Abstract
BACKGROUND
Subclinical inflammation induced by persistent exposure to lipopolysaccharide (LPS) is found in some clinical conditions such as obesity or diabetes. This study aimed to investigate the effect of recurrent LPS exposure on inflammatory markers, oxidative stress balance and cardiac and renal fibrosis in male rats.
METHODS
Male Wistar rats were divided into control and LPS-treated. LPS (10 mg/kg/week) was injected intraperitoneally. After 4 weeks, left ventricles and kidneys were homogenized and stained with hematoxylin and eosin (H&E) and Masson trichrome for histological examination. Serum levels of nitrite, interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α) were measured and total thiol, malondialdehyde (MDA), superoxide dismutase (SOD) and catalase were evaluated in the heart and kidney homogenates.
RESULTS
Serum inflammatory markers were higher in LPS group than control (nitrite: 37.0 ± 2.2 vs. 25.5 ± 1.9 µmol/l; IL-6: 84 ± 3 vs. 98.0 ± 4.4 pg/ml; TNF-α: 75.5 ± 4.9 vs. 85.3 ± 4.7 pg/ml; respectively, P < 0.050). Evaluation of total thiol concentration (heart: 10.0 ± 0.9 vs. 22.5 ± 1.2; kidney: 7.0 ± 0.5 vs. 27.8 ± 3.1 nmol/g tissue, respectively), catalase (heart: 0.18 ± 0.03 vs. 0.66 ± 0.04; kidney: 0.17 ± 0.03 vs. 0.73 ± 0.03, U/g tissue, respectively) and SOD (heart: 8.01 ± 0.70 vs. 12.3 ± 0.4; kidney: 7.02 ± 0.60 vs. 12.0 ± 0.2, U/g tissue, respectively) showed lower levels in LPS-treated group compared to control; while MDA concentration in LPS group was higher than control (P < 0.05). Histopathological examination in LPS-treated group indicated infiltration of inflammatory cells and more collagen deposition in left ventricle wall and kidney compared to control group.
CONCLUSION
We concluded that in clinical conditions with chronic LPS, cardiac and renal fibrosis occurs even in absence of preceding tissue injury due to imbalances in oxidative stress.
Keywords: Inflammation, Lipopolysaccharide, Oxidative Stress, Heart, Kidney
Introduction
Inflammation is a part of complex biological and adaptive immune response of body tissues to harmful stimuli such as irritation that plays a central role in metabolism in a variety of organisms.1 Inflammatory abnormalities are demonstrated in allergic reactions and some pathological conditions. Moreover, in some diseases including cancer, obesity, atherosclerosis, and ischemic heart disease, inflammatory processes are the origin of etiology.2-5
Inflammation is classified as acute or chronic. Acute inflammation is a short-term process, appears within a few minutes or hours to remove injurious stimulus.6 Chronic inflammation can be caused by excessive calorie consumption, elevated blood glucose levels, and oxidative stress. The danger of chronic low grade inflammation is its silent nature with destructive power and characterized by increasing in the systemic concentrations of cytokines such as interleukins (ILs), C-reactive protein (CRP) and tumor necrosis factor-α (TNF-α).7 Several clinical observations indicated that chronic low-grade inflammation contributes in the pathogenesis of many diseases, including obesity, atherosclerosis and cardiovascular disease (CVD).8-11
The murine endotoxin or lipopolysaccharide (LPS) model is a known model of inflammation which is important in understanding the inflammatory response. LPS, an endotoxin of gram-negative bacteria, via toll-like receptor 4 (TLR4), induces inflammation in the rodent.12 Heart can be used as a target tissue for TLR4 receptor.13 The effect of gram negative bacteria on myocardium has been reported in human and different animal models of inflammation or bacteremia.14-16 It is indicated that low levels of LPS activate cardiac myocytes and depress cardiac contractility as well as apoptosis by activating the renin angiotensin system. On the other hand, recurrent exposure to subclinical LPS may alter left ventricular structure and function.17 Therefore, we hypothesized that chronic exposure to low level of LPS may induce cardiac and kidney fibrosis.
In the present study, we used a chronic low grade inflammation model, which is very close to clinical conditions with chronic low-grade inflammation, by recurrent exposure to LPS, and evaluated its effects on serum inflammatory markers and cardiac fibrosis in male rats.
