Abstract
Human stromal (mesenchymal) stem cells (hMSC) represent a group of non-hematopoietic stem cells present in the bone marrow stroma and the stroma of other organs including subcutaneous adipose tissue, placenta, and muscles. They exhibit the characteristics of somatic stem cells of self-renewal and multi-lineage differentiation into mesoderm-type of cells, e.g., to osteoblasts, adipocytes, chondrocytes and possibly other cell types including hepatocytes and astrocytes. Due to their ease of culture and multipotentiality, hMSC are increasingly employed as a source for cells suitable for a number of clinical applications, e.g., non-healing bone fractures and defects and also non-skeletal degenerative diseases like heart failure. Currently, the numbers of clinical trials that employ MSC are increasing. However, several biological and biotechnological challenges need to be overcome to benefit from the full potential of hMSC. In this current review, we present some of the most important and recent advances in understanding of the biology of hMSC and their current and potential use in therapy.
Human bone marrow-derived stromal stem cells (hMSC) (also known as skeletal stem cells, mesenchymal stem cells) are a group of clonogenic cells that are present among the bone marrow stroma as well as the stroma of other organs. hMSC are capable of multilineage differentiation into mesoderm-type cells such as osteoblasts, adipocytes and chondrocytes1 and possibly, but still controversially, non-mesoderm type cells like neuronal cells or hepatocytes.2,3 Moreover, hMSC provide supportive stroma for growth and differentiation of hematopoeitic stem cells (HSC) and hematopoiesis.4 Recently, MSC has been employed in an increasing number of cell-based therapies for treating skeletal and non-skeletal chronic degenerative diseases. The aim of this review is to provide an update on the biology of hMSC and their current and potential uses in therapy.
Biological characteristics of hMSC
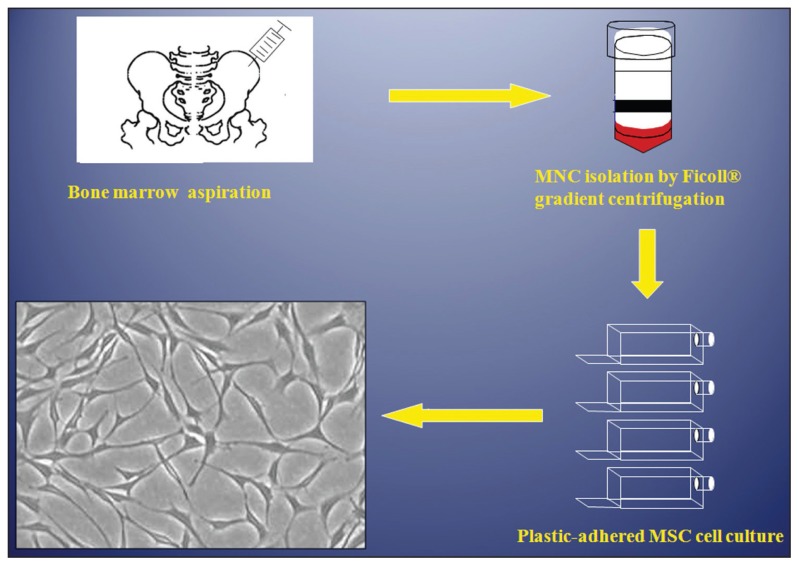
hMSC are fusiform, fibroblast-like cells that form colonies when cultured at a low density5–7 (Figure 1). hMSC exhibit characteristic surface markers being negative for hematopoietic cell markers: CD34−, CD45−, CD14− and positive for CD29+, CD73+, CD90+, CD105+, CD166+ and CD44+.8–10 Unfortunately, these markers are not specific for MSC and are expressed in a number of other mesodermal cells. Therefore, MSC are usually defined operationally as cells capable of ex vivo differentiation to osteoblastic, adipocytic and chondrocytic cells (i.e. multipotential) or forming bone and bone marrow organ— “an ossicle” upon transplantation subcutaneously in immune-deficient mice (Figure 2a).11 Traditionally, MSC have been isolated from bone marrow low-density mononuclear cell populations based on their selective adherence to plastic surfaces (Figure 1).7,12,13 hMSC have also been isolated using antibody-based cell selection employing a number of antibodies (e.g. Stro-1,14,15 CD146 (MCAM),16 CD200 and CD271).17, 18
Figure 1.
Standard isolation procedure for bone marrow derived human stromal (mesenchymal) stem cells (MSC). The cells are established in cultures based on their characteristic plastic surface adherence ability.
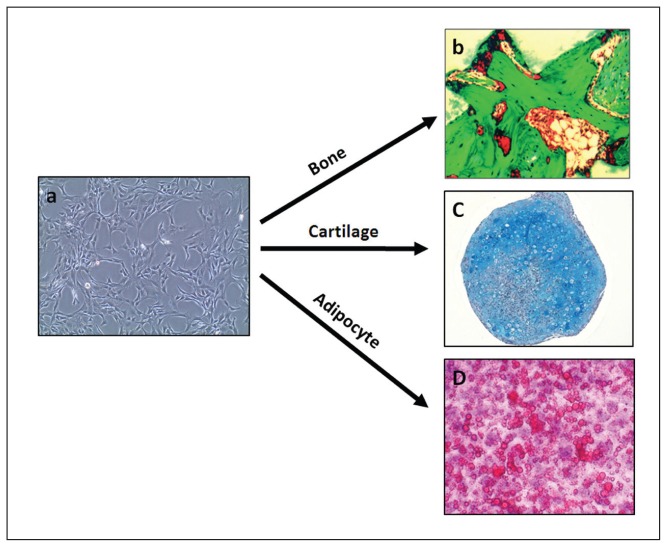
Figure 2.
