Abstract
The discovery of the molecular mechanisms underlying the circadian clock, which functions in virtually every cell throughout the body to coordinate biological processes to anticipate and better adapt to daily rhythmic changes in the environment, is one of the major biomedical breakthroughs in the 20th century. Twenty years after this breakthrough, the biomedical community is now at a new frontier to incorporate the circadian clock mechanisms into many areas of biomedical research, as studies continue to reveal an important role of the circadian clock in a wide range of biological functions and diseases. A forefront of this exciting area is the research of interactions between the clock and energy metabolism. In this review, we summarize animal and human studies linking disruptions of the circadian clock, either environmental or genetic, to metabolic dysfunctions associated with obesity, diabetes, and other metabolic disorders. We also discuss how these advances in circadian biology may pave the way to revolutionize clinical practice in the era of precision medicine.
Keywords: circadian clock, energy metabolism, obesity, diabetes
Introduction
Most living organisms have an internal circadian clock that regulates diverse 24-hour rhythms such as body temperature, sleep/wake, and feed/fast cycles. This central clock in mammals is located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus [1]. While normally entrained to the 24-hour light/dark cycle as the earth rotates on its axis on a daily basis, the SCN central clock continues to provide endogenous cycles even in the absence of any external light/dark or other diurnal 24-hour environmental signals. The endogenously generated rhythms have a period of about a day (e.g. 23~25-hour cycles), and thus the internal clock is referred to as a “circadian” (about a day) clock. Many organisms are programmed to be active and thus feed during the night (nocturnal) while others are active during the light portion of the 24-hour day (diurnal). Humans are by nature diurnal, and an evolutionary history over millions of years have led humans and their humanoid ancestors to be active during the light (day) and inactive or asleep during the dark (night) portion of the day. Alas, with the ability to make light at night, especially with the invention and use of the electric light bulb 100+ years ago, humans often decide to ignore Mother Nature and to become a diurnal/nocturnal organism with many individuals in modern society being active (and eating) during the dark as well as the day. In many cases, we even reverse our natural diurnality by being active (and eating) all night long and sleeping (or at least attempting to sleep) in the light portion of the day (e.g., shift workers).
While disrupted circadian rhythms, as in shift work, have been associated with adverse health effects for many years, our understanding of the mechanisms by which disrupted circadian regulation could impact health took a giant leap forward with the identification and cloning of circadian clock genes, first in flies and later in mice [2–7]. This led to the discovery of the core molecular mechanism of circadian clocks, a transcriptional/translational feedback loop, in which transcriptional suppressors PER and CRY repress the activity of transcriptional activators CLOCK and BMAL1, shutting down their own transcription and generating ~24-hour rhythms in the expression levels of clock genes as well as clock-controlled genes [8]. Equally important, and quite unexpected was the discovery that the molecular clock was soon found in almost all the cells of the body [9–11] and this molecular timing system was regulating the rhythmic expression of about 10% of all expressed genes in a given tissue in a tissue-specific fashion and collectively over 50% of the genome [12–15]. The significance of this discovery has likely contributed greatly to the awarding of the 2017 Nobel Prize in Physiology or Medicine to the trio of circadian biologists who cloned the first circadian clock gene, period (per), in fruit flies in 1984 (Michael Young, Michael Rosbash, and Jeff Hall). The fact that the circadian clock is operating in every tissue implies that “for many tissues correct timing is important enough to warrant keeping track of it locally”, as Young noted in his article published by Scientific American in 2000 [16]. Observations in many areas of biomedical research have since supported this prediction and elucidated molecular cascades that link the molecular clock to metabolism, endocrine regulation, immune functions, cell proliferation/differentiation, sleep/wake, psychiatric/cognitive functions, and many other biological processes in various organs and tissues [17, 18]. These advances have led to new frontiers of circadian biomedical research, where we are beginning to understand how circadian programs are coordinated within and across tissues/organs in health and dis-coordinated in disease.
In this review, we present findings from studies in animals and humans demonstrating effects of disrupted circadian rhythms on metabolic balance and body weight regulation. This area of physiology is perhaps the most “mature” area for understanding how circadian clock genes interact with other gene networks of cellular functions. We then expand our focus and briefly summarize the links between disrupted circadian clocks and other diseases. We conclude by discussing the challenges and opportunities that have been made available by the exciting scientific breakthroughs over the past few decades to incorporate circadian mechanisms into standard clinical practice for improved clinical outcomes and overall human health.
Animal models
An early significant finding to support a role of the molecular clock in body weight regulation and metabolic disorders came from our study of the ClockΔ19 mutant mice [19]. ClockΔ19 is a dominant-negative mutant allele identified in a mutagenesis screen in mice [5], which led to the discovery of the positive arm of the transcriptional/translational core clock machinery. ClockΔ19 mice have a much weakened clock (low rhythmic output) with a long circadian period, and this mouse model has been used extensively over the past two decades as a primary genetic model that has directly linked the molecular clock machinery to many aspects of physiology. In 2005, we found that homozygous ClockΔ19 mutant mice were obese and showed signs of metabolic syndrome, including hyperleptinemia, hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia [19]. This finding directly linked the circadian machinery to metabolic functions and body weight regulation. Importantly, feeding rhythms in these animals are blunted: mutant animals eat roughly the same amount during the day and night, while the wildtype animals eat more (~70–80% of the daily food consumption) during the night as is typical for nocturnal animals, implying that disruptions of the circadian feeding rhythm and eating during the wrong time of the day are associated with obesity [19]. The finding that a genetic mutation in a core circadian clock gene leads to obesity and metabolic dysfunction has allowed the biomedical community to quickly tie the clock machinery with metabolic pathways, revealing highly intertwined clock-metabolic networks [20] particularly in metabolic tissues and organs. For example, accumulating evidence has now highlighted a role of the circadian clock machinery in the differentiation and function of the adipose tissue, providing a mechanistic view of the circadian metabolic program (for a review, see [21]). Together, these advances represent a key step towards “a unified ‘systems’ map of metabolic disease” [22].
