Abstract
Although necrosis is recognized as the main mode of cell death induced by acetaminophen (APAP) overdose in animals and humans, more recently an increasing number of publications, especially in the herbal medicine and dietary supplement field, claim an important contribution of apoptotic cell death in the pathophysiology. However, most of these conclusions are based on parameters that are not specific for apoptosis. Therefore, the objective of this review was to re-visit the key signaling events of receptor-mediated apoptosis and APAP-induced programmed necrosis and critically analyze the parameters that are being used as evidence for apoptotic cell death. Both qualitative and quantitative comparisons of parameters such as Bax, Bcl-2, caspase processing and DNA fragmentation in both modes of cell death clearly show fundamental differences between apoptosis and cell death induced by APAP. These observations together with the lack of efficacy of pan-caspase inhibitors in the APAP model strongly supports the conclusion that APAP hepatotoxicity is dominated by necrosis or programmed necrosis and does not involve relevant apoptosis. In order not to create a new controversy, it is important to understand how to use these “apoptosis” parameters and properly interpret the data. These issues are discussed in this review.
Keywords: acetaminophen, drug hepatotoxicity, apoptosis, programmed necrosis, DNA fragmentation, caspase activation
1. INTRODUCTION
Apoptosis, a form of cell death different from the commonly recognized necrosis, was first described in the 1970s by Kerr and coworkers (Kerr et al., 1972). However, it took another 20 years until major progress was made in understanding the specific signaling mechanisms of apoptosis including the discovery of caspases in the 1990s (Green and Kroemer, 1998; Thornberry, 1997). As a consequence, the number of publications describing apoptotic cell death in virtually every disease process including liver diseases, increased exponentially. This culminated in the prevailing hypothesis that apoptosis is the dominant form of cell death for most liver diseases (Guicciardi and Gores, 2005). A typical example of this development was hepatic ischemia-reperfusion injury, where initial studies only recognized necrosis mainly caused by inflammatory cells (Jaeschke, 1998). However, only a few years later, the injury appeared to be exclusively caused by apoptosis (Neuman, 2001; Rudiger et al., 2003). Although subsequent studies questioned these conclusions and demonstrated convincingly that cell death during hepatic ischemia-reperfusion is caused by necrosis and that apoptosis is of very limited relevance (Gujral et al., 2001; Yang et al., 2014), the idea that apoptosis is somehow important prevails until today (Cao et al., 2016). For acetaminophen (APAP) hepatotoxicity, a slightly different story developed. Whereas early studies suggested necrosis (Mitchell et al., 1973), only few studies surfaced that implicated a relevant contribution of apoptosis (El-Hassan et al., 2003; Ray and Jena, 2000; Ray et al., 1996). In addition, direct comparison between apoptosis and APAP-induced cell death showed very clearly that APAP-induced liver injury is caused by necrosis and not apoptosis (Gujral et al., 2002). Today, none of the main laboratories investigating mechanisms of APAP hepatotoxicity suggest a role for apoptosis in the pathophysiology (Iorga et al., 2017; Jaeschke et al., 2012; Hinson et al., 2010; Ramachandran and Jaeschke, 2018).
However, during the last few years a rapidly increasing number of studies, especially in the natural product literature, are being published that again claim that apoptosis is an important part of the pathophysiology (e.g, Ahmed et al., 2016; Cao et al., 2018; Dong et al., 2014; Hu et al., 2017; Hong et al., 2012; Li et al., 2013; Sharma et al., 2011; Song et al., 2014; Wang et al., 2010; Wang et al., 2017, Wang et al., 2018; Zhang et al., 2017; Zhao et al., 2012). Although most of these studies do not appear to directly challenge previous reports regarding the lack of apoptosis, they mainly ignore the pertinent literature and simply conclude based on the measurement of a few, assumed apoptosis parameters that the natural product that is being tested is protecting due to its anti-apoptotic effect (e.g. Ahmed et al., 2016; Cao et al., 2018; Dong et al., 2014; Hu et al., 2017; Hong et al., 2012; Li et al., 2013; Sharma et al., 2011; Song et al., 2014; Wang et al., 2010; Wang et al., 2017, Wang et al., 2018; Zhang et al., 2017; Zhao et al., 2012). Unfortunately, this is perpetuating a fundamentally wrong conclusion, which not only questions the proposed mechanism of protection of a particular natural product but runs the risk that many other studies follow their lead. Therefore, the objective of this review is to re-visit the mechanisms of apoptosis and how this can be studied in APAP toxicity.
2. SIGNALING MECHANISMS OF APOPTOTIC CELL DEATH IN HEPATOCYTES
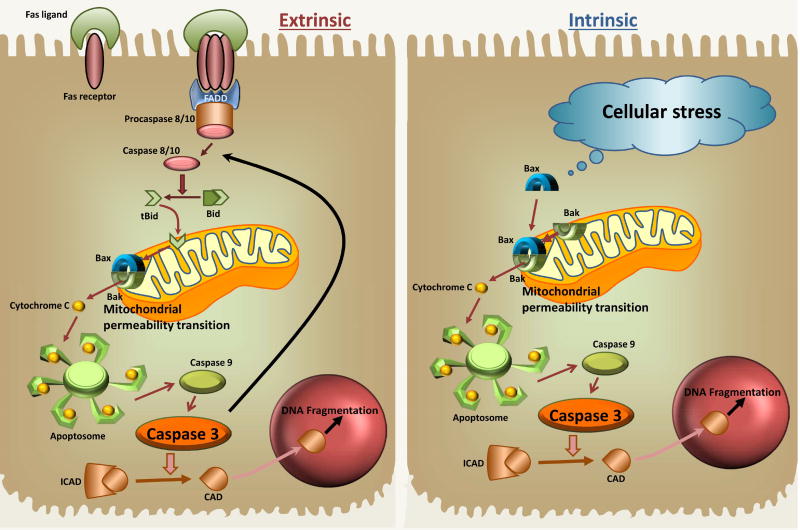
Apoptosis is characterized by very tightly regulated signaling mechanisms (Guicciardi and Gores, 2005; Schattenberg et al., 2006). There are 2 principal pathways of apoptosis induction: the extrinsic (cell death receptor) pathway and the intrinsic (mitochondrial pathway). Both pathways trigger the activation of intracellular proteases and endonucleases, which are responsible for the breakdown of the cell (Figure 1). In the case of the extrinsic pathway, a ligand (e.g., FasL) binds to the death receptor (e.g., Fas receptor), which triggers the trimerization of the receptor and the formation of a death-inducing signaling complex consisting of the cytoplasmic death domain of the receptor, an adapter molecule (e.g. FADD) and procaspase-8 or -10. Through autocatalytic processing, the active caspase is generated, which can cleave the pro-apoptotic Bcl-2 family member Bid to form tBid. This truncated form of Bid translocates to the mitochondria and facilities together with Bax and Bak the formation of pores in the outer mitochondrial membrane (MOMP), which enable the release of intermembrane proteins. Critical for the progression of apoptotic signaling is the mitochondrial release of cytochrome c, which facilitates the formation of the apoptosome and the activation of caspase-9 and subsequently caspase-3. The active caspase-3 can promote procaspase-8 cleavage and further amplify the pro-apoptotic signaling loop through the mitochondria and it can cleave downstream targets to continue the apoptosis pathway. One of the targets of caspase-3 is the inhibitor of caspase-activated DNase (ICAD). Cleavage of ICAD liberates CAD for translocation to the nucleus causing nuclear DNA cleavage into inter-nucleosomal fragments of 180 base pairs and multiples thereof (Nagata et al., 1998). The intrinsic pathway of apoptosis is activated mainly by internal signals such as Bax activation and its translocation to the mitochondria and then follows the same signaling events downstream of MOMP formation as the extrinsic pathway. Hepatocytes generally require mitochondria to amplify the death signal and execute apoptosis (type II cell) (Scaffidi et al., 1998). However, a very strong signal at the Fas receptor can lead to sufficient caspase-8 activation, which in turn directly activates enough caspase-3 to cause apoptotic cell death (type I cell) (Schüngel et al., 2009).
Figure 1. Signaling Mechanisms of Apoptotic Cell death.
The extrinsic pathway requires activation of a death receptor, such as the Fas receptor, which triggers the formation of a death-inducing signaling complex with FADD and procaspase-8 or -10. Generation of the active caspase then results in cleavage of Bid to tBid, which translocates to mitochondria and initiates the mitochondrial outer membrane permeabilization facilitated by Bax and Bak, which triggers the release of cytochrome c. This enables formation of the apoptosome and subsequent activation of caspase-9 and caspase-3. Active caspase-3 cleaves ICAD to release CAD, which translocates to the nucleus and initiates nuclear DNA fragmentation. Caspase 3 can also promote procaspase-8 cleavage and further amplify the pro-apoptotic signaling loop through the mitochondria. The intrinsic pathway for apoptosis is activated by cellular stress signals, which activate Bax and its translocation to the mitochondria, which then follows signaling events similar to the extrinsic pathway downstream of the MPT.
