Abstract
We have systematically studied the physiological responses elicited by amino acids from the principal taste organ of the Drosophila head. Although the detection and coding of sugars and bitter compounds have been examined extensively in this organism, little attention has been paid to the physiology of amino acid taste. We find that one class of sensilla, the S sensilla, yield the strongest responses to amino acids, although these responses were much weaker than the most robust responses to sugar or bitter compounds. S sensilla are heterogeneous in their amino acid responses, and amino acids differ in the responses they elicit from individual sensilla. Tryptophan elicited relatively strong responses from S sensilla, and these responses were eliminated when bitter-sensing neurons were ablated. Although tryptophan yielded little if any response in a behavioral paradigm, phenylalanine elicited a relatively strong response in the same paradigm and had a different physiological profile, supporting the notion that different amino acids are differentially encoded by the repertoire of taste neurons.
Keywords: Drosophila, labellum, amino acids, physiology, taste, gustation
Introduction
Animals depend on taste systems to evaluate food sources. An animal must determine the nutritive value of a potential food source, as well as the presence of toxins that it might contain. Accurate assessment of the benefits and risks of ingestion is essential to feeding decisions, which in turn are critical to fitness.
Drosophila provides an excellent model system in which to analyze the cellular basis of taste (Liman et al., 2014; Freeman et al., 2015), including the basis of taste in agricultural pest insects that cause enormous damage to the world’s food supply (van der Goes van Naters et al., 2006). The principal taste organ on the Drosophila head is the labellum, which contains 31 taste sensilla (Figure 1A). Each sensillum contains a pore at the tip and is innervated by two to four taste neurons. When the sensillum makes contact with a food source, tastants from the food enter through the pore and activate the neurons of the sensillum. There are other taste sensilla on the tarsal segments of the legs, the pharynx, and the margin of the wing.
Figure 1. Labellar sensilla respond to mixes of amino acids.

(A) The Drosophila labellum, each half of which contains ~31 sensilla. Each sensillum is designated by a letter, indicating whether it is small (S), intermediate (I), or large (L), and a number, indicating its position. The positions are stereotyped, although there is some variation. Sensilla of different size classes are differentially shaded. Taken from Weiss et al. (2011) (B) Amino acids are grouped into mixes of amino acids, with each amino acid present in the mix at a 10mM concentration. The total concentration in each mix thus varies from 40mM to 60mM. (C) Amino acids in Mix A elicited the strongest electrophysiological responses to select S sensilla. The total number of recordings was 965: n=318 from S sensilla; n=279 from I sensilla; n=368 from L sensilla. (D) Sample traces, all taken from one individual S7 sensillum. A control trace with the diluent, TCC, is shown at the bottom. The arrow at the beginning of the trace indicates the contact artifact. Scale bar indicates 10mV, 100 ms.
The taste sensilla on the labellum fall into three morphological classes: short (S), intermediate (I), and long (L). There are 11 S sensilla, 11 I sensilla, and 9 L sensilla, which occupy stereotyped positions on each bilaterally symmetric half of the labellum (Figure 1A). These neurons send axons to the subesophageal zone (SEZ) of the CNS.
Sugars, which taste sweet to humans, and toxins, which taste bitter, elicit appetitive and aversive responses, respectively, in Drosophila. The cellular basis of these responses have been examined in detail (Meunier et al., 2000; Dahanukar et al., 2001; Meunier et al., 2003; Scott 2004; Thorne et al., 2004; Dahanukar et al., 2007; Jiao et al., 2007; Kwon et al., 2011; Weiss et al., 2011). Responses to salt and to water, i.e. osmolarity, have also been analyzed (Liu et al., 2003; Cameron et al., 2010; Chen et al., 2010).
Amino acids are found ubiquitously in the environment, but little is known about the taste responses they elicit from Drosophila. Amino acids are the building blocks and breakdown products of protein. There are twenty standard amino acids that are specified by the genetic code. Some of these amino acids can be synthesized by the fly, but several “essential” amino acids must be consumed: arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine. Essential amino acids are critical for egg production (Sang et al., 1961), and a diet lacking amino acids has been shown to impair larval development and to reduce adult lifespan (Lee et al., 2013). In the wild, amino acids may be consumed as free amino acids or through protein-rich food sources, such as yeast.
Electrophysiological responses of labellar taste cells to several amino acids have been documented in the fleshfly Boettcherisca peregrina and the blowfly Phormia regina (Shiraishi et al., 1970). In the tsetse fly Glossina fuscipes fuscipes, a systematic physiological analysis of amino acid detection revealed strong responses to many amino acids in a pair of sensilla on the leg (Van Naters et al., 1998). Weaker responses were found in the Drosophila leg to two of three amino acids tested; these responses were limited to a single sensillum on the foreleg that also responds to sugars and a subset of bitter compounds (Ling et al., 2014).
The molecular basis of amino acid response has been analyzed in most detail in mammals, where a heterodimeric G-protein coupled receptor (GPCR) consisting of T1R1 and T1R3 subunits plays a role in amino acid recognition (Li et al., 2002). A recent study of responses to several amino acids in leg neurons of Drosophila has revealed a requirement for the Ionotropic Receptor (IR) gene IR76b (Ganguly et al., 2017). Likewise, IR76b has been found to be required for behavioral response of Drosophila larvae to amino acids (Croset et al., 2016). Taste receptors of the Gustatory receptor (Gr) family have been shown to be required for detection of canavanine, a non-proteinogenic amino acid (Lee et al., 2012; Shim et al., 2015).
