Abstract
BACKGROUND AND OBJECTIVES
Sickle cell disease (SCD) is a group of hereditary chronic anemias that manifest essentially as painful crisis and susceptibility to infection. Neonatal screening is a preventive action that reduces the rates of mortality due to complications arising from infections by encouraging early prophylactic penicillin use and pneumococcal vaccination. The purpose of this pilot study was to set up a neonatal screening protocol at a lower cost than one that uses commercially available screening kits.
DESIGN AND SETTING
Pilot study conducted over 1 year in two Tunis maternity hospitals.
PATIENTS AND METHODS
Samples from 9148 newborns were collected using paper printed using a common office printer to collect blood spots from the newborns. A lab-prepared agarose gel for isoelectrofocusing (IEF) was used to test the dried blood samples from these newborns.
RESULTS
The IEF on lab-prepared agarose gels was efficient since it was able to detect the main abnormal Hbs previously identified in the Tunisian population (HbS, HbC, HbO, and HbG). Furthermore, when data collected in this screening program were compared with the previously established national data, no statistically significant differences were found. After analysis, results were given back to the families of the patients, and the major Hb cases were directed to one of the hemoglobinopathies specialized centers, where at-risk couples benefited from genetic counselling and were informed about the possibility of prenatal diagnosis.
CONCLUSION
This pilot experiment demonstrated the feasibility of SCD neonatal detection using a lower cost method as well as detection of other main structural Hb variants.
Sickle cell disease (SCD) is a group of autosomal recessive disorders characterized by the production of abnormal (S or sickle) hemoglobin (Hb). Heterozygotes (A/S) are silent carriers with no clinical manifestations, whereas the HbS in homozygotes (S/S) precipitates in certain circumstances, transforming the red blood cells into rigid cells that aggregate and cause SCD. SCD is a chronic hematologic disease that manifests essentially as a painful crisis during vaso-occlusive events and increased infection susceptibility. These events can be life threatening. Considering its high frequency and difficulties in management, SCD represents as a major public health problem in many parts of the world, especially in the African and south Mediterranean areas. In Tunisia (North Africa), the SCD carrier rate is around 1.9%, but it is not uniformly distributed across the country; in some regions, this rate can reach a level as high as 7.7%.1
Like any hereditary disease, the management of SCD is essentially preventive, with multiple levels of action. The first level of prevention is prenatal diagnosis (PND), which prevents new cases. This began in Tunisia 15 years ago. Despite of the net decrease in the number of new cases, PND is unable to reach many at-risk families in which parents are homozygous and at risk of having a child with SCD. This particularly affects those living in remote areas of the country because of the lack of information and awareness. Secondary prevention is neonatal screening. In the first 3 years of life, children with SCD are at great risk of infection with Streptococcus pneumoniae, which has a case-fatality rate as high as 30%.2,3 Trials of early prophylactic use of penicillin reduce the incidence of mortality due to complications arising from infections.4 This is complemented by aggressive vigilance for routine immunization and pneumococcal vaccination before the child reaches 2 years of age.5,6 The detection of carriers (sickle cell trait) helps early follow-up and adequate genetic counseling to sensitize parents to the possibility of PND.
Many attempts at screening have been successfully conducted around the world,7–9 but the initiation of such programs in African countries is particularly discouraged by the high cost of screening kits that are available on the market. We have therefore tried to implement a lower cost technique to detect SCD cases at an early stage with significantly lower costs. We present a complete protocol intended for this purpose conducted as a pilot study conducted in two maternity hospitals of Tunis (the capital) during the course of 1 year. The purpose of this experiment was to assess the feasibility of a neonatal screening protocol that encompasses all steps—from sample collection to the delivery of results.
PATIENTS AND METHODS
Samples were collected at the maternity centers on blotting paper (Whatman grade BFC 180 cat no. 9535–9362). The paper was printed using a common office printer, with three circles for the blood drops and also an information range. Each newborn was pricked in the heel with a sterile microlance. Each dried blood card was accompanied with an information sheet with information on the baby’s and parent’s identities, address, and phone number. This step was performed at the laboratory where the data were centralized. Each newborn was given a number, and the information collected was computerized.