Materials and Methods
Twenty male Wistar rats (8 weeks old; 200-220 g weight) were purchased from animal house of Mashhad University of Medical Sciences, Mashhad, Iran. The animals were kept under standard conditions (temperature 22 ± 2 °C and 12h light/dark cycle) with free access to food and water. Working with the animals was performed in accordance with approved animal protocols and guidelines established by local ethical committee on animal research. The animals were divided into two experimental groups: control and LPS-treated (n = 10 in each group).
LPS was purchased from Sigma (Sigma chemical Co., USA) and dissolved in physiological saline solution. For induction of subclinical inflammation, the rats received LPS (10 mg/kg/week) intraperitoneally for 4 weeks.18 Control group received 2 ml/kg/week of saline instead of LPS. After 4 weeks, the animals were anaesthetized with diethyl ether and blood samples were taken for further analysis. Then, they were sacrificed, and left ventricle of hearts and right kidneys were dissected, washed with cold saline and kept in 10% formalin solution for histological examination. Right ventricles and left kidneys were stored at -70 °C for tissue marker measurements. Serums were separated by blood centrifugation at 3000 g for 20 min. Then, the samples were stored at −70 °C for further analyses.
Heart and kidney tissues were homogenized in phosphate-buffered saline (PBS) buffer and the supernatant was used for enzyme-linked immunosorbent assay (ELISA). Levels of nitrite in the tissue homogenates and serum were measured using griess reagent method (Promega Co., USA) as previously described.19-21 Serum IL-6 and TNF-α levels were measured with standard ELISA kits (ebioscience Co., San Diego, CA, USA) according to the manufacturer instructions. The techniques were conducted using automated ELISA Reader (BioTek Instruments, USA). Tissue’s total thiol content, malondialdehyde (MDA), and the activity of superoxide dismutase (SOD) and catalase were measured in homogenate solutions.
MDA which is the index of lipid peroxidation was measured as previously described.22 2 ml from reagent of thiobarbituric acid (TBA)/trichloroacetic acid (TCA)/ Hydrochloric acid (HCl) and 1 ml of tissue homogenate were mixed. Then, they were centrifuged within 3000×g and the absorbance was measured at 535 nm.
For measurement of total thiol concentration, 1 ml of tris-ethylenediamine tetraacetic acid (EDTA) buffer (pH = 8.6) was added to tissue homogenate and absorbance was read at 412 nm against tris-EDTA (TE) buffer alone. Then, 20 μl of 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) was added to solution and kept at room temperature for 15 minutes. Then, absorbance was read again. The absorbance of DTNB reagent was also read as a blank.23
SOD and catalase activities were measured using a Ransod kit (Randox Laboratory, UK) by a method which was previously described.24
The left ventricles of heart and right kidneys were put in 10% buffered formalin for 24-72 hours prior to processing and paraffin following a routine procedure. Then, samples with 5µm thickness were prepared and stained with hematoxylin and eosin (H&E) and Masson's trichrome, and were examined by light microscopy. Fibrosis was quantified in five fields per animal, and expressed as the percentage of fibrous tissue area stained with Masson trichrome. The fibrotic area was quantified using NIH image software (ImageJ).
All data were expressed as means ± standard error (SE). The data was analyzed using the SPSS software (version 20, IBM Corporation, Armonk, NY, USA). Independent sample t-test was used for comparison of data between groups. Differences were considered statistically significant when P < 0.050.
Results
Body weight: There was no significant difference in initial body weight between the groups. Over the course of the 4 weeks study, the animals gained body weight, and LPS-treated group gained less body weight compared to the normal group (230.0 ± 5.2 vs. 245.4 ± 3.7 g) (P < 0.050).