Multipotentiality of human stromal (mesenchymal) stem cells (MSC). Under proper conditions, MSCs can form (a) bone when implanted subcutaneously in immune deficient mouse coupled with hydroxyapatite/tricalicum phosphate (HA/TCP) as carrier, (b) cartilage when cells cultured in vitro as cell aggregates in presence of transforming growth factor B or (c) fat when treated in vitro with insulin, dexamethasone and rosiglitazone.
Other MSC-like cells obtained from different tissues
Populations with MSC-like phenotype have been isolated from different tissues including peripheral blood,19 umbilical cord blood,20 synovial membranes,21 adipose tissue,22 lung,23 fetal liver,24 dental pulp25,26 and deciduous teeth.27 In particular adipose tissue-derived MSC cultured from fat tissue aspirates obtained during liposuction procedures represent a good source for obtaining large number of hMSC.28 Tissue-specific MSC share some basic morphological and differentiation characteristics with bone marrow-derived MSC. However, these cells are not identical and differences have been reported in their “genetic signature” as determined by global analysis of their transcriptomes.29–31
From the laboratory to the clinic
The emerging field of regenerative medicine holds promise for treating a variety of degenerative and age-related diseases, where no specific or effective treatment is currently available, by transplanting biologically competent mature cells and tissues or by stimulating tissue-resident stem cells. Stem cells in general and MSC in particular with their versatile growth and differentiation potential, are ideal candidates for use in regenerative medicine protocols and are currently making their way into clinical trials. However, successful use of MSC in therapy requires developing well-defined methods for MSC cell isolation, growth and differentiation. The following sections cover progress achieved in understanding the biology of MSC relevant for their clinical use.
Isolation of hMSC prospectively based on specific criteria
The current standard procedure for isolating hMSC based on plastic adherence to cell culture plates, results in heterogeneous cell cultures comprised of MSC and other tissue specific cells. Thus, there is a need for identifying surface markers that can be employed in isolating hMSC prospectively. We have employed several approaches to identify hMSC-specific markers. Using DNA microarray technology, we have identified a set of genes (a molecular signature) predictive for “stemness” phenotype as evaluated by in vivo criteria. 32 We have also employed state-of-the-art mass spectrometry-based proteomic methods to identify novel plasma membrane-associated protein makers.33 These global methods provide a large number of novel candidate marker genes and proteins that are currently being verified and tested for their usefulness in isolating homogenous populations of hMSC needed for clinical applications.
Limited in vitro cell growth and replicative senescence of hMSC
The clinical use of hMSC requires the availability of a large number of functionally competent cells with a stable phenotype and genotype. This is usually achieved by long-term ex vivo culturing of hMSC. However, hMSC, in contrast to embryonic stem cells or cancer cells, exhibit a limited capacity for ex vivo growth, a phenomenon known as “in vitro replicative senescence”.34 Also, the proliferative capacity of hMSC is dependent on donor age and thus compromises the ability to generate enough number of cells from elderly donors.34 Several approaches have been tried to improve ex vivo growth of hMSC using an enriched culture media with the addition of relevant growth factors, e.g. fibroblast growth factor 2 (FGF-2).35 We have also demonstrated that genetic over-expression of human telomerase reverse transcriptase gene (hTERT) in hMSC increases their telomerase activity and abolishes replicative senescence phenotype.36 However, genetic manipulation of hMSC is not desirable for cells to be used in clinical transplantation and alternative methods for ex vivo enhancement of hMSC growth, e.g. use of small chemical molecules with proliferation-enhancing abilities or a enhancing cell growth by using a combination of growth factors represent alternative approaches that are being tested.
Directing differentiation of MSC into specific lineages
While the multi-potentiality of MSC is the basis for using the cells for generating differentiated cells for cell replacement therapy, protocols that direct the differentiation of hMSC into a specific lineage are still inefficient and require improvement. Several approaches have been employed to direct the differentiation of MSC to a particular lineage. For example, differentiation into bone-forming osteoblastic cells has been achieved as ex vivo treatment with a mixture of growth factors (e.g. bone morphogenetic protein [BMP] or transforming growth factor [TGF]-β) to enhance osteoblast differentiation.37,38
Development of “off-the-shelf” MSC for allogeneic transplantation
Our current experience with autologus hMSC transplantation in clinical trials shows that it does not result in immunological problems. However, allogeneic hMSC transplantation is more clinically relevant since it allows the development of “off-the-shelf “ allogeneic cells ready for use in therapy. hMSC are hypoimmunogenic and thus allogeneic hMSC transplantation may be possible. hMSC express intermediate levels of HLA major histocompatibility complex (MHC) class I molecules, low levels of HLA class II antigens and no expression of co-stimulatory molecules e.g. CD40, CD40L, CD80 or CD86.39,40 Also, MSC have been reported to possess immunosuppressive properties in vitro as they inhibit T-cell alloreactivity induced in mixed lymphocyte cultures or by nonspecific factors.41,42 In addition, MSC inhibit the secretion of TNF-β and IL-10 secretion by dendritic cells and therefore directs the immune response toward more anti-inflammatory/tolerant phenotype.43 In vivo, the immunosuppressive effect of MSC has been shown by their ability to prolong histo-incompatible skin graft survival.44 These immunoregulatory characteristics are the basis of using hMSC in the treatment of graf-versus-host (GvH) disease.