The relationships between the functioning of the circadian clock and body weight regulation are bidirectional. Mice that are obese due to feeding on a high-fat diet show altered diurnal patterns of locomotor and feeding rhythms such that a significant portion of locomotor activity and food intake occur during the light portion of the day (i.e., the “wrong” time for nocturnal animals) [23, 24]. This association between obesity and eating at the wrong time of the day in mice fed a high fat diet makes a striking resemblance to that observed in the in the ClockΔ19 mice. Similar observations have been made in genetic models of obesity. Mice genetically deficient in leptin (i.e., ob/ob mice) or its receptor (i.e., db/db mice) are obese and diabetic, and intriguingly, both models show significantly attenuated diurnal rhythms in the locomotor activity [25, 26]. Leptin indeed appears to be a central link between circadian food intake and body weight regulation. As a hormone important for the regulation of food intake and energy homeostasis, circulating levels of leptin in humans exhibit diurnal variations [27], which is independent of the sleep/wake and fasting/feeding cycles as it persisted under a constant routine protocol [28]. Animal studies have demonstrated that the leptin rhythms are dependent on the SCN [29] and the transcription of the leptin gene in adipocytes are under direct control of the circadian clock machinery [30]. More recent data suggest that leptin modulates gastric vagal afferent (VGA) mechanosensitivity in a circadian fashion [31]. Obesity induced by a high-fat diet blunts the circadian variation in the leptin modulation of VGA signaling, which may contribute to arrhythmic eating behavior seen in the high-fat diet fed mice [31]. Furthermore, circadian disruptions due to clock gene deficiency or chronic repetitive shits of the light/dark cycle lead to leptin resistance in mice [30]. Together, these findings have important implications for body weight regulation since obesity and disruptions in circadian rhythms may be reciprocally contributing to dysfunctions in both regulatory systems and exacerbate the negative health consequences.
The findings from a number of different animal models indicate that the time of day at which feeding occurs may be important for body weight regulation. Indeed, when comparing mice fed on a high-fat diet only during the light phase of the day (i.e., the “wrong” time) to those fed on the same diet but only during the dark phase (i.e., the “right” time) of day, we found that the wrong-time-fed mice gained weight at a faster pace and the differences became significant in only 2 weeks [32]. Importantly, the amount of daily food intake and the activity levels were not significantly different between the two groups of animals [32]. These observations have provided early implications that when to eat, in addition to what and how much, is important for body weight regulation. It is interesting to note that, unlike the master circadian clock in the SCN, peripheral clocks in metabolic organs, such as the fat body in flies and the liver in mammals, can be entrained to feeding schedules [33–35]. Hence, wrong-time feeding creates a conflict between the transcriptional output of the local clock and systemic neuroendocrine signals from the master clock, which may contribute to a modulated metabolic output that can be reflected by a reprogrammed circadian transcriptome [33, 36].
Follow up studies by Panda and colleagues further demonstrated that mice fed on a time-restricted schedule during the dark phase of the day were protected from obesity, glucose intolerance, hyperinsulinemia, leptin resistance, and steatohepatitis that occur in mice fed on high-fat or high-sugar diets [37, 38]. Mice on time-restricted feeding showed these metabolic benefits without reducing the total amount of caloric intake, but exhibited changes in the liver and serum metabolome that suggested an improved energy expenditure [37, 38]. Remarkably, by switching to time-restricted dark-phase feeding, mice with diet-induced obesity and type 2 diabetes showed reduced body weight and reversed progression of metabolic diseases [38]. These observations are similar to earlier findings that time-restricted feeding during the dark reduced weight gain in obese rats with deficient leptin receptors without changing their total caloric intake [39]. Together, these findings in different animal models suggest restricting feeding to the right time of the day is both preventive and therapeutic to metabolic insults induced by unhealthy diet and genetic deficiency.
Studies in humans
The importance of feeding time in body weight regulation suggested by animal studies has led to studies in humans to understand the effects on body weight when calories are consumed at different times of the day. Garaulet, Scheer, and colleagues have studied the effects of mealtime in 420 subjects (49.5% female) participating in a 20-week weight-loss treatment program and found that subjects who ate their daily main meal at earlier times of the day showed a more rapid loss of body weight [40]. Remarkably, similar to what has been observed in animal models, early eaters and late eaters showed similar energy intake, dietary composition, and estimated energy expenditure [40], suggesting a better metabolic balance in early eaters. Importantly, associations between a late mealtime and reduced effectiveness of bariatric surgery in the treatment of severe obesity have been reported in a group of 270 patients [41]. Furthermore, a later “chronotype” (i.e., habitual timing of sleep/wake and fast/feed) has been associated with higher body mass index and body fat content in the general population [42, 43], providing further support for a link between the late eating time and an adverse outcome in body weight regulation in normal as well as obese subjects. It is important to note that, due to individual differences in circadian timing in humans, eating at a late clock time in individuals whose circadian timing is significantly later than normal does not necessarily equal to eating at a “wrong time” of the day. A recent study addressed this particular issue by using the timing of melatonin release as a marker of the endogenous circadian time, and showed that increased body fat content was associated with the circadian timing, rather than the clock time, of food intake [44].
