I. Introduction
Dietary supplements, as defined by the Dietary Supplement Health and Education Act of 1994 (DSHEA) in the United States, are products intended to supplement the diet that contain vitamins, minerals, amino acids, other dietary substances, and/or herbs or other botanicals (Abdel-Rahman et al., 2011). They are further defined as only including products intended for ingestion, not representing a conventional food or complete nutritional source, and requiring labeling as a dietary supplement. The DSHEA legislation was aimed at balancing access to dietary supplements with public safety and amended the Federal Food, Drug, and Cosmetic Act (FD&C Act) to provide a clear definition of dietary supplements and a regulatory framework for evaluating safety and claims associated with activity (Abdel-Rahman et al., 2011). The focus herein is on a subset of dietary supplements, those containing herbs or other botanicals, that are collectively referred to as botanical dietary supplements or, simply, botanicals. It is important to note that use patterns and regulatory guidance for botanical dietary supplements differ around the world. While this manuscript focuses primarily on the United States, recent reviews offer comparisons of global regulatory paradigms (Enioutina et al., 2017; Low et al., 2017). Furthermore, the challenges in assessing the safety of botanical dietary supplements discussed within are universally relevant.
Botanical dietary supplements are generally available as whole plants, plant parts, powdered plant material, or plant extracts. These supplements are marketed in various forms, including as powders, tablets, capsules, gummies, teas, tinctures, and essential oils. A variety of botanical dietary supplements are used in complementary and integrative health practices (https://nccih.nih.gov/health/integrative-health). Although there is overlap in the botanical species used in dietary supplements and other forms of complementary medicine, such as Ayurveda and Traditional Chinese Medicine, the applications can vary widely, and safety considerations associated with these practices are beyond the scope of the current work.
Botanical dietary supplements are widely available in the United States. According to the 2012 National Health Information Survey, an estimated 18% of adults in the United States used dietary supplements that were not vitamin- or mineral-based (Clarke et al., 2015). Although this value is not specific to botanical dietary supplements (also includes fish oil, glucosamine, etc.), it provides an informative estimate about consumer use of these types of products. This is consistent with findings from the 2007 National Health Information Survey, in which an estimated 18% of adults and 4% of children used non-vitamin, non-mineral natural products at least once during the 12 months preceding the survey (Barnes et al., 2008). According to the Dietary Supplement Label Database, there are currently over 20,000 dietary supplements in the botanical ingredient category available in the United States marketplace (https://dsld.nlm.nih.gov/dsld/). Furthermore, it was estimated that approximately $7.5 billion was spent on botanical dietary supplements in 2016 (Smith et al., 2017). Extensive use of botanical dietary supplements, combined with a paucity of toxicity data, has fueled interest in developing approaches for ensuring the safety of botanical dietary supplements (Job et al., 2016; Marcus, 2016; van Breemen, 2015).
Many different stakeholders recognize the importance of ensuring the quality and safety of botanical dietary supplements (e.g., the public, suppliers and manufacturers, regulators, healthcare providers, researchers). This endeavor is multi-dimensional and involves consideration of the chemical properties and toxicological profiles of the raw botanical ingredient(s), excipients present in the finished product, and reagents involved in the processing or manufacturing of the finished product, as well as possible sources of contamination at any step along the process from harvest of the raw ingredients to storage of the finished product (Figure 1).
Figure 1.