In order to characterize the cellular basis of amino acid response in the Drosophila labellum, we used single-unit electrophysiology to measure systematically the response of all 31 labellar sensilla to 19 amino acids. We found no responses as strong as the most robust responses to sugars or bitter compounds, but we found a pattern of excitatory responses that we document here. S sensilla yielded the strongest responses and were heterogeneous in their response profiles. Different amino acids elicited different responses from individual S sensilla, suggesting that amino acid identity could in principle be coded combinatorially from the firing patterns they evoke. Tryptophan elicited relatively strong responses from the S sensilla. We found no responses to tryptophan in S sensilla in which bitter-sensing neurons were ablated, suggesting that these neurons are required in these sensilla for tryptophan response. Tryptophan, despite its relatively strong physiological response, yielded little if any behavioral response in a CAFÉ assay. Phenylalanine, which elicited a stronger behavioral response, also elicited physiological responses from S sensilla, but with a different profile than tryptophan. The distinct profiles of phenylalanine and trytophan support the possibility that different amino acids are differentially encoded by the taste neuron repertoire.
Results
A screen of labellar sensilla for electrophysiological responses to amino acids
As a means of determining which, if any, of the 31 taste sensilla of the labellum responded strongly to amino acids, we used single-unit electrophysiology. We focused on the 20 amino acids that are specified by the genetic code, excluding tyrosine, which is less soluble than the others. Rather than test ~600 individual pairwise combinations of sensilla and amino acids, we initially tested each of the 31 sensillum types with each of four mixes of amino acids, i.e. 124 combinations. Mix A contained six amino acids that had previously been found to elicit strong electrophysiological responses from taste sensilla of the leg of the tsetse fly (Figure 1B) (Van Naters et al., 1998). These amino acids included five with hydrophobic side chains, Val, Ile, Leu, Met, and Trp, in addition to Cys. Mix B included Phe, Ala, Gly, and Pro. Mix C contained the five amino acids with charged side chains: Arg, His, Lys, Asp, and Glu. Mix D contained four amino acids with polar uncharged side chains: Ser, Thr, Asn, Gln. This analysis was designed as a screen and each amino acid was tested at a 10 mM concentration; as a corollary the total amino acid concentrations in the four mixes were not identical and ranged from 40 mM to 60 mM. The analysis comprised a total of 965 single-unit recordings.
The strongest responses were found among S sensilla (Figure 1C,D). S sensilla were heterogeneous in their responses to amino acids, in agreement with results found with bitter compounds and ammonia (Weiss et al., 2011; Delventhal et al., 2017). For example, S7 and S9 gave stronger excitatory responses to Mix A than did S4 or S8, similar to the pattern found with bitter compounds and ammonia.
Mix A elicited responses from a number of S sensilla in this screen, including the strongest response found in this survey (S9; 22.0 spikes/s ± 7.4 spikes/s, n=6). Mix B also elicited some moderate responses. Less excitation was found with Mixes C or D.
I sensilla and L sensilla yielded few if any responses to any mix (Figure 1C). Accordingly, we focused the rest of our study on the S sensilla. We acknowledge that: i) sensilla that did not respond in this screen might respond to amino acids at higher concentrations; ii) there may be antagonistic interactions between amino acids in a mix, thereby masking responses that would have been elicited by individual amino acids; iii) responses might depend on the internal state of the fly, which we tested next.
There is evidence that the internal nutritional state of the fly alters its behavioral responses to amino acids (Toshima et al., 2012; Kudow et al., 2017). There is also evidence that fasting affects gene expression in chemosensory organs (Farhadian et al., 2012). We asked whether starvation increased electrophysiological responses to amino acids. We focused the analysis on five S sensilla that are more convenient to record from than others and that showed a range of responses in our initial screen: S5, S6, S7, S9 and S10. (Figure S1A). We tested each of these sensilla with all four mixes of amino acids to see whether starvation increased the responses observed in control flies or induced responses not observed in control flies.
We starved flies for 24h and then measured responses. There were no differences between starved and control responses (Figure S1B, p>0.05; n= 9–14). We note that the starvation protocol led to debilitation and lethality: on the order of half of the recordings from starved flies were unsuccessful. We acknowledge the formal possibility that the successful recordings are from a subpopulation of flies that are more resistant to the effects of starvation.
S sensilla respond to tryptophan and some other individual amino acids
Having identified S sensilla as yielding the greatest responses to amino acid mixtures, and Mix A as eliciting the most excitation from S sensilla, we next asked whether S sensilla responded to individual amino acid constituents of Mix A. Again we focused on S5, S6, S7, S9, and S10, and tested all six of the amino acid constituents of Mix A at 10 mM concentrations, each at a pH ranging from 6.8 to 6.9 (n=30 sensillum-amino acid combinations) (Figure 2A).
Figure 2. Tryptophan elicited a response from S sensilla.
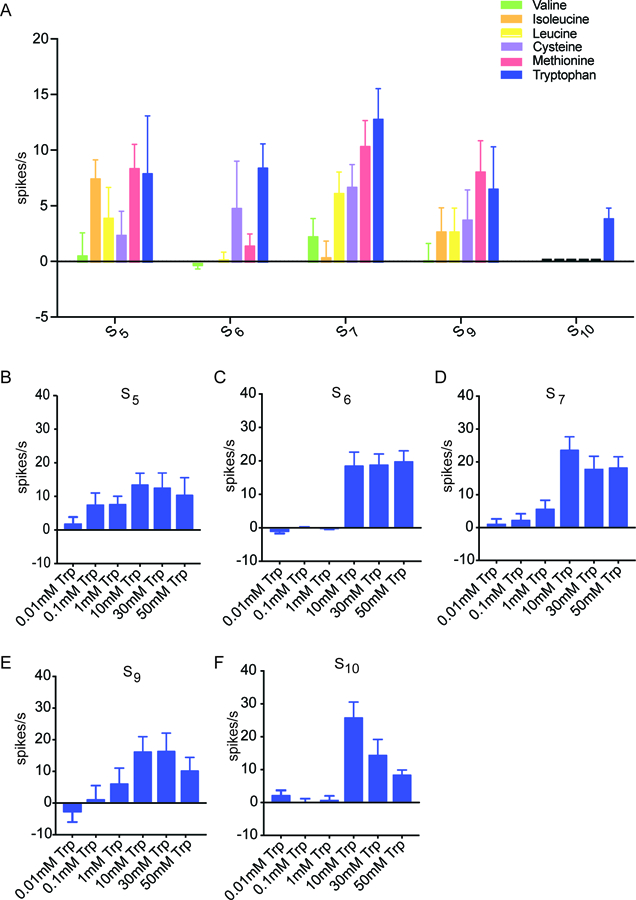
(A) Responses of S sensilla to the individual amino acids of Mix A at 10 mM concentrations (n=12–18). (B-F) Responses of each of the five indicated S sensilla to tryptophan. Sensilla can differentiate varying concentrations of tryptophan (n=7–14).