From a historical perspective, it is interesting to note that, in the 1950s, taking a large portion of the daily caloric intake (>25%) at a time much later than normal had been described in a group of obese individuals seeking weight loss treatment [45]. This abnormal eating pattern, termed night eating disorder (NED), is characterized by recurrent episodes of night eating, either consuming a large number of calories after dinnertime or eating after awakening from sleep at night. NED is estimated to occur in ~1.5% of the U.S. population, and the prevalence is significantly increased in obese patients, particularly during times of life stress and weight gain [46]. It is interesting to note that non-obese NED patients are overall very similar to obese NED patients in the self-reported eating behaviors, sleep quality, and mood, but are strikingly younger (by ~9 years) [47], implying that NED may contribute to the risk of obesity later in life. Future longitudinal studies are needed to test this intriguing hypothesis.
While associations between mealtime and body weight observed in the above-mentioned studies do not establish causality, recent studies have directly tested metabolic outcomes of distributing caloric intake at different times of the day. In an elegant study, Jakubowicz and colleagues compared obese women who were randomly assigned into two isocaloric weight-loss groups with high caloric intake occurring at either breakfast or dinner for 12 weeks [48]. They found consuming a large breakfast and a small dinner caused greater weight loss and greater improvements in multiple metabolic parameters compared to having a small breakfast and a large dinner [48]. In addition, a randomized, crossover clinical trial found that delaying dinner time close to the habitual bedtime, when circulating melatonin levels are rising, led to impaired glucose tolerance [49]. Interestingly, genetic variations in MTNR1B, a gene encoding a melatonin receptor, have been associated with risk of type 2 diabetes [50, 51], and subjects carrying the diabetic risk allele of MTNR1B showed augmented impairment of glucose tolerance when the dinner time was delayed close to the time of daily rise of melatonin levels [49]. These findings thus establish a causal role of late mealtime in adverse metabolic consequences and imply a potential underlying mechanism involving the circadian melatonin rhythms.
In addition to eating at a particularly late time of day, wrong-time feeding can result from frequent shifts of activity/rest (and thus feed/fast) schedules due to work and social demands. According to a 2004 survey by the Bureau of Labor Statistics, 17.7% of US workers usually work alternate shifts that fall at least partially outside the daytime shift range [52]. The irregular work schedule is often associated with eating at a time of the day when a person would normally not be eating [53, 54]. Thus, the frequent occurrence of eating at the wrong time of day, in addition to factors such as unhealthy diets and insufficient sleep, could contribute to the higher risks of obesity, diabetes, and cardiovascular diseases observed in shift workers [53, 55]. In addition to shift work, eating time can be influenced by work and social demands in the form of “social jet lag”, a phrase used to describe weekly circadian phase shifts for an earlier sleep/wake time during workdays to accommodate work/social schedules and a later sleep/wake time during weekends, equivalent to traveling across time zones twice a week [56]. In a large study of Europeans, ~70% of the participants were found to have a social jet lag of at least 1 hour, and a more severe social jet lag was associated with a higher body mass index [57]. Thus, frequent circadian phase shifts may be linked to adverse effects on body weight regulation, similar to what has been found in those eating at the wrong time of day. In rats, adverse metabolic outcomes, including an increased weight gain, can result from a weekly “shift work” scenario, in which the rats are kept awake during the light phase 8 hours a day, 5 days a week, and are allowed to sleep ad libitum during the 2-day “weekends” [58]. Interestingly, these deficits were prevented by restricting food availability to the dark phase, suggesting that metabolic dysfunctions associated with chronic circadian phase shifts can be largely attributable to feeding at the wrong time of the day [58]. How much wrong-time eating contributes to the risk of obesity in shift workers and individuals with severe social jet lag remains an interesting topic for future studies.
Influence of the sleep/wake cycle
In humans, hunger and appetite show circadian rhythms that can be desynchronized from the sleep/wake cycles [59]. Nevertheless, time of feeding is influenced to a large extent by the sleep/wake cycle, since, except for rare parasomnias, we do not eat while asleep. Sleep/wake influences body weight regulation and metabolic outcomes beyond its role in determining the timing of feeding. It has long been noted that insufficient sleep is associated with a higher body mass index and an increased risk of diabetes (for reviews, see [60–63]), similar to the circadian misalignment caused by a late chronotype, shift work, or social jet lag. In many cases, it is difficult to separate out the effects of circadian disruptions and sleep disturbances. Shift work and late sleep timing often lead to insufficient sleep [64, 65], which could be a contributing factor to the risk of obesity and metabolic dysfunction. Conversely, activity and eating can occur at the wrong circadian time when sleep time is restricted to a short window of time [66]. Under such conditions, the circadian phase of awakening (as measured by the time in relation to an endogenous timing marker, the rhythm in circulating melatonin levels) has been correlated with insulin insensitivity, indicating that circadian misalignment contributes to the metabolic challenge induced by restricted sleep time [66]. Interestingly, ClockΔ19 mice, while overweight, also show a significant reduction in sleep of ~2 hours a day [67]. Indeed, sleep and the circadian clock are deeply embedded with each other at the molecular level, as mutations or deletions in almost all clock genes have been found to altered sleep architecture and/or homeostasis, in addition to changes in sleep timing [68]. Thus, disruptions in the circadian rhythmicity and sleep often accompany each other, making it difficult to dissect the exact contribution of each process to metabolic health.