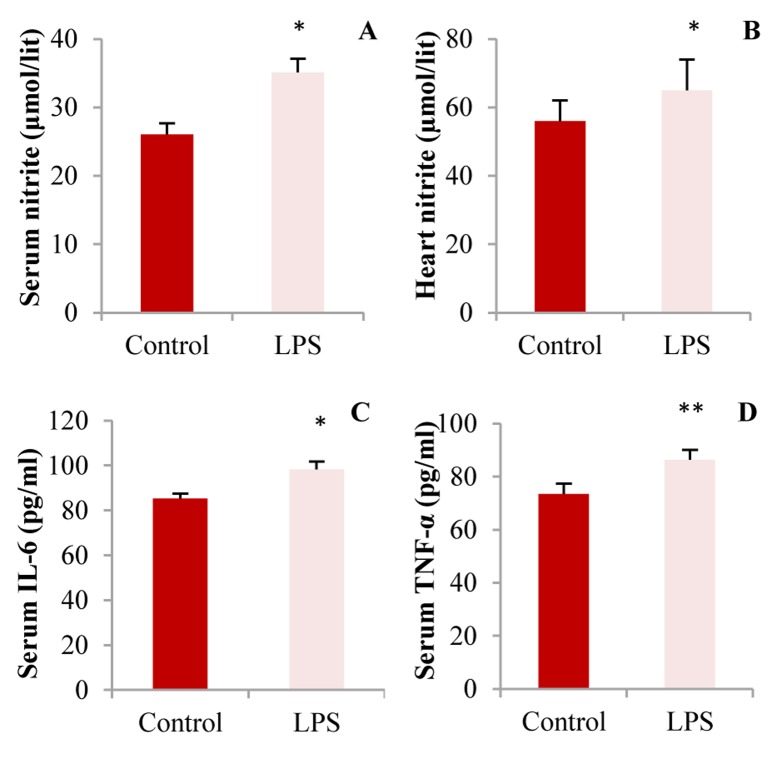
Effect of recurrent LPS administration on serum and heart nitrite levels: As shown in figure 1, significant increase in serum nitrite concentrations were observed in LPS group as compared to control (37.0 ± 2.2 vs. 25.5 ± 1.9 µmol/l; respectively, P < 0.050). Nitrite levels in the heart tissue of LPS-treated group showed a higher level than control, although it was not statistically significant (P = 0.090).
Figure 1.
A: Serum nitrite; B: Heart tissue nitrite; C: Interleukine (IL)-6; and D: Serum tumor necrosing factor (TNF)-α concentrations in control and lipopolysaccharide (LPS)-treated groups, (* P < 0.050 and ** P < 0.010 compared to control, n = 10 in each group)
Effect of LPS administration on serum IL-6 and TNF-α levels: Figure 1 illustrated the effect of LPS administration on serum IL-6 and TNF-α levels. Serum TNF-α and IL-6 concentrations were significantly higher in LPS-treated group compared to control (IL-6: 84 ± 3 vs. 98.0 ± 4.4; TNF-α: 75.5 ± 4.9 vs. 85.3 ± 4.7 pg/ml; respectively, (P < 0.050).
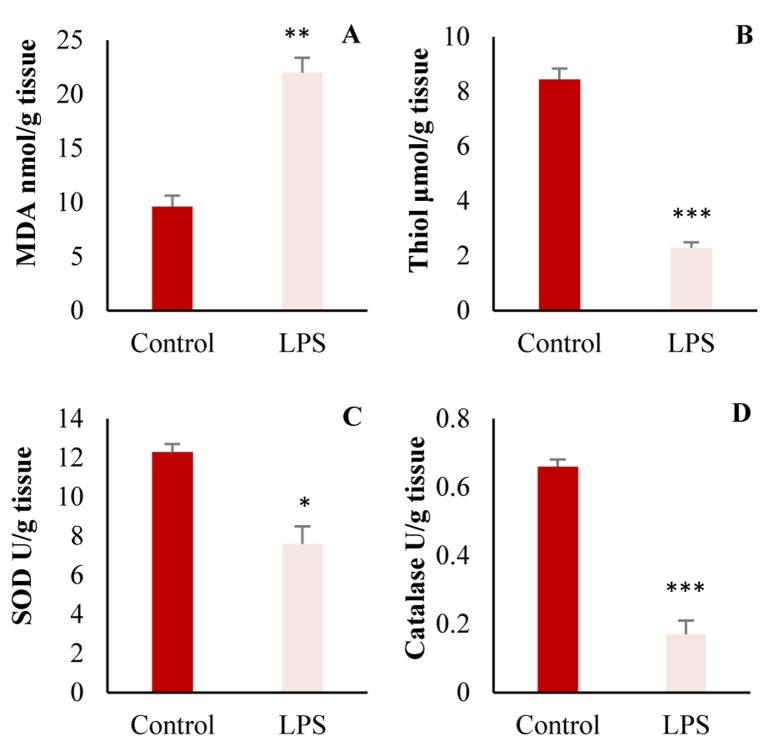
Tissue oxidant and antioxidant factor: As shown in figure 2, MDA contents in heart tissues of LPS-treated group were significantly higher than control (P = 0.010), while, total thiol concentration, catalase and SOD were significantly reduced after LPS treatment (P = 0.010).