Is it safe to transplant hMSC?
There are concerns that transplanted, culture-expanded hMSC may undergo spontaneous transformation and lead to cancer.45 The concept of the transformation potential of MSC is based mainly on extrapolations from studies performed on murine MSCs that exhibit spontaneous malignant transformation in long-term culture. However, spontaneous transformation of cultured hMSC has not been reported. Although Rubio et al46 reported that cultured adipose-tissue derived MSC from children exhibited spontaneous transformation in long-term culture, the results of this study were later shown to be false and caused by spurious contamination with an osteosarcoma cell line cultured simultaneously in the lab.47,48 Another area of concern is the possible role of administered hMSC in promoting growth of a latent tumor. Some studies have demonstrated that MSC can be recruited to the stroma of developing tumors when systemically infused in animal models for glioma, colon carcinoma, ovarian carcinoma, Karposi sarcoma, and melanoma.49 Conversely, the current clinical experience with hMSC transplantation, though limited, has been safe with an absence of cancer transformation. In conclusion, human MSC obtained from healthy individuals do not readily transform in culture and are safe for transplantation. However, further studies are needed to develop a set of safety criteria for predicting normal behavior of MSC employed in clinical programs.
What is the mechanism of tissue repair achieved by hMSC?
It has been thought that the therapeutic potential of hMSC is based on their ability to differentiate into a particular lineage cells that replace damaged cells and contribute to tissue regeneration. However, recent studies suggest that important therapeutic effects of MSC are mediated by their secreted factors (i.e. paracrine effects). 50,51 This mechanism may explain the intriguing observation of the presence of therapeutically-relevant effects after systemic or local transplantation of hMSC in a number of animal models of tissue injury (e.g. myocardial infarction, ischemic brain injury, in spite of the presence of low tissue engraftment). Several putative secreted factors with anti-inflammatory, anti-apoptotic and immune-modulatory effects are known to be produced by MSC.51–53 Identifying novel regeneration-promoting factors produced by MSC suitable for clinical use, is currently an active area of research in several laboratories.
Clinical application of hMSC in tissue regeneration
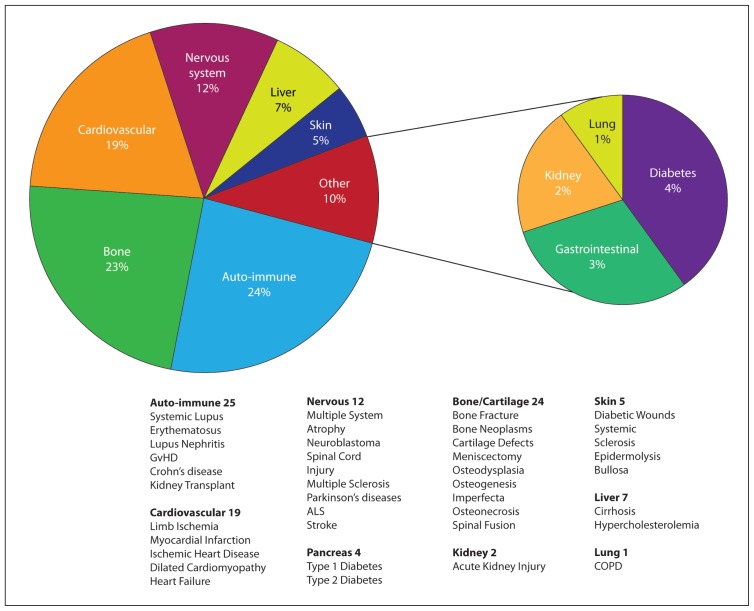
hMSC have been employed in an increasing number of clinical trials that range from individual case reports, patient series, and non-randomized as well as randomized clinical trials n skeletal and non-skeletal tissue regeneration. Tables 1 and 2 are a list of clinical trials in which hMSC have been employed. In the website of the National Institutes of Health, USA (http://clinicaltrials.gov), approximately 95 clinical trials are registered and covers a diverse indication (e.g. bone diseases, cardiovascular diseases and other rare pathologies such as amyotrophic lateral sclerosis (ALS), Hurler syndrome and metachromatic leukodystrophy). Results from these clinical trials are expected to have a major impact on the treatment of several disease conditions (Figure 3).
Table 1.
