Abstract
Background
Portal hypertensive gastropathy (PHG) is a common anomaly with potential for bleeding found in portal hypertension. Blood ammonia levels correlate well with liver disease severity and existence of portosystemic shunts. Increased ammonia results in vasodilation and hepatic stellate cell activation causing and exacerbating portal hypertension.
Objective
To assess the relation of blood ammonia to the presence and severity of portal hypertensive gastropathy in cirrhosis.
Methods
This cross-sectional study included 381 cirrhotics undergoing screening for esophageal varices (EV) divided into a portal hypertensive gastropathy group (203 patients with EV and PHG), esophageal varix group (41 patients with EV but no PHG), and control group (137 patients with no EV or PHG). A full clinical examination, routine laboratory tests, abdominal ultrasonography, child score calculation, and blood ammonia measurement were performed for all patients.
Results
Blood ammonia, portal vein, splenic vein, and splenic longitudinal diameters were significantly higher and platelet counts lower in patients with EV and EV with PHG than controls. Patients having EV with PHG had significantly higher bilirubin and ammonia than those with EV but no PHG. Severe PHG was associated with significantly higher ammonia, EV grades, and superior location and a lower splenic longitudinal diameter than mild PHG. The PHG score showed a positive correlation with blood ammonia and a negative correlation with splenic longitudinal diameter.
Conclusions
Blood ammonia levels correlate with the presence, severity, and score of portal hypertensive gastropathy in cirrhosis suggesting a causal relationship and encouraging trials of ammonia-lowering treatments for the management of severe PHG with a tendency to bleed.
1. Introduction
Portal hypertensive gastropathy (PHG) is a gastric mucosal anomaly commonly found in patients with portal hypertension [1]. It is more common and more aggressive in patients with cirrhosis than in those with portal hypertension due to noncirrhotic conditions [2].
The key element for the development of PHG is portal hypertension causing vascular congestion of the gastric mucosa with a resultant activation of cytokines, growth factors, and hormones that maintain and further the pathology which is characterized by capillary and venule dilatation, as well as congestion and tortuosity of the submucosal venules together with possible lamina propria stromal fibrosis and edema [3–5].
The endoscopic appearance is characterized by a mosaic or snake-skin-like appearance most commonly at the gastric body and fundus which may be associated with the presence of red spots [6].
PHG has been classified into mild and severe forms by different classification systems with the mild form in general having an appearance of mosaic-like pattern, scarlatiniform rash, superficial reddening, and snake-skin pattern, and the severe form displaying added red marks of variable diameter, with or without bleeding [7, 8]. The Baveno scoring system also classifies PHG into mild and severe using a score calculation that incorporates the presence or absence of gastric antral vascular ectasia (GAVE) [9]. This system has been found to be accurate in reflection of the bleeding risk from PHG among cirrhotic patients [10].
PHG is accountable for approximately 8% of nonvariceal upper gastrointestinal bleeding in hepatic patients [11]. Acute bleeding may infrequently complicate PHG especially when severe, yet chronic gastric mucosal bleeding leading to recurrent iron deficiency anemia is much more common and occurs in around 11–25% of cases [6, 7, 12, 13].
The major risk factors for PHG bleeding are increased duration, extent, and severity together with advanced cirrhosis, and eradication of esophageal varices by sclerotherapy or band ligation [3, 14].
Blood ammonia levels have been found to correlate well with the severity of liver disease and existence of portosystemic shunts, especially esophageal varices [15]. Accumulation of ammonia in splanchnic vessels in cases of liver function impairment results in vasodilatation and increased portal blood flow bringing about portal hypertension [16, 17]. There are indications that ammonia also affects endoplasmic reticulum dynamics and induces oxidative stress, both of which result in hepatic stellate cell (HSC) activation with production of multiple proinflammatory, contractile, and profibrogenic genes and proteins. A trial involving removal of ammonia in vitro, and lowering ammonia in vivo, has documented a downregulation of HSC activation markers with resulting reestablishment of HSC biology, suggesting that ammonia is a possible target for therapy directed at lowering portal hypertension [18].
PHG is a common manifestation of portal hypertension, and therefore, we aimed at finding out if blood ammonia levels correlate with the presence and severity of portal hypertensive gastropathy in cirrhotic patients.
2. Patients and Methods
This cross-sectional study was conducted on 381 cirrhotic patients positive for HCV or HBV attending the Tropical Medicine Endoscopy Unit, Tanta University, for endoscopic screening of esophageal varices. The study was conducted during the period from 7/1/2017 to 2/28/2018, 2018. The sample size of 381 was calculated based on a previous study by Kumar et al., who found the prevalence of portal hypertensive gastropathy in patients with cirrhosis to be 55%, with 5% precision and 95% confidence level [19]. Patients were excluded from the study if they had cardiac or kidney failure, hepatic encephalopathy or coma, active gastrointestinal bleeding, a history of bleeding during the previous two weeks to inclusion in the study, hepatocellular carcinoma, or portal vein thrombosis or were taking lactulose. Patients with factors affecting the portal pressure and gastric mucosal vascularity and blood flow such as those taking beta blockers and those for whom a previous intervention for varices (sclerotherapy, endoscopic band ligation, or scleroligation) or PHG (argon plasma coagulation or gastric mucosal band ligation) had been performed were also excluded.
A total of 381 patients were enrolled in the study. They were divided into three groups: (1) the portal hypertensive gastropathy group—203 patients with esophageal varices (EV) and PHG, (2) the esophageal varix group—41 patients with EV but no PHG, and (3) the control group—137 patients with no EV or PHG.