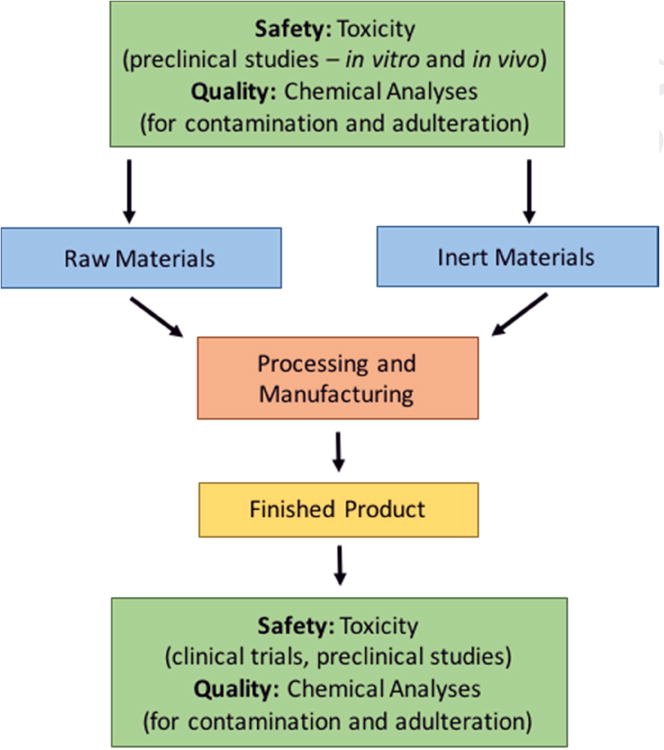
Multi-dimensional considerations that protect against inconsistencies in botanical product quality and integrity.
A complicating factor in the evaluation of botanical quality and safety is their inherent complexity. Botanical dietary supplements are typically complex mixtures and can display a high degree of variability (both natural and introduced) (Tanko et al., 2005). Sources of variability in finished products can range from compositional differences between batches of raw materials to differences in processing and manufacturing of the source biomass (Pferschy-Wenzig and Bauer, 2015). The constituents of individual batches may differ based on factors such as geographical location where the plant material is grown (e.g., altitude (Rieger et al., 2008), climate (Melito et al., 2016) and time and growth stage at harvest (Galasso et al., 2014; Pacifico et al., 2016). Furthermore, the processes and practices that individual manufacturers use are often unique to the company and proprietary, so while batch-to-batch variation within a company may be minimal, variability between products that are nominally the same from different companies may be considerable. Due to this widespread variability, chemical evaluation of composition has joined botanical morphology as an important tool in the manufacture, study and regulation of these products (Schilter et al., 2003). Standardization, a process that measures and adjusts the amount of and ratio between key constituents has been adopted as a means of controlling batch-to-batch variability.
Due to the complexity and variation in botanical dietary supplement composition, there are significant issues with comparing test articles across studies and, therefore, reproducibility in botanicals research is a challenge. Protocols for dietary supplement research must take many different factors into consideration, including populations (generalizability), responders vs. non-responders, timing and duration of exposure, endpoints of concern, dose levels, and earlier phase studies. Data from exploratory clinical trials and studies of natural products have been highly inconsistent, and there are many problems replicating the effects of botanicals that have been reported in the literature. NIH’s National Center for Complementary and Integrative Health (NCCIH) has long recognized these issues, and has had a natural product integrity policy in place since 2007 (https://nccih.nih.gov/research/policies/naturalproduct.htm) and a requirement to address composition of research materials in grant applications has been added to NIH-wide grant policies (https://www.nih.gov/research-training/rigor-reproducibility). Clinical research to evaluate the efficacy of a botanical product in “curing, treating, or mitigating a disease” shifts the botanical from the dietary supplement category to the drug category and must be conducted under the umbrella of the Investigational New Drug process administered by the Food and Drug Administration/Center for Drug Evaluation and Research (FDA/CDER). The clinical research process requires exhaustive characterization of product composition, but compositional details are proprietary and known only to the study sponsor and the FDA.
Due to many inconsistencies in study design and knowledge about product composition, there is a low level of confidence in published data on potential biological targets of botanical dietary supplements (Landis et al., 2012). As part of efforts to standardize botanical quality, there are now many publications that set out minimal quality standards for botanical raw materials and very simple finished products. These include the European Pharmacopoeia, the United States Pharmacopeia (USP), Pharmacopoeia of the People’s Republic of China, the Hong Kong Materia Medica Standards, and others. These science-based, quality monographs for botanicals contain specifications on the identity, content and composition, purity, and performance of individual botanicals. These publications set out specifications and tests for use in Good Manufacturing Practice (GMP) settings, but are not mandatory for dietary supplement products in the U.S. (www.usp.org).