Examples of published clinical trials using human stromal stem cell therapy in skeletal disorders.
| System | Indication | Sample size | Cell type | Study design | Delivery route | Outcome | Ref | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Skeletal | Bone | Tibial non-union fracrure | 20 | Autologous bone marrow | Prospective study | Percutanous injection | Clinical and radiological bone union following in 15 out of 20 patients | 57 |
| Large bone diaphysis defects (tibia, ulna, humerus) | 4 | Autologous MSC’s cultured and seeded on porous hydroxyapatite scaffold | Case report | Local implantation | Complete fusion between the implant and the host bone occurred 5 to 7 months after surgery. In all patients at the last follow-up (6 to 7 years post surgery), a good integration of the implants was maintained | 58 | ||
| Atrophic tibial diaphysea non-union sites | 60 | Autologous bone marrow | Prospective study | Percutanous injection | Bone unions were obtained in 53 patients after 6 months. | 64 | ||
| Steroid-induced osteonecrosis of the femoral head | 3 | Autologous MSC’s cultured with beta-tricalcium phosphate | Case report | Intrafemoral | Osteonecrosis did not progress and early bone regeneration was observed. | 65 | ||
| Femoral head osteonecrosis | 13 | Autologous bone marrow | Phase I clinical trialI | ntrafemoral injection | Significant reduction in pain and in joint symptoms within the bone-marrow-graft group At twenty-four months, five of the eight hips in the control group had deteriorated to stage III, whereas only one of the ten hips in the bone-marrowgraft group had progressed to this stage | 66 | ||
| Unicameral bone cysts | 79 | Bone marrow | Clinical trial | Intra cystic | No clear differences in healing compared with steroid injection | 93 | ||
| Mandibular defect | 1 | Autologous bone marrow seeded on titanium mesh cage scaffold filled with bone mineral blocks and BMP 7 | Case report | Local implantation | In-vivo skeletal scintigraphy showed bone remodelling and mineralisation inside the mandibular transplant both before and after transplantation. CT provided radiological evidence of new bone formation. | 94 | ||
| Cartilage | Full-thickness articular cartilage defects in human patella | 2 | Autologous bone marrow stromal cells | Case report | Local implantation | Evidence for promotion of repair of articular cartilage defects | 61 | |
| Achondroplasia, congenital pseudarthrosis | 3 | Autologous MSC’s mixed with platelet rich plasma | Case report | Local injection | Acceleration of bone regeneration during distraction osteogenesis | 62 | ||
Table 2.
Examples of published clinical trials using human stromal stem cell therapy in non-skeletal disorders.
| System | Indication | Sample size | Cell type | Study design | Delivery route | Outcome | Ref | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Non-Skeletal | Cardiovascular | Acute myocardial infarction | 34\35 | Allogenic MSCs | Placebo controlled clinical trial | Intracoronary | Increased left ventricular ejection fraction and left ventricular end diastolic volume, improve contractility and enhanced infarct viability | 76 |
| Acute myocardial infarction | 53 | Allogenic MSCs (Prochymal) | Double-blind, placebo-controlled | Intravenous | Significant improvement of ejection fraction in hMSC-treated group | 77 | ||
| Nephrology | Acute kidney injury (post-operative) | 15 | Autologous MSCs | Phase 1 Trial | Intraarterial | Renal function was well preserved postoperatively, and none of the patients required hemodialysis. | 87 | |
| Gastro-intestinal | Perianal fistulas (Crohn disease) | 24\25 | Allogenic adiposetissue MSCs | Phase II Randomized controlled trial | Intravenous | AD-MSC induced higher rates of healing when used together with fibrin glue compared to fibrin glue alone | 91 | |
| Neurology | Multiple system atrophy | 11\18 | Autologous MSCs | case-control study | Intra-arterial × 1 Intravenous ×3 |
Delayed the progression of neurological deficits with achievement of functional improvement | 82 | |
| Stroke | 16\36 | Autologous MSC | Randomised, Placebo controlled | Intravenous | Improve recovery after stroke, improvements in the Barthel index and modified Rankin score | 81 | ||
| Multiple systems | Steroid-resistant, severe, acute GvHD | 55 | Autologous MSCs | phase II study | Intravenous | Better survival in patients with complete response (54.5%) | 68 | |
| Acute GvHD | 32 | Allogenic MSCs | Randomized clinical trial | Intravenous | 77% of patients fully responded, 17% partially responded | 69 | ||
| Lupus nephritis | 16 | Allogeneic umbilical cord MSCs | Phase I clinical trial | Intravenous | Improved Systemic activity Index and renal function | 95 | ||
Abbreviations: GvHD, Graft versus host disease. MSC’s, Mesenchymal Stem Cells. SLE, Systemic lupus erythromatosis
Figure 3.
Examples of ongoing or completed clinical trials using stromal stem cell. Data were collected from National Institute of Health (NIH), USA, clinical trials registery on (March 2011). Categorization was based on information provided in the trial summary.