Tryptophan elicited the greatest mean response among the 30 combinations, from S7: 12.9 spikes/s ± 2.8 spikes/s; n=18. Tryptophan evoked a response from all five S sensilla tested. Methionine elicited a response that was comparable to that of tryptophan from S5, S7 and S9, but little or no response from S6 or S10. Isoleucine produced a response when tested with S5 but little or no response with the other S sensilla. Valine elicited little or no activity from any of these sensilla at the tested concentration. Thus individual amino acids have different profiles when tested against the S sensilla. These results indicate that the identity of some amino acids could in principle be encoded by the pattern of activity they elicit from individual sensilla.
Moreover, the various S sensilla differed in their profiles when tested against these individual amino acids. For example, S7 responded to most of these amino acids, whereas S10 responded only to tryptophan. These results suggest that the molecular underpinnings of amino acid response vary among the S sensilla, as is found for bitter responses.
A previous cluster analysis of the responses of S sensilla to bitter compounds identified two classes, S-a and S-b (Weiss et al., 2011). The same division into S-a and S-b emerged from a cluster analysis based on their expression of Gr–GAL4 drivers (Weiss et al., 2011). (Two other S sensilla, S4 and S8, did not respond to bitter compounds or express Gr-GAL4 drivers that are expressed in bitter neurons and thus did not fall into the S-a or S-b classes in either the functional or molecular cluster analysis.) The S-a class included S6, S7, and S10 sensilla, whereas S5 and S9 fell into S-b.
Accordingly, we asked whether S5, S6, S7, S9 and S10 also divided into S-a and S-b classes on the basis of their responses to the individual amino acids of Mix A. We carried out a cluster analysis using 13 clustering algorithms and did not find convincing support for such clustering. One interpretation of these results is that the molecular underpinnings of amino acid response differ from those of bitter responses, as discussed below.
Responses of S sensilla show different dose-dependence to tryptophan
To confirm and extend our finding that S sensilla respond to tryptophan, we carried out a dose-response analysis. We tested six increasing concentrations of tryptophan, ranging across more than three orders of magnitude, against S5, S6, S7, S9, and S10.
We found that the lowest doses produced small responses, whereas higher doses produced greater responses (Figure 2B–F). However, the dose dependence differed among sensillum types. For example, the S5 sensillum showed a lower response threshold than the S6 sensillum.
We note that we were surprised that the response of S10 to 50 mM tryptophan appeared lower than to a 10 mM concentration. However, declining spike rates at higher tastant concentrations have been observed in several other insect species (Marion-Poll et al., 2002; Calas et al., 2006). All of these experiments followed a standard protocol in which sensilla are stimulated successively with progressively greater doses of tastant, and it is possible that these apparent declines represent adaptation of neurons during the course of the experiments.
A kokumi substance does not increase the response of S sensilla to tryptophan
In addition to sweet, bitter, salty, sour, and umami taste, there is evidence for an additional taste modality called kokumi, which is sometimes described as “heartiness” (Ohsu et al., 2010). We were interested in the possibility that kokumi substances, which enhance certain taste responses in mammals, might enhance the response of S sensilla to tryptophan. In mammals, there is evidence that kokumi substances induce responses in taste cells that express a receptor, Calcium-sensing receptor (CaSR), which may respond to amino acids in taste cells (Bystrova et al., 2010; Ohsu et al., 2010). Accordingly, we asked whether the kokumi substance γ-glutamyl-cysteinyl-glycine (γ-glu-cys-gly), when mixed with 10 mM tryptophan, would elicit a greater response than tryptophan alone. We found the response of S5, S6, S7, S9, and S10 sensilla to tryptophan were not enhanced by the addition of γ-glu-cys-gly (Figure 3).
Figure 3. A kokumi substance did not increase the response rate of selected S sensilla to tryptophan.
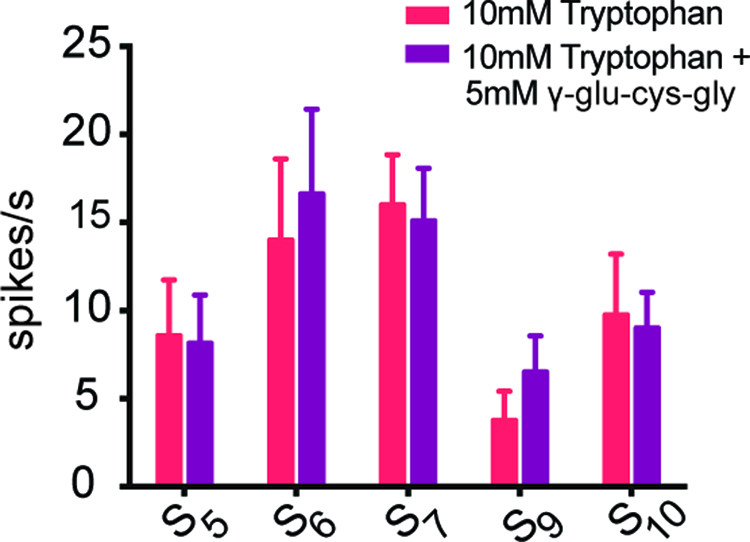
The spike rate did not increase when 5 mM γ-glutamyl-cysteinyl-glycine (γ-glu-cys-gly) was added to tryptophan (n=8–14).