In 2016, the National Toxicology Program (NTP) hosted a workshop entitled “Addressing Challenges in the Assessment of Botanical Dietary Supplement Safety”. The goal of the workshop was to bring together stakeholders with varying expertise and discuss best strategies for: 1) developing methods for assessing phytoequivalence of botanicals, 2) identifying the active constituent(s) or patterns of biological response of botanicals, and 3) assessing the absorption, distribution, metabolism, and elimination (ADME) of botanicals (NTP, 2016). This manuscript will serve to expand on the three primary challenges associated with botanical supplement safety including the complexity of botanical dietary supplements, the struggle for reproducibility in botanicals research, and the regulatory issues relevant to botanicals; additional companion manuscripts will expand further on the three key topics outlined above (Catlin et al., submitted; Roberts et al., submitted; Waidyanatha et al., submitted).
II. The Complexity of Botanical Dietary Supplements
The complexity of botanical dietary supplements presents a significant challenge in evaluating toxicity and maintaining product quality and integrity. As outlined in the introduction, there are multiple elements and processes that contribute to this complexity. These elements include the plant or plants that represent the “dietary ingredient(s)” referenced in 201(ff) of the FD&C Act; “components” defined in the Current Good Manufacturing Practice (cGMP) regulations (21 CFR 111) as “any substance intended for use in the manufacture of a dietary supplement, including those that may not appear in the finished batch of the dietary supplement”; and contaminants (e.g., drug, bacteria, pesticide, glass, lead). The “process” is defined here as the series of steps (often proprietary) that a manufacturer takes to get from raw materials to the finished product. Finally, storage conditions from the time a product is produced to its eventual consumption also factor into safety, integrity, and quality considerations.
Defining what is meant by the terms “quality” and “integrity” in the context of botanical dietary supplements also contributes to confusion in the field. According to the cGMP regulations, “quality means that the dietary supplement consistently meets the established specifications for identity, purity, strength, and composition, and limits on contaminants, and has been manufactured, packaged, labeled, and held under conditions to prevent adulteration under section 402(a)(1), (a)(2), (a)(3), and (a)(4) of the act.” Unlike quality, botanical integrity is not a regulatory concept. Instead, botanical integrity emerged from work by the NCCIH and Office of Dietary Supplements (ODS) to implement policy on botanical study materials used in research, and involves the integration of botany, chemistry, and bioactivity of the test articles (Simmler et al., 2015). Later sections of this manuscript will address the topics of quality and integrity in more detail. The remainder of this section will delve into the sources of complexity and variability of botanical dietary supplements.
Botanical raw material(s) provide a good starting place for discussion of the complexity of botanical dietary supplements. Plants are complex entities, and their complexity is believed to be a result of natural selection and evolution (Weng, 2014). In a recent review on plant communication, Simpraga et al. (2016) refer to plants as “chemical factories”, which produce numerous structurally-diverse primary and secondary metabolites. Whereas primary metabolites have a role in basic plant functions (e.g., growth, photosynthesis and reproduction), secondary metabolites represent a diverse group of chemicals that are not involved in primary functions (Simpraga et al., 2016). Secondary metabolites can be divided into four major classes based on their biosynthetic pathway: terpenoids, fatty acid derivatives (commonly known as “green leaf volatiles”), phenylpropanoids/benzenoids derived from phenylalanine, and “other” amino acid derivatives (Dudareva et al., 2013; Simpraga et al., 2016). Secondary metabolites exhibit a broad range of bioactivities, often serving to defend against herbivores, insects, and abiotic factors such as heat, ultraviolet radiation, and oxidative stress (Dicke et al., 2009; Jones and Firn, 1991). Furthermore, many bioactive constituents from plants have been exploited by humans for use as pesticides, pharmaceuticals, poisons, or in other consumer products (Pohlit et al., 2011; Xie and Zhou, 2017). The complex composition of secondary metabolites is thought to be responsible for both the purported therapeutic effects of botanical dietary supplements as well as their toxicity. Roberts et al. (submitted) discusses the need for analytical methods that are capable of efficiently evaluating the active constituents (and subsequent toxicity/safety) of numerous botanical products. Bioassay-guided fractionation (BGF), one of the predominant methods for identification of biologically-active constituents, is a useful tool, but becomes limited when the mechanism of action is unknown. Newer methods are being developed to overcome these limitations, which will ultimately improve safety evaluations of botanical products on the market (Roberts et al., submitted).