Because a number of cytokines and stress signals can activate the apoptotic pathways, it is critical to have checkpoints in the process in order to avoid accidental activation. FLICE-inhibitory proteins can prevent death receptor activation (Krueger et al., 2001) and inhibitors of apoptosis (IAPs) in the cytosol can bind to certain pro-caspases and activated caspases and prevent further activation (Marivin et al., 2012; Wang and Lin, 2013). On the other hand, Second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/Diablo) can be released from mitochondria and inactivate IAPs to allow apoptotic signaling to progress (Fulda, 2015). The intermembrane proteins apoptosis-inducing factor (AIF) and endonuclease G can also be released through the outer membrane pores but do not appear to be critical for caspase-dependent apoptosis (van Gurp et al., 2003) but can contribute to DNA fragmentation during non-apoptotic cell death, e.g. APAP toxicity (Bajt et al., 2006). Additional regulators of both the extrinsic and the intrinsic pathway are the anti-apoptotic molecules Bcl-2 and Bcl-xL, which act mainly through heterodimerization of pro-apoptotic Bcl-2 family members like Bax and Bak in the outer mitochondrial membrane, which prevents the outer membrane permeabilization (Chao and Korsmeyer, 1998).
Thus, apoptotic signaling pathways are well defined and can be measured by a number of parameters including caspase activities, pro-caspase processing, cell morphology changes, DNA fragmentation, translocation of pro-apoptotic factors to the mitochondria and release of mitochondrial intermembrane proteins into the cytosol or translocation to the nucleus. Some of the parameters are relatively specific, e.g. caspase activation; however, it is important to keep a quantitative perspective between these parameters and the extent of cell death. This is best accomplished by using a positive control for apoptosis such as Fas- or TNF-induced apoptosis. In addition, caspase inhibitors are highly effective in preventing apoptosis and should be used to demonstrate the relevance of apoptosis in the overall cell death (Bajt et al., 2000).
3. EXPERIMENTAL EVIDENCE FOR APOPTOSIS IN ACETAMINOPHEN-INDUCED CELL DEATH
3.1 Morphology
Apoptosis was initially defined by characteristic morphological features including cell shrinkage, chromatin condensation and margination, nuclear fragmentation, membrane blebbing and the breakdown of the cell into apoptotic bodies (Kerr et al., 1972) (Figure 2). However, the morphological characteristics of APAP-induced cell death are fundamentally different and include cell swelling, karyolysis, cell contents release and inflammation (Gujral et al., 2002) (Figure 2). Importantly, the apoptotic bodies generated during apoptosis will be removed through phagocytosis by macrophages or neighboring cells thereby preventing the initiation of an inflammatory response (Ravichandran, 2010).
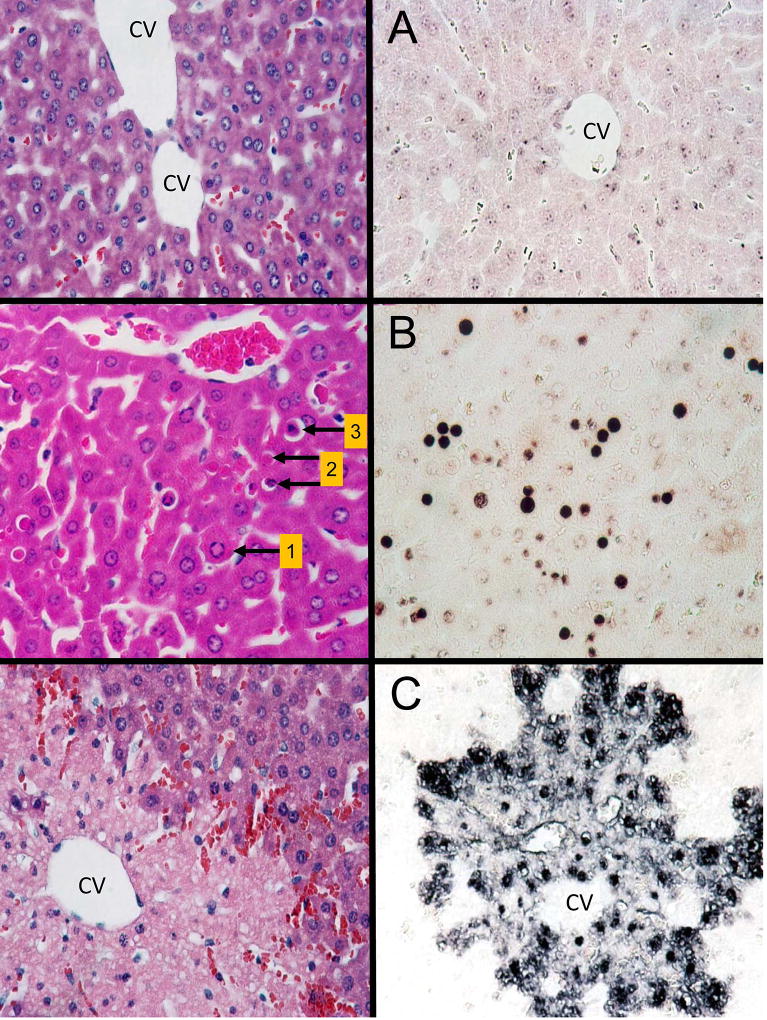
Figure 2. Representative H&E and TUNEL-stained liver sections.
A. Liver sections from untreated mice were stained with hematoxylin and eosin (left panel) and the TUNEL assay (right panel). CV, central vein. B. Liver sections from an animal treated with galactosamine/endotoxin for 6 h (TNF-mediated apoptosis). Arrows in the H&E section mark examples of hepatocytes in different stages of apoptotic cell death. Arrow 1: cell shrinkage and chromatin margination (early stage of apoptosis). Arrow 2: marks 2 apoptotic bodies with and without chromatin present (late stages of apoptosis). Arrow 3: marks a shrinking hepatocyte with the characteristic halo and condensed chromatin. The right panel shows the characteristic TUNEL-staining of apoptotic cells. C. Liver sections of an acetaminophen-treated mouse (300 mg/kg, 6 h). The H&E-stained section shows a confluent area of necrosis around the central vein (CV) with extensive loss of nuclei. The right panel shows the characteristic TUNEL staining of necrotic cells with staining of nuclei that are still present and extensive cytoplasmic staining. (Images are reproduced with permission from Gujral et al., 2002).
In general, if apoptosis is a relevant contributor to the overall cell death in the liver, the apoptotic cells are easily identifiable with light microscopy (×100 or ×200) in H&E stained liver sections (Figure 2). However, some investigators prefer a higher magnification (×1000) or even use electron microscopy (×8,000) (Ray et al., 1996). This allows a better view of the morphology of individual cells. However, the caveat is that due to regular cell turnover in the liver, at any given time there are thousands of cells undergoing apoptosis. Thus, it is always possible to find individual apoptotic cells using higher magnification. However, the very miniscule field of view of electron microscopy makes it very difficult to quantitatively assess the percentage of apoptotic cells in the total liver, and if these cells are part of the pathophysiology or just regular cell turnover.
The classical mechanism of apoptosis applies mainly to the turnover of individual cells or removal of cells during development (Jacobson et al., 1997). Under these conditions, cellular organelles remain intact and no intracellular content is released. However, under pathophysiological conditions, when a number of contiguous cells undergo apoptosis, the process cannot be completed. Instead, due to declining cellular ATP levels, the cells switch from apoptosis to secondary necrosis characterized by organelle swelling and cell content release (Bajt et al., 2000; Jaeschke and Lemasters, 2003; Ogasawara et al., 1993) and subsequently an inflammatory response. The fundamental difference between APAP-induced oncotic necrosis and secondary necrosis is that only during secondary necrosis extensive caspase activities are detectable and pancaspase inhibitors prevent both apoptosis and the following secondary necrosis (Jaeschke et al., 2004).
3.2 Nuclear DNA Fragmentation
Most of the confusion regarding mode of cell death comes from misinterpretation of presumed biochemical apoptosis parameters as none of the recent papers show any evidence of apoptotic morphology. It was recognized very early that APAP hepatotoxicity causes genomic DNA fragmentation as indicated by a DNA ladder on agarose gel (Ray et al., 1990; Shen et al., 1991). Since this did not go along with the morphological evidence of apoptosis (cell shrinkage, apoptotic bodies formation), it was initially still considered as necrosis (Ray et al., 1993). However, eventually the conclusion emerged that this reflects actually apoptosis (Ray et al., 1996, 2000). We measured DNA strand breaks using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and DNA fragmentation using an anti-histone ELISA assay (Lawson et al., 1999; Gujral et al., 2002). However, since this did not correlate with caspase activity increases, we concluded that this DNA damage cannot be caused by caspase-activated DNase, the classical enzyme responsible for DNA fragmentation during apoptosis (Lawson et al., 1999). Follow-up studies documented that APAP-induced DNA damage is dependent on mitochondrial dysfunction (Cover et al., 2005) and that the mitochondrial intermembrane proteins AIF and endonuclease G translocate to the nucleus where they cause DNA fragmentation (Bajt et al., 2006, 2011). In fact, the direct comparison of TNF-mediated apoptosis and APAP-induced cell death showed the similarities in developing TUNEL-positive cells and inducing DNA fragmentation between both modes of cell death but also fundamental differences in the appearance of TUNEL staining (nuclear staining in apoptosis and cytosolic staining after APAP) (Jaeschke et al., 2011; Jaeschke and Lemasters, 2003). This difference is also reflected by direct analysis of the molecular weights of the DNA fragments, which showed mainly inter-nucleosomal DNA fragments after apoptosis and mostly higher molecular weight fragments after APAP (Jahr et al., 2001); however, evidence for limited inter-nucleosomal DNA fragments was also observed (Cover et al., 2005). The larger versus more lower molecular weight DNA fragments explains the difference in TUNEL staining between APAP hepatotoxicity with staining of the nucleus and the cytosol and TNF-mediated apoptosis with the more selective nuclear staining (Figure 2) (Jaeschke and Lemasters, 2003; Jaeschke et al., 2011). In addition, the fundamental difference is that apoptosis-induced DNA fragmentation is completely eliminated by a caspase inhibitor (Cover et al., 2005; Jaeschke et al., 1998) whereas APAP-induced DNA fragmentation is not affected by caspase inhibitors (Cover et al., 2005; Gujral et al., 2002; Lawson et al., 1999). Thus, despite the similarities of some of these parameters, there are fundamental differences to consider when interpreting the results; importantly, these parameters do not reflect apoptosis in APAP hepatotoxicity.