Lack of response to tryptophan in S sensilla expressing diphtheria toxin
S sensilla contain a neuron that responds to bitter compounds, and robust responses to bitter compounds can be recorded readily. Responses to sugar compounds are much less robust and more difficult to record consistently from most S sensilla. We wondered whether ablation of the bitter-responding cell of S sensilla would eliminate responses to tryptophan. To address this question we used Gr66a-GAL4, a construct that is expressed in bitter neurons, to drive expression of diphtheria toxin (UAS-DTA). The Gr66a-GAL4 and UAS-DTA constructs were each backcrossed five times into our control wild type stock prior to use, in order to minimize genetic background effects. We have previously used these constructs successfully to ablate responses to bitter compounds (Delventhal et al., 2017).
To confirm the ablation in the present experiments we tested responses to caffeine, a well-characterized bitter compound. As expected, we found that expression of diphtheria toxin in the bitter neuron eliminated response to caffeine (Figure 4A,B).
Figure 4. Lack of response to tryptophan in S sensilla expressing diphtheria toxin.
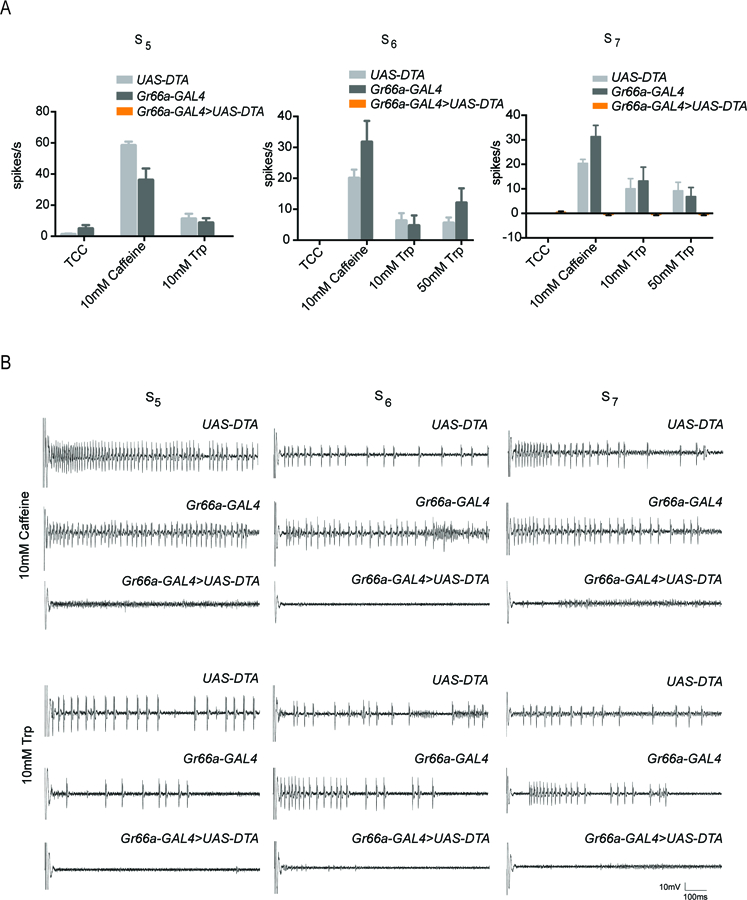
(A) Ablation of Gr66a+ -expressing bitter neurons in S5, S6, and S7 sensilla with a Gr66a-GAL4 driver and UAS-DTA (diphtheria toxin). No response is observed to caffeine or tryptophan in the sensilla that have undergone ablation. (n=5–8). (B) Sample electrophysiological traces. Scale bar indicates 10mV, 100 ms.
We then tested tryptophan and found no response in any S5, S6, or S7 sensillum, i.e. 0.0 ± 0.0 spikes/s (n = 5 for each sensillum type at each concentration). The simplest interpretation of these results is that response to tryptophan depends on the bitter neuron. However, we note that the response to the parental control genotypes (Gr66a-GAL4 and UAS-DTA) was low, despite the repeated backcrossing, and only in the case of the S7 sensillum at 10 mM tryptophan was the reduction in response significant with respect to both of these controls (p<0.0001, two-way ANOVA with Bonferroni’s multiple comparison correction). We note finally that since sugar responses are difficult to record reliably from most of these sensilla, we used salt solutions as positive controls to ensure that the expression of toxin did not ablate all neurons in the sensilla (see Materials and Methods; Hiroi et al., 2004; Delventhal et al., 2017).
Behavioral responses to tryptophan and phenylalanine
We tested the behavioral response to tryptophan in a Capillary Feeding (CAFÉ) assay (Figure 5A). Flies were first starved overnight and then given a choice between two solutions in separate capillary tubes: a mixture of 10 mM tryptophan and 1mM sucrose, and 1 mM sucrose alone. Sucrose was used in both solutions to stimulate feeding; in preliminary experiments without sucrose we found minimal feeding. In each assay we tested a small population of flies consisting predominantly of mated females, on the grounds that mated females may have an elevated need for amino acids to support egg production. The volume of each solution consumed was determined after 4h. A preference index (PI) was calculated as: PI = [(volume of 10 mM tryptophan and 1 mM sucrose consumed) – (volume of 1 mM sucrose consumed)]/total volume consumed. Thus the PI could in principle range from +1 (complete preference for the solution containing tryptophan) to −1 (complete aversion to the solution containing tryptophan).
Figure 5. Phenylalanine elicits a behavioral response and a response from S sensilla.