A detailed history was taken; a full clinical examination, routine laboratory tests, and pelvic-abdominal ultrasonography were performed for all participating patients after obtaining an informed written consent. Child-Pugh scores were calculated for each patient.
To overcome potential bias of results, study collaborators who were not involved in the upper endoscopy procedures screened and enrolled the participants, and all endoscopists were blinded to the study outcome.
2.1. Upper GI Endoscopy
Upper GI endoscopy was performed for all the patients. Classification of varices was performed according to the Japanese classification [20]. This involved recording of location (L), form (F), color (C), and red color signs (RC) of the varices. Classification and scoring of portal hypertensive gastropathy were performed according to the Baveno II scoring system; the mild mucosal mosaic pattern was given 1 point, and the severe pattern, 2 points. Isolated red markings were given 1 point, and confluent markings, 2. The presence of gastric antral vascular ectasia (GAVE) was noted and a further 2 points added to the score when flat or slightly raised, red stripes were seen radiating from the pylorus to the antrum and body of the stomach. PHG is considered mild when the score is ≤3 and severe at a score ≥ 4 [9].
2.2. Measurement of Blood Ammonia Level
A venous blood sample was collected from each patient after a minimum of 6 hours of fasting and 9 hours of abstinence from smoking. All patients had been instructed not to perform any physical exercise the day before.
The samples were taken at the laboratory, put in EDTA-containing sterile specimen tubes, and separated within 15 minutes of collection to be analyzed immediately. These precautions are necessary as the ammonia content of standing blood increases spontaneously due to generation and release of ammonia from red blood cells and due to deamination of amino acids by enzymes in the blood. Analysis was performed by the enzymatic method using reagents manufactured by BIOLABO SAS, Maizy, France.
2.3. Statistical Analysis
Statistical analysis was carried out using SPSS, version 15 for Microsoft Windows (SPSS, Chicago, Illinois, USA). The statistical data were reported as mean ± SD, frequencies (number), and percentages when appropriate. A comparison of numerical variables between the study groups was performed using one-way analysis of variance test followed by post hoc Tukey's multiple comparison test when data were normally distributed. Student's t-test was used to compare independent samples from two groups when the samples were normally distributed. Pearson's correlation was used to quantify the association between continuous variables. To compare categorical data, the χ2 test was performed. P values less than 0.05 (two-tailed) were considered statistically significant.
2.4. Ethical Considerations
All participating subjects provided written informed consents. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. The study was approved by the Ethics Committee of the Faculty of Medicine, Tanta University (approval code 31588/06/17).
3. Results
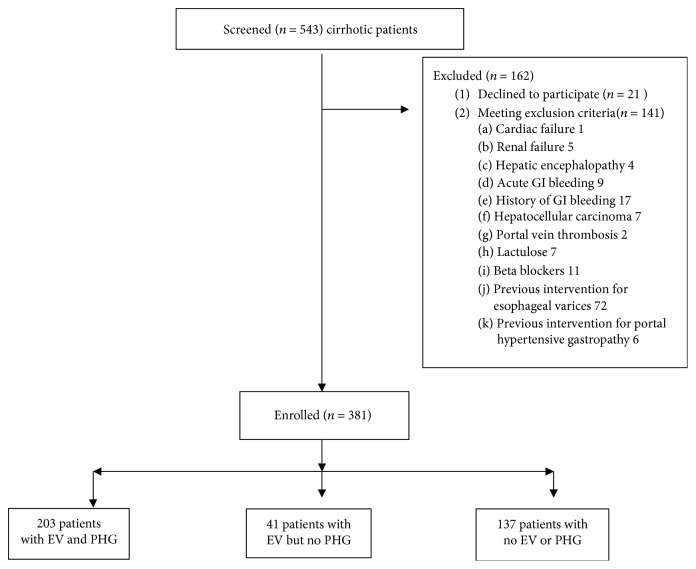
In total, 543 cirrhotic patients attending the Tropical Medicine Endoscopy Unit, Tanta University, were screened for participation in this study. 162 were excluded due to the presence of exclusion criteria in 141 patients and refusal to participate by 21. Thus, 381 cirrhotic patients were enrolled in this cross-sectional study; they were 258 males (67.7%) and 123 females (32.3%) (Figure 1). Testing for HCV RNA was positive for 377, whereas only 4 patients were HBV DNA positive. The participants were divided into three groups with no significant differences regarding distribution of age, sex, or Child-Pugh class: (1) the portal hypertensive gastropathy group—203 (53.28%) patients with EV and PHG, (2) the esophageal varices group—41 (10.76%) patients with EV but no PHG, and (3) the control group—137 (35.96%) patients with neither EV nor PHG (Table 1).
Figure 1.
Study analysis population. n: number of patients; EV: esophageal varices; PHG: portal hypertensive gastropathy.
Table 1.