3.3 Role of Bcl-2 family members
Bcl-2 (B-cell lymphoma 2) family members can have both pro- and anti-apoptotic functions (Chao and Korsmeyer, 1998). Bcl-2 itself is considered anti-apoptotic due to its location in the outer mitochondrial membrane and its capacity to heterodimerize with the pro-apoptotic Bax protein (Yin et al., 1994), which can form pores in the outer membrane and promote apoptotic cell death by releasing cytochrome c (Scorrano and Korsmeyer, 2003). Bcl-2 levels in normal hepatocytes are generally very low in mice or humans (Charlotte et al., 1994; Rodriguez et al., 1996). Bcl-2 overexpressing mice are highly resistant to Fas receptor-induced apoptosis (Rodriguez et al., 1996), however, the same mice showed only a minor initial delay in injury by 6 h after APAP but a severe aggravation of the necrosis by 24 h (Adams et al., 2001). Although the mechanisms of this effect of Bcl-2 overexpression in hepatocytes remained unclear, the fact that the transgenic mice had evidence of a higher oxidant stress (higher glutathione disulfide levels) (Adams et al., 2001) is consistent with previous studies showing pro-oxidant properties of Bcl-2 (Steinman, 1995). Thus, Bcl-2 overexpression effectively blocks the progression of apoptotic signaling during apoptosis but may have unexpected off-target pro-oxidant effects during non-apoptotic cell death.
More recently, it became popular to measure Bcl-2 mRNA and protein levels during APAP hepatotoxicity (Ahmed et al., 2016; Cao et al., 2018; Dong et al., 2014; Hu et al., 2017; Hong et al., 2012; Li et al., 2013; Sharma et al., 2011; Song et al., 2014; Wang et al., 2017, Wang et al., 2018; Zhang et al., 2017; Zhao et al., 2012). Interestingly, most of these reports show high Bcl-2 expression in controls and declining levels during APAP toxicity (Ahmed et al., 2016; Cao et al., 2018; Dong et al., 2014; Hu et al., 2017; Hong et al., 2012; Li et al., 2013; Sharma et al., 2011; Song et al., 2014; Wang et al., 2017, Wang et al., 2018; Zhang et al., 2017; Zhao et al., 2012). Treatment with natural products appears to reverse this decline (Ahmed et al., 2016; Cao et al., 2018; Dong et al., 2014; Hu et al., 2017; Hong et al., 2012; Li et al., 2013; Sharma et al., 2011; Song et al., 2014; Wang et al., 2017, Wang et al., 2018; Zhang et al., 2017; Zhao et al., 2012). This is then interpreted as evidence for apoptotic cell death. However, there are serious concerns with these types of isolated data. First, there is no verification that the authors actually measured Bcl-2 especially because early reports have indicated that there is little to no expression of Bcl-2 in control hepatocytes (Charlotte et al., 1994; Rodriguez et al., 1996). Second, it is not proven that Bcl-2 expression levels have anything to do with the mechanism of cell death; it may just be a secondary effect of the modified injury. Third, a fundamental contradiction of this idea is that APAP can cause extensive “apoptotic” cell death despite the presence of high levels of Bcl-2, which actually should prevent apoptosis. On the other hand, it is argued that only a partial recovery of depleted Bcl-2 levels caused by a natural product will prevent apoptotic cell death. It is difficult to reconcile these contradictory arguments. Thus, Bcl-2 expression levels, especially when the data contradict relevant studies on this subject (Charlotte et al., 1994; Rodriguez et al., 1996), are insufficient to draw any conclusions regarding apoptotic cell death during APAP hepatotoxicity.
Bax (Bcl-2-associated X protein) is one of the pro-apoptotic Bcl-2 family members located predominantly in the cytosol. Under stress conditions, Bax can translocate to the outer mitochondrial membrane where it can homo- or heterodimerize with Bak and form pores in the outer membrane to release intermembrane proteins such as cytochrome c and promote apoptosis (Gross et al., 1998; Wolter et al., 1997). Interestingly, Bax translocation to the mitochondria was observed as early as 2 h after APAP overdose (Adams et al., 2001; Bajt et al., 2008; El-Hassan et al., 2003; Jaeschke and Bajt, 2006) (Figure 3). Mitochondrial Bax translocation appears to be dependent on the activation of c-jun N-terminal kinase (JNK) activation (Gunawan et al., 2006). Bax was responsible for the initial release of intermembrane proteins including AIF and endonuclease G, which translocate to the nucleus (Bajt et al., 2006, 2008). The pathophysiological importance of Bax in APAP hepatotoxicity was shown by the temporary inhibition of nuclear DNA fragmentation and delayed cell death in Bax-deficient mice (Bajt et al., 2008). However, Bax deficiency did not affect the main mitochondrial oxidant stress and peroxynitrite formation, which resulted in the later opening of the mitochondrial membrane permeability transition pore (MPTP) and matrix swelling with outer membrane rupture; a second wave of AIF and endonuclease G release still triggered DNA fragmentation and cell necrosis (Bajt et al., 2008). Thus, Bax has a temporary effect on APAP-induced cell necrosis by causing a delay in the progression of the injury process (Bajt et al., 2008).
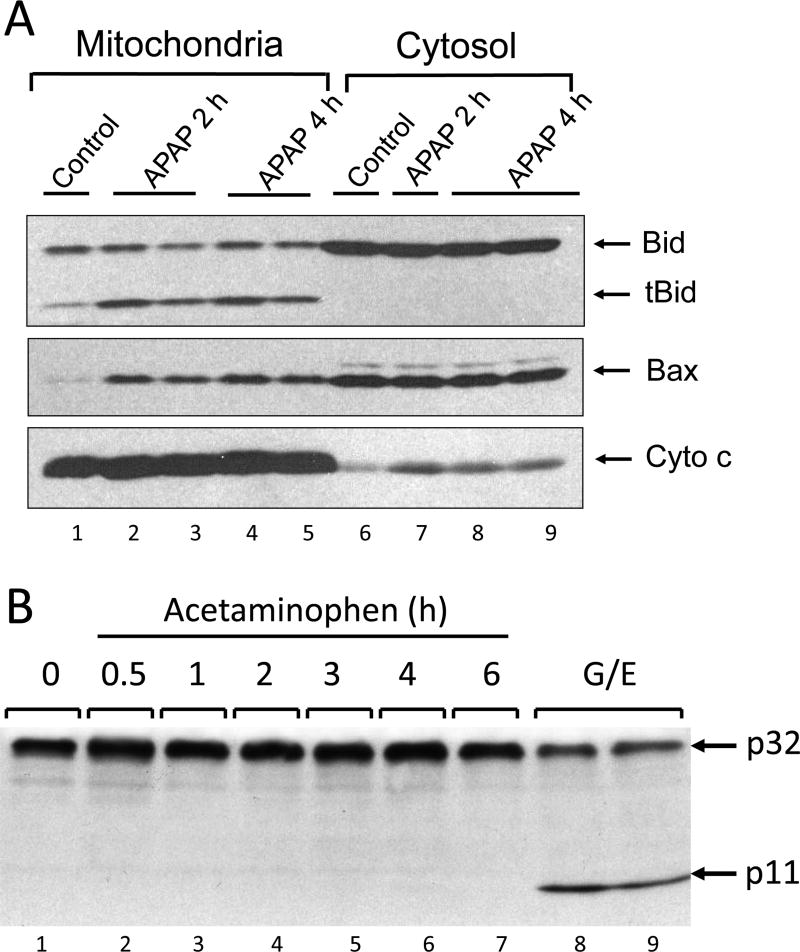
Figure 3. Bcl-2 Family Members and Caspase-3 during Acetaminophen-induced Cell Death.
A. Western blot of Bcl-2 family members (Bax, Bid and the truncated form of Bid) and cytochrome c in the cytosol and in mitochondria of a control mouse liver and after treatment of the animals with 300 mg/kg acetaminophen (2 and 4h). B. Western blot of pro-caspase-3 and the cleaved fragment (p11) representing activation in the liver of an untreated mouse, after various times (0.5–6 h) of acetaminophen treatment (300 mg/kg), and after galactosamine/endotoxin (G/E; positive control for apoptosis) treatment for 6 h. [Images are reproduced with permission from Jaeschke and Bajt, 2006 (A) and Gujral et al., 2002 (B)].
Similar to Bcl-2, Bax mRNA and protein expression at the whole liver level have been used as evidence for apoptosis during APAP-induced liver injury (Cao et al., 2018; Dong et al., 2014; Hu et al., 2017; Hong et al., 2012; Li et al., 2013; Sharma et al., 2011; Song et al., 2014; Wang et al., 2017, Wang et al., 2018; Zhang et al., 2017; Zhao et al., 2012). However, this conclusion is highly questionable for several reasons. First, Bax is expressed in all cells in sufficient quantities to trigger cell death if initiated properly. Second, Bax translocation to the mitochondria inducing the permeabilization of the outer membrane is the critical event for apoptosis. Thus, Bax protein expression changes without further supporting evidence, especially at the whole liver level, do not justify the conclusion that apoptotic cell death is involved in the pathophysiology of APAP hepatotoxicity. Because of the alleged opposite effect of Bcl-2 and Bax, recent studies have reported changes in the Bcl-2-to-Bax ratio as evidence of apoptosis (Hu et al., 2017; Wang et al., 2018). Again, the ratio is not more informative than the individual proteins, which are not reliable indicators of apoptosis.