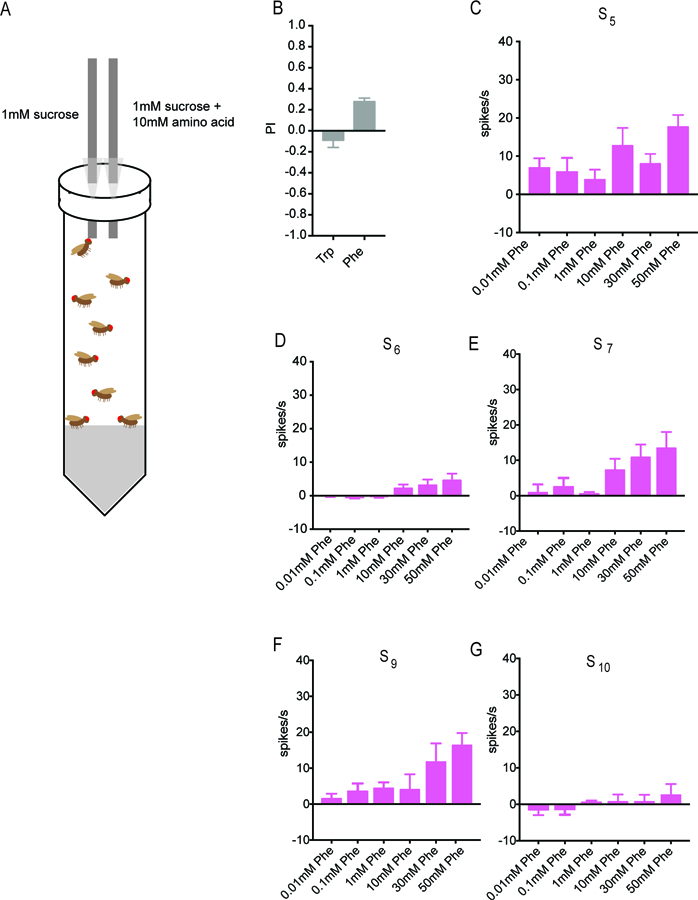
(A) CAFÉ assay for measuring the behavioral preference between a solution containing sucrose alone and a solution containing sucrose and an amino acid. Taken from Delventhal et al. (2017). (B) Little if any preference was shown in the case of tryptophan (n=32), but phenylalanine elicited a stronger behavioral response (n=76). (C-G) Responses of each of the five indicated S sensilla to phenylalanine. Some sensilla can differentiate varying concentrations of phenylalanine (n=10–12).
The presence of tryptophan elicited little if any response from flies (Figure 5B; PI = −0.09 ± 0.07; n=32). We asked whether the assay were sufficiently sensitive to detect responses to any amino acid. We therefore screened the other 18 amino acids at 10 mM concentrations (not shown) and found that the amino acid with the greatest mean PI in this CAFÉ analysis was phenylalanine (0.28 ± 0.03; n=76)(Fig. 5B). Phenylalanine also yielded the largest mean response of any amino acid in an electrophysiological study of taste neurons in the tsetse fly leg (Van Naters et al., 1998). Phenylalanine was a component of Mix B, which elicited substantial physiological responses from S sensilla (Figure 1C and S1B). We therefore tested phenylalanine against the S sensilla that we had tested previously with tryptophan.
Phenylalanine does in fact elicit responses from S sensilla (Figure 5C–G). The sensitivities of the five sensilla are not, however, identical to those for tryptophan. For example S9 shows substantially greater responses than S10 to phenylalanine at higher concentrations, but S9 and S10 are comparable to each other in their responses to tryptophan (Figure 2E,F). These results support the possibility that different amino acids are differentially encoded by the ensemble of taste neurons. We note finally that we were surprised to find an apparent response of S5, albeit weak, to a 0.01 mM concentration of phenylalanine; while further analysis will be required to investigate this observation in more detail, it is interesting that S5 also had a lower response threshold to tryptophan than did other S sensilla (Figure 2), and that tsetse also responds to this low concentration of phenylalanine (Van Naters et al., 1998).
Discussion
In this study we have carried out a systematic analysis of the physiological responses of the Drosophila labellum to the standard amino acids. The analysis has provided new insight into an understudied problem: how amino acids are detected and encoded by the taste system.
Our electrophysiological analysis of amino acid responses is unprecedented in scope, in that we documented the responses elicited by amino acids from each of the 31 taste sensilla of the primary taste organ of the fly head. The study has revealed that individual amino acids elicit different responses from different sensilla, and that individual sensilla respond differently to various amino acids. These results suggest the possibility that the identity of amino acids may be signaled via combinatorial coding.
It is interesting that the strongest responses to amino acids were from S sensilla. S sensilla also produced the strongest responses to bitter compounds and to ammonia. I sensilla, by contrast, yielded strong responses to a number of bitter compounds, but we found no strong responses to amino acids. L sensilla produced strong responses to neither bitter compounds nor amino acids; the lack of amino acid responses in L sensilla agrees with results of an earlier study (Dahanukar et al., 2007). S sensilla, besides being shorter than the other classes, are located more medially on the labellum. It would be interesting to determine if they make more contact than other sensillar classes with certain kinds of food sources, such as food sources with particular textures or viscosity.
Among the S sensilla, responses were heterogeneous. Particularly conspicuous was the lack of responses from S4 or S8 sensilla. This unresponsiveness to amino acids is consistent with the lack of responses of S4 or S8 to any of 16 bitter compounds tested (Weiss et al., 2011). These results raise interesting questions about the function of these exceptional sensilla in sensory coding. S4 and S8 appear morphologically similar to other S sensilla but do not express any of 33 Gr-GAL4 drivers that are expressed in bitter neurons (Weiss et al., 2011). These sensilla do show expression of a small number of IR-GAL4 drivers (Koh et al., 2014), which could underlie response to another kind of sensory stimuli.
The S sensilla that responded to bitter compounds divided into two classes, S-a and S-b (Weiss et al., 2011). Our analysis of amino acid responses of S sensilla does not reveal the same division. The division of bitter responses may derive primarily from the segregation of Gr receptors, which showed a similar division into S-a and S-b classes. There is evidence that amino acid responses depend on certain IR receptors (Croset et al., 2016; Ganguly et al., 2017), which may be distributed among sensilla independently of Gr receptors; it will be interesting to carry out an extensive analysis of electrophysiological responses to amino acids in the labella of IR mutants. In any case, the differences among individual sensilla in their response profiles to different amino acids suggests that they may also vary in the amino acid receptors they contain. The differences among S sensilla in the dose-dependence of responses to tryptophan, and to phenylalanine, may also reflect differences in the molecular composition of S sensilla.