More recent studies have attempted to separate out the effects of a disrupted circadian organization from inadequate sleep by scheduling the time of sleep/wake across different circadian phases. Under a so-called “forced desynchrony” protocol in which sleep/wake and feed/fast cycles are scheduled into 28-hour days and thus are decoupled from the endogenous circadian timing system, subjects showed decreased leptin, increased glucose, and increased insulin levels on the days when eating and sleep occurred ~12-hour apart from habitual times [69]. While this study could not rule out the effects of a decreased sleep efficiency (i.e., percent time spent in sleep when in bed) during circadian misalignment, statistical analysis controlling the effects of lower sleep efficiency suggested an independent effect of circadian misalignment [69]. Only recently have sleep-independent contributions of the circadian misalignment to metabolic dysfunctions been directly assessed [70]. In this study by Van Cauter and colleagues, subjects were restricted to only 5 hours of sleep each day, which occurred either during the middle of habitual bedtime (i.e., circadian alignment) or 8.5 hours later during the daytime (i.e., circadian misalignment). Compared to unrestricted nights, 5-hour sleep restriction resulted in a decreased insulin sensitivity in both groups, but the decrease was doubled in circadian misaligned subjects compared to circadian aligned subjects, even though both groups slept almost identical amount during the 5-hour sleep window. This observation demonstrates an augmented metabolic disruption that is induced by circadian misalignment independent of sleep loss [70], providing critical evidence that challenges in metabolic balance and body weight regulation under conditions such as shift work, late chronotype, and social jet lag are likely to result from independent contributions of both insufficient sleep and circadian misalignment. Therefore, strategies that mitigate the impact of both sleep and circadian disruptions are needed in order to combat these health challenges sufficiently.
Clock and other diseases
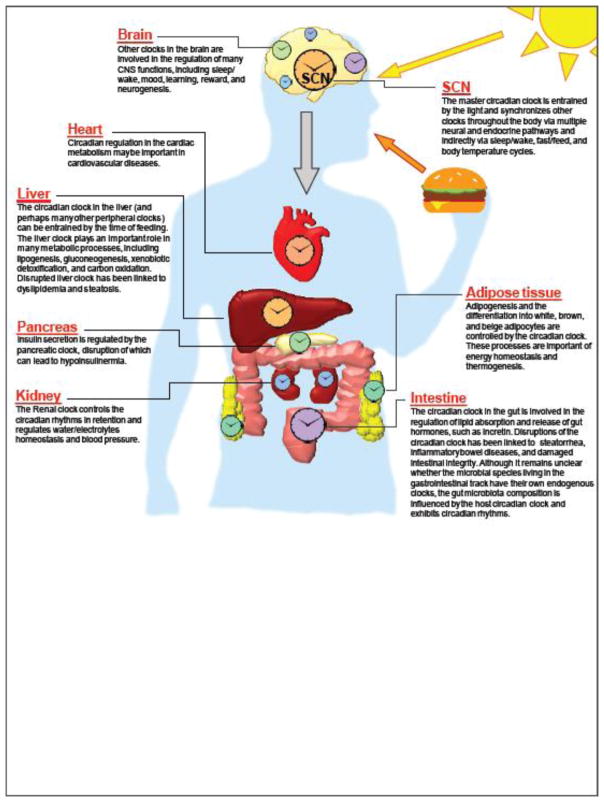
Critically, since the clock is functional in tissues across the body in addition to metabolic organs (Figure 1), the impact of circadian organization and disorganization to health and disease reaches far beyond metabolic balance and body weight regulation. Studies in both humans and animal models have linked circadian disruptions, either genetic (e.g., mutations in clock genes) or environmental (e.g., frequent circadian phase shifts), to a range of diseases, including cardiovascular disease, inflammatory bowel disease, other immune-related disorders (e.g., rheumatoid arthritis and asthma), various cancers, neurodegenerative disorders, psychiatric illness, cognitive impairment, and many others [17, 18]. In many cases, the circadian disruption acts as a “second hit” that exacerbates pathological insult. For example, disruptions of the circadian clock worsen toxin-induced colitis [71] and alcohol-induced “gut leakiness”, a deterioration in gastrointestinal barrier integrity [72]. Similarly, mutations in core clock genes promote tumorigenesis of radiation-induced lymphoma [73] as well as genetically induced lung adenoma and adenocarcinoma [74]. These genetic studies, in particular, begin to tie the clock machinery directly to the molecular cascades in the pathogenesis of tissue-specific disease states [18]. Similar to the bidirectional relationships between clock and energy metabolism, many of the links between the circadian clock and disease-relevant pathways are also bidirectional. For instance, the oncogene Myc modulates the expression of a core clock gene, Bmal1, and this process is dysregulated in cancer leading to facilitated energy metabolism of tumor cells [75]. In turn, the degradation of MYC protein is regulated by the core clock protein CRY and a circadian clock modulator FBXL3 in a ubiquitin protein ligase complex [76]. Taken together, these findings suggest that the circadian clock is linked to many cellular pathways key to disease pathology.