Understanding the sources of variability for constituents such as metabolites and how that variability might affect toxicity is an important part of determining safety of botanicals. The amount of and ratio between marker constituents (i.e., compounds typically used to authenticate the botanical of interest) can vary in botanical raw material based on geography, climate, environmental stress, and growth stage at harvest. This phenomenon of plants containing different chemical profiles based on environmental factors is known as phytochemical variation (Dhami and Mishra, 2015). Binns et al. (2002) assessed phytochemical variation in Echinacea angustifolia by measuring 28 constituents in plants from nine different regions in the United States. Significant differences were observed among the chemical profiles, with some constituents contributing more to the variation than others, and 5 of the constituent concentrations correlating with latitudinal variation. Seeds from each of the 9 regions were grown in identical, controlled conditions; therefore, observed chemical differences were attributed to genetic variation in the plants from regional selection pressures. Similar findings were noted in a study of black cohosh (Actaea racemosa) gathered from 11 wild populations across the United States and grown in a common location (Al-Amier et al., 2006). In another study that specifically assessed the effects of climate on botanical constituents, Kaur et al. (2016) grew five medicinal plants (Hypericum perforatum, Matricaria chamomilla, Thymus vulgaris, Cynara cardunculus, and Echinacea purpurea) from seed in two different altitudes and climates (305 meters and subtropic vs. 1730 meters and temperate) and found that there were significant differences in phenol and flavonoid concentrations between plants grown in the two locations. The effects of temperature stress on the plant metabolome, and subsequent cellular, metabolic, and physiological processes, were extensively reviewed by Guy et al. (2008), who reported that both heat- and cold-stress induced alterations in the metabolic activity of plants. Some of these changes included alterations in sugar metabolism (both heat- and cold-stress), shifts in carbohydrate metabolism (both heat- and cold-stress), and increased flavonoid metabolism (cold-stress). Interestingly, it was concluded that colder temperatures had a much stronger impact on plant metabolism than warmer temperatures (Guy et al., 2008).
Constituent concentrations can also differ between plant parts (i.e., leaves, flowers, roots, seeds, stems, bark); therefore, for some botanicals, only certain parts of the plants should be harvested. In the case of Ginkgo biloba, the leaves should be the only plant part harvested as they contain the desirable concentrations of the active botanical constituents associated with Ginkgo biloba’s purported mechanism of action (e.g. flavone glycosides, terpene lactones, ginkgolic acids) (USP, 2015). Similarly, the active constituents in Panex ginseng, ginsenosides, are found predominantly in the main and lateral root and root hairs, which should be the only plant parts collected during harvest (USP, 2015). Adulteration of P. ginseng products with leaf or flower extracts is known to be a problem, particularly in China, and analytical chemistry techniques (i.e. HPLC) are more commonly being utilized to identify adulterated root extracts (Wang, 2009). With Echinacea purpurea, on the other hand, extracts can be prepared from either the aerial parts (the aboveground purple coneflower, harvested while flowering) or from freshly harvested or dried roots; the ratio between and amount of marker constituents are considered to be sufficiently similar between parts (USP, 2015).