Additional Bcl-2 family members such as Bid, Bcl-xL, Bik and Bad have only been investigated superficially in the APAP hepatotoxicity model. It was shown that Bid is cleaved early during APAP-induced liver injury and the truncated form of Bid (tBid) translocates to the mitochondria (El-Hassan et al., 2003; Jaeschke and Bajt, 2006) (Figure 3). Although there is no evidence of caspase activation during APAP hepatotoxicity (Gujral et al., 2002; Lawson et al., 1999), Bid cleavage may be caused by calpains (Mandic et al., 2002), which have been shown to be activated during APAP-induced liver injury (Limaye et al., 2006). However, a critical pathophysiological role of Bid has not been demonstrated. Based on the fact that tBid supports the formation of Bax pores, it can be assumed that deficiency of Bid will at best cause a temporary delay in DNA fragmentation and injury similar to Bax deficiency (Bajt et al., 2008). APAP has been shown to reduce Bcl-xL expression and a number of natural products have attenuated this reduction in Bcl-xL expression (Ahmed et al., 2016; Dong et al., 2014; Ray et al., 1999, 2006; Ray and Jena, 2000). However, these results are merely correlative and do not support any causative role of Bcl-xL in APAP-induced cell death. Likewise, the protein expression of other Bcl-2 family members, e.g. Mcl-1, Bik, and Bad, after APAP overdose showed changes that correlated or inversely correlated with cell death but the pathophysiological role of these proteins has not been tested (Jang et al., 2015; Shinohara et al., 2010; Williams et al., 2015).
Taken together, the only Bcl-2 family member that has been shown to have a limited impact on APAP-induced hepatocellular necrosis is Bax; all other pro- or anti-apoptotic family members show some correlations with the injury but no clear impact on cell death. Nevertheless, given the role of Bax in the pathophysiology, anti-apoptotic Bcl-2 members such as Bcl-xL and Mcl-1 can be expected to have some impact on necrotic cell death. However, hepatic protein expression changes of these Bcl-2 family members alone are insufficient to support the hypothesis that there is relevant apoptotic cell death after APAP overdose.
3.4 Caspase Activation
Caspases are directly or indirectly responsible for all aspects of apoptotic signaling in the cell. Thus, procaspase processing and caspase enzyme activities are the most specific indicators of apoptotic cell death (Julien and Wells, 2017; Thornberry, 1997). Early studies assessing caspase activation could not find any evidence for increased caspase-3 activity, the main executioner caspase, during APAP toxicity in vivo (Adams et al., 2001; Lawson et al., 1999). This was measured by both enzyme activity and procaspase-3 processing (Adams et al., 2001; Lawson et al., 1999) (Figure 3). Importantly, pan-caspase inhibitors had no effect on APAP-induced liver injury (Gujral et al., 2002; Lawson et al., 1999; Jaeschke et al., 2006). This led to the conclusion that caspase-dependent apoptotic cell death is not involved in APAP hepatotoxicity (Gujral et al., 2002; Lawson et al., 1999; Jaeschke et al., 2006). Unfortunately, a caveat of using a caspase inhibitor such as ZVAD-fmk, which is a highly effective suicide substrate, is the fact that these types of inhibitors are only soluble in dimethyl sulfoxide (DMSO). However, DMSO is a potent inhibitor of cytochrome P450 and can block APAP toxicity even at relatively low concentrations in vivo (Jaeschke et al., 2006; Kelava et al., 2010). If this fact is overlooked and proper solvent controls are not included, then protective effects of caspase inhibitors against APAP toxicity can be observed (El-Hassan et al., 2003; Hu and Colletti, 2010), even in the verified absence of caspase activation (El-Hassan et al., 2003). However, as has been shown repeatedly, caspase inhibitors are readily absorbed and are highly effective within less than an hour during TNF- or Fas receptor-induced apoptosis (Bajt et al., 2000, 2001; Jaeschke et al., 1998, 2000) and therefore can be given right before the onset of the injury. Thus, in order to avoid the effect of DMSO on the oxidative metabolism of APAP, we generally treat animals 2–3 h after APAP (Gujral et al., 2002; Lawson et al., 1999; Jaeschke et al., 2006). This is sufficient to block apoptotic signaling with these irreversible suicide substrates. However, these inhibitors dissolved in DMSO are only effective against APAP toxicity as pretreatment (El-Hassan et al., 2003; Hu and Colletti, 2010), which really assesses the inhibitory effect of the solvent on drug metabolism and not the effect of the caspase inhibitor (Jaeschke et al., 2006, 2011). Thus, the lack of relevant caspase activation and the absence of any inhibitory effect on the toxicity by highly potent caspase inhibitors clearly indicate that there is no relevant apoptotic cell death contributing to APAP-induced liver injury. These findings in mice also apply to primary human hepatocytes (Xie et al., 2014), human HepaRG cells (McGill et al., 2011) and patients (Antoine et al., 2012; McGill et al., 2012a).
A recent study by Kucera et al. (2017) comparing APAP-induced cell death in cultured mouse and rat hepatocytes demonstrated the much higher susceptibility of mouse hepatocytes to APAP and the absence of caspase activation. In contrast, the delayed GSH depletion and lower injury in cultured rat hepatocytes compared to the mouse was accompanied by a modest increase in caspase-3 activity (Kucera et al., 2017). Overall, these data are consistent with in vivo studies where similar hepatic GSH depletion and even protein adduct formation was seen in mice when compared to rats treated with 3-to-4 times higher doses of APAP. Protein adducts were quantitatively less in the liver and in mitochondria in rats, however, with no liver injury (McGill et al., 2012b). Importantly, there was no activation of JNK and no nitrotyrosine staining, suggesting the absence of the critical mitochondrial oxidant stress in rats (McGill et al., 2012b). These observations indicate that if the central event of the mechanism of APAP toxicity in mouse and human hepatocytes, i.e. the mitochondrial oxidant stress, is absent or is prevented, cultured cells may undergo late apoptosis. This conclusion is supported by our earlier studies with APAP in mouse hepatocytes where there was necrotic cell death caused by oxidant stress and the MPTP opening (Bajt et al., 2004; Kon et al., 2004). However, when necrosis was prevented by pharmacological interventions that affected the MPTP opening, further incubation of the cells resulted in caspase activation and apoptosis (Kon et al., 2004, 2007). The reason for this secondary apoptosis is likely the fact that when the end stages of necrotic signaling are blocked, there is still the cellular stress of reactive metabolite and mitochondrial protein adducts formation, which eventually can trigger an intrinsic apoptotic cell death. However, it has to be kept in mind that this type of apoptotic cell death is not the primary mechanism of APAP-induced cell death but a secondary response to inhibition of necrotic signaling.
Despite this overwhelming evidence against apoptosis as the primary mode of cell death after APAP overdose, more recently it became more popular to demonstrate caspase involvement in APAP hepatotoxicity through western blotting. A reduction of pro-caspase-3, -8 and -9 and the appearance of cleaved fragments of these caspases is increasingly been used as evidence for caspase activation (Cao et al., 2018; Dong et al., 2014; Hong et al., 2012; Sharma et al., 2011; Song et al., 2014; Wang et al., 2010; Wang et al., 2017; Zhang et al., 2017). Although these parameters can be indicators of the presence of activated caspases, the important aspect is that there is a low baseline turnover of hepatocytes in every liver; this turnover is accomplished by apoptosis. Thus, extensive overexposure of any western blot of liver tissue can reveal some minor caspase activation, which is always detectable by caspase processing even in untreated controls (Cao et al., 2018; Dong et al., 2014; Hong et al., 2012; Song et al., 2014; Wang et al., 2010; Wang et al., 2017; Zhang et al., 2017). However, when compared to pathophysiologically relevant apoptotic liver injury during TNF- or Fas receptor-induced apoptosis with its 30-to-100-fold increase in caspase-3 activities and extensive processing of pro-caspases and the appearance of the cleaved fragments (Lawson et al., 1999; Bajt et al., 2000, 2001; Jaeschke et al., 1998, 2000), the effects observed on these parameters during an APAP overdose is negligible despite the fact that the APAP-induced injury is much more severe (Lawson et al., 1999; Gujral et al., 2002) (Figure 3). Thus, in order to properly interpret these types of western blot data, it is critical to directly compare them to a positive control of apoptosis, which will give the necessary perspective. In addition, the measurement of caspase activities and the use of a caspase inhibitor can provide further support for the absence of apoptosis.