Use of hMSC for skeletal tissue regeneration and non-skeletal repair and regeneration
hMSC possess the ability for osteoblast and chondrocyte differentiation and thus can potentially be used in skeletal tissue regeneration. Several animal studies have demonstrated the efficacy of MSC in treatment of segmental bone defects in rat and sheep where autologous bone marrow–derived MSCs were expanded in culture, then injected directly at the site of injury or after loading onto synthetic or natural biomaterial (i.e. scaffold).54–56 Human clinical case reports have demonstrated the success of autologus MSC in the treatment of large bone defects in patients with defective fracture healing57–60 and cartilage defects.61–63 In a recent study of atrophic tibial diaphyseal nonunion fractures, percutaneous autologous bone marrow stem cells were injected into60 atrophic non-union fractures of the tibia and the investigators reported positive effects that were correlated with the number of implanted hMSC.64 Also, promising preliminary results for treatment of femoral head osteonecrosis have been reported.65,66 Table 1 summarizes some of clinical trials using hMSC for bone regeneration. A number of clinical trials where hMSC has been employed for enhancing non-skeletal repair and are summarized in Table 2 and Figure 3. Both autologous and allogeneic MSC have been employed.
Graft-versus-host disease (GvHD) disease
GvHD is a potentially fatal disease that develops in the context of allogenic hematopoietic stem cell transplantation. In a pilot study, MSC was employed to treat 9 patients (8 patients with steroid refractory acute GvHD and 1 patient with chronic GvHD) where hMSC obtained from a 2 HLA identical sibling, 5 haploidentical donor and 4 HLA-mismatched donors, and resulted in the clinical recovery in 6 of the 8 patients.67 The beneficial effects of autologous hMSC transplantation have been also observed in a phase II clinical trial of 55 children and adult patients with acute severe and steroid resistant GvHD. Infusion of MSC was safe, and resulted in higher survival rates in patients with complete response (54.5%) and significantly lower transplantation-related mortality in patients with partial or no response (45.5%).68 In a recent randomized clinical trial of 32 patients with grade II–IV GvHD that either received intravenous autologous MSC (2 or 8 million MSCs/kg) or standard therapy, 77% of patients exhibited complete response and no MSC infusion-related toxicities were observed.69 In addition to using BM-derived MSC, Fang et al. also reported some success using human adipose tissue-derived hMSC for treatment of steroid-refractory GvHD.70,71 Currently several phase II and III clinical trials are being conducted to confirm these encouraging initial results and to define the role of MSC transplantation as either the primary therapy or as adjuvant therapy.
Heart diseases
MSC transplantation for enhancing myocardial tissue regeneration following acute myocardial infarction or chronic ischemic heart failure is an important area of regenerative medicine and has been initiated based on the positive therapeutic effects of marrow-derived MSC demonstrated in preclinical animal models.72–75 For example, in a porcine myocardial infarction (MI) model, bone-marrow derived MSCs injected directly into the myocardium were found to efficiently engraft into the myocardium and to significantly reduce infarct size, wall thinning, and contractile dysfunction.72 In humans, a placebo-controlled clinical study of intra-coronary injection of autologous MSC in 69 patients with acute myocardial infarction where MSC was injured within 12 hours after the onset of symptoms. No side effects or toxicity were reported during the 6-month follow-up and beneficial effects of increased left ventricular ejection fraction and Left ventricular end diastolic volume, improved contractility and enhanced infarct viability were reported.76 In a recent study, Hera et al performed a double-blind, placebo-controlled, dose-ranging (0.5, 1.6, and 5 million hMSC cells per kg) safety trial of intravenous allogeneic hMSCs conducted in 53 patients with anterior myocardial infarction with a follow up period of 6 months. No side effects of therapy were observed and global symptom score and ejection fraction (an estimated of left ventricular function) were significantly improved in the hMSC-treated group compared to controls.77 Results from these initial studies are encouraging and demonstrate the need for confirmation in larger randomized clinical trials.
Neurological diseases
Preclinical studies have demonstrated the importance of post-injury neurogenesis for clinical recovery in animal models of stroke and that local transplantation of MSCs enhanced this process.78–80 A recent study in rats has demonstrated the beneficial effects of administering allogenic MSC during the acute phase of ischemic stroke in improving neurological recovery and decreasing brain damage. The beneficial effects have been reported to be obtained regardless of the administration route (intravenous or intracarotid).78 Recently, the results of an open-label, observer blinded clinical trial in 52 patients with severe middle cerebral artery territory infarct have been reported. Patients were randomly allocated to receive intravenous autologous ex vivo cultured MSC (5×107 cells/patient) (MSC group) or standard therapy (control group) and both groups were followed for 5 years. The mortality rate in the MSC-treated group was lower and some improvement in the functional recovery in MSC-treated group was observed.81