The purity of both the initial botanical ingredients as well as the finished product is important in determining product integrity. Factors that can affect the quality and toxicity of the botanical raw material include contaminants, which can occur due to soil conditions (e.g., metals), treatment (e.g., pesticides), harvesting (e.g., co-harvesting non-target plant species or other materials – soil, insects), or storage (e.g., bacteria or fungi) of the plant material (Wang and Huang, 2015). A study of botanical dietary supplements containing milk thistle, including whole and powdered seed, cut and powdered herb, ground seed in tea bags, extracts, gels, and capsules, found that a significant number of the samples tested contained fungal contaminants (Tournas et al., 2013). The highest levels of fungi were found on milk thistle seeds (whole) and cut herb material, and numerous mycotoxins, including Aspergillus, Penicilium, and Alternia, were identified in the tested supplements. Natural toxins are also found in many medicinal herbs; for example, compounds such as pyrrolizidine alkaloids are naturally occurring in comfrey, among other plants, and are toxic to humans and livestock (Roeder, 1995). Pyrrolizidine alkaloids are known hepatotoxins and have also been associated with neurotoxicity, pneumotoxicity, embryo-fetal toxicity, and carcinogenicity (Fu et al., 2001; Neuman et al., 2015; Seaman, 1987; Yan et al., 2016). There are four plant families that commonly contain high levels of pyrrolizidine alkaloids, including Asteraceae, Boraginacceae, Leguminosae, and Orchidaceae; however, over 660 unique pyrrolizidine alkaloids and their derivatives have been identified in thousands of different plants (Chou and Fu, 2006; Roeder, 1995; Roeder et al., 2015; Stegelmeier et al., 1999; Yan et al., 2016). The presence of pyrrolizidine alkaloids in other botanical products, such as teas, may sometimes occur due to unintentional co-harvesting of weeds containing pyrrolizidine alkaloids alongside the targeted plant (i.e. chamomile) (Mathon et al., 2014).
Contaminants in finished botanical products may be introduced during the manufacturing and preparation processes, and can include undeclared synthetic substances (e.g. pharmaceuticals and related compounds) and metals. Intentional adulteration of botanical products with pharmaceutical chemicals is a significant concern worldwide (Liu and Lu, 2017; Rocha et al., 2016; Sullivan and Crowley, 2006). Examples of pharmaceutical adulterants of botanicals include stimulants, anti-depressants, appetite suppressors, and laxatives in weight-loss supplements, anabolic steroids in muscle-promoting supplements, and phosphodiesterase-5 (PDE-5) inhibitors in supplements marketed for enhancement of sexual performance (Rocha et al., 2016).
Plant identification is extremely important in maintaining product integrity, and identification techniques become more complicated the further a product gets from the original source material (the more the plant material undergoes processing). Many of the anatomical and biological features of botanicals are lost during extraction and manufacturing processes, and precise identification becomes increasingly dependent on chemistry rather than botanical morphology. DNA barcoding has been proposed as a plant identification method for botanical research and regulation. The technique has been utilized by the New York Botanical Garden in an effort to identify a universally-accepted barcode for each plant (http://sciweb.nybg.org/Science2/DNABank.asp) and by the Urban Barcode Project as part of an effort to identify the extensive species of plants in New York City (http://www.dnabarcoding101.org/programs/ubp/). The use of this newer technology for identification and authentication of botanicals, particularly extracts, in dietary supplements has significant challenges. For example, the DNA fragments in extracts may be degraded, of poor quality, and/or possibly contaminated with secondary metabolites due to the extraction processes or with DNA from fillers or excipients of plant origin. Also, there is currently no universal DNA barcode or a DNA library of plant extracts large enough for reliable and accurate comparisons (Cowan and Fay, 2012; Fabricant and Hilmas, 2015).