Patient baseline characteristics.
| Parameter | PHG (n = 203) | EV (n = 41) | Controls (n = 137) | P value |
|---|---|---|---|---|
| Age, years (mean, SD) | 53.57 ± 6.61 | 55 ± 8.72 | 52.33 ± 11.06 | 0.923 |
| Sex: male, n (%) | 142 (69.95) | 31 (75.61) | 85 (62.04) | 0.456 |
| Etiology of cirrhosis, n (%) | ||||
| Hepatitis C virus | 199 (98.03) | 41 (100) | 137 (100) | 0.471 |
| Hepatitis B virus | 4 (1.97) | 0 (0) | 0(0) | |
| Ascites, n (%) | 90 (44.33) | 17 (41.46) | 39 (28.47) | 0.063 |
| History of hepatic encephalopathy, n (%) | 28 (13.79) | 4 (9.76) | 6 (4.38) | 0.089 |
| Hemoglobin (g/dL) | 11.15 ± 1.81 | 10.99 ± 1.52 | 11.31 ± 2.53 | 0.726 |
| WBCs (×109/L) | 5.23 ± 2.22 | 5.15 ± 2.09 | 5.93 ± 2.60 | 0.180 |
| Platelets (×109/L) | 101.70 ± 31.34a | 100 ± 30.61c | 125.54 ± 36.25 | <0.001∗ |
| ALT (U/L) | 43.34 ± 17.49 | 35.54 ± 15.69 | 39.28 ± 23.34 | 0.129 |
| AST (U/L) | 62.20 ± 26.62 | 53.34 ± 30.71 | 56.64 ± 29.67 | 0.308 |
| Serum albumin (g/dL) | 3.03 ± 0.56 | 3.05 ± 0.42 | 3.24 ± 0.61 | 0.086 |
| Total serum bilirubin (mg/dL) | 1.83 ± 1.24a,b | 1.29 ± 0.55 | 1.31 ± 0.92 | 0.007∗ |
| International normalized ratio | 1.29 ± 0.24 | 1.22 ± 0.19 | 1.24 ± 0.30 | 0.449 |
| Creatinine (mg/dL) | 0.97 ± 0.21 | 0.94 ± 0.27 | 0.96 ± 0.24 | 0.781 |
| Blood ammonia (μg/dL) | 162.12 ± 58.17a,b | 153.04 ± 59.41c | 103.23 ± 72.76 | <0.001∗ |
| Child-Pugh class, n (%) | ||||
| Class A | 97 (47.79) | 22 (53.66) | 77 (56.20) | 0.067 |
| Class B | 66 (32.51) | 17 (41.46) | 44 (32.12) | |
| Class C | 40 (19.70) | 2 (4.88) | 16 (11.68) | |
| Portal vein diameter (mm) | 13.64 ± 1.81a | 13.42 ± 1.49c | 11.75 ± 2.11 | <0.001∗ |
| Splenic longitudinal diameter (cm) | 15.84 ± 1.69a | 15.75 ± 1.74c | 14.28 ± 2.65 | <0.001∗ |
| Splenic vein diameter (mm) | 11.19 ± 1.20a | 11.10 ± 0.92c | 8.55 ± 1.91 | <0.001∗ |
| Esophageal varices, n (%) | 203 (100) | 41 (100) | — | — |
∗Statistically significant at P < 0.05; aP < 0.05 when compared with controls; bP < 0.05 when compared with esophageal varices; cP < 0.05 when compared with controls. ALT: alanine aminotransferase; AST: aspartate aminotransferase: WBCs, white blood cells; EV: esophageal varices; PHG: portal hypertensive gastropathy.
Patient baseline characteristics and demographic data are presented in Table 1. Platelet counts were significantly lower among patients with EV and PHG as well as those with EV only than in controls (P < 0.001). We found the group with EV and PHG to have significantly higher serum bilirubin levels than either controls or patients with EV only (P = 0.007) (Table 1).
Blood ammonia was significantly higher among patients with EV than controls and among patients with EV and PHG than among those with EV only as well as controls (P = 0.001) (Table 1).
The portal vein, splenic vein, and splenic longitudinal diameters were significantly higher in patients with EV and PHG as well as those with EV only than in controls (P < 0.001) (Table 1).
By using the Baveno II PHG scoring system to classify our 203 patients with PHG, we found that 149 (73.4%) had mild PHG (score ≤ 3) and 54 (26.6%) had severe PHG (score ≥ 4) (Table 2).
Table 2.
Relationship of PHG severity with demographic, clinical, and endoscopic characteristics.
| Parameter | Portal hypertensive gastropathy severity (n = 203) | P value | |||
|---|---|---|---|---|---|
| Mild (n = 149) |
Severe (n = 54) |
||||
| n | (%) | n | (%) | ||
| Sex | |||||
| Male | 105 | 70.47 | 37 | 68.52 | 0.965 |
| Female | 44 | 29.53 | 17 | 31.48 | |
| Child-Pugh class | |||||
| Class A | 76 | 51.01 | 21 | 38.89 | 0.539 |
| Class B | 41 | 27.52 | 25 | 46.29 | |
| Class C | 32 | 21.47 | 8 | 14.82 | |
| EV grading | |||||
| Grade 1 | 56 | 37.58 | 25 | 46.29 | 0.002∗ |
| Grade 2 | 68 | 45.64 | 13 | 24.07 | |
| Grade 3 | 25 | 16.78 | 12 | 22.22 | |
| Grade 4 | 0 | 0.00 | 4 | 7.41 | |
| EV red color sign (RC) | |||||
| RC0 (none) | 84 | 56.38 | 38 | 70.37 | 0.063 |
| HCS (hematocystic spots) | 20 | 13.42 | 0 | 0.00 | |
| CRS (cherry red spots) | 8 | 5.37 | 4 | 7.41 | |
| RWM (red wale markings) | 37 | 24.83 | 12 | 22.22 | |
| EV form (F) | |||||
| F1 | 84 | 56.38 | 29 | 53.70 | 0.973 |
| F2 | 44 | 29.53 | 16 | 29.63 | |
| F3 | 21 | 14.09 | 9 | 16.67 | |
| Risk signs | |||||
| No | 93 | 62.42 | 38 | 70.37 | 0.578 |
| Yes | 56 | 37.58 | 16 | 29.63 | |
| EV location (L) | |||||
| LI (inferior) | 96 | 64.43 | 25 | 46.29 | 0.013∗ |
| LM (medialis) | 48 | 32.22 | 21 | 38.89 | |
| LS (superior) | 5 | 3.36 | 8 | 14.82 | |
| EV color (C) | |||||
| Cw (white varices) | 101 | 67.78 | 41 | 75.93 | 0.535 |
| Cb (blue varices) | 48 | 32.22 | 13 | 24.07 | |
| PHG mosaic pattern | |||||
| No | 20 | 13.42 | 0 | 0.00 | <0.001∗ |
| Mild | 121 | 81.21 | 16 | 29.63 | |
| Severe | 8 | 5.37 | 38 | 70.37 | |
| PHG red markings | |||||
| No | 121 | 81.21 | 8 | 14.82 | <0.001∗ |
| Isolated | 28 | 18.79 | 13 | 24.07 | |
| Confluent | 0 | 0.00 | 33 | 61.11 | |
| GAVE | |||||
| No | 133 | 89.26 | 46 | 85.19 | 0.729 |
| Yes | 16 | 10.74 | 8 | 14.81 | |
∗Statistically significant at P < 0.05. EV: esophageal varices; PHG: portal hypertensive gastropathy; GAVE: gastric antral vascular ectasia.