The use of MSC in therapy has been reported in a few other neurological diseases. Multiple system atrophy (MSA) is a sporadic, progressive, adult-onset neurodegenerative disorder associated with varying degrees of symptoms of Parkinsonism, autonomic dysfunction, and cerebellar ataxia with no available drug treatment. Lee et al evaluated the feasibility and safety of therapy of autologous MSC infusion in 29 patients with MSA and reported that MSC transplantation delayed the progression of neurological deficits as well as significantly greater functional improvement in the MSC-treated patients and with no serious adverse effects related to MSC therapy.82
Renal diseases
As mentioned above, the anti-inflammatory effects of transplanted MSC may offer a novel therapeutic strategy in acute and chronic kidney disease and also for treatment of renal allograft rejection.83 In pre-clinical studies, the beneficial effects of MSC infusion on repair of acute renal damage have been reported. In an acute renal failure (ARF) mouse model, injection of allogeneic BM-derived MSCs protected cisplatin-treated mice from renal function impairment and severe tubular injury. Donor cells were shown to localize within the tubular epithelial lining suggesting that MSC engrafted within the damaged kidney.84 Lange et al85 reported that in a rat ARF model, MSC-treated animals had both significantly better renal functions and lower injury scores. The specificity of MSC effects were studied by Togel et al86 in rats with ischemia-reperfusion-induced acute renal failure. The authors reported that intra-carotid artery administration of MSC after the onset of renal ischemia resulted in significantly improved renal functions compared with animals treated with fibroblasts. Also, preliminary results of a phase I clinical trial of autologous MSC in 5 patients with post operative acute kidney injury (AKI) have shown that administration of autologous MSC was safe and some beneficial effects as renal function was well preserved postoperatively and none of the patients required hemodialysis.87
Other diseases
Systemic lupus erythromatosis (SLE) is a systemic multi-organ autoimmune disease that involves the cardiovascular system, joints and kidneys. The pathogenesis of SLE has been attributed to T cell deficiencies, polyclonal B-cell activation, macrophage dysfunction and environmental factors such as hormonal disturbances. 88 Also, defects in hematopoietic stem cells have been suggested as a pathological mechanism underlying SLE.89 The effects of allogeneic intravenous infusion of umbilical cord-derived MSC (1×106 cells/kg body weight) in 15 patients with SLE patients has been recently published and demonstrated safety, improvement of clinical symptoms and stabilization of inflammatory markers.90
Another inflammatory condition where MSC has been employed is Crohn disease. Results of a phase II randomized controlled trial in patients with Crohn disease-related perianal fistulas refractory to conventional therapies have been reported.91 Intravenous (2×107 cells) autologous adipose-tissue derived and expanded MSCs were given to 24 patients along with fibrin glue (FG) and the effects were compared to 25 control patients that received FG alone. The administration of MSC in combination with FG was not associated with any side effects and was associated higher rates of healing. MSC have been tried for treatment of recto-vaginal fistula in a recent case report.92
Uncontrolled and commercial stem cell therapy (stem cell tourism)
The enthusiastic media coverage of recent developments in stem cell biology and the positive initial results of stem cell-based clinical trials, have created unrealistic public understanding of the nature of the available stem cell therapies. Many patients and their families think that it is possible to treat a large number of chronic degenerative diseases using stem cells. The situation became complicated due to the wide use of the internet as a source for medical information and the establishment of several commercial companies that offer uncontrolled stem cell therapies. These companies employ stem cells from a wide variety of sources, including embryonic stem cells, and they claim that stem cells can be employed to treat all diseases! These commercial companies usually provide therapy that is very expensive and without any proof for efficacy. As mentioned above, stem cell therapy for chronic degenerative conditions is experimental therapy and should not be provided outside clinical trials in academic institutions. Patients should be discouraged from receiving stem cell therapies from commercial companies. The International Stem Cell Research Society (ISCRS) has responded to the increasing demands for objective information and guidance regarding stem cell therapy by publishing a guide to patients and their families (please see: http://www.isscr.org/clinical_trans/patient_handbook.html).
Conclusions and future perspectives
The potential for MSC use in therapy is enormous and positive results have been obtained in preclinical animal models of human diseases and from phase I/II clinical trials with very promising preliminary results. We think that combining basic research studies identifying the mechanisms controlling of MSC cell proliferation and differentiation with well designed and controlled clinical trials, will bring major advances in realizing the potential of MSC-based therapy for treatment of chronic and degenerative diseases.
Acknowledgment
This manuscript was supported by grants from; King Abdulaziz City for Science and Technology (09-BIO740-20), and from the Lundbeck foundation, Denmark.