One of the biggest challenges in plant identification is that most consumer materials are extracts and not biomass, or plant material. Many extracts claim to be standardized, or adjusted to contain a defined amount of the known marker compound(s) or active constituents, which theoretically helps ensure batch-to-batch reproducibility. Extracts may also be quantified or normalized; the method used depends on whether the active constituent is known or not (Garg et al., 2012; Ong, 2004; Zeng et al., 2011). Different approaches to extract preparation may alter the properties of the final product (i.e. inconsistencies in the final amount of marker compound), which ultimately makes characterization of the final product difficult. There are different types of extracts, including native extracts, liquid extracts, and extracts processed using a carrier. Native extracts are dried, while liquid extracts are extracted via a solvent, or extracts that were processed using a carrier. Numerous diverse approaches to extract preparation can result in significantly different final products and make comparing extracts and finished products very complicated. Accurate identification of a specific botanical product is dependent on knowledge of the type of extract (e.g., dried or liquid) and the manufacturing process used to create the extract. Ultimately, the development and application of more precise identification methods for fully characterizing the materials (e.g., metabolomics) may be required (Azwanida, 2015; Kellogg et al., 2017). Mattoli et al. (2006) determined that electrospray mass spectrometry (ESI-MS) was a reliable method for developing a metabolomic fingerprint for plant extracts, making it a potentially useful tool for identifying and comparing botanical extracts.
An illustrative example of inconsistencies in botanical dietary supplement composition can be found in Ginkgo biloba extract. Standard, high quality Ginkgo biloba extract formulations contain a range of compounds, including: ginkgolides (2.5-4.5%), bilobalide (2.6-3.2%), flavonol glycosides (22-26%), proanthocyanidins (1-10%), and ginkgolic acids (< 5 ppm) (USP, 2015). The Ginkgo biloba plant produces different levels of these compounds depending on the season, as the desired levels of these measured compounds only occur during the fall. In the spring, Ginkgo biloba leaves have much higher levels of flavonoid glycosides and much lower levels of terpene lactones, which results in significant variability in the components of Ginkgo biloba extract supplements (Lobstein et al., 1991; van Beek and Lelyveld, 1992). Additionally, Ginkgo biloba extract may be subject to economic adulteration, where lower-cost plant materials or single chemicals are added to increase bulk material; examples of adulterants include Saphora japonica and pure flavanol (Chandra et al., 2011). Simple analytical methods, such as measuring total flavonol aglycones, can be easily fooled by this type of adulteration, thus necessitating the use of pattern and fingerprint analysis to accurately determine what is in the final product (Chandra et al., 2011).
Toward the goals of understanding how to compare across complex botanical products and how chemical differences in botanical extracts relate to biological activity, the NTP developed multiple case studies (i.e., Ginkgo biloba extract, black cohosh extract, and Echinacea purpurea extract) that work through evaluation of sufficient similarity among related products. For the Ginkgo biloba extract (GBE) case study, the NTP analyzed levels of different components in numerous samples (both unfinished extracts and finished products), and demonstrated that there was significant variability among products, which led to noted differences in biological and toxicological effects of the samples (Catlin et al., submitted). Catlin et al. (submitted) evaluated 26 different GBE samples for similarity in chemistry and biological responses as compared to a reference sample (i.e., the NTP sample that was evaluated in 90-day and 2-year toxicity and carcinogenicity studies). Untargeted and targeted chemical analyses were used to compare chemical composition of the samples. The NTP had previously identified the liver as a target of GBE exposure (NTP, 2013b; Rider et al., 2014), and therefore assessed the biological activity of the different GBE samples in vitro in primary human hepatocytes and a subset of samples were evaluated in vivo in 5-day rat studies. Overall, this case study found that 62% of the GBE samples (including NIST standard reference material and finished products known to contain high quality GBE) were sufficiently similar to a reference sample, 27% were different from the reference sample, and 12% were borderline (considered similar or different based on the evaluation method used).