We note that while a number of essential amino acids elicited responses from some of the S sensilla, responses were also elicited by cysteine, a non-essential amino acid. The mean response to cysteine was in fact greater than that to valine, an essential amino acid, in most cases. These results suggest that the need to consume a particular amino acid does not predict perfectly the magnitude of the physiological responses it elicits.
The greatest mean physiological response we recorded was to tryptophan. Why tryptophan? Tryptophan is an essential amino acid that is a precursor to the neurotransmitter serotonin as well as melatonin (Murch 2000). It is found in a variety of fruits at varying concentrations, e.g. 10x higher in banana than apple (Kader 1978; Dahinog 1982–1983; Islam 2015), and its level is likely to vary with the extent of fruit maturation (MacRae 1992) and decomposition. Tryptophan is synthesized by yeast and by a variety of bacteria. We do not know whether its presence indicates the presence of nutrients or, alternatively, that the nutrients in a food source have been largely depleted by microbes that produce tryptophan.
Overall the amino acid responses we recorded from labellar sensilla of Drosophila are much lower than those recorded in the tsetse fly Glossina fuscipes fuscipes (Van Naters et al., 1998). Tsetse responses to amino acids ranged up to ~280 spikes/s, a value higher than that observed for any amino acid, bitter compound, or sugar tested on any taste sensillum of Drosophila. The recordings from the tsetse fly were made from a different taste organ, the leg. When tsetse lands on a human, sensilla on the fly legs can taste the skin. Human sweat contains amino acids, so it is possible that amino acid taste confirms the suitability of a feeding source for tsetse and provides information the fly needs to act upon quickly.
The strongest responses to individual amino acids documented here (on the order of 15 spikes/s) are also much lower than the strongest responses elicited by individual bitter compounds (~60 spikes/sec) and sugars (~75 spikes/s) in studies of the Drosophila labellum, using the same genetic strain and similar procedures (Dahanukar et al., 2007; Weiss et al., 2011). The responses to amino acids also show a good deal of variability (most strikingly, the responses to 10 mM tryptophan in S10 shown in Figure 2A were substantially lower than those in Figure 2F). It is possible that the weakness and variability of amino acid responses reflect a lower salience of amino acid cues for the fly compared to sugars, which may signal the presence of a food rich in many nutrients, and bitter compounds, which signal the presence of a toxin that can cause death.
Which neuron in the S sensilla of the fly senses tryptophan? When we ablated the bitter-sensing neuron of these sensilla we found a complete absence of any spikes in response to tryptophan. The simplest interpretation of these results is that the bitter neuron mediates the response. However, we cannot formally exclude the possibility that the bitter neuron is required to support the activity of another neuron in the same sensillum that in fact transduces the amino acid signal.
If tryptophan is in fact encoded by bitter neurons, we might expect it, like bitter compounds, to have a negative valence. In this case the presence of tryptophan might signal the presence of a food source whose nutrients were largely depleted, or conceivably a food source contaminated with a toxic microbe that produces high levels of tryptophan. We did not observe a strong aversive response in the CAFÉ assay. However, confident evaluation of the valence of tryptophan in a natural context will require testing at a broad range of concentrations, in different taste paradigms, both singly and in combination with other chemosensory cues. We note that responses in the CAFÉ assay are likely to be influenced not only by the labellar sensilla, but by other sensilla as well, including pharyngeal sensilla and perhaps leg sensilla. It would also be interesting to explore the behavioral response to tryptophan as a function of the internal state of the animal; the internal state has been found to influence the behavioral response to other amino acids (Toshima et al., 2012; Ganguly et al., 2017). We note finally that behavioral responses to complex mixtures of amino acids may be stronger than responses to individual amino acids.
Although we have found that the tryptophan response of S sensilla depends on bitter neurons, we do not claim that our findings can be generalized to all other amino acids, or to all other sensilla, on either the labellum or the leg. It will be of special interest to determine the cellular basis of the electrophysiological and behavioral response to phenylalanine, which has a positive valence in our CAFÉ assay (Figure 5B). Amino acids are a diverse class of molecules, and taste sensilla on the fly are diverse in both their anatomical and molecular properties. In larger fly species, different amino acids were found to stimulate different cell types in labellar taste sensilla (Shiraishi et al., 1970). A recent study found that some, but not all, neurons of the Drosophila leg that responded to 100 mM serine also responded to sucrose, supporting the idea that the cellular basis of amino acid sensation is complex (Ganguly et al., 2017). Our study provides a foundation that should be useful in defining the molecular and cellular basis of this complexity.
Materials and Methods
Drosophila stocks
Flies were grown on standard cornmeal-agar medium at 25°C in a 50% humidity-controlled room. Canton-S (CS) flies were used for electrophysiological recordings and behavioral assays, unless indicated otherwise. Gr66a-GAL4 (Weiss et al., 2011), Gr64f-GAL4 (Dahanukar et al., 2007), and UAS-DTA (Bloomington Stock Center, Stock 25039) flies were outcrossed into wCS for five generations before electrophysiological recordings. We have previously used these constructs successfully to ablate responses to bitter compounds (Delventhal et al., 2017).
Electrophysiology
Single-sensillum recordings were performed using the tip-recording method (Hodgson et al., 1955; Delventhal et al., 2014). Electrophysiological recordings were performed on female flies. After eclosion, 10 female flies and 2 males were transferred to a fresh food vial and aged 2–11 days.
For recordings, a glass capillary filled with Beadle-Ephrussi Ringer solution (B&E) was inserted into the dorsal thorax of the fly, continued through the neck, and extended into the proboscis, thereby granting stable access to the labellar sensilla. A second glass pipette containing the experimental tastant was connected to an amplifier with a silver wire and acted as the recording electrode. The recording electrode with tastant was presented to the individual sensillum and recording began upon contact.