Figure 1. Coordinated circadian timing in tissues across the human body.
In humans, a master circadian clock located in the SCN of the brain synchronizes downstream clocks in cells throughout the body, regulating a wide range of tissue-specific functions. Health problems may occur when the synchronization and coordination of the circadian programs among tissues are disrupted.
This new and fast-growing area of research integrating circadian biology as a key component of biomedical research, although still in its infancy, is expected to pave the way for the use of circadian principles for the treatment and prevention of various diseases. Circadian transcriptomic studies have indeed revealed a large number of rhythmically expressed genes that are targets and metabolic enzymes of best-selling drugs [14], providing support to earlier circadian pharmacokinetics observations that an optimal treatment outcome can benefit from drug administration at a certain time of the day [77, 78]. In addition, an improved understanding of the interrelationships between the circadian clock and disease pathways could reveal novel treatment targets for drug development, as demonstrated by a recent study showing that treating obese mice with a clock-targeting drug controls body weight and alleviates metabolic dysfunctions in a clock-dependent manner [79]. In addition, a study in flies has demonstrated that caloric-restriction-induced lifespan extension requires an intact clock [80], adding new insights into the long-known bidirectional links between the circadian clock and aging [81–83], a strong risk factor for a wide range of diseases [84]. Thus, from a disease prevention perspective, it is important to further explore how a robust and well-coordinated circadian system may contribute to healthy aging and how a disrupted circadian clock system may contribute to age-related diseases as well as to the process of aging itself.
Circadian coordination beyond tissues and organs
A critical concept in understanding the role of circadian clock in health and disease is that the circadian synchronization and coordination, which lead to the harmonious timing across molecular pathways within a cell, across cells of related functions in a tissue, and across different tissues and organs throughout our body, are important for maintaining good health. Recent studies have extended this concept of circadian synchronization and coordination beyond the cells of our body. The gut microbiota, the collective community of thousands of microbial species living in the mammalian gastrointestinal tract, has been shown to interact with the digestive, metabolic, immune, and even neuropsychiatric and cognitive functions of the host, and a disrupted microbiota has been linked to a wide range of diseases [85, 86]. Interestingly, disruption of the circadian clock by weekly light-dark inversions results in a deviation from the normal microbiota in mice [87], similar to later findings in animals with genetic defects of clock genes [88–90]. Indeed, the gut microbial composition varies with a diurnal rhythm that is regulated by the host circadian clock and feeding time [89–92]. Conversely, peripheral clocks and the circadian transcriptome are modulated in the absence of gut microbiota [91, 93, 94]. While most of the microbiota studies focused on the intestinal microbiota, studies have demonstrated that altered microbiota at other parts of the gastrointestinal tract, such as the oral cavity, have also been linked to obesity and metabolic dysfunction [95–97]. A recent randomized, crossover study examined microbiota in the saliva samples from young, healthy women and found that the salivary microbial relative abundance and diversity exhibited circadian rhythms and the timing of the rhythms were influenced by the mealtime [98].
At this time, it is not clear whether at least some microbial species living in the mammalian gastrointestinal tract can endogenously generate their own ~24-hour rhythms in the absence of any timing cue from the host organism [99, 100]. This is a distinct possibility since it is known that the freely living cyanobacteria are capable of generating endogenous circadian rhythms [101]. Whether or not some microbial groups in the gastrointestinal tract have their own internal circadian clocks, these recent observations of interactions between gut microbiota and host circadian rhythms raise an intriguing hypothesis that many of the adverse effects of clock disruption may be due, in part, to the impact of an altered diversity and/or composition of gut microbiota. This is likely true at least for metabolic dysfunctions induced by jet lag, as transplantation of the fecal material from jet-lagged human subjects to germ-free mice leads to obesity and glucose intolerance similar to circadian disrupted mice [89]. Whether the gut microbiota and the pathways through which it interacts with the host can be a point of intervention to mitigate adverse health consequences associated with circadian disruptions, such as in shift work and social jet lag, remains an interesting and important subject for future studies.