Figure 2.
Comparison of A: malondialdehyde (MDA); B: Total thiol concentration; C: Superoxide dismutase (SOD); and D: Catalase in the heart of control and lipopolysaccharide (LPS)-treated groups, (n = 10 in each group, * P < 0.050; ** P < 0.010; *** P < 0.001 compared to control group)
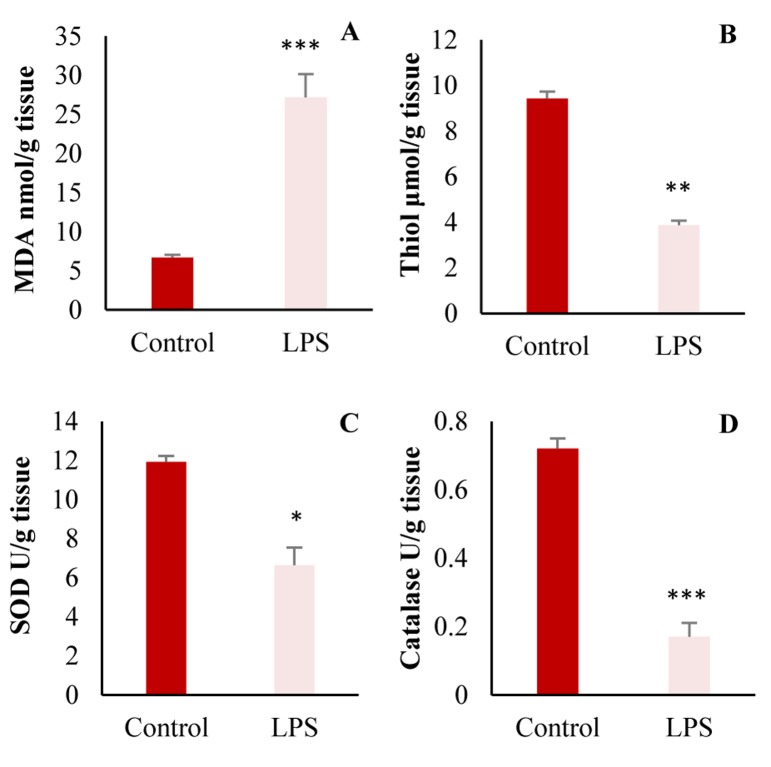
The same results were also observed in oxidant and antioxidant markers in the kidney (Figure 3).
Figure 3.