There was a significant difference between mild and severe PHG patients regarding the grading of the accompanying EV (P = 0.002), with a higher percentage of EV grades 1, 3, and 4 among the severe cases. Of interest was the absence of grade 4 EV among the patients with mild PHG (Table 2).
A significant difference in EV location was noticed between mild and severe PHG patients (P = 0.013), with severe cases having higher percentages of association with locus superior EV than their counterparts with mild PHG (14.82% versus 3.36%, resp.) (Table 2).
We compared between mild and severe cases of PHG with regard to multiple variables and found no difference between them in respect to patient age, blood count, liver function tests, Child-Pugh scores, or portal vein diameter. On the other hand, cases with severe PHG had significantly higher levels of blood ammonia (192.69 ± 59.87 versus 151.39 ± 54.37 μg/dL) (P = 0.026) and significantly lower splenic longitudinal diameters (P = 0.001) (Table 3).
Table 3.
Relationship of portal hypertensive gastropathy severity with different variables.
| Parameter | Portal hypertensive gastropathy severity (n = 203) | P value | |
|---|---|---|---|
| Mild (n = 149) |
Severe (n = 54) |
||
| Mean ± SD | Mean ± SD | ||
| Age, years | 53.43 ± 7.71 | 58.00 ± 8.15 | 0.076 |
| Hemoglobin (g/dL) | 10.86 ± 1.62 | 11.97 ± 2.11 | 0.055 |
| Platelets (×109/L) | 103.68 ± 32.44 | 96.08 ± 28.44 | 0.458 |
| WBCs (×109/L) | 5.05 ± 2.22 | 5.74 ± 2.23 | 0.341 |
| AST (U/L) | 63.59 ± 29.48 | 58.23 ± 16.26 | 0.538 |
| ALT (U/L) | 43.16 ± 17.54 | 43.85 ± 18.03 | 0.905 |
| Serum albumin (g/dL) | 3.06 ± 0.59 | 2.95 ± 0.52 | 0.578 |
| Total serum bilirubin (mg/dL) | 1.92 ± 1.35 | 1.59 ± 0.89 | 0.413 |
| International normalized ratio | 1.33 ± 0.26 | 1.18 ± 0.17 | 0.056 |
| Creatinine (mg/dL) | 0.98 ± 0.20 | 0.95 ± 0.26 | 0.672 |
| Child-Pugh score | 7.19 ± 2.17 | 7.31 ± 1.93 | 0.863 |
| Blood ammonia (μg/dL) | 151.39 ± 54.37 | 192.69 ± 59.87 | 0.026∗ |
| Portal vein diameter (mm) | 13.85 ± 1.68 | 13.07 ± 2.11 | 0.184 |
| Splenic longitudinal diameter (cm) | 16.31 ± 1.59 | 14.51 ± 1.26 | 0.001∗ |
| Splenic vein diameter (mm) | 11.39 ± 1.08 | 10.64 ± 1.39 | 0.052 |
∗Statistically significant at P < 0.05. ALT: alanine aminotransferase; AST: aspartate aminotransferase; WBCs: white blood cells.
The PHG score itself was significantly higher with higher locations of the varices (P = 0.046) (Table 4).
Table 4.
Relationship of portal hypertensive gastropathy score with demographic, clinical, and endoscopic characteristics.