Conclusions
The continuing accumulation of evidence linking the molecular clock machinery to a wide range of cellular pathways that play a central role in many diseases has now raised a new challenge to today’s circadian biomedical community: how to take advantage of these enormous advances and utilize them in clinical practice, particularly in the age of precision and personalized medicine. At the forefront of this challenge is the lack of reliable and easily accessible circadian biomarkers. Even in areas where some progress has been made towards using circadian principles in clinical practice, such as in the case of circadian pharmacokinetics [77], the full potential of circadian-timed treatment cannot be achieved without knowing precisely the internal circadian timing of the patient, especially given the diversity in human chorotypes [102]. Although transcriptomic and metabolomic studies have demonstrated in principle that it is possible to infer endogenous timing by accessing the omics at only one time point [103, 104], very limited progress has been made toward developing cost-effective technologies that rely on a smaller set of biomarkers and can be applied in a convenient manner in the clinic on a routine basis. Furthermore, it has not been demonstrated whether such methods can provide useful information regarding the exact nature of circadian disruptions, such as lost or reprogrammed rhythmicity of disease-relevant pathways as well as internal desynchrony among organs, which can be used to aid disease diagnosis or define characteristic subpopulations of certain diseases. Nevertheless, with the rapid advances in systems biology, information technologies, and wearable or implantable biosensors, it can be expected that it will be possible in the near future to continuously monitor an array of circadian biomarkers in real-time to assist medical professionals in many aspects of clinical practice from diagnosis to treatment and patient care. Perhaps the awarding of the Nobel Prize in Physiology or Medicine will inspire circadian researchers to more rapidly advance this new frontier of medicine, circadian medicine, so that it can be fully integrated into standard clinical practice, a necessity for medicine to be truly precise and personalized.
Highlights.
A disrupted the circadian clock is linked to adverse outcomes in energy metabolism.
Circadian coordination across tissues are important for health and disease.
Precision medicine may benefit from incorporation of circadian timing.
Acknowledgments
This work is supported by the National Institute on Aging of the NIH (Project Number 2P01AG011412-18A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annual review of physiology. 2010;72:551–77. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:2112–6. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312:752–4. doi: 10.1038/312752a0. [DOI] [PubMed] [Google Scholar]
- 4.Zehring WA, Wheeler DA, Reddy P, Konopka RJ, Kyriacou CP, Rosbash M, et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984;39:369–76. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- 5.Vitaterna MH, King DP, Chang AM, Kornhauser JM, Lowrey PL, McDonald JD, et al. Mutagenesis and Mapping of a Mouse Gene, Clock, Essential for Circadian Behavior. Science (New York, NY) 1994;264:719–25. doi: 10.1126/science.8171325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–53. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoch MP, Song EJ, Chang AM, Vitaterna MH, Zhao Y, Wilsbacher LD, et al. Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell. 1997;89:655–67. doi: 10.1016/s0092-8674(00)80246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The News and Editorial Staffs. Breakthroughs of the Year: The Runners-Up. Science (New York NY) 1998;282:2157–61. [PubMed] [Google Scholar]
- 9.Giebultowicz JM, Hege DM. Circadian clock in Malpighian tubules. Nature. 1997;386:664. doi: 10.1038/386664a0. [DOI] [PubMed] [Google Scholar]
- 10.Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science (New York, NY) 1997;278:1632–5. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 11.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–37. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 12.Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- 13.Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002:109. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:9305–19. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young MW. The Tick-Tock of the Biological Clock. Scientific American; 2000. [DOI] [PubMed] [Google Scholar]
- 17.Summa KC, Turek FW. Molecular Clocks throughout Body, Not Just Brain, Keep Tissues Humming. Scientific American; 2015. [Google Scholar]
- 18.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science (New York, NY) 2016;354:994–9. doi: 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 19.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science (New York, NY) 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang P, Turek FW. Timing of meals: when is as critical as what and how much. American journal of physiologyEndocrinology and metabolism. 2017;312:E369–e80. doi: 10.1152/ajpendo.00295.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Froy O, Garaulet M. The circadian clock in white and brown adipose tissue: mechanistic, endocrine and clinical aspects. Endocrine reviews. 2018 doi: 10.1210/er.2017-00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staels B. When the Clock stops ticking, metabolic syndrome explodes. Nat Med. 2006;12:54–5. doi: 10.1038/nm0106-54. discussion 5. [DOI] [PubMed] [Google Scholar]
- 23.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–21. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Pendergast JS, Branecky KL, Yang W, Ellacott KL, Niswender KD, Yamazaki S. High-fat diet acutely affects circadian organisation and eating behavior. The European journal of neuroscience. 2013;37:1350–6. doi: 10.1111/ejn.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW. Altered sleep regulation in leptin-deficient mice. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2006;290:R894–R903. doi: 10.1152/ajpregu.00304.2005. [DOI] [PubMed] [Google Scholar]