A: Malondialdehyde (MDA); B: Total thiol; C: Superoxide dismutase (SOD); and D: Catalase in the kidney (* P < 0.050; ** P < 0.010; *** P < 0.001 compared to control group)
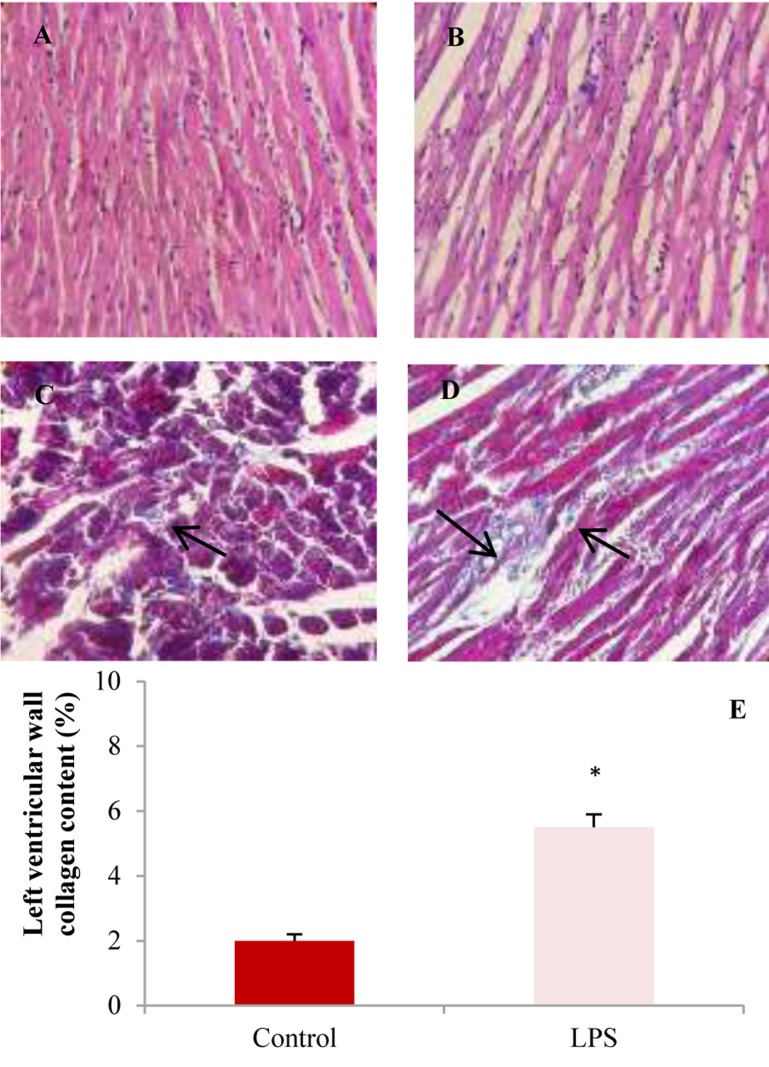
Histopathological findings: Figures 4-6 illustrate representative examples of photomicrographs of H&E and Masson tichrome stained of the left ventricular tissues and kidneys in control and LPS-treated groups.
Figure 4.
The light micrograph of left ventricles in control and lipopolysaccharide (LPS)-treated groups. A: Control group with normal architecture [×20, hematoxylin and eosin (H&E)]. B: Recurrent LPS treatment [(10 mg/kg/week for 4 weeks (×20, H&E)] showing infiltration of inflammatory cells, edema and disarrangement of fibers. Masson trichrome staining of left ventricular muscles of control (C) and LPS (D) groups show more collagen deposition (blue color, black arrows) in LPS-treated group. Blue color indicates collagen fibers. E: Left ventricular wall fibrosis showed higher collagen content (%) in LPS group compared to control (* P < 0.050 compared to control).
Figure 5.
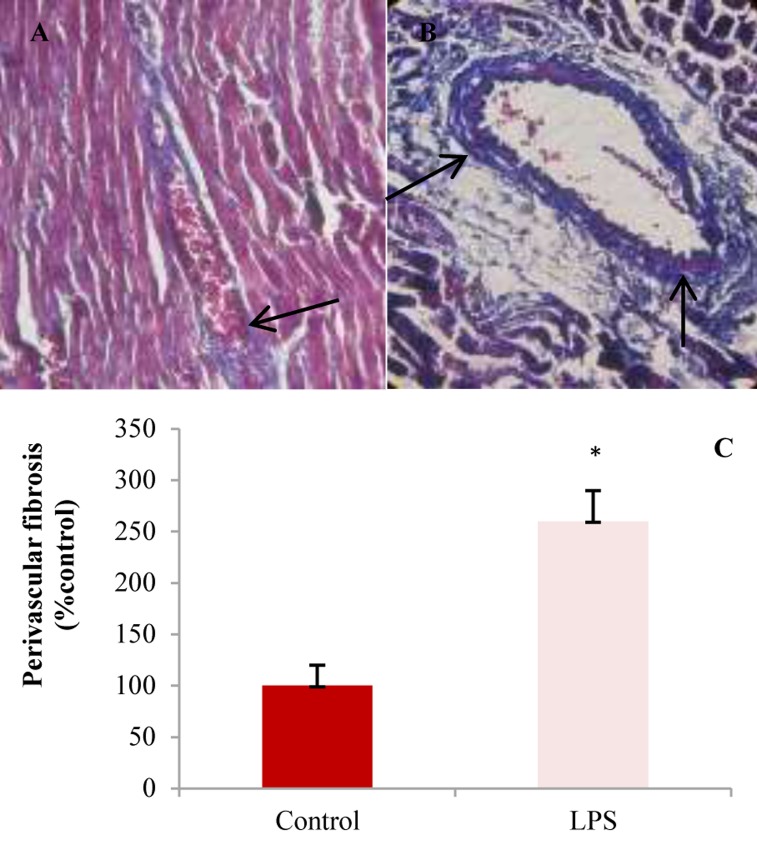
Perivascular fibrosis around left anterior descending coronary artery stained by Masson trichrome. Blue color indicates collagen deposition. A: Control; B: Lipopolysaccharide (LPS); C: Percent of perivascular fibrosis in experimental groups (* P < 0.050 compared to LPS)
As illustrated in figure 6, the cardiac muscle fibers in control animals have normal histopathological features with single, oval and centrally located nuclei of cardiomyocytes and regularly arranged myofibers. The left ventricle of LPS-treated group showed infiltration of inflammatory cells, disarrangement of fibers, edema, and dilation of blood vessels. Masson’s trichrome stained samples showed a significant increase in collagen deposition in left ventricle wall of LPS group compared to control (Figure 5).
