [DOI] [PubMed] [Google Scholar]
- 6.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: Are the answers in sight? Nat Rev Genet. 2008;9(2):102–14. doi: 10.1038/nrg2290. https://doi.org/10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 7.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103(7):2257–61. doi: 10.1073/pnas.0510565103. https://doi.org/10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin M, Duan B, Hu J, Yu H, Sheng H, Gao H, et al. Decreased expression of miR-193a-3p is associated with poor prognosis in colorectal cancer. Oncol Lett. 2017;14(1):1061–7. doi: 10.3892/ol.2017.6266. https://doi.org/10.3892/ol.2017.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J, Yan XR, Liu T, Han XB, Yu JJ, Liu SH, et al. MicroRNA-552 promotes tumor cell proliferation and migration by directly targeting DACH1 via the Wnt/beta-catenin signaling pathway in colorectal cancer. Oncol Lett. 2017;14(3):3795–802. doi: 10.3892/ol.2017.6600. https://doi.org/10.3892/ol.2017.6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang H, Liu J, Chen Y, Ma C, Li B, Hao T. Up-regulation of mir-10b predicate advanced clinicopathological features and liver metastasis in colorectal cancer. Cancer Med. 2016;5(10):2932–41. doi: 10.1002/cam4.789. https://doi.org/10.1002/cam4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colden M, Dar AA, Saini S, Dahiya PV, Shahryari V, Yamamura S, et al. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2017;8(1):e2572. doi: 10.1038/cddis.2017.15. https://doi.org/10.1038/cddis.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun P, Shen Y, Gong JM, Zhou LL, Sheng JH, Duan FJ. A new microRNA expression signature for cervical cancer. Int J Gynecol Cancer. 2017;27(2):339–43. doi: 10.1097/IGC.0000000000000863. https://doi.org/10.1097/IGC.0000000000000863. [DOI] [PubMed] [Google Scholar]
- 13.Martini G, Troiani T, Cardone C, Vitiello P, Sforza V, Ciardiello D, et al. Present and future of metastatic colorectal cancer treatment: A review of new candidate targets. World J Gastroenterol. 2017;23(26):4675–88. doi: 10.3748/wjg.v23.i26.4675. https://doi.org/10.3748/wjg.v23.i26.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atreya CE, Yaeger R, Chu E. Systemic therapy for metastatic colorectal cancer: From current standards to future molecular targeted approaches. Am Soc Clin Oncol Educ Book. 2017;37:246–56. doi: 10.1200/EDBK_175679. https://doi.org/10.14694/EDBK_175679. [DOI] [PubMed] [Google Scholar]
- 15.Nilsson I, Hoffmann I. Cell cycle regulation by the Cdc25 phosphatase family. Prog Cell Cycle Res. 2000;4:107–14. doi: 10.1007/978-1-4615-4253-7_10. https://doi.org/10.1007/978-1-4615-4253-7_10. [DOI] [PubMed] [Google Scholar]
- 16.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75(4):791–803. doi: 10.1016/0092-8674(93)90498-f. https://doi.org/10.1016/0092-8674(93)90498-F. [DOI] [PubMed] [Google Scholar]
- 17.Yin XM. Signal transduction mediated by Bid, a pro-death Bcl-2 family proteins, connects the death receptor and mitochondria apoptosis pathways. Cell Res. 2000;10(3):161–7. doi: 10.1038/sj.cr.7290045. https://doi.org/10.1038/sj.cr.7290045. [DOI] [PubMed] [Google Scholar]
- 18.Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95(9):4997–5002. doi: 10.1073/pnas.95.9.4997. https://doi.org/10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol. 2001;117(2):333–40. doi: 10.1046/j.0022-202x.2001.01409.x. https://doi.org/10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- 20.LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor - induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8(3):274–81. doi: 10.1038/nm0302-274. https://doi.org/10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 21.Sahai E. Mechanisms of cancer cell invasion. Curr Opin Genet Dev. 2005;15(1):87–96. doi: 10.1016/j.gde.2004.12.002. https://doi.org/10.1016/j.gde.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9(8):893–904. doi: 10.1038/ncb1616. https://doi.org/10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 23.Zheng H, Takahashi H, Murai Y, Cui Z, Nomoto K, Niwa H, et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26(5A):3579–83. [PubMed] [Google Scholar]
- 24.Oku N, Sasabe E, Ueta E, Yamamoto T, Osaki T. Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 gamma2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 2006;66(10):5251–7. doi: 10.1158/0008-5472.CAN-05-4478. https://doi.org/10.1158/0008-5472.CAN-05-4478. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, D’Souza T, Morin PJ. Claudin-3 and claudin-4 expression in ovarian epithelial cells enhances invasion and is associated with increased matrix metalloproteinase-2 activity. Cancer Res. 2005;65(16):7378–85. doi: 10.1158/0008-5472.CAN-05-1036. https://doi.org/10.1158/0008-5472.CAN-05-1036. [DOI] [PubMed] [Google Scholar]