| Parameter | Portal hypertensive gastropathy score (n = 203) | P value | |
|---|---|---|---|
| n | Mean ± SD | ||
| Sex | |||
| Male | 142 | 1.89 ± 1.05 | 0.954 |
| Female | 61 | 1.87 ± 1.13 | |
| Hepatitis C virus | |||
| No | 4 | 1.00 ± 0.83 | 0.408 |
| Yes | 199 | 1.89 ± 1.07 | |
| Hepatitis B virus | |||
| No | 199 | 1.89 ± 1.08 | 0.611 |
| Yes | 4 | 1.50 ± 0.71 | |
| Ascites | |||
| No | 133 | 1.75 ± 1.04 | 0.334 |
| Yes | 90 | 2.05 ± 1.09 | |
| History of hepatic encephalopathy | |||
| No | 175 | 1.84 ± 1.02 | 0.486 |
| Yes | 28 | 2.14 ± 1.35 | |
| Child-Pugh class | |||
| Class A | 97 | 1.75 ± 0.99 | 0.716 |
| Class B | 66 | 2.00 ± 1.27 | |
| Class C | 40 | 2.00 ± 0.94 | |
| EV grading | |||
| Grade 1 | 81 | 1.85 ± 1.27 | 0.616 |
| Grade 2 | 81 | 1.75 ± 0.85 | |
| Grade 3 | 37 | 2.11 ± 1.05 | |
| Grade 4 | 4 | 3.00 ± 0.95 | |
| EV red color sign (RC) | |||
| RC0 (none) | 122 | 1.93 ± 1.17 | 0.912 |
| HCS (hematocystic spots) | 20 | 1.60 ± 0.55 | |
| CRS (cherry red spots) | 12 | 1.67 ± 1.16 | |
| RWM (red wale markings) | 49 | 1.92 ± 0.99 | |
| EV form (F) | |||
| F1 | 113 | 1.82 ± 1.19 | 0.780 |
| F2 | 60 | 1.87 ± 0.83 | |
| F3 | 30 | 2.14 ± 1.07 | |
| EV risk signs | |||
| No | 131 | 1.88 ± 1.16 | 0.965 |
| Yes | 72 | 1.89 ± 0.90 | |
| EV location (L) | |||
| LI (inferior) | 121 | 1.77 ± 0.94 | 0.046∗ |
| LM (medialis) | 69 | 1.82 ± 1.13 | |
| LS (superior) | 13 | 3.33 ± 1.16 | |
| EV color (C) | |||
| Cw (white varices) | 142 | 1.89 ± 1.13 | 0.954 |
| Cb (blue varices) | 61 | 1.87 ± 0.92 | |
∗Statistically significant at P < 0.05. EV: esophageal varices; GAVE: gastric antral vascular ectasia.
We further studied the correlation between the PHG score and differential variables which revealed a significant positive correlation with blood ammonia and hemoglobin levels, and a significant negative correlation with the splenic longitudinal diameter (P < 0.05) (Table 5).
Table 5.
Correlation between portal hypertensive gastropathy score and different variables.
| Parameter | Portal hypertensive gastropathy score (n = 203) |
|
|---|---|---|
| R | P value | |
| Age (years) | 0.187 | 0.194 |
| Hemoglobin (g/dL) | 0.325 | 0.021∗ |
| Platelets (×109/L) | −0.127 | 0.378 |
| WBCs (×109/L) | 0.114 | 0.431 |
| AST (U/L) | −0.066 | 0.651 |
| ALT (U/L) | −0.014 | 0.922 |
| Serum albumin (g/dL) | −0.072 | 0.620 |
| Total serum bilirubin (mg/dL) | −0.080 | 0.581 |
| International normalized ratio (INR) | −0.137 | 0.342 |
| Creatinine (mg/dL) | 0.086 | 0.555 |
| Child-Pugh score | −0.228 | 0.111 |
| Blood ammonia (μg/dL) | 0.295 | 0.037∗ |
| Portal vein diameter (mm) | −0.142 | 0.325 |
| Splenic longitudinal diameter (cm) | −0.383 | 0.006∗ |
| Splenic vein diameter (mm) | −0.233 | 0.104 |
∗Statistically significant at P < 0.05. WBCs: white blood cells; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
4. Discussion
Portal hypertensive gastropathy (PHG) is a common gastric mucosal anomaly in patients with cirrhosis and portal hypertension [1]. The condition carries a potential risk of acute and chronic gastrointestinal bleeding among these patients [13]. Evidence suggests that the key factor for the development of PHG is portal hypertension [3].
Increased blood ammonia in cases of liver function impairment is a pathogenetic factor for the production of portal hypertension and has been suggested as a possible target for portal hypertension lowering therapy [16–18]. In the light of this data, we aimed at finding out if blood ammonia levels correlate with severity of portal hypertensive gastropathy in cirrhotic patients.
Platelet counts were significantly lower among patients with EV and PHG as well as those with EV alone than in controls (P < 0.001). It has been found by other recent studies that low platelet counts are associated with the presence of varices in viral cirrhotic patients [21–23].
Patients with EV and PHG in our study were found to have significantly higher serum bilirubin levels than either controls or patients with EV only (P = 0.007) indicating that the presence of PHG is related to severity of the hepatic disease. This finding is similar to that of the HALT-C Trial in 2006, which recorded that bilirubin was significantly higher among PHG patients than those without [24]. However, contrary to the HALT-C Trial, we did not record any significant worsening in albumin or INR levels.
Several authors have found a correlation between the presence of PHG and Child-Pugh stage [2, 6, 25]. In this study, there were no significant differences in Child class between the cases with or without PHG. This is congruent with the results of Spina et al., Primignani et al., Merkel et al., and Bellis et al. [8, 13, 26, 27].
Blood ammonia was significantly higher among patients with EV than controls (P = 0.001). Other studies have found this relation between the presence of varices and elevated blood ammonia and even suggested a blood ammonia cutoff value for prediction of variceal presence [15, 28–30]. This can be explained by the role ammonia plays in production of portal hypertension and hence development of varices [18].
Blood ammonia levels were significantly higher among patients with EV and PHG (162.12 ± 58.17 μg/dL) than among those with EV but no PHG as well as the cirrhotic controls (153.04 ± 59.41 and 103.23 ± 72.76 μg/dL, resp.) (P = 0.001). It is known that PHG pathogenesis is associated with a hyperdynamic state brought about by portal hypertension in which there is an increased splanchnic and total gastric blood flow and most probably a decreased gastric mucosal blood flow [31]. Ammonia has been postulated to be part of a vicious circle in which elevation of ammonia results from the presence of portal hypertension and portosystemic collaterals and then leads to a further increase in portal hypertension [32]. It has also been suggested that PHG is associated with increased portal hypertension [24]. This could explain the higher levels of ammonia in cases with both PHG and EV and suggests a causal relationship.