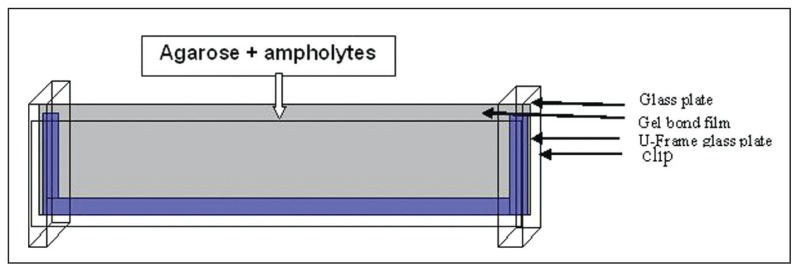
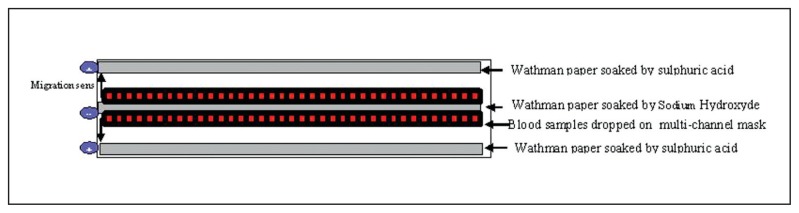
The gels for isoelectrofocusing (IEF) were prepared with agarose (Sigma-A4905, St. Loius, MO, USA)-0.5 g in 45 mL of distilled water to swell for 1 hour. This solution was then boiled until it became clear. One milliliter of ampholyte 5,7–9 (Sigma 89096, 95632, St. Loius, MO, USA was added when the temperature reached 70°C. The mixture was then poured into a previously prepared device that was an assembly of two glass plates: a U-frame (125×260 mm) and a 125×260-mm plate (Amersham Bioscience, Uppsala, Sweden), between which was inserted a gel bond (Lonza, Rockland, ME, USA) (Figures 1, 2). Samples were cut to form a 5-mm diameter pellet and left to elute in a microplate titration in potassium cyanide (100 mM) for at least 2 hours. Migration was carried out on a Multiphor II device (Amersham, Uppsala, Sweden). Two anodes made up by a band (5×260 mm) of Whatman paper soaked in sulfuric acid (0. 05M) were placed on the edges of the gel, and a cathode consisting of the same paper band soaked in sodium hydroxide (0.1M) was placed in the middle of the gel (Figure 2). The samples were dropped onto multichannel masks with a pipette. The migration was carried out at 12°C, at a constant power (18 W) and a maximum voltage of 1500 V. After focusing (voltage stabilization), the gel was quickly read and then put in 15% TCA (trichloracetic acid) for 20 minutes and then rinsed with water for 1 hour and dried overnight at room temperature. Once dry, the gel was stained by a specific Hb colorant (TTF3, Sébia, Lisses, France) with water, oxygen, and distilled water for 20 minutes. After rinsing, the gel was dried in an oven at 70°C for 2 hours.
Figure 1.
Device for agarose gel preparation.
Figure 2.
Gel preparation for IEF.
High-performance liquid chromatography (HPLC) is a second technique that confirms the Hb defect detected by IEF. The system uses the Hb VARIANT II (Hb testing system, BIO RAD Augusta, GA, USA) using VARIANTTM II HbA2/HbA1C dual kit (Hercules, CA). This check was performed on the same type of pellet as used for IEF, but eluted in 1000 mL of wash solution provided by the manufacturer.
Parents with a newborn carrying an Hb defect were invited to attend our lab. Venous blood was collected from the family members and analyzed by IEF and/or HPLC.
RESULTS
Samples from 9148 newborns were collected over 1 year in two Tunis maternity hospitals. In the tested samples, 4 homozygous (Hb SS, Hb CC, thalassemia [thal] major) or double heterozygous (HbSC) and 233 heterozygous carriers were detected; among them were 4 beta-globin structural variants (HbS, HbC, HbO, HbD and 1 alpha-globin structural variant (HbG) (Table 1). All abnormal samples identified by IEF on agarose gel were verified and confirmed by HPLC. Family members were invited to the lab to check the Hb status of the parents. Of the 237 families, 161 attended our lab, and 9 among them were at-risk couples: 4 SS risk, 2 S/beta-thal risk, 2 beta-thal risk and 1 CC risk.
Table 1.
Detected cases with abnormal hemoglobin by isoelectric focusing on agarose gel.