4. ACETAMINOPHEN-INDUCED APOPTOTIC CELL DEATH IN HEPATOMA CELL LINES
Human hepatoma cell lines such as HepG2, HuH7, SK-Hep1, and others have been extensively used for drug toxicity studies including APAP toxicity (Donato et al., 2008). Interestingly, exposing hepatoma cells to 5–20 mM APAP for 24–48 h consistently causes apoptotic cell death (Boulares et al., 2002, 2004; Kass et al., 2003; Manov et al., 2004). The apoptotic signaling in these cells include extensive caspase activation, translocation of Bax to mitochondria, the cytoplasmic release of cytochrome c and Smac/DIABLO, chromatin condensation and nuclear DNA fragmentation (Boulares et al., 2002; Kass et al., 2003; Macanas-Pirard et al., 2005; Manov et al., 2004). Apoptosis in hepatoma cell lines appears to be mainly caused by the intrinsic pathway, as caspase activation and DNA fragmentation are post-mitochondrial events (Boulares et al., 2002). Overall, there is little doubt that APAP triggers apoptotic cell death in these hepatoma cells. However, the important question is whether these mechanisms of cell death have any relevance for primary hepatocytes or the liver in vivo? The fundamental difference between primary hepatocytes and hepatoma cells is the dramatically lower expression of cytochrome P450 enzymes (Westerink and Schoonen, 2007), which are responsible for the metabolic activation of APAP to generate the reactive metabolite NAPQI and initiate the toxicity. Hence it was shown that APAP only causes minimal GSH depletion, low protein adducts formation and minimal mitochondrial dysfunction in HepG2 cells (McGill et al., 2011) compared to primary human hepatocytes (Xie et al., 2014). In addition, N-acetylcysteine, which is the standard of care for APAP overdose in humans (Rumack and Bateman, 2012) and is also highly effective in animals (James, et al., 2003; Saito et al., 2010), does not prevent APAP-induced apoptosis in HepG2 cells (Manov et al., 2004). HepG2 cells could be sensitized to APAP-induced cell death by transfection of CYP2E1, which resulted in greater GSH depletion and protein adducts formation and more severe mitochondrial dysfunction (Bai and Cederbaum, 2004). However, in addition to the now prevalent necrosis, there was still evidence of apoptotic cell death (Bai and Cederbaum, 2004). Thus, in general, hepatoma cell lines are not good models for studying mechanisms of APAP hepatotoxicity because most of these findings cannot be translated to the human pathophysiology. One notable exception from this rule is the human hepatoma cell line HepaRG (McGill et al., 2011; Rodrigues et al., 2016). Although there are also differences in gene expression from primary human hepatocytes, the gene expression profile of HepaRG cells is more closely related to human hepatocytes than HepG2 cells (Yokoyama et al., 2018). In particular, the expression of drug metabolizing enzymes is close to human hepatocytes and much higher compared to HepG2 cells (Anthérieu et al., 2012; Yokoyama et al., 2018). Therefore, HepaRG cells show many similarities in cell signaling in response to APAP, which include extensive GSH depletion, protein adducts formation, oxidant stress and mitochondrial dysfunction, and they die by necrosis and not apoptosis (McGill et al., 2011). Thus, HepaRG cells are the only hepatoma cell model that is relevant for studying mechanisms of APAP hepatotoxicity.
5. MECHANISM OF APAP-INDUCED CELL DEATH: PROGRAMMED NECROSIS
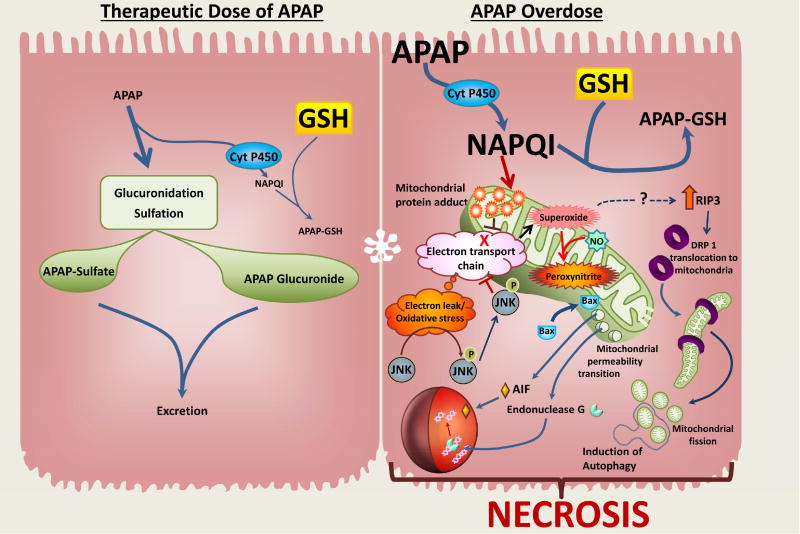
The morphology of APAP-induced liver injury clearly suggests an oncotic necrotic cell death (Figure 2). These morphological features are caused by biochemical signaling pathways that start with the mainly Cyp2E1-mediated formation of the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI), which can be detoxified by cellular GSH but also leads to low levels of protein adducts formation by binding to cysteine residues in cellular proteins (McGill and Jaeschke, 2013). However, after an overdose of APAP, NAPQI formation is substantially increased leading to extensive GSH depletion and enhanced protein binding, especially to mitochondrial proteins (McGill and Jaeschke, 2013; Ramachandran and Jaeschke, 2017). Mitochondrial protein adducts formation inhibits the electron transport chain and causes electron leakage, which triggers the activation of redox-sensitive components of the mitogen-activated protein kinase (MAPK) cascade (Du et al., 2015; Han et al., 2013). Ultimately, this leads to the phosphorylation and mitochondrial translocation of c-jun N-terminal kinase (JNK) (Hanawa et al., 2008). This event further inhibits the electron flow in the mitochondria and increases the electron leakage causing a substantial amplification of the oxidant stress and also peroxynitrite formation (Win et al., 2016), which are critical for the mechanism of cell death (Du et al., 2016). The oxidant stress triggers the opening of the MPTP resulting in the collapse of the membrane potential and cessation of ATP production (Kon et al., 2004). In addition, early mitochondrial translocation of Bax, which is potentially induced by JNK activation (Gunawan et al., 2006), causes outer mitochondrial membrane permeabilization that facilitates release of intermembrane membrane proteins such as AIF, endonuclease G, cytochrome c, and Smac/DIABLO (Bajt et al., 2006, 2008). After the MPTP, which triggers matrix swelling and outer membrane rupture, a second and more permanent wave of intermembrane proteins is released (Bajt et al., 2008). AIF and endonuclease G can translocate to the nucleus and cause DNA fragmentation (Bajt et al., 2006) generating fragments of various molecular weight size (Jahr et al., 2001; Cover et al., 2005). The critical importance of AIF in nuclear DNA fragmentation and cell death has been shown with AIF-deficient mice (Bajt et al., 2011) and with pharmacological interventions that inhibit nuclear AIF translocation (Nagy et al., 2010; Liang et al., 2012). The MPTP in mitochondria and nuclear DNA fragmentation are critical, terminal events of APAP-induced cell death (Figure 4).
Figure 4. Signaling Mechanisms of Acetaminophen-induced Liver Cell Death.
Therapeutic doses of acetaminophen (APAP) are metabolized by glucuronidation and sulfation with a minor amount converted by cytochrome P450 into a reactive metabolite NAPQI. Cellular stores of glutathione scavenge these levels of NAPQI without any deleterious consequences. In case of an APAP overdose, glucuronidation and sulfation are overwhelmed and large amounts of APAP are metabolized through the cytochrome P450 system to generate high levels of NAPQI. This then depletes cellular glutathione and forms protein adducts, especially on mitochondria, which then inhibits the electron transport chain (ETC), resulting in leakage of electrons and oxidative stress. This then activates the MAP kinase c-jun N-terminal kinase (JNK), which translocates to the mitochondria and further induces generation of superoxide from the ETC, which, along with nitric oxide results in formation of the highly reactive peroxynitrite. The amplified oxidative stress, coupled with translocation of Bax from the cytosol to the mitochondria results in induction of the mitochondrial permeability transition, and release of AIF and endonuclease G into the cytosol. Their translocation to the nucleus then results in DNA fragmentation. APAP overdose also causes the upregulation of RIP3, which induces DRP1 translocation to mitochondria and mitochondrial fission and mitophagy. These events subsequently lead to necrotic cell death.
More recently it was recognized that TNF receptor-mediated signaling can cause one of three effects in cells: NF-kB activation, caspase-mediated apoptosis or necroptosis (Grootjans et al., 2017). Necroptosis depends on the activation of receptor interacting serine/threonine kinase 1(RIP1), RIP3 and mixed lineage kinase domain like pseudokinase (MLKL) (Grootjans et al., 2017). In the multi-protein complex of the necrosome, which includes both RIP1 and RIP3, RIP3 is responsible for the phosphorylation of MLKL (Sun et al., 2012). The phosphorylated MLKL translocates to the cell membrane and causes membrane permeabilization (Zhang et al., 2016). In APAP hepatotoxicity, increased RIP3 expression was noted (Ramachandran et al., 2013; Deutsch et al., 2015; Li et al., 2014). In addition, interventions that eliminated RIP1 (Dara et al., 2015; Zhang et al., 2014) and RIP3 (Ramachandran et al., 2013; Deutsch et al., 2015; Li et al., 2014) showed protection against the toxicity but animals deficient in MLKL were not protected (Dara et al., 2015). Furthermore, some studies questioned the role of RIP3 (Dara et al., 2015) and TNF-α (Boess et al., 1998) in the pathophysiology. Together, these data clearly indicate that APAP-induced cell death cannot be defined as necroptosis. Nevertheless, the intracellular mechanisms identified over the last decade demonstrate that this is not just a random destruction of the cell but certainly follows signaling pathways leading to cell death (Ramachandran and Jaeschke, 2018). Therefore, the term programmed necrosis is appropriate for this type of cell death (Dara et al., 2016).