The portal vein, splenic vein, and splenic longitudinal diameters were significantly higher in patients with EV and PHG as well as those with EV only than in controls (P < 0.001), which is expected as these are indicators of raised portal pressure; however, we found no difference in these parameters between patients having PHG and those without PHG in contrary to the HALT-C Trial [24]. Zardi et al. concluded their study in 2015 with there being no relation between splenic vein diameter and PHG [25].
By using the Baveno II PHG scoring system to classify our 203 patients with PHG, we found that 149 (73.4%) had mild PHG (score ≤ 3) and 54 (26.6%) had severe PHG (score ≥ 4). This more frequent pattern of mild PHG is consistent with the recorded prevalence range of mild PHG (29%–57%) and of severe PHG (9%–46%) among patients with portal hypertension [1].
There was no difference between mild or severe PHG regarding Child-Pugh class as also recorded by Abbasi et al. and Primignani et al. [13, 33]. Esophageal varix form, color, red color signs, or presence of risky bleeding signs were also not significantly different between the two PHG forms. This is the opposite to the findings of Taranto et al. who reported that PHG severity was closely related to the hemorrhagic risk of esophageal varices and Child-Pugh criteria [34].
A significant difference was recorded between mild and severe PHG patients regarding the grading of the accompanying EV (P = 0.002), with a higher percentage of large EV (grades 3 and 4) with the severe form of PHG than with the mild form (29.9% versus 16.78%). Of interest was the absence of grade 4 EV among the patients with mild PHG pointing to the relationship between severity of both PHG and portal hypertension and the similar mechanism for development of EV and PHG in response to increased portal pressure. Multiple previous studies have also reached this same interpretation which is supported by the strong correlation between severity of PHG and hepatic venous pressure gradient (HVPG) [13, 24, 26, 33, 35]. Kim et al. in their study on 331 cirrhotic patients noted that patients with severe PHG had significantly raised HVPG than those with either mild or no gastropathy [35].
Regarding the location of the EV (P = 0.013), severe PHG cases had higher percentages of association with locus superior EV than their counterparts with mild PHG (14.82% versus 3.36%, resp.) (P = 0.013), and the PHG score itself was significantly higher with higher locations of the varices (P = 0.046), a further indication of the relation with increased portal pressure.
Severe PHG can cause acute or chronic bleeding in cirrhotic patients, yet we found no significant decrease in hemoglobin levels among patients with severe PHG. The reason may be the exclusion of patients with active gastrointestinal bleeding or a history of bleeding during the previous two weeks to the study to eliminate the effect on blood ammonia levels.
Child-Pugh class and liver function tests were no different statistically in cases with severe PHG than in those with mild PHG indicating that worsening and progression of the condition is not affected by worsening of the liver condition. Primignani et al. also reported an unexpected low prevalence of severe gastropathy in patients with Child C class [13]. Taranto et al. in 1994 concluded that the severity of the mosaic-like appearance correlated with the severity of liver dysfunction yet made no such conclusion regarding the presence or appearance of the gastric mucosal red spots [34].
Cases with severe PHG had significantly higher levels of blood ammonia than those with mild PHG (192.69 ± 59.87 μg/dL versus 151.39 ± 54.37 μg/dL, resp.) (P = 0.026). We recorded a significant positive correlation between PHG scores and blood ammonia levels (P = 0.037). Increased ammonia in splanchnic vessels in cases of liver function impairment results in vasodilatation and increased portal blood flow bringing about portal hypertension [16, 17]. The increased levels may also cause such an increase in the gastric blood flow aggravating the vascular congestion characteristic of PHG and provoking cytokines, growth factors, and hormones to further worsen the condition. Ammonia is also known to affect endoplasmic reticulum dynamics and induce oxidative stress [18]. To our knowledge, no other studies have been performed to study the relationship between ammonia levels and severity of PHG; therefore, we could not compare our results with any others.
Significantly lower splenic longitudinal diameters were recorded among our patients with severe PHG than those with mild PHG (P = 0.001). This is in disagreement with the results recorded by Kim et al. whose patients with severe PHG had higher splenic diameters than those with mild PHG or no PHG [35].
A significantly negative correlation of the PHG score was found with the splenic longitudinal diameter (P = 0.006). This may be due to the effect of factors other than portal hypertension that maintain and further the pathology of PHG such as cytokines, growth factors, and hormones [3–5]. Even though the development of PHG is related to the presence of portal hypertension, there is generally no direct correlation with the level of the portal pressure [36].
Another study from Egypt by Nashaat et al. found that mean splenic bipolar diameter (MSBD) showed a significantly positive correlation with the presence of OV but not with PHG [37].
There is controversy regarding the correlation of splenic size with portal pressure as Nedredal et al. [38] state that spleen size does not correlate well with portal venous pressure in contrary to splenic stiffness which may be another explanation for our finding.
We recorded an unexpected significant positive correlation between PHG scores and serum hemoglobin levels; this may be related to the lower spleen size and therefore to lower phagocytic activity resulting in a decreased incidence of anemia among our patients with severe PHG having no present or recent related gastric mucosal bleeding.
The limitation of this study was the absence of a patient group with portal hypertensive gastropathy and no esophageal varices.
5. Conclusions
Blood ammonia levels correlate with the presence, severity, and score of portal hypertensive gastropathy in cirrhosis suggesting a causal relationship and encouraging trials of ammonia-lowering treatments for the management of severe PHG with a tendency to bleed.