| Hemoglobin abnormalities | AS | AC | AO | AD | AG | Weak HbA rate | CC | SC | TT |
|---|---|---|---|---|---|---|---|---|---|
| Number of cases | 176 | 34 | 13 | 2 | 8 | 141 | 2 | 1 | 1 |
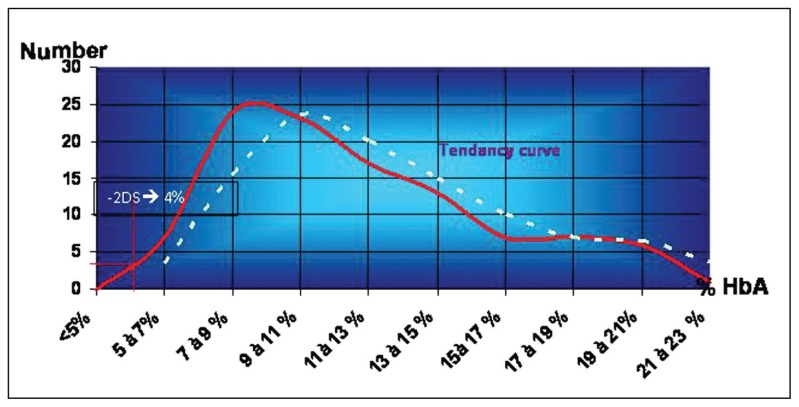
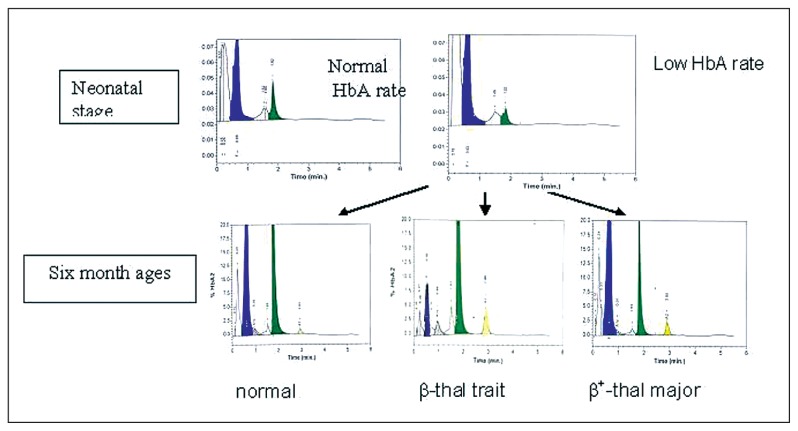
In addition to detection of structural variants, it was noted by visual estimation that the HbA fraction was smaller in some samples. To estimate this variation, samples presenting with a visually reduced HbA fraction were tested by HPLC, and the HbA rate was compared with the control group values. The control group consisted of 200 samples of newborn blood spots. They were tested by both methods: IEF and HPLC. The average values of HbA at birth were 11.67 (3.97%); values lower than 3.8% (2SD<mean) were considered as a weak HbA rate (Figure 3). In the light of these results, the samples for which the HbA rate was less than 4% were retained as having a low HbA and were submitted for family study. The results showed that children with low HbA at birth could be normal, beta-thal minor or beta+-thal major (Figure 4). Comparison between results obtained by IEF and those obtained by HPLC for the control group showed identical results for both methods concerning the structural variants (S, C, O-Arab and others.), while the detection of a weak HbA rate was much better by HPLC than by IEF, where visual detection was subjective in this case.
Figure 3.
Distribution of HbA rate in a control group of newborns.
Figure 4.
Hemoglobin HPLC profile in neonates and six months babies; and different evolutions of HPLC profile for low HbA rate at neonatal stage.
DISCUSSION
Our results demonstrate the feasibility of obtaining a neonatal diagnosis of hemoglobinopathies at a lower cost. This protocol, inspired by an earlier published technique,10 helps reduce program costs, acting on two levels: the first one concerns sample collection, for which we used a Whatman A4 paper sheet instead of the trade-available paper, thereby significantly reducing the cost of collecting samples. The second economizing factor is the use of lab-prepared agarose gels for IEF, the cost of which corresponds to one fifth of the screening kit price on the market. In this way, we significantly reduced the usual product cost by one tenth; the usual product cost often constitutes a major funding difficulty in managing a project.
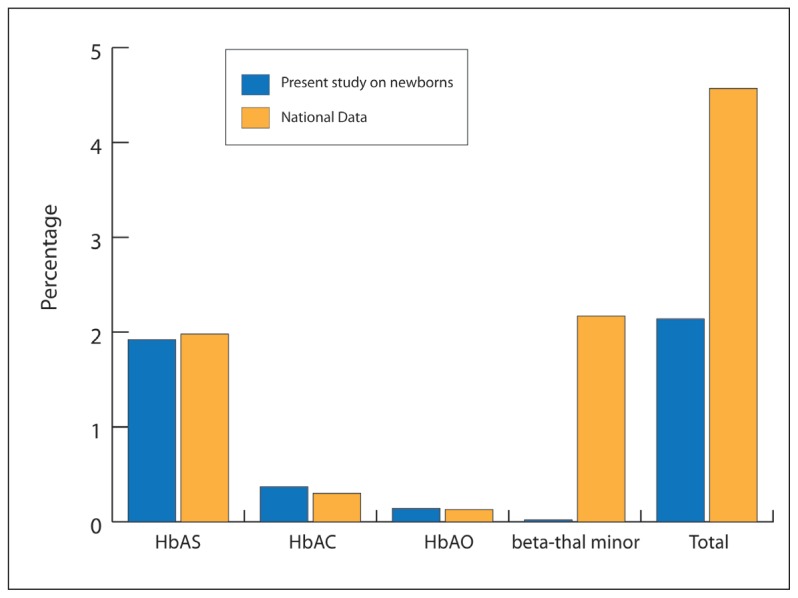
As a second technique used to confirm the IEF result before meeting with the families, HPLC could be avoided if the laboratory does not have enough funds for this. Also, the family study could be done directly after analysis of the IEF results. The IEF on lab-prepared agarose gels displays its efficiency here, since it was able to detect the main abnormal hemoglobins previously identified in the Tunisian population (HbS, HbC, HbO, and HbG). Furthermore, when comparing data collected in this screening program with the previously established national data,1 no statistically significant differences were found (Figure 5). The only observed divergence was with the beta-thalassemia carriers, but this result was expected given that the protocol was not designed for beta-thal detection. Nevertheless, this study has contributed to the determination of the normal HbA rate range in the neonatal period.