- 26.Grosbellet E, Dumont S, Schuster-Klein C, Guardiola-Lemaitre B, Pevet P, Criscuolo F, et al. Circadian phenotyping of obese and diabetic db/db mice. Biochimie. 2016;124:198–206. doi: 10.1016/j.biochi.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 27.Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. The Journal of clinical investigation. 1996;97:1344–7. doi: 10.1172/JCI118551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. The Journal of clinical endocrinology and metabolism. 2005;90:2537–44. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalsbeek A, Fliers E, Romijn JA, La Fleur SE, Wortel J, Bakker O, et al. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–85. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- 30.Kettner Nicole M, Mayo Sara A, Hua J, Lee C, Moore David D, Fu L. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metabolism. 2015;22:448–59. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kentish SJ, Vincent AD, Kennaway DJ, Wittert GA, Page AJ. High-Fat Diet-Induced Obesity Ablates Gastric Vagal Afferent Circadian Rhythms. The Journal of Neuroscience. 2016;36:3199–207. doi: 10.1523/JNEUROSCI.2710-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring, Md) 2009;17:2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu K, DiAngelo Justin R, Hughes Michael E, Hogenesch John B, Sehgal A. The Circadian Clock Interacts with Metabolic Physiology to Influence Reproductive Fitness. Cell Metabolism. 2011;13:639–54. doi: 10.1016/j.cmet.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & development. 2000;14:2950–61. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science (New York, NY) 2001;291:490–3. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 36.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21453–8. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong Eric A, Gill S, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metabolism. 2012;15:848–60. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaix A, Zarrinpar A, Miu P, Panda S. Time-Restricted Feeding Is a Preventative and Therapeutic Intervention against Diverse Nutritional Challenges. Cell Metabolism. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mistlberger RE, Lukman H, Nadeau BG. Circadian Rhythms in the Zucker Obese Rat: Assessment and Intervention. Appetite. 1998;30:255–67. doi: 10.1006/appe.1997.0134. [DOI] [PubMed] [Google Scholar]
- 40.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37:604–11. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Lozano T, Vidal J, de Hollanda A, Scheer F, Garaulet M, Izquierdo-Pulido M. Timing of food intake is associated with weight loss evolution in severe obese patients after bariatric surgery. Clinical nutrition (Edinburgh, Scotland) 2016;35:1308–14. doi: 10.1016/j.clnu.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. The Journal of clinical endocrinology and metabolism. 2015;100:1494–502. doi: 10.1210/jc.2014-3754. [DOI] [PubMed] [Google Scholar]
- 43.Arora T, Taheri S. Associations among late chronotype, body mass index and dietary behaviors in young adolescents. International journal of obesity (2005) 2015;39:39–44. doi: 10.1038/ijo.2014.157. [DOI] [PubMed] [Google Scholar]
- 44.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106:1213–9. doi: 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stunkard AJ, Grace WJ, Wolff HG. The night-eating syndrome. The American journal of medicine. 1955;19:78–86. doi: 10.1016/0002-9343(55)90276-x. [DOI] [PubMed] [Google Scholar]
- 46.McCuen-Wurst C, Ruggieri M, Allison KC. Disordered eating and obesity: associations between binge-eating disorder, night-eating syndrome, and weight-related comorbidities. Annals of the New York Academy of Sciences. 2017 doi: 10.1111/nyas.13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marshall HM, Allison KC, O’Reardon JP, Birketvedt G, Stunkard AJ. Night eating syndrome among nonobese persons. The International journal of eating disorders. 2004;35:217–22. doi: 10.1002/eat.10241. [DOI] [PubMed] [Google Scholar]
- 48.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring, Md) 2013;21:2504–12. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 49.Lopez-Minguez J, Saxena R, Bandin C, Scheer FA, Garaulet M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: A randomized, cross-over study. Clinical nutrition (Edinburgh, Scotland) 2017 doi: 10.1016/j.clnu.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nature genetics. 2009;41:82–8. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in MTNR1B influence fasting glucose levels. Nature genetics. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMenamin TM. A Time to Work: Recent Trends in Shift Work and Flexible Schedules. Monthly Labor Review. 2007;130:3–15. [Google Scholar]
- 53.Knutsson A. Health disorders of shift workers. Occupational Medicine. 2003;53:103–8. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- 54.Lennernas M, Hambraeus L, Akerstedt T. Shift related dietary intake in day and shift workers. Appetite. 1995;25:253–65. doi: 10.1006/appe.1995.0060. [DOI] [PubMed] [Google Scholar]
- 55.Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutrition research reviews. 2010;23:155–68. doi: 10.1017/S0954422410000016. [DOI] [PubMed] [Google Scholar]
- 56.Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. Handbook of experimental pharmacology. 2013:311–31. doi: 10.1007/978-3-642-25950-0_13. [DOI] [PubMed] [Google Scholar]
- 57.Roenneberg T, Allebrandt Karla V, Merrow M, Vetter C. Social Jetlag and Obesity. Current Biology. 2012;22:939–43. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 58.Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151:1019–29. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- 59.Scheer FAJL, Morris CJ, Shea SA. The Internal Circadian Clock Increases Hunger and Appetite in the Evening Independent of Food Intake and Other Behaviors. Obesity (Silver Spring, Md) 2013;21:421–3. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. European Journal of Endocrinology. 2008;159:S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel SR, Hu FB. Short Sleep Duration and Weight Gain: A Systematic Review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and Quality of Sleep and Incidence of Type 2 Diabetes. A systematic review and meta-analysis. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proceedings of the National Academy of Sciences. 2011;108:15609–16. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Åkerstedt T, Wright KP., Jr Sleep Loss and Fatigue in Shift Work and Shift Work Disorder. Sleep Medicine Clinics. 2009;4:257–71. doi: 10.1016/j.jsmc.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. Journal of biological rhythms. 2013;28:141–51. doi: 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]