To quantify the response, the number of action potentials (spikes) was counted over a 500 ms period, starting 200 ms after contact. To calculate the number of spikes/s, we multiplied the number of spikes over the 500 ms period by two. A low and/or high salt stimulus (50mM NaCl and 400 mM NaCl, respectively) was used as a positive control at the beginning and end of the recording session for each sensillum to ensure that at least one gustatory receptor neuron was functional (Hiroi et al., 2004, Delventhal et al., 2017). No more than 11 tastants were presented to each sensillum, with minimum of 1 minute between each presentation. Traces were recorded using LabView (National Instruments, Austin, TX) and analyzed using MATLAB.
Tastants were acquired from Sigma-Aldrich at the highest available purity. Tastants were dissolved in 30mM tricholine citrate (TCC), which inhibits water neuron activity (Wieczorek et al., 1989). Responses to the TCC diluent alone were subtracted to yield the indicated values. Solutions were stored long-term as aliquots in −20°C, kept at 4°C when in use, and discarded after one week of use.
CAFÉ assay
The CAFÉ assay was performed as described in Delventhal et al. (Delventhal et al., 2017), similar to the assay described originally (Ja et al., 2007). A chamber was prepared by filling a 50 ml plastic Falcon conical tube with 30 ml of 2% agarose. Two holes were punched into the cap, and shortened 1 ml pipette tips were inserted through the holes partially into the chamber. Calibrated glass capillary tubes (Drummond Scientific Company, Catalog #2–000-001) were filled with liquid food by capillary action and inserted into the chamber through the pipette tips. Two tubes with liquid food were present in each chamber: one with 1 mM sucrose alone and the other with 1 mM sucrose and 10mM of an individual amino acid.
For the assay, 13 female and 2 male flies (all 7 days old) were introduced into the CAFÉ chamber, and starved overnight in a 25°C, 50% humidity-controlled room. Two capillary tubes were introduced the next morning, and flies were given four hours to ingest the liquid food. Both individual capillaries must have had at least 0.05μL consumed and a total consumption volume of 0.18μL for a PI calculation; only a small fraction of vials did not meet this criterion. Average values ± SEM are given.
Supplementary Material
(A) The labellar sensilla, with the tested S sensilla highlighted in red. Adapted from (Weiss et al., 2011) and from Figure 1A. (B) Starving the flies for 24 hours did not increase the response rate to the mixes of amino acids (p > 0.5, n =9–12; t-test with a Bonferroni-Dunn multiple comparisons correction). The negative values observed in S5 with Mix C are likely to arise stochastically: values are obtained by subtracting the response to the TCC diluent from the response to the amino acids in TCC, and variance in the response to TCC alone is likely to exceed any excitatory effect of Mix C on the sensillum.
Acknowledgments:
We thank members of the Carlson lab for help, discussion, and comments on the manuscript. The work was supported by grants from the NIH to J.C.
Contributor Information
Joori Park, Dept. of Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06511.
John R. Carlson, Dept. of Molecular, Cellular and Developmental Biology, Yale University, New Haven, CT 06511.
References
- Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD & Kolesnikov SS (2010). Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci, 123, 972–982. [DOI] [PubMed] [Google Scholar]
- Calas D, Thiery D & Marion-Poll F (2006). 20-hydroxyecdysone deters oviposition and larval feeding in the European grapevine moth, Lobesia botrana. J Chem Eco, 32, 11, 2443–2454. [DOI] [PubMed] [Google Scholar]
- Cameron P, Hiroi M, Ngai J & Scott K (2010). The molecular basis for water taste in Drosophila. Nature, 7294, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Wang QX & Wang ZR (2010). The Amiloride-Sensitive Epithelial Na+ Channel PPK28 Is Essential for Drosophila Gustatory Water Reception. J Neurosci, 30, 18, 6247–6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Schleyer M, Arguello JR, Gerber B & Benton R (2016). A molecular and neuronal basis for amino acid sensing in the Drosophila larva. Sci Rep, 6, 34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahanukar A, Foster K, van der Goes van Naters, W. M. & Carlson, J. R. (2001). A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nature Neurosci, 4, 12, 1182–1186. [DOI] [PubMed] [Google Scholar]
- Dahanukar A, Lei YT, Kwon JY & Carlson JR (2007). Two Gr genes underlie sugar reception in Drosophila. Neuron, 56, 3, 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahinog MJ, Rafols ED, Laspinas V, Lau HKF, and Boyde TRC (1982–1983). Amino Acid Composition of Vegetables and Fruits from the Philippines. Bull Phil Biochem Soc, 5, 6, 31–39. [Google Scholar]
- Delventhal R, Kiely A & Carlson JR (2014). Electrophysiological recording from Drosophila labellar taste sensilla. J Vis Exp, 84, e51355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delventhal R, Menuz K, Joseph R, Park J, Sun JS & Carlson JR (2017). The taste response to ammonia in Drosophila. Sci Rep, 7, 43754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadian SF, Suarez-Farinas M, Cho CE, Pellegrino M & Vosshall LB (2012). Post-fasting olfactory, transcriptional, and feeding responses in Drosophila. Physiol Behav, 105, 2, 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EG & Dahanukar A (2015). Molecular neurobiology of Drosophila taste. Curr Opin Neurobiol, 34, 140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Pang L, Duong VK, Lee A, Schoniger H, Varady E & Dahanukar A (2017). A Molecular and Cellular Context-Dependent Role for Ir76b in Detection of Amino Acid Taste. Cell Rep, 18, 3, 737–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi M, Meunier N, Marion-Poll F & Tanimura T (2004). Two Antagonistic Gustatory Receptor Neurons Responding to Sweet-Salty and Bitter Taste in Drosophila. J. Neurobiol, 61, 3, 333–342. [DOI] [PubMed] [Google Scholar]