APAP-induced liver injury in vivo is characterized by centrilobular necrosis (Figure 2). The zonation of the injury is caused by the higher cytochrome P450 levels and lower GSH content in this zone making it more susceptible to the APAP-induced events (McGill and Jaeschke, 2013). This means the discussed mechanisms do not occur to the same degree in every cell of the liver lobule (Ni et al., 2013). The severity of the insult and the number of mitochondria affected by the described signaling mechanisms is gradually reduced from the centrilobular towards the periportal region (Ni et al., 2013). Thus, adaptive mechanisms such as autophagy to remove damaged mitochondria and protein adducts (Ni et al., 2012, 2016) and mitochondrial biogenesis to restore mitochondrial mass (Du et al., 2017) can limit cell death and promote regeneration around the necrotic area. These events add to the complexity of the intracellular signaling mechanisms of APAP-induced programmed necrosis (Figure 4).
Although the absence of a relevant apoptotic cell death in APAP hepatotoxicity is supported by many different parameters, the question remains why there is no caspase activation despite mitochondrial Bax translocation and outer mitochondrial membrane permeabilization with release of cytochrome c and Smac/DIABLO (Adams et al., 2001; Bajt et al., 2008, Knight and Jaeschke, 2002). It was suggested that the declining ATP levels after APAP overdose in fasted animals (Jaeschke, 1990) were responsible for the prevention of caspase-3 activation (Antoine et al., 2010). In fact, fed animals showed a minor caspase activation (5% apoptosis; 95% necrosis) based on cleaved versus full length cytokeratin-18; although a pan-caspase inhibitor reduced the caspase activities, there was no protection based on plasma ALT activities (Antoine et al., 2010). This temporary minor increase of caspase-3 activation in fed mice could be confirmed but this was only observed in an outbred strain and was independent of hepatic ATP levels (Williams et al., 2011). Again, a pan-caspase inhibitor did not protect (Williams et al., 2011). These data indicated that under certain circumstances APAP may trigger a minor increase in caspase activity. Interestingly, a more recent study suggests that the heparin sulfate proteoglycan syndecan-1 inhibits caspase activation and late injury in cells surrounding the area of necrosis (Nam et al., 2017). These cells are also most active for mitochondrial biogenesis (Du et al., 2017) suggesting that the prevention of apoptosis by syndecan-1 and the stimulation of mitochondrial biogenesis promote regeneration and recovery (Du et al., 2017; Nam et al., 2017).
6. CONCLUSIONS
When certain biochemical parameters are being selected, it appears that there is plenty of evidence for apoptotic cell death during APAP-induced liver injury. However, when the lack of specificity of these parameters for apoptosis is considered, it is very obvious that the support is actually weak at best. In addition, if APAP hepatotoxicity is directly compared to a positive control for apoptosis and a selective intervention such as a caspase inhibitor is being used with the appropriate solvent control, it is quite clear that apoptosis is not a quantitatively relevant mode of cell death during APAP-induced liver injury. Although many of these studies and arguments against apoptosis have been discussed in the past, the question remains why an increasing number of recent studies, especially in the field of natural product testing, appear to not consider the entire literature on this subject. There is currently no scientific rationale or sound experimental evidence to support the hypothesis that apoptosis is critically involved in APAP hepatotoxicity in any relevant animal model, primary human hepatocytes or humans. One can only speculate what the attraction may be to favor apoptosis without experimental support. It appears that the desire to provide more data by measuring additional, easily accessible parameters may be at the heart of the problem despite that these extra data do not provide any meaningful insight into the pathophysiology. In order not to go backwards and create questionable narratives, it is important that both authors and reviewers are more aware of these problems. Only if authors are held to higher standards and either pay more attention to the entire literature or provide solid experimental evidence against the established view if they disagree, will we make progress in understanding the mechanisms of protection of natural products.
Supplementary Material
Highlights.
-
-
Acetaminophen overdose causes severe liver injury in mice and in humans
-
-
Morphological evidence suggests mainly necrotic not apoptotic cell death
-
-
There is no relevant caspase activation and no protection with caspase inhibitors
-
-
Mitochondrial Bax translocation triggers the release of AIF and endonuclease G
-
-
Nuclear DNA fragmentation and the MPTP opening are causing necrotic cell death
Acknowledgments
This work was supported in part by a National Institutes of Health grant R01 DK102142, and by grants from the National Institute of General Medical Sciences (P20 GM103549 and P30 GM118247) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST DISCLOSURE
The authors declare that there is no conflict of interest.
References
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol. 2001;60:907–15. doi: 10.1124/mol.60.5.907. [DOI] [PubMed] [Google Scholar]
- Ahmed MM, Al-Obosi JA, Osman HM, Shayoub ME. Overexpression of Aldose Reductase Render Mouse Hepatocytes More Sensitive to Acetaminophen Induced Oxidative Stress and Cell Death. Indian J Clin Biochem. 2016;31:162–70. doi: 10.1007/s12291-015-0517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthérieu S, Chesné C, Li R, Guguen-Guillouzo C, Guillouzo A. Optimization of the HepaRG cell model for drug metabolism and toxicity studies. Toxicol In Vitro. 2012;26:1278–85. doi: 10.1016/j.tiv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–9. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoine DJ, Williams DP, Kipar A, Laverty H, Park BK. Diet restriction inhibits apoptosis and HMGB1 oxidation and promotes inflammatory cell recruitment during acetaminophen hepatotoxicity. Mol Med. 2010;16:479–90. doi: 10.2119/molmed.2010.00126. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bai J, Cederbaum AI. Adenovirus mediated overexpression of CYP2E1 increases sensitivity of HepG2 cells to acetaminophen induced cytotoxicity. Mol Cell Biochem. 2004;262:165–76. doi: 10.1023/b:mcbi.0000038232.61760.9e. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Cover C, Lemasters JJ, Jaeschke H. Nuclear translocation of endonuclease G and apoptosis-inducing factor during acetaminophen-induced liver cell injury. Toxicol Sci. 2006;94:217–25. doi: 10.1093/toxsci/kfl077. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetylcysteine. Toxicol Sci. 2004;80:343–9. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Lawson JA, Vonderfecht SL, Gujral JS, Jaeschke H. Protection against Fas receptormediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol Sci. 2000;58:109–17. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- Bajt ML, Ramachandran A, Yan HM, Lebofsky M, Farhood A, Lemasters JJ, Jaeschke H. Apoptosis-inducing factor modulates mitochondrial oxidant stress in acetaminophen hepatotoxicity. Toxicol Sci. 2011;122:598–605. doi: 10.1093/toxsci/kfr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt ML, Vonderfecht SL, Jaeschke H. Differential protection with inhibitors of caspase-8 and caspase-3 in murine models of tumor necrosis factor and Fas receptor-mediated hepatocellular apoptosis. Toxicol Appl Pharmacol. 2001;175:243–52. doi: 10.1006/taap.2001.9242. [DOI] [PubMed] [Google Scholar]
- Boess F, Bopst M, Althaus R, Polsky S, Cohen SD, Eugster HP, Boelsterli UA. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology. 1998;27:1021–9. doi: 10.1002/hep.510270418. [DOI] [PubMed] [Google Scholar]
- Boulares AH, Ren T. Mechanism of acetaminophen-induced apoptosis in cultured cells: roles of caspase-3, DNA fragmentation factor, and the Ca2+ and Mg2+ endonuclease DNAS1L3. Basic Clin Pharmacol Toxicol. 2004;94:19–29. [PubMed] [Google Scholar]
- Boulares AH, Zoltoski AJ, Stoica BA, Cuvillier O, Smulson ME. Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol. 2002;90:38–50. doi: 10.1034/j.1600-0773.2002.900108.x. [DOI] [PubMed] [Google Scholar]
- Cao L, Quan XB, Zeng WJ, Yang XO, Wang MJ. Mechanism of hepatocyte apoptosis. J Cell Death. 2016;9:19–29. doi: 10.4137/JCD.S39824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao P, Sun J, Sullivan MA, Huang X, Wang H, Zhang Y, Wang N, Wang K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int J Biol Macromol. 2018;111:1133–9. doi: 10.1016/j.ijbiomac.2018.01.139. [DOI] [PubMed] [Google Scholar]
- Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- Charlotte F, L'Herminé A, Martin N, Geleyn Y, Nollet M, Gaulard P, Zafrani ES. Immunohistochemical detection of bcl-2 protein in normal and pathological human liver. Am J Pathol. 1994;144:460–5. [PMC free article] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–87. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Dara L, Johnson H, Suda J, Win S, Gaarde W, Han D, Kaplowitz N. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015;62:1847–57. doi: 10.1002/hep.27939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dara L, Liu ZX, Kaplowitz N. Questions and controversies: the role of necroptosis in liver disease. Cell Death Discov. 2016;2:16089. doi: 10.1038/cddiscovery.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch M, Graffeo CS, Rokosh R, Pansari M, Ochi A, Levie EM, Van Heerden E, Tippens DM, Greco S, Barilla R, Tomkotter L, Zambirinis CP, Avanzi N, Gulati R, Pachter HL, Torres- Hernandez A, Eisenthal A, Daley D, Miller G. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 2015;6:e1759. doi: 10.1038/cddis.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato MT, Lahoz A, Castell JV, Gómez-Lechón MJ. Cell lines: a tool for in vitro drug metabolism studies. Curr Drug Metab. 2008;9:1–11. doi: 10.2174/138920008783331086. [DOI] [PubMed] [Google Scholar]