- 66.Eckel RH, Depner CM, Perreault L, Markwald RR, Smith MR, McHill AW, et al. Morning Circadian Misalignment during Short Sleep Duration Impacts Insulin Sensitivity. Current biology: CB. 2015;25:3004–10. doi: 10.1016/j.cub.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Naylor E, Bergmann BM, Krauski K, Zee PC, Takahashi JS, Vitaterna MH, et al. The circadian clock mutation alters sleep homeostasis in the mouse. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:8138–43. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Franken P. A role for clock genes in sleep homeostasis. Current opinion in neurobiology. 2013;23:864–72. doi: 10.1016/j.conb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America. 2009:106. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leproult R, Holmback U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–9. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. American journal of physiologyRegulatory, integrative and comparative physiology. 2008;295:R2034–40. doi: 10.1152/ajpregu.00118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, et al. Disruption of the Circadian Clock in Mice Increases Intestinal Permeability and Promotes Alcohol-Induced Hepatic Pathology and Inflammation. PloS one. 2013;8:e67102. doi: 10.1371/journal.pone.0067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 74.Papagiannakopoulos T, Bauer M, Davidson S, Heimann M, Subbaraj L, Bhutkar A, et al. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell metabolism. 2016;24:324–31. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altman BJ, Hsieh AL, Sengupta A, Krishnanaiah SY, Stine ZE, Walton ZE, et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metab. 2015;22:1009–19. doi: 10.1016/j.cmet.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huber AL, Papp SJ, Chan AB, Henriksson E, Jordan SD, Kriebs A, et al. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Molecular cell. 2016;64:774–89. doi: 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Griffett K, Burris TP. The mammalian clock and chronopharmacology. Bioorganic & medicinal chemistry letters. 2013;23:1929–34. doi: 10.1016/j.bmcl.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dallmann R, Okyar A, Lévi F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends in Molecular Medicine. 2016;22:430–45. doi: 10.1016/j.molmed.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 79.He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23:610–21. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katewa SD, Akagi K, Bose N, Rakshit K, Camarella T, Zheng X, et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2016;23:143–54. doi: 10.1016/j.cmet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turek FW, Penev P, Zhang Y, van Reeth O, Zee P. Effects of age on the circadian system. Neuroscience and biobehavioral reviews. 1995;19:53–8. doi: 10.1016/0149-7634(94)00030-5. [DOI] [PubMed] [Google Scholar]
- 82.Banks G, Nolan PM, Peirson SN. Reciprocal interactions between circadian clocks and aging. Mammalian genome: official journal of the International Mammalian Genome Society. 2016;27:332–40. doi: 10.1007/s00335-016-9639-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mattis J, Sehgal A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends in endocrinology and metabolism: TEM. 2016;27:192–203. doi: 10.1016/j.tem.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Niccoli T, Partridge L. Ageing as a risk factor for disease. Current biology: CB. 2012;22:R741–52. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 85.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–70. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, et al. Circadian disorganization alters intestinal microbiota. PloS one. 2014;9:e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, et al. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcoholism, clinical and experimental research. 2016;40:335–47. doi: 10.1111/acer.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thaiss Christoph A, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler Anouk C, et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell. 2014;159:514–29. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 90.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:10479–84. doi: 10.1073/pnas.1501305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell host & microbe. 2015;17:681–9. doi: 10.1016/j.chom.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Microbiome. Cell Metabolism. 2014;20:1006–17. doi: 10.1016/j.cmet.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153:812–27. doi: 10.1016/j.cell.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 94.Thaiss Christoph A, Levy M, Korem T, Dohnalová L, Shapiro H, Jaitin Diego A, et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell. 167:1495–510.e12. doi: 10.1016/j.cell.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Goodson JM, Groppo D, Halem S, Carpino E. Is obesity an oral bacterial disease? Journal of dental research. 2009;88:519–23. doi: 10.1177/0022034509338353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takeshita T, Kageyama S, Furuta M, Tsuboi H, Takeuchi K, Shibata Y, et al. Bacterial diversity in saliva and oral health-related conditions: the Hisayama Study. Scientific reports. 2016;6:22164. doi: 10.1038/srep22164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeigler CC, Persson GR, Wondimu B, Marcus C, Sobko T, Modeer T. Microbiota in the oral subgingival biofilm is associated with obesity in adolescence. Obesity (Silver Spring, Md) 2012;20:157–64. doi: 10.1038/oby.2011.305. [DOI] [PubMed] [Google Scholar]
- 98.Collado MC, Engen PA, Bandin C, Cabrera-Rubio R, Voigt RM, Green SJ, et al. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2018;32:2060–72. doi: 10.1096/fj.201700697RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paulose JK, Wright JM, Patel AG, Cassone VM. Human Gut Bacteria Are Sensitive to Melatonin and Express Endogenous Circadian Rhythmicity. PloS one. 2016;11:e0146643. doi: 10.1371/journal.pone.0146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Johnson CH, Zhao C, Xu Y, Mori T. Timing the day: what makes bacterial clocks tick? Nature reviews. Microbiology. 2017;15:232–42. doi: 10.1038/nrmicro.2016.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kondo T, Strayer CA, Kulkarni RD, Taylor W, Ishiura M, Golden SS, et al. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:5672–6. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US - Influence of age and sex. PloS one. 2017;12:e0178782. doi: 10.1371/journal.pone.0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kasukawa T, Sugimoto M, Hida A, Minami Y, Mori M, Honma S, et al. Human blood metabolite timetable indicates internal body time. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15036–41. doi: 10.1073/pnas.1207768109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ueda HR, Chen W, Minami Y, Honma S, Honma K, Iino M, et al. Molecular-timetable methods for detection of body time and rhythm disorders from single-time-point genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11227–32. doi: 10.1073/pnas.0401882101. [DOI] [PMC free article] [PubMed] [Google Scholar]