Figure 5.
Comparison of data collected in the present screening program and the previously established national data concerning hemoglobinopathies carrier frequency. (The percentage comparison were done by the Fisher test and P was considered as nonsignificant when it exceeded .05)
Among the detected cases, 71% of the families could be contacted for carrying out a family-based study; the remaining families could not be contacted because of incorrect telephone numbers or having changed addresses, and these families were contacted in another manner, such as in pediatric centers in hospital settings. After analysis, results were given back to the families. The major Hb cases were directed to one of the specialized hemoglobinopathy centers, whereas genetic counseling was offered to at-risk couples, who could benefit from being informed of the possibility of a PND.
In conclusion, this pilot study demonstrates the feasibility of SCD neonatal detection and also the detection of other main structural Hb variants, with very good results. It also detected beta-thal cases, but this was not always possible since this method is not adapted for this purpose. This protocol presents the advantage of being of low cost and is therefore accessible to families in countries where financial resources are limited, such as African countries, where hemoglobinopathies are very frequent. Once the laboratory protocol is set up, the next step is to provide the specialized centers for the care of patients with hemoglinapathies needed to benefit from neonatal screening. In fact, while the drugs for prophylactic antibiotherapy are available and currently used for major SCD cases, the immunization program is still limited to routine vaccination and we need to convince the authorities to support pneumococcal vaccination. When this is done, we need to generalize this program for the whole country, beginning with the high-risk regions.
REFERENCES
- 1.Fattoum S. Hemoglobinopathies in Tunisia. An updated review of the epidemiologic and molecular data. Tunis Med. 2006;84:687–96. [PubMed] [Google Scholar]
- 2.Zarkowsky HS, Gallagher D, Gill FM, Wang WC, Falletta JM, Lande WM, et al. Bacteremia in sickle hemoglobinopathies. J Pediatr. 1986;109:579–85. doi: 10.1016/s0022-3476(86)80216-5. [DOI] [PubMed] [Google Scholar]
- 3.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983–2002. Pediatr. 2009;154:541–5. doi: 10.1016/j.jpeds.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 4.Tsevat J, Wong JB, Pauker SG, Steinberg MH. Neonatal screening for sickle cell disease: A cost-effectiveness analysis. J Pediatr. 1991;118:546–54. doi: 10.1016/s0022-3476(05)83375-x. [DOI] [PubMed] [Google Scholar]
- 5.Serjeant GR. Sickle-cell disease. Lancet. 1997;350:725–30. doi: 10.1016/S0140-6736(97)07330-3. [DOI] [PubMed] [Google Scholar]
- 6.Olney RS. Preventing morbidity and mortality from sickle cell disease. A public health perspective. Am J Prev Med. 1999;16:116–21. doi: 10.1016/s0749-3797(98)00140-8. [DOI] [PubMed] [Google Scholar]
- 7.Etienne-Julan M, Saint Martin C. The sickle cell disease in the french west indies. Revue Française des Laboratoires. 2005:374. [Google Scholar]
- 8.Bardakjian J, Benkerrou M, Bernaudin F, Briard ML, Ducrocq R. Le dépistage néonatal de la drépanocytose en France métropilitaine. Arch Pédiatr. 2007;7:1261–3. doi: 10.1016/s0929-693x(00)00140-8. [DOI] [PubMed] [Google Scholar]
- 9.Rahimy MC, Ganbo A, Ahouignan G, Alihonou E. Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol. 2009;62:46–8. doi: 10.1136/jcp.2008.059113. [DOI] [PubMed] [Google Scholar]
- 10.Ducrocq R, Pascaud O, Bévier A, Finet C, Benkerrou M, Elion J. Strategy linking several analytical methods of neonatal screening for sickle cell disease. J Med Screen. 2001;8:8–14. doi: 10.1136/jms.8.1.8. [DOI] [PubMed] [Google Scholar]