REFERENCES
- 1.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19(3):180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 2.Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113(12):1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luk JM, Wang PP, Lee CK, Wang JH, Fan ST. Hepatic potential of bone marrow stromal cells: development of in vitro co-culture and intra-portal transplantation models. J Immunol Methods. 2005;305(1):39–47. doi: 10.1016/j.jim.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Dexter TM. Haemopoiesis in long-term bone marrow cultures. A review. Acta Haematol. 1979;62(5–6):299–305. doi: 10.1159/000207593. [DOI] [PubMed] [Google Scholar]
- 5.Owen M. Marrow stromal stem cells. J Cell Sci Suppl. 1988;10:63–76. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea- pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Luria EA, Panasyuk AF, Friedenstein AY. Fibroblast colony formation from monolayer cultures of blood cells. Transfusion. 1971;11(6):345–349. doi: 10.1111/j.1537-2995.1971.tb04426.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdallah BM, Haack-Sorensen M, Burns JS, et al. Maintenance of differentiation potential of human bone marrow mesenchymal stem cells immortalized by human telomerase reverse transcriptase gene despite [corrected] extensive proliferation. Biochem Biophys Res Commun. 2005;326(3):527–538. doi: 10.1016/j.bbrc.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Foster LJ, Zeemann PA, Li C, Mann M, Jensen ON, Kassem M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23(9):1367–1377. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 10.Dominici M, Le BK, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah BM, Ditzel N, Kassem M. Assessment of bone formation capacity using in vivo transplantation assays: procedure and tissue analysis. Methods Mol Biol. 2008;455:89–100. doi: 10.1007/978-1-59745-104-8_6. [DOI] [PubMed] [Google Scholar]
- 12.Kassem M, Mosekilde L, Eriksen EF. 1,25-dihydroxyvitamin D3 potentiates fluoride-stimulated collagen type I production in cultures of human bone marrow stromal osteoblast-like cells. J Bone Miner Res. 1993;8(12):1453–1458. doi: 10.1002/jbmr.5650081207. [DOI] [PubMed] [Google Scholar]
- 13.Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996;11(3):312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- 14.Gronthos S, Graves SE, Ohta S, Simmons PJ. The STRO -1+ fraction of adult human bone marrow contains the osteogenic precursors. Blood. 1994;84(12):4164–4173. [PubMed] [Google Scholar]
- 15.Stenderup K, Justesen J, Eriksen EF, Rattan SI, Kassem M. Number and proliferative capacity of osteogenic stem cells are maintained during aging and in patients with osteoporosis. J Bone Miner Res. 2001;16(6):1120–1129. doi: 10.1359/jbmr.2001.16.6.1120. [DOI] [PubMed] [Google Scholar]
- 16.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Delorme B, Ringe J, Gallay N, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2007 doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 18.Buhring HJ, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Ann N Y Acad Sci. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153(5):1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72(2):135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- 21.De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44(8):1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189(1):54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 23.in ‘t Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003;88(8):845–852. [PubMed] [Google Scholar]
- 24.Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98(8):2396–2402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 25.Gronthos S, Robey PG, Boyde A, Shi S. Human dental pulp stem cells (DPSCs): Characterization and developmental potential. J Bone Miner Res. 2001;16:S265. [Google Scholar]
- 26.Otaki S, Ueshima S, Shiraishi K, et al. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007 doi: 10.1016/j.cellbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Miura M, Gronthos S, Zhao M, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindroos B, Suuronen R, Miettinen S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 29.Djouad F, Bony C, Haupl T, et al. Transcriptional profiles discriminate bone marrow-derived and synovium-derived mesenchymal stem cells. Arthritis Res Ther. 2005;7(6):R1304–R1315. doi: 10.1186/ar1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Yamada Y, Fujimoto A, Ito A, Yoshimi R, Ueda M. Cluster analysis and gene expression profiles: a cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials. 2006;27(20):3766–3781. doi: 10.1016/j.biomaterials.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Larsen KH, Frederiksen CM, Burns JS, Abdallah BM, Kassem M. Identifying a molecular phenotype for bone marrow stromal cells with in vivo bone-forming capacity. J Bone Miner Res. 2010;25(4):796–808. doi: 10.1359/jbmr.091018. [DOI] [PubMed] [Google Scholar]
- 33.Foster LJ, Zeemann PA, Li C, Mann M, Jensen ON, Kassem M. Differential expression profiling of membrane proteins by quantitative proteomics in a human mesenchymal stem cell line undergoing osteoblast differentiation. Stem Cells. 2005;23(9):1367–1377. doi: 10.1634/stemcells.2004-0372. [DOI] [PubMed] [Google Scholar]
- 34.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Walsh S, Jefferiss C, Stewart K, Jordan GR, Screen J, Beresford JN. Expression of the developmental markers STRO -1 and alkaline phosphatase in cultures of human marrow stromal cells: regulation by fibroblast growth factor (FGF)-2 and relationship to the expression of FGF receptors 1–4. Bone. 2000;27(2):185–195. doi: 10.1016/s8756-3282(00)00319-7. [DOI] [PubMed] [Google Scholar]
- 36.Simonsen JL, Rosada C, Serakinci N, et al. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat Biotechnol. 2002;20(6):592–596. doi: 10.1038/nbt0602-592. [DOI] [PubMed] [Google Scholar]
- 37.Luu HH, Song WX, Luo X, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–677. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 38.Noel D, Gazit D, Bouquet C, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells. 2004;22(1):74–85. doi: 10.1634/stemcells.22-1-74. [DOI] [PubMed] [Google Scholar]
- 39.Le BK, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HL A expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31(10):890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 40.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells 6. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 41.Di NM, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 42.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 43.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 44.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 45.Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43(11):1018–1023. doi: 10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65(8):3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 47.de la FR, Bernad A, Garcia-Castro J, Martin MC, Cigudosa JC. Retraction: Spontaneous human adult stem cell transformation. Cancer Res. 2010;70(16):6682. doi: 10.1158/0008-5472.CAN-10-2451. [DOI] [PubMed] [Google Scholar]