Data Availability
The authors' institution does not allow public data access.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Thuluvath P. J., Yoo H. Y. Portal hypertensive gastropathy. The American Journal of Gastroenterology. 2002;97(12):2973–2978. doi: 10.1111/j.1572-0241.2002.07094.x. [DOI] [PubMed] [Google Scholar]
- 2.Sarin S. K., Sreenivas D. V., Lahoti D., Saraya A. Factors influencing development of portal hypertensive gastropathy in patients with portal hypertension. Gastroenterology. 1992;102(3):994–999. doi: 10.1016/0016-5085(92)90188-5. [DOI] [PubMed] [Google Scholar]
- 3.Cubillas R., Rockey D. C. Portal hypertensive gastropathy: a review. Liver International. 2010;30(8):1094–1102. doi: 10.1111/j.1478-3231.2010.02286.x. [DOI] [PubMed] [Google Scholar]
- 4.De B. K., Das T. K. Color Doppler and laser velocimetry studies in the assessment of portal hemodynamics and severity of chronic liver disease. Indian Journal of Gastroenterology. 2002;21(5):173–175. [PubMed] [Google Scholar]
- 5.Ohta M., Yamaguchi S., Gotoh N., Tomikawa M. Pathogenesis of portal hypertensive gastropathy: a clinical and experimental review. Surgery. 2002;131(1):S165–S170. doi: 10.1067/msy.2002.119499. [DOI] [PubMed] [Google Scholar]
- 6.Merli M., Nicolini G., Angeloni S., Gentili F., Attili A. F., Riggio O. The natural history of portal hypertensive gastropathy in patients with liver cirrhosis and mild portal hypertension. The American Journal of Gastroenterology. 2004;99(10):1959–1965. doi: 10.1111/j.1572-0241.2004.40246.x. [DOI] [PubMed] [Google Scholar]
- 7.McCormack T. T., Sims J., Eyre-Brook I., et al. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26(11):1226–1232. doi: 10.1136/gut.26.11.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spina G. P., Arcidiacono R., Bosch J., et al. Gastric endoscopic features in portal hypertension: final report of a consensus conference, Milan, Italy, September 19, 1992. Journal of Hepatology. 1994;21(3):461–467. doi: 10.1016/S0168-8278(05)80329-0. [DOI] [PubMed] [Google Scholar]
- 9.Sarin S. K. Diagnostic issues: portal hypertensive gastropathy and gastric varices. In: DeFranchis R., editor. Portal Hypertension II. Proceedings of the Second Baveno International Consensus Workshop on Definitions, Methodology and Therapeutic Strategies; 1996; Baveno, Italy. pp. 30–54. [Google Scholar]
- 10.Stewart C. A., Sanyal A. J. Grading portal gastropathy: validation of a gastropathy scoring system. The American Journal of Gastroenterology. 2003;98(8):1758–1765. doi: 10.1111/j.1572-0241.2003.07595.x. [DOI] [PubMed] [Google Scholar]
- 11.Gostout C. J., Viggiano T. R., Balm R. K. Acute gastrointestinal bleeding from portal hypertensive gastropathy: prevalence and clinical features. The American Journal of Gastroenterology. 1993;88(12):2030–2033. [PubMed] [Google Scholar]
- 12.D'Amico G., Montalbano L., Traina M., et al. Natural history of congestive gastropathy in cirrhosis. Gastroenterology. 1990;99(6):1558–1564. doi: 10.1016/0016-5085(90)90458-D. [DOI] [PubMed] [Google Scholar]
- 13.Primignani M., Carpinelli L., Preatoni P., et al. Natural history of portal hypertensive gastropathy in patients with liver cirrhosis. Gastroenterology. 2000;119(1):181–187. doi: 10.1053/gast.2000.8555. [DOI] [PubMed] [Google Scholar]
- 14.Sarin S. K., Shahi H. M., Jain M., Jain A. K., Issar S. K., Murthy N. S. The natural history of portal hypertensive gastropathy: influence of variceal eradication. The American Journal of Gastroenterology. 2000;95(10):2888–2893. doi: 10.1111/j.1572-0241.2000.03200.x. [DOI] [PubMed] [Google Scholar]
- 15.Tarantino G., Citro V., Esposito P., et al. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterology. 2009;9(1, article 21) doi: 10.1186/1471-230X-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benoit J. N., Barrowman J. A., Harper S. L., Kvietys P. R., Granger D. N. Role of humoral factors in the intestinal hyperemia associated with chronic portal hypertension. American Journal of Physiology-Gastrointestinal and Liver Physiology. 1984;247(5):G486–G493. doi: 10.1152/ajpgi.1984.247.5.G486. [DOI] [PubMed] [Google Scholar]
- 17.Benoit J. N., Granger D. N. Splanchnic hemodynamics in chronic portal hypertension. Seminars in Liver Disease. 1986;6(04):287–298. doi: 10.1055/s-2008-1040611. [DOI] [PubMed] [Google Scholar]
- 18.Jalan R., de Chiara F., Balasubramaniyan V., et al. Ammonia produces pathological changes in human hepatic stellate cells and is a target for therapy of portal hypertension. Journal of Hepatology. 2016;64(4):823–833. doi: 10.1016/j.jhep.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A., Mishra S. R., Sharma P., Sharma B. C., Sarin S. K. Clinical, laboratory, and hemodynamic parameters in portal hypertensive gastropathy: a study of 254 cirrhotics. Journal of Clinical Gastroenterology. 2010;44(4):294–300. doi: 10.1097/MCG.0b013e3181b37ea1. [DOI] [PubMed] [Google Scholar]
- 20.Idezuki Y., Japanese Research Society for Portal Hypertension General rules for recording endoscopic findings of esophagogastric varices (1991) World Journal of Surgery. 1995;19(3):420–422. doi: 10.1007/BF00299178. [DOI] [PubMed] [Google Scholar]