Figure 6.
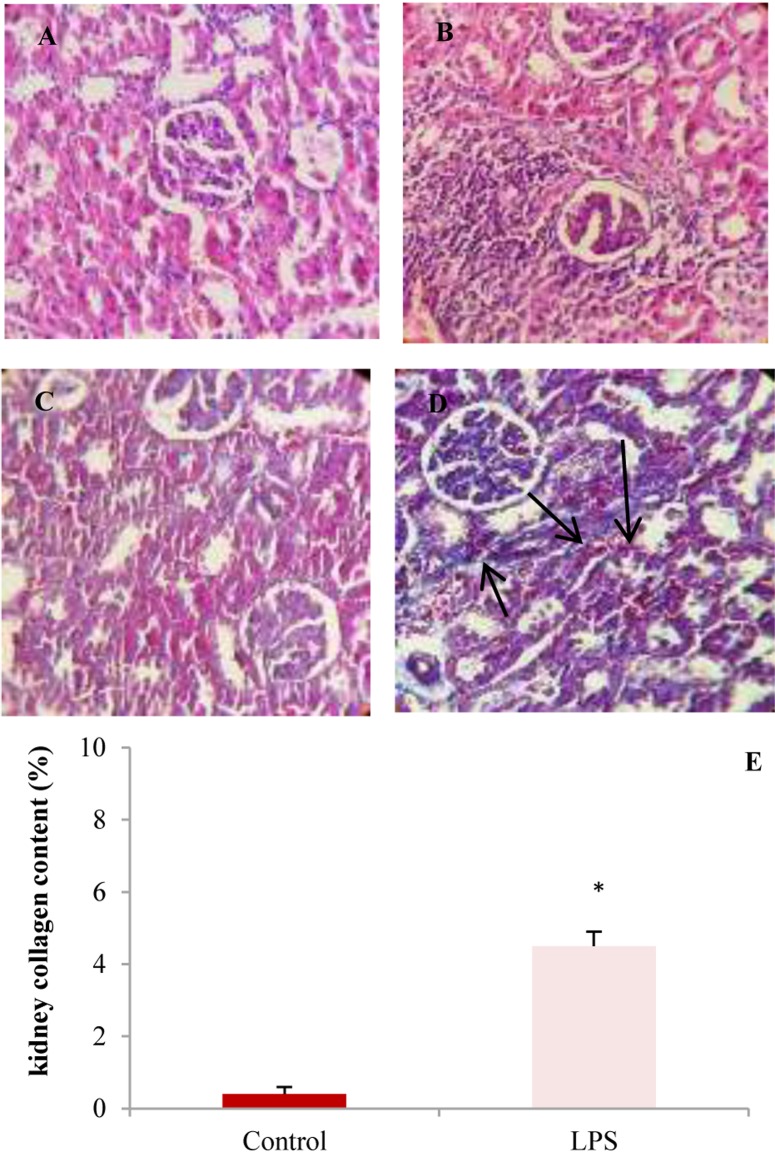
Images A and B are hematoxylin and eosin (H&E)-stained sections demonstrating infiltration of inflammatory cells in kidneys from lipopolysaccharide (LPS)-treated rats. Masson's trichrome stained sections demonstrate collagen deposition (blue color, black arrows) in the kidneys from rats in control and LPS groups (C and D). E: Percent of collagen content in the kidneys in experimental groups (* P < 0.010 compared to control)
Histological evaluation of renal tissue demonstrated a marked increase in inflammatory cells and more fibrotic tissue and collagen content in LPS group than control (Figure 6).