III. Issues with Reproducibility in Botanicals Research
In June 2012, the U.S. National Institute of Neurological Disorders and Stroke (NINDS) gathered numerous stakeholders to review and discuss the lack of transparency and adequate reporting in preclinical research (Landis et al., 2012). There has been a push to improve scientific standards in preclinical research, including research on botanical dietary supplements, as inconsistent findings have been noted in both preclinical and clinical studies exploring efficacy and/or benefits of botanical dietary supplements. The report from the 2012 NINDS meeting concluded that the predictive value and reliability of preclinical research is significantly reduced when the experimental design, study conduct, and data analysis techniques are not substantially described. To address these issues, they offered a series of recommendations for information that should be reported in preclinical studies (e.g., sample size, randomization methods, and data analysis details) (Landis et al., 2012). The issues with preclinical studies identified in the NINDS review are applicable to botanicals research, and, furthermore, preclinical and clinical studies with botanical dietary supplements have the added challenge of complex and variable test articles, as described previously (see Section II). Considering the nature of botanical dietary supplements, identification and characterization of botanical test articles used in preclinical and clinical research is critical. However, Wolsko et al., (2005) found that in a survey of published clinical trials on popular botanicals (i.e., Echinacea, garlic, Ginkgo biloba, saw palmetto, or St. John’s wort), only 15% (12 of 81 studies identified) reported testing to assess the content of the botanical, and only 4% (3 studies) provided enough information to compare the measured content to expected content.
The NCCIH has supported large-scale studies of common botanical dietary supplements to assess efficacy that had previously been reported in more limited clinical trials (Hopp, 2015). One of the clinical studies supported by NCCIH evaluated the efficacy of Hypericum perforatum, or St. John’s wort, in comparison to sertraline (Zoloft) and a placebo. No evidence of efficacy of St. John’s wort was observed; in fact, both St. John’s wort and sertraline had lower efficacy than the placebo (Davidson et al., 2002). In another NCCIH-supported evaluation, Actaea racemosa (black cohosh) and Trifolium pratense (red clover), which are commonly used to alleviate symptoms of menopause, were compared to more standard hormone replacement therapies (specifically conjugated equine estrogens) and a placebo, and neither botanical was determined to be more effective than the placebo (Geller et al., 2009). A study of Echinacea angustifolia, which is marketed to both prevent and alleviate cold symptoms, demonstrated that there was no perceived benefit of Echinacea angustifolia treatment relative to placebo (Turner et al., 2005). Lastly, a large clinical trial conducted between 2000 and 2008, termed the “Ginkgo Evaluation of Memory (GEM) Study”, evaluated the effectiveness of Ginkgo biloba in diminishing the onset of dementia and Alzheimer’s disease in elderly participants. Clinical trial patients consumed a Ginkgo biloba extract or placebo twice daily, and Ginkgo biloba extract was determined to be ineffective at lowering the rates of dementia and Alzheimer’s disease (DeKosky et al., 2008). In all four of these cases, previously reported literature from clinical trials indicated that there may be benefits to using these products (Frei-Kleiner et al., 2005; Linde et al., 1996; Oken et al., 1998; Schoop et al., 2006); however, the NCCIH-supported studies were unable to replicate those findings. The largely negative findings from NCCIH-supported clinical trials of botanicals prompted a critical evaluation of potential contributing factors. In order to strengthen conclusions from future human efficacy studies with botanicals, it was suggested that clinical trials should be informed by preclinical data on molecular mechanisms of the botanical under study - with identification of bioactive chemicals and molecular targets, preclinical safety data, and pharmacokinetics data in humans (Kuszak et al., 2016). These conclusions have led to a reevaluation of funding priorities by NCCIH, and a movement away from large clinical trials to study disease treatment and towards a better understanding of the molecular interactions of botanicals with biological systems (Hopp, 2015; Kuszak et al., 2016).