- Hodgson ES, Lettvin JY & Roeder KD (1955). Physiology of a Primary Chemoreceptor Unit. Science, 122, 3166, 417–418. [DOI] [PubMed] [Google Scholar]
- Islam J, Shirakawa H, Nguyen TK, Aso H, and Komai M (2015). Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Bioscience, 13, 2016, 56–59. [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T & Benzer S (2007). Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA, 104, 20, 8253–8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Moon SJ & Montell C (2007). A Drosophila gustatory receptor required for the responses to sucrose, glucose, and maltose identified by mRNA tagging. Proc Natl Acad Sci USA, 104, 35, 14110–14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader A, Stevens MA, Albright M, and Morris LL (1978). Amino Acid Composition and Flavor of Fresh Market Tomatoes as Influenced by Fruit Ripeness When Harvested. J Ameri Soc Hort Sci, 103, 4, 541–544. [Google Scholar]
- Koh TW, He Z, Gorur-Shandilya S, Menuz K, Larter NK, Stewart S & Carlson JR (2014). The Drosophila IR20a clade of ionotropic receptors are candidate taste and pheromone receptors. Neuron, 83, 4, 850–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudow N, Miura D, Schleyer M, Toshima N, Gerber B & Tanimura T (2017). Preference for and learning of amino acids in larval Drosophila. Biol Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA & Carlson JR (2011). Molecular and cellular organization of the taste system in the Drosophila larva. J Neurosci, 31, 43, 15300–15309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC & Micchelli CA (2013). Development and characterization of a chemically defined food for Drosophila. PLoS One, 8, 7, e67308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kang MJ, Shim J, Cheong CU, Moon SJ & Montell C (2012). Gustatory receptors required for avoiding the insecticide L-canavanine. J Neurosci, 32, 4, 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Staszewski L, Xu H, Durick K, Zoller M & Adler E (2002). Human receptors for sweet and umami taste. Proc Natl Acad Sci USA, 99, 7, 4692–4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Zhang YV & Montell C (2014). Peripheral coding of taste. Neuron, 81, 5, 984–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling F, Dahanukar A, Weiss LA, Kwon JY & Carlson JR (2014). The molecular and cellular basis of taste coding in the legs of Drosophila. J Neurosci, 34, 21, 7148–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Johnson WA & Welsh MJ (2003). Drosophila DEG/ENaC pickpocket genes are expressed in the tracheal system, where they may be involved in liquid clearance. Proc Natl Acad Sci USA, 100, 4, 2128–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae EA, and Redgwell RJ (1992). Amino Acids in kiwifruit. New Zealand Journal of Crop and Horicultural Science, 20, 329–336. [Google Scholar]
- Marion-Poll F & Descoins C (2002). Taste detection of phytoecdysteroids in larvae of Bombyx mori, Spodoptera littoralis and Ostrinia nubilalis. J Insect Physiol, 48, 4, 467–476. [DOI] [PubMed] [Google Scholar]
- Meunier N, Ferveur JF & Marion-Poll F (2000). Sex-specific non-pheromonal taste receptors in Drosophila. Curr Biol, 10, 24, 1583–1586. [DOI] [PubMed] [Google Scholar]
- Meunier N, Marion-Poll F, Rospars JP & Tanimura T (2003). Peripheral coding of bitter taste in Drosophila. J Neurobiol, 56, 2, 139–152. [DOI] [PubMed] [Google Scholar]
- Murch S, KrishnaRag S, and Saxena P (2000). Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Reports, 19, 7, 698–704. [DOI] [PubMed] [Google Scholar]
- Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N & Eto Y (2010). Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem, 285, 2, 1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang JH & King RC (1961). Nutritional Requirements of Axenically Cultured Drosophila Melanogaster Adults. Journal of Experimental Biology, 38, 4, 793–809. [Google Scholar]
- Scott K (2004). The sweet and bitter of mammalian taste. Curr Opin Neurobiol, 14, 4, 423–427. [DOI] [PubMed] [Google Scholar]
- Shim J, Lee Y, Jeong YT, Kim Y, Lee MG, Montell C & Moon SJ (2015). The full repertoire of Drosophila gustatory receptors for detecting an aversive compound. Nature Communications, 6, 8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A & Kuwabara M (1970). The effects of amino acids on the labellar hair chemosensory cells of the fly. J Gen Physiol, 56, 6, 768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S & Amrein H (2004). Taste perception and coding in Drosophila. Curr Biol, 14, 12, 1065–1079. [DOI] [PubMed] [Google Scholar]
- Toshima N & Tanimura T (2012). Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J Exp Biol, 215, Pt 16, 2827–2832. [DOI] [PubMed] [Google Scholar]
- van der Goes van Naters W & Carlson JR (2006). Insects as chemosensors of humans and crops. Nature, 444, 7117, 302–307. [DOI] [PubMed] [Google Scholar]
- Van Naters WMV & Den Otter CJ (1998). Amino acids as taste stimuli for tsetse flies. Physiological Entomology, 23, 3, 278–284. [Google Scholar]
- Weiss LA, Dahanukar A, Kwon JY, Banerjee D & Carlson JR (2011). The molecular and cellular basis of bitter taste in Drosophila. Neuron, 69, 2, 258–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek H & Wolff G (1989). The Labellar Sugar Receptor of Drosophila. Journal of Comparative Physiology, 164, 6, 825–834. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The labellar sensilla, with the tested S sensilla highlighted in red. Adapted from (Weiss et al., 2011) and from Figure 1A. (B) Starving the flies for 24 hours did not increase the response rate to the mixes of amino acids (p > 0.5, n =9–12; t-test with a Bonferroni-Dunn multiple comparisons correction). The negative values observed in S5 with Mix C are likely to arise stochastically: values are obtained by subtracting the response to the TCC diluent from the response to the amino acids in TCC, and variance in the response to TCC alone is likely to exceed any excitatory effect of Mix C on the sensillum.