Discussion
Our results showed that chronic low grade inflammation increased heart and kidney factors of oxidative stress, and reduced antioxidative markers. In addition, histopathological examination indicated increased inflammatory cells and collagen deposition in heart and kidney tissues.
In this study, we used subclinical LPS model. The rodent has capacity to tolerate recurrent LPS injection without lethality and this makes them as suitable model for chronic model. Study by Lew et al.,17 revealed that weekly injection of 10 mg/kg LPS intraperitoneally increased serum LPS levels which is comparable to human;25,26 and induced a level found in several human conditions.8
Previous studies demonstrated that LPS exacerbates hepatic, renal, and cardiac fibrosis in preexisting abnormalities. In the liver in bile duct ligation model or after hepatic injury, fibrosis was promoted by LPS from gut microbial.27
Renal fibrosis was also promoted by TLR4 activation in urethral ligation model.28 Cardiac fibrosis was also exacerbated in myocarditis.29 In this study, we found that low grade inflammation induced cardiac and renal fibrosis without preexisting heart or kidney injury. Our results were in agreement with that of Lew et al. who used LPS and induced subclinical inflammation in mice.17 They found that after two and four weeks of LPS administration, collagen fraction area in the left ventricle was higher compared with control group. In addition, they showed that LPS increased cardiac IL-6 and nitric oxide (NO) synthase expression. They also examined if cardiac fibroblasts are target of LPS, and showed that incubation of heart fibroblast with LPS for 48 hours dose-dependently increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) messenger ribonucleic acid (mRNA) expression. In our study, although we did not measure inflammatory cytokine expression, however, we found that LPS administration increased serum and cardiac nitrite, serum IL-6 and TNF-α concentrations. IL-6 has a role in fibrosis in different organs by differentiation of fibroblasts to myofibroblasts which produces more collagen.30 In addition, cardiac and renal fibroblasts increased IL-6 expression after exposure to LPS.
Transforming growth factor-β (TGF-β) and TNF-α are common mediators of tissue fibrosis in pathological conditions.31 Since TGF-β is not involved in cardiac fibrosis in our model,17 we did not measure it. Interestingly, we showed that oxidant/antioxidant balance altered in the heart and kidney in subclinical LPS. Higher MDA concentration, lower SOD and catalase activity and total thiol concentration suggest that lipid peroxidation increased and antioxidant enzyme was suppressed after recurrent LPS. It is indicated that LPS promotes the production of inflammatory cytokines that in turn leads to excessive production of free radicals and oxidative stress. It is indicated that higher reactive oxygen species (ROS) is associated with tissue fibrosis in chronic renal failure and myocardial infarction.32 In the present study, we used LPS for induction of chronic inflammation. It should be considered that in several common diseases in human such as rheumatoid arthritis (RA) and atherosclerosis, the exact mechanism for induction of chronic inflammation is different, and this is the limitation of study. On the other hand, we did not measure LPS level and some serum markers such as TGF-β in serum and thus, we cannot compare it with human plasma level in the same condition.
Conclusion
We concluded that conditions with chronic low grade inflammation which is common in high-fat diet, metabolic syndrome or smoking are associated with cardiac fibrosis as a primary effect which may lead to heart failure (HF) with preserved ejection fraction in chronic condition. Further studies in different models of inflammatory disease especially in chronic condition such as rheumatoid should be done to approve our findings and to find the exact mechanism of inflammation-induced fibrosis.
Acknowledgments
The authors are thankful to vice chancellor of Mashhad University of Medical Sciences for their financial support (grant number: 941425).
Footnotes
Conflicts of Interest
Authors have no conflict of interests.