- 48.Garcia S, Bernad A, Martin MC, Cigudosa JC, Garcia-Castro J, de la FR. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res. 2010;316(9):1648–1650. doi: 10.1016/j.yexcr.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 49.Lazennec G, Jorgensen C. Concise review: adult multipotent stromal cells and cancer: risk or benefit? Stem Cells. 2008;26(6):1387–1394. doi: 10.1634/stemcells.2007-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 51.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Togel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292(5):F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 53.Rose RA, Jiang H, Wang X, et al. Bone marrow-derived mesenchymal stromal cells express cardiac-specific markers, retain the stromal phenotype, and do not become functional cardiomyocytes in vitro. Stem Cells. 2008;26(11):2884–2892. doi: 10.1634/stemcells.2008-0329. [DOI] [PubMed] [Google Scholar]
- 54.Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49(3):328–337. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 55.Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18(9):959–963. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 56.Viateau V, Guillemin G, Bousson V, et al. Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25(6):741–749. doi: 10.1002/jor.20352. [DOI] [PubMed] [Google Scholar]
- 57.Goel A, Sangwan SS, Siwach RC, Ali AM. Percutaneous bone marrow grafting for the treatment of tibial non-union. Injury. 2005;36(1):203–206. doi: 10.1016/j.injury.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 58.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 59.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 60.Tilley S, Bolland BJ, Partridge K, et al. Taking tissue-engineering principles into theater: augmentation of impacted allograft with human bone marrow stromal cells. Regen Med. 2006;1(5):685–692. doi: 10.2217/17460751.1.5.685. [DOI] [PubMed] [Google Scholar]
- 61.Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: two case reports. Cell Transplant. 2004;13(5):595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- 62.Kitoh H, Kitakoji T, Tsuchiya H, et al. Transplantation of marrow-derived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis--a preliminary result of three cases. Bone. 2004;35(4):892–898. doi: 10.1016/j.bone.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 63.Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16(6):571–577. doi: 10.1053/jars.2000.4827. [DOI] [PubMed] [Google Scholar]
- 64.Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi: 10.2106/JBJS.D.02215. [DOI] [PubMed] [Google Scholar]
- 65.Kawate K, Yajima H, Ohgushi H, et al. Tissue-engineered approach for the treatment of steroid-induced osteonecrosis of the femoral head: transplantation of autologous mesenchymal stem cells cultured with beta-tricalcium phosphate ceramics and free vascularized fibula. Artif Organs. 2006;30(12):960–962. doi: 10.1111/j.1525-1594.2006.00333.x. [DOI] [PubMed] [Google Scholar]
- 66.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. Surgical technique. J Bone Joint Surg Am. 2005;87(Suppl 1(Pt 1)):106–112. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 67.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 68.Le BK, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 69.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15(7):804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Fang B, Song YP, Liao LM, Han Q, Zhao RC. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38(5):389–390. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 71.Fang B, Song Y, Lin Q, et al. Human adipose tissue-derived mesenchymal stromal cells as salvage therapy for treatment of severe refractory acute graft-vs.-host disease in two children. Pediatr Transplant. 2007;11(7):814–817. doi: 10.1111/j.1399-3046.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 72.Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002;73(6):1919–1925. doi: 10.1016/s0003-4975(02)03517-8. [DOI] [PubMed] [Google Scholar]
- 73.varez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425(6961):968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 74.Dai W, Hale SL, Martin BJ, et al. Allogeneic mesenchymal stem cell transplantation in post-infarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 75.Noiseux N, Gnecchi M, Lopez-Ilasaca M, et al. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14(6):840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 76.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 77.Hare JM, Traverse JH, Henry TD, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gutierrez-Fernandez M, Rodriguez-Frutos B, Alvarez-Grech J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 79.Pavlichenko N, Sokolova I, Vijde S, et al. Mesenchymal Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- 80.Wakabayashi K, Nagai A, Sheikh AM, et al. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88(5):1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- 81.Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28(6):1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 82.Lee PH, Kim JW, Bang OY, Ahn YH, Joo IS, Huh K. Autologous mesenchymal stem cell therapy delays the progression of neurological deficits in patients with multiple system atrophy. Clin Pharmacol Ther. 2008;83(5):723–730. doi: 10.1038/sj.clpt.6100386. [DOI] [PubMed] [Google Scholar]
- 83.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15(7):1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 85.Lange C, Togel F, Ittrich H, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68(4):1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 86.Togel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289(1):F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 87.Anna G, John D, Jean F, et al. Initial report on a phase I clinical trial: Prevention and treatment of post-operative Acute Kidney Injury with allogeneic Mesenchymal Stem Cells in patients who require on-pump cardiac surgery. Cellular Therapy and Transplantation. 2008 Dec 24;(2):31–35. 2008;1(2, 24 Dec 2008):31–35. [Google Scholar]
- 88.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 89.Ikehara S, Good RA, Nakamura T, et al. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc Natl Acad Sci U S A. 1985;82(8):2483–2487. doi: 10.1073/pnas.82.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H, Zeng X, Sun L. Allogenic bone-marrow-derived mesenchymal stem cells transplantation as a novel therapy for systemic lupus erythematosus. Expert Opin Biol Ther. 2010;10(5):701–709. doi: 10.1517/14712591003769816. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52(1):79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Olmo D, Herreros D, De-La-Quintana P, et al. Adipose-derived stem cells in Crohn’s rectovaginal fistula. Case Report Med. 2010;2010:961758. doi: 10.1155/2010/961758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang CH, Stanton RP, Glutting J. Unicameral bone cysts treated by injection of bone marrow or methylprednisolone. J Bone Joint Surg Br. 2002;84(3):407–412. doi: 10.1302/0301-620x.84b3.12115. [DOI] [PubMed] [Google Scholar]
- 94.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364(9436):766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 95.Sun L, Wang D, Liang J, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]