- Dong D, Xu L, Han X, Qi Y, Xu Y, Yin L, Liu K, Peng J. Effects of the total saponins from Rosa laevigata Michx fruit against acetaminophen-induced liver damage in mice via induction of autophagy and suppression of inflammation and apoptosis. Molecules. 2014;19:7189–206. doi: 10.3390/molecules19067189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016;10:148–56. doi: 10.1016/j.redox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Ramachandran A, McGill MR, Mansouri A, Asselah T, Farhood A, Woolbright BL, Ding WX, Jaeschke H. Induction of mitochondrial biogenesis protects against acetaminophen hepatotoxicity. Food Chem Toxicol. 2017;108:339–50. doi: 10.1016/j.fct.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du K, Xie Y, McGill MR, Jaeschke H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity. Expert Opin Drug Metab Toxicol. 2015;11:1769–79. doi: 10.1517/17425255.2015.1071353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, Lee PC, Hinton RH, Price SC, Kass GE. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–29. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- Fulda S. Smac mimetics as IAP antagonists. Semin Cell Dev Biol. 2015;39:132–8. doi: 10.1016/j.semcdb.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Green D, Kroemer G. The central executioners of apoptosis: caspases or mitochondria? Trends Cell Biol. 1998;8:267–71. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24:1184–95. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–85. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi ME, Gores GJ. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54:1024–33. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–8. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Han D, Dara L, Win S, Than TA, Yuan L, Abbasi SQ, Liu ZX, Kaplowitz N. Regulation of drug-induced liver injury by signal transduction pathways: critical role of mitochondria. Trends Pharmacol Sci. 2013;34:243–53. doi: 10.1016/j.tips.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–77. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SW, Lee HS, Jung KH, Lee H, Hong SS. Protective effect of fucoidan against acetaminophen-induced liver injury. Arch Pharm Res. 2012;35:1099–105. doi: 10.1007/s12272-012-0618-5. [DOI] [PubMed] [Google Scholar]
- Hu B, Colletti LM. CXC receptor-2 knockout genotype increases X-linked inhibitor of apoptosis protein and protects mice from acetaminophen hepatotoxicity. Hepatology. 2010;52:691–702. doi: 10.1002/hep.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JN, Liu Z, Wang Z, Li XD, Zhang LX, Li W, Wang YP. Ameliorative effects and possible molecular mechanism of action of black ginseng (Panax ginseng) on acetaminophen-mediated liver injury. Molecules. 2017;22(4) doi: 10.3390/molecules22040664. pii: E664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorga A, Dara L, Kaplowitz N. Drug-induced liver injury: cascade of events leading to cell death, apoptosis or necrosis. Int J Mol Sci. 2017;18(5) doi: 10.3390/ijms18051018. pii: E1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–54. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–41. [PubMed] [Google Scholar]
- Jaeschke H. Mechanisms of reperfusion injury after warm ischemia of the liver. J Hepatobiliary Pancreat Surg. 1998;5:402–8. doi: 10.1007/s005340050064. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–6. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Farhood A, Cai SX, Tseng BY, Bajt ML. Protection against TNF-induced liver parenchymal cell apoptosis during endotoxemia by a novel caspase inhibitor in mice. Toxicol Appl Pharmacol. 2000;169:77–83. doi: 10.1006/taap.2000.9035. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- Jaeschke H, Gujral JS, Bajt ML. Apoptosis and necrosis in liver disease. Liver Int. 2004;24:85–9. doi: 10.1111/j.1478-3231.2004.0906.x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–57. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Farhood A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology. 2011;53:718–9. doi: 10.1002/hep.23940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr S, Hentze H, Englisch S, Hardt D, Fackelmayer FO, Hesch RD, Knippers R. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–65. [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci. 2003;75:458–67. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- Jang YH, You DH, Nam MJ. Protective effects of HGF gene-expressing human mesenchymal stem cells in acetaminophen-treated hepatocytes. Growth Factors. 2015;33:319–25. doi: 10.3109/08977194.2015.1080695. [DOI] [PubMed] [Google Scholar]
- Julien O, Wells JA. Caspases and their substrates. Cell Death Differ. 2017;24:1380–9. doi: 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass GE, Macanas-Pirard P, Lee PC, Hinton RH. The role of apoptosis in acetaminopheninduced injury. Ann N Y Acad Sci. 2003;1010:557–9. doi: 10.1196/annals.1299.103. [DOI] [PubMed] [Google Scholar]
- Kelava T, Cavar I, Culo F. Influence of small doses of various drug vehicles on acetaminopheninduced liver injury. Can J Physiol Pharmacol. 2010;88:960–7. doi: 10.1139/y10-065. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181:133–41. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- Kon K, Ikejima K, Okumura K, Aoyama T, Arai K, Takei Y, Lemasters JJ, Sato N. Role of apoptosis in acetaminophen hepatotoxicity. J Gastroenterol Hepatol. 2007;22(Suppl 1):S49–52. doi: 10.1111/j.1440-1746.2007.04962.x. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–54. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kučera O, Endlicher R, Rychtrmoc D, Lotková H, Sobotka O, Červinková Z. Acetaminophen toxicity in rat and mouse hepatocytes in vitro. Drug Chem Toxicol. 2017;40:448–456. doi: 10.1080/01480545.2016.1255953. [DOI] [PubMed] [Google Scholar]
- Lawson JA, Fisher MA, Simmons CA, Farhood A, Jaeschke H. Inhibition of Fas receptor (CD95)-induced hepatic caspase activation and apoptosis by acetaminophen in mice. Toxicol Appl Pharmacol. 1999;156:179–86. doi: 10.1006/taap.1999.8635. [DOI] [PubMed] [Google Scholar]
- Li G, Chen JB, Wang C, Xu Z, Nie H, Qin XY, Chen XM, Gong Q. Curcumin protects against acetaminophen-induced apoptosis in hepatic injury. World J Gastroenterol. 2013;19:7440–6. doi: 10.3748/wjg.v19.i42.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Feng JM, Wang Y, Li XH, Chen XX, Su Y, Shen YY, Chen Y, Xiong B, Yang CH, Ding J, Miao ZH. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 2014;5:e1278. doi: 10.1038/cddis.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YL, Zhang ZH, Liu XJ, Liu XQ, Tao L, Zhang YF, Wang H, Zhang C, Chen X, Xu DX. Melatonin protects against apoptosis-inducing factor (AIF)-dependent cell death during acetaminophen-induced acute liver failure. PLoS One. 2012;7(12):e51911. doi: 10.1371/journal.pone.0051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limaye PB, Bhave VS, Palkar PS, Apte UM, Sawant SP, Yu S, Latendresse JR, Reddy JK, Mehendale HM. Upregulation of calpastatin in regenerating and developing rat liver: role in resistance against hepatotoxicity. Hepatology. 2006;44:379–88. doi: 10.1002/hep.21250. [DOI] [PubMed] [Google Scholar]
- Macanas-Pirard P, Yaacob NS, Lee PC, Holder JC, Hinton RH, Kass GE. Glycogen synthase kinase-3 mediates acetaminophen-induced apoptosis in human hepatoma cells. J Pharmacol Exp Ther. 2005;313:780–9. doi: 10.1124/jpet.104.081364. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, Shoshan MC. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–13. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manov I, Hirsh M, Iancu TC. N-acetylcysteine does not protect HepG2 cells against acetaminophen-induced apoptosis. Basic Clin Pharmacol Toxicol. 2004;94:213–25. doi: 10.1111/j.1742-7843.2004.pto940504.x. [DOI] [PubMed] [Google Scholar]
- Marivin A, Berthelet J, Plenchette S, Dubrez L. The Inhibitor of Apoptosis (IAPs) in Adaptive Response to Cellular Stress. Cells. 2012;1:711–37. doi: 10.3390/cells1040711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Jaeschke H. Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res. 2013;30:2174–87. doi: 10.1007/s11095-013-1007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012a;122:1574–83. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Williams CD, Xie Y, Ramachandran A, Jaeschke H. Acetaminophen-induced liver injury in rats and mice: comparison of protein adducts, mitochondrial dysfunction, and oxidative stress in the mechanism of toxicity. Toxicol Appl Pharmacol. 2012b;264:387–94. doi: 10.1016/j.taap.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–82. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J Pharmacol Exp Ther. 1973;187:185–94. [PubMed] [Google Scholar]
- Nagata S, Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Nagy G, Szarka A, Lotz G, Dóczi J, Wunderlich L, Kiss A, Jemnitz K, Veres Z, Bánhegyi G, Schaff Z, Sümegi B, Mandl J. BGP-15 inhibits caspase-independent programmed cell death in acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 2010;243:96–103. doi: 10.1016/j.taap.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Nam EJ, Hayashida K, Aquino RS, Couchman JR, Kozar RA, Liu J, Park PW. Syndecan-1 limits the progression of liver injury and promotes liver repair in acetaminophen-induced liver injury in mice. Hepatology. 2017;66:1601–15. doi: 10.1002/hep.29265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman MG. Apoptosis in diseases of the liver. Crit Rev Clin Lab Sci. 2001;38:109–66. doi: 10.1080/20014091084182. [DOI] [PubMed] [Google Scholar]
- Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–32. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, McGill MR, Chao X, Du K, Williams JA, Xie Y, Jaeschke H, Ding WX. Removal of acetaminophen protein adducts by autophagy protects against acetaminophen-induced liver injury in mice. J Hepatol. 2016;65:354–62. doi: 10.1016/j.jhep.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni HM, Williams JA, Jaeschke H, Ding WX. Zonated induction of autophagy and mitochondrial spheroids limits acetaminophen-induced necrosis in the liver. Redox Biol. 2013;1:427–32. doi: 10.1016/j.redox.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunage R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–9. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H. Mechanisms of acetaminophen hepatotoxicity and their translation to the human pathophysiology. J Clin Transl Res. 2017;3(Suppl 1):157–69. doi: 10.18053/jctres.03.2017S1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Jaeschke H. Acetaminophen toxicity novel insights into mechanisms and future perspectives. Gene Expr. 2018;7:17–21. doi: 10.3727/105221617X15084371374138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207:1807–17. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SD, Jena N. A hepatotoxic dose of acetaminophen modulates expression of BCL-2, BCLX(L), and BCL-X(S) during apoptotic and necrotic death of mouse liver cells in vivo. Arch Toxicol. 2000;73:594–606. doi: 10.1007/s002040050013. [DOI] [PubMed] [Google Scholar]
- Ray SD, Kamendulis LM, Gurule MW, Yorkin RD, Corcoran GB. Ca2+ antagonists inhibit DNA fragmentation and toxic cell death induced by acetaminophen. FASEB J. 1993;7:453–63. doi: 10.1096/fasebj.7.5.8462787. [DOI] [PubMed] [Google Scholar]
- Ray SD, Kumar MA, Bagchi D. A novel proanthocyanidin IH636 grape seed extract increases in vivo Bcl-XL expression and prevents acetaminophen-induced programmed and unprogrammed cell death in mouse liver. Arch Biochem Biophys. 1999;369:42–58. doi: 10.1006/abbi.1999.1333. [DOI] [PubMed] [Google Scholar]
- Ray SD, Mumaw VR, Raje RR, Fariss MW. Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate pretreatment. J Pharmacol Exp Ther. 1996;279:1470–83. [PubMed] [Google Scholar]
- Ray SD, Patel N, Shah N, Nagori A, Naqvi A, Stohs SJ. Pre-exposure to a novel nutritional mixture containing a series of phytochemicals prevents acetaminophen-induced programmed and unprogrammed cell deaths by enhancing BCL-XL expression and minimizing oxidative stress in the liver. Mol Cell Biochem. 2006;293:119–36. doi: 10.1007/s11010-006-9235-2. [DOI] [PubMed] [Google Scholar]
- Ray SD, Sorge CL, Raucy JL, Corcoran GB. Early loss of large genomic DNA in vivo with accumulation of Ca2+ in the nucleus during acetaminophen-induced liver injury. Toxicol Appl Pharmacol. 1990;106:346–51. doi: 10.1016/0041-008x(90)90254-r. [DOI] [PubMed] [Google Scholar]
- Rodrigues RM, Heymans A, De Boe V, Sachinidis A, Chaudhari U, Govaere O, Roskams T, Vanhaecke T, Rogiers V, De Kock J. Toxicogenomics-based prediction of acetaminopheninduced liver injury using human hepatic cell systems. Toxicol Lett. 2016;240:50–9. doi: 10.1016/j.toxlet.2015.10.014. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Matsuura K, Khatib K, Reed JC, Nagata S, Vassalli P. A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J Exp Med. 1996;183:1031–6. doi: 10.1084/jem.183.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149–59. [PubMed] [Google Scholar]
- Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila) 2012;50:91–98. doi: 10.3109/15563650.2012.659252. [DOI] [PubMed] [Google Scholar]
- Saito C, Zwingmann C, Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–54. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liver Int. 2006;26:904–11. doi: 10.1111/j.1478-3231.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- Schüngel S, Buitrago-Molina LE, Nalapareddy Pd, Lebofsky M, Manns MP, Jaeschke H, Gross A, Vogel A. The strength of the Fas ligand signal determines whether hepatocytes act as type 1 or type 2 cells in murine livers. Hepatology. 2009;50:1558–66. doi: 10.1002/hep.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Korsmeyer SJ. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem Biophys Res Commun. 2003;304:437–44. doi: 10.1016/s0006-291x(03)00615-6. [DOI] [PubMed] [Google Scholar]
- Sharma S, Singh RL, Kakkar P. Modulation of Bax/Bcl-2 and caspases by probiotics during acetaminophen induced apoptosis in primary hepatocytes. Food Chem Toxicol. 2011;49:770–9. doi: 10.1016/j.fct.2010.11.041. [DOI] [PubMed] [Google Scholar]
- Shen W, Kamendulis LM, Ray SD, Corcoran GB. Acetaminophen-induced cytotoxicity in cultured mouse hepatocytes: correlation of nuclear Ca2+ accumulation and early DNA fragmentation with cell death. Toxicol Appl Pharmacol. 1991;111:242–54. doi: 10.1016/0041-008x(91)90028-d. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. Silencing glycogen synthase kinase-3beta inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem. 2010;285:8244–55. doi: 10.1074/jbc.M109.054999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E, Fu J, Xia X, Su C, Song Y. Bazhen decoction protects against acetaminophen induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. PLoS One. 2014;9:e107405. doi: 10.1371/journal.pone.0107405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman HM. The Bcl-2 oncoprotein functions as a pro-oxidant. J Biol Chem. 1995;270:3487–90. [PubMed] [Google Scholar]
- Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–27. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Thornberry NA. The caspase family of cysteine proteases. Br Med Bull. 1997;53:478–90. doi: 10.1093/oxfordjournals.bmb.a011625. [DOI] [PubMed] [Google Scholar]
- van Gurp M, Festjens N, van Loo G, Saelens X, Vandenabeele P. Mitochondrial intermembrane proteins in cell death. Biochem Biophys Res Commun. 2003;304:487–97. doi: 10.1016/s0006-291x(03)00621-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hu JN, Yan MH, Xing JJ, Liu WC, Li W. Caspase-Mediated Anti-Apoptotic Effect of Ginsenoside Rg5, a Main Rare Ginsenoside, on Acetaminophen-Induced Hepatotoxicity in Mice. J Agric Food Chem. 2017;65:9226–36. doi: 10.1021/acs.jafc.7b03361. [DOI] [PubMed] [Google Scholar]
- Wang AY, Lian LH, Jiang YZ, Wu YL, Nan JX. Gentiana manshurica Kitagawa prevents acetaminophen-induced acute hepatic injury in mice via inhibiting JNK/ERK MAPK pathway. World J Gastroenterol. 2010;16:384–91. doi: 10.3748/wjg.v16.i3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Lin B. Inhibitor of apoptosis proteins (IAPs) as regulatory factors of hepatic apoptosis. Cell Signal. 2013;25:1970–80. doi: 10.1016/j.cellsig.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu J, Zhang X, Zhao S, Zou K, Xie J, Wang X, Liu C, Wang J, Wang Y. Seabuckthorn berry polysaccharide extracts protect against acetaminophen induced hepatotoxicity in mice via activating the Nrf-2/HO-1-SOD-2 signaling pathway. Phytomedicine. 2018;38:90–7. doi: 10.1016/j.phymed.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Westerink WM, Schoonen WG. Cytochrome P450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol In Vitro. 2007;21:1581–91. doi: 10.1016/j.tiv.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Williams CD, Koerner MR, Lampe JN, Farhood A, Jaeschke H. Mouse strain-dependent caspase activation during acetaminophen hepatotoxicity does not result in apoptosis or modulation of inflammation. Toxicol Appl Pharmacol. 2011;257:449–58. doi: 10.1016/j.taap.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Ni HM, Haynes A, Manley S, Li Y, Jaeschke H, Ding WX. Chronic Deletion and Acute Knockdown of Parkin Have Differential Responses to Acetaminophen-induced Mitophagy and Liver Injury in Mice. J Biol Chem. 2015;290:10934–46. doi: 10.1074/jbc.M114.602284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win S, Than TA, Min RW, Aghajan M, Kaplowitz N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 2016;63:1987–2003. doi: 10.1002/hep.28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–92. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, McGill MR, Dorko K, Kumer SC, Schmitt TM, Forster J, Jaeschke H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol Appl Pharmacol. 2014;279:266–74. doi: 10.1016/j.taap.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Antoine DJ, Weemhoff JL, Jenkins RE, Farhood A, Park BK, Jaeschke H. Biomarkers distinguish apoptotic and necrotic cell death during hepatic ischemia/reperfusion injury in mice. Liver Transpl. 2014;20:1372–82. doi: 10.1002/lt.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature (Lond) 1994;369:321–23. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama Y, Sasaki Y, Terasaki N, Kawataki T, Takekawa K, Iwase Y, Shimizu T, Sanoh S, Ohta S. Comparison of drug metabolism and its related hepatotoxic effects in HepaRG, cryopreserved human hepatocytes, and HepG2 cell cultures. Biol Pharm Bull. 2018;41:722–732. doi: 10.1248/bpb.b17-00913. [DOI] [PubMed] [Google Scholar]
- Zhang YF, He W, Zhang C, Liu XJ, Lu Y, Wang H, Zhang ZH, Chen X, Xu DX. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 2014;225:445–53. doi: 10.1016/j.toxlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Zhang J, Song Q, Han X, Zhang Y, Zhang Y, Zhang X, Chu X, Zhang F, Chu L. Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int Immunopharmacol. 2017;47:95–105. doi: 10.1016/j.intimp.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang Y, He W, Sun L. Necrosome core machinery: MLKL. Cell Mol Life Sci. 2016;73:2153–63. doi: 10.1007/s00018-016-2190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Cong X, Zheng L, Xu L, Yin L, Peng J. Dioscin, a natural steroid saponin, shows remarkable protective effect against acetaminophen-induced liver damage in vitro and in vivo. Toxicol Lett. 2012;214:69–80. doi: 10.1016/j.toxlet.2012.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.