- 21.Schepis F., Cammà C., Niceforo D., et al. Which patients with cirrhosis should undergo endoscopic screening for esophageal varices detection? Hepatology. 2001;33(2):333–338. doi: 10.1053/jhep.2001.21410. [DOI] [PubMed] [Google Scholar]
- 22.Nada L., Samira E. F., Bahija B., Adil I., Nourdine A. Noninvasive predictors of presence and grade of esophageal varices in viral cirrhotic patients. The Pan African Medical Journal. 2015;20:p. 145. doi: 10.11604/pamj.2015.20.145.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abd-Elsalam S., Habba E., Elkhalawany W., et al. Correlation of platelets count with endoscopic findings in a cohort of Egyptian patients with liver cirrhosis. Medicine. 2016;95(23, article e3853) doi: 10.1097/MD.0000000000003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana R. J., Sanyal A. J., Mehta S., et al. Portal hypertensive gastropathy in chronic hepatitis C patients with bridging fibrosis and compensated cirrhosis: results from the HALT-C Trial. The American Journal of Gastroenterology. 2006;101(5):983–992. doi: 10.1111/j.1572-0241.2006.00461.x. [DOI] [PubMed] [Google Scholar]
- 25.Zardi E. M., Ghittoni G., Margiotta D., Viera F. T., di Matteo F., Rossi S. Portal hypertensive gastropathy in cirrhotics without varices: a case-control study. European Journal of Gastroenterology & Hepatology. 2015;27(1):91–96. doi: 10.1097/MEG.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 26.Merkel C., Schipilliti M., Bighin R., et al. Portal hypertension and portal hypertensive gastropathy in patients with liver cirrhosis: a haemodynamic study. Digestive and Liver Disease. 2003;35(4):269–274. doi: 10.1016/S1590-8658(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 27.Bellis L., Nicodemo S., Galossi A., et al. Hepatic venous pressure gradient does not correlate with the presence and the severity of portal hypertensive gastropathy in patients with liver cirrhosis. Journal of Gastrointestinal and Liver Diseases. 2007;16(3):273–277. [PubMed] [Google Scholar]
- 28.El-Hefny N., Hareedy M., Abdel-Aal R. Blood ammonia level and other non invasive parameters as indicators of portosystemic collaterals in cirrhotic patients. Thesis submitted for partial fulfillment of MSC degree in internal medicine. Assiut University; 2013. [Google Scholar]
- 29.Montasser M. F., Abdella H. M., Samy A. H. Evaluation of venous ammonia level, splenic longitudinal diameter, portal vein and splenic vein diameters as non-invasive indicators for the presence of portosystemic collaterals in Egyptian cirrhotic patients. Open Journal of Gastroenterology. 2014;04(06):265–274. doi: 10.4236/ojgas.2014.46039. [DOI] [Google Scholar]
- 30.Ali A. A., Badawy A. M., Sonbol A. A., Ayad M. E. Study of the relationship between blood ammonia level and esophageal varices in patients with liver cirrhosis. Afro-Egyptian Journal of Infectious and Endemic Diseases. 2015;5:78–85. [Google Scholar]
- 31.Gjeorgjievski M., Cappell M. S. Portal hypertensive gastropathy: a systematic review of the pathophysiology, clinical presentation, natural history and therapy. World Journal of Hepatology. 2016;8(4):231–262. doi: 10.4254/wjh.v8.i4.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bode J. G., Peters-Regehr T., Gressner A. M., Häussinger D. De novo expression of glutamine synthetase during transformation of hepatic stellate cells into myofibroblast-like cells. The Biochemical Journal. 1998;335(3):697–700. doi: 10.1042/bj3350697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbasi A., Bhutto A. R., Butt N., Munir S. M., Dhillo A. K. Frequency of portal hypertensive gastropathy and its relationship with biochemical, haematological and endoscopic features in cirrhosis. Journal of the College of Physicians and Surgeons–Pakistan. 2011;21(12):723–726. [PubMed] [Google Scholar]
- 34.Taranto D., Suozzo R., Romano M., et al. Gastric endoscopic features in patients with liver cirrhosis: correlation with esophageal varices, intra-variceal pressure, and liver dysfunction. Digestion. 2004;55(2):115–120. doi: 10.1159/000201135. [DOI] [PubMed] [Google Scholar]
- 35.Kim M. Y., Choi H., Baik S. K., et al. Portal hypertensive gastropathy: correlation with portal hypertension and prognosis in cirrhosis. Digestive Diseases and Sciences. 2010;55(12):3561–3567. doi: 10.1007/s10620-010-1221-6. [DOI] [PubMed] [Google Scholar]
- 36.Wong F. Portal hypertensive gastropathy. Gastroenterology & Hepatology. 2007;3(6):428–473. [PMC free article] [PubMed] [Google Scholar]
- 37.Nashaat E. H., Abd- Elaziz H., Sabry M., Ibrahim A. A. Non-endoscopic predictors of esophageal varices and portal hypertensive gastropathy. Nature and Science. 2010;8(6):43–50. [Google Scholar]
- 38.Nedredal G. I., Yin M., McKenzie T., et al. Portal hypertension correlates with splenic stiffness as measured with MR elastography. Journal of Magnetic Resonance Imaging. 2011;34(1):79–87. doi: 10.1002/jmri.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors' institution does not allow public data access.