REFERENCES
- 1.Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147(2):227–35. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asgharzadeh F, Rouzbahani R, Khazaei M. Chronic low-grade inflammation: Etiology and its effects. J Isfahan Med Sch. 2016;34(379):408–21. [Google Scholar]
- 3.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 4.Shalapour S, Karin M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J Clin Invest. 2015;125(9):3347–55. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahergorabi Z, Khazaei M. The relationship between inflammatory markers, angiogenesis, and obesity. ARYA Atheroscler. 2013;9(4):247–53. [PMC free article] [PubMed] [Google Scholar]
- 6.Burgoyne JR, Mongue-Din H, Eaton P, Shah AM. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111(8):1091–106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 7.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 8.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–44. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 9.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34(1):39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: A review on proposed mechanisms. Cell Biochem Funct. 2016;34(8):533–45. doi: 10.1002/cbf.3229. [DOI] [PubMed] [Google Scholar]
- 11.Sallam N, Khazaei M, Laher I. Effect of moderate-intensity exercise on plasma C-reactive protein and aortic endothelial function in type 2 diabetic mice. Mediators Inflamm. 2010;2010:149678. doi: 10.1155/2010/149678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beutler B, Rietschel ET. Innate immune sensing and its roots: The story of endotoxin. Nat Rev Immunol. 2003;3(2):169–76. doi: 10.1038/nri1004. [DOI] [PubMed] [Google Scholar]
- 13.Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104(3):271–80. doi: 10.1172/JCI6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: Myocardial depression in sepsis and septic shock. Crit Care. 2002;6(6):500–8. doi: 10.1186/cc1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8(5):376–88. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119(10):2868–78. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew WY, Bayna E, Molle ED, Dalton ND, Lai NC, Bhargava V, et al. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS One. 2013;8(4):e61057. doi: 10.1371/journal.pone.0061057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lew WY, Bayna E, Dalle Molle, Contu R, Condorelli G, Tang T. Myocardial fibrosis induced by exposure to subclinical lipopolysaccharide is associated with decreased miR-29c and enhanced NOX2 expression in mice. PLoS One. 2014;9(9):e107556. doi: 10.1371/journal.pone.0107556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmi S, Sallam NA, Rahman MM, Teng X, Hunter AL, Moien-Afshari F, et al. Sulfaphenazole treatment restores endothelium-dependent vasodilation in diabetic mice. Vascul Pharmacol. 2008;48(1):1–8. doi: 10.1016/j.vph.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Khazaei M, Fallahzadeh AR, Sharifi MR, Afsharmoghaddam N, Javanmard SH, Salehi E. Effects of diabetes on myocardial capillary density and serum angiogenesis biomarkers in male rats. Clinics (Sao Paulo) 2011;66(8):1419–24. doi: 10.1590/S1807-59322011000800019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nematollahi S, Nematbakhsh M, Haghjooyjavanmard S, Khazaei M, Salehi M. Inducible nitric oxide synthase modulates angiogenesis in ischemic hindlimb of rat. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2009;153(2):125–9. doi: 10.5507/bp.2009.021. [DOI] [PubMed] [Google Scholar]
- 22.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9(6):515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinzadeh H, Sadeghnia HR. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharm Sci. 2005;8(3):394–9. [PubMed] [Google Scholar]
- 24.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 25.Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, et al. Resilience to bacterial infection: Difference between species could be due to proteins in serum. J Infect Dis. 2010;201(2):223–32. doi: 10.1086/649557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–7. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seki E, De Minicis, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13(11):1324–32. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 28.Pulskens WP, Rampanelli E, Teske GJ, Butter LM, Claessen N, Luirink IK, et al. TLR4 promotes fibrosis but attenuates tubular damage in progressive renal injury. J Am Soc Nephrol. 2010;21(8):1299–308. doi: 10.1681/ASN.2009070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blyszczuk P, Kania G, Dieterle T, Marty RR, Valaperti A, Berthonneche C, et al. Myeloid differentiation factor-88/interleukin-1 signaling controls cardiac fibrosis and heart failure progression in inflammatory dilated cardiomyopathy. Circ Res. 2009;105(9):912–20. doi: 10.1161/CIRCRESAHA.109.199802. [DOI] [PubMed] [Google Scholar]
- 30.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10(1):15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 32.Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51(2):319–25. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]