As NCCIH has moved away from large-scale clinical trials, exploratory clinical trials of natural products have become higher priority research topics. One of the goals of the NCCIH is to design exploratory trials such that the results, whether positive or negative, provide information of high scientific use and support decisions about further development and/or testing of natural products. It has been proposed that the data from these studies should be utilized to fill gaps in scientific knowledge and provide the information necessary to develop competitive, full-scale clinical trials of botanical dietary supplements (https://nccih.nih.gov/grants/funding/clinicaltrials). Ideally, the data from these future studies will help to confirm the link between the impact of a natural product on the biological signature (i.e., mechanism of action) and demonstrate an association between the change in the biological signature and subsequent clinical outcomes.
The “product integrity policy”, put into place in 2005 by the NCCIH, has a goal of requiring investigators to provide evidence that both their biologically-active test agents and their placebos proposed for study are of high enough quality to ensure that the research can be reproduced (Kuszak et al., 2016). One example of where this policy has been successfully utilized is the case of Dulcamara, which was being evaluated as a chemotherapeutic agent. In the initial stages of these studies, the incorrect botanical material was initially sent to researchers (Solanum dulcamara was acquired instead of the correct test material, Kalanchoe gastonis-bonnieri), however the mistake was caught prior to the research being performed due to the stringent integrity policy (C. Hopp, unpublished). Another example is that of differences in cinnamon products on the market; multiple species of cinnamon are commercially available and the levels of both coumarin and cinnamaldehyde, the active ingredients in cinnamon, vary greatly between different products. NCCIH became aware of this problem due to the product integrity policy, and, after testing numerous products, determined that they could not find a powdered cinnamon product that was a single species. It was discovered, however, that cinnamon sticks are generally more genetically pure than the powdered form. A third example is the difficulty of determining what manufacturers are referring to when the term “green tea” is used. Green tea products can contain highly concentrated amounts of a small number of catechins (the major polyphenolic compounds in green tea), and it has proven to be challenging to get a single sample that is genetically homogenous. The goal of these policies to enhance integrity is to gain more accurate and precise data reporting and to ensure proper identification of the botanical material being studied.
Another important consideration in comparing across studies (including both preclinical animal toxicity studies and clinical trials) assessing a common botanical dietary supplement is understanding the ADME properties of the specific botanical product being evaluated in each system. Knowledge of internal dose, tissue distribution, and kinetics in animal models provides context for comparing results from an animal toxicity assessment to what might be expected in humans taking botanical dietary supplements at recommended doses. However, there is very little ADME data available for botanicals and the limited data available often focuses on a single marker constituent, which may or may not reflect the active constituent(s). Waidyanatha et al. (submitted) address the key challenges and a proposed path forward in generating ADME data for botanicals.
Standards for botanical quality are implemented and maintained by the United States Pharmacopeia (USP). The mission of the USP is to improve global health through public standards and related programs that help ensure the quality, safety, and benefit of natural medicines (including botanical supplements) and foods. Well-characterized materials need to be utilized when performing high quality research studies on botanicals, and the monographs provided by the USP help ensure botanical quality by detailing the standards that ensure proper botanical identity. Some of the different standards developed by the USP include the dietary supplement compendium, the Harvard Medical School compendium, and the USP-National Formulary (USP-NF), a joint compendium containing monographs on pharmaceutical and dietary ingredient standards (in the USP) and monographs on excipients (in the NF) (http://www.usp.org/). Another resource that is useful in understanding potential safety concerns associated with botanical dietary supplements is the European Food and Safety Authority (EFSA) compendium, which contains detailed information on compounds in botanicals that may present a risk to human health (EFSA, 2012).
The USP continues to develop public quality standards for botanical dietary supplements as information becomes available. Prior to developing a quality monograph, the USP performs an admission evaluation, a process that incorporates safety evaluations to assess if a dietary ingredient is associated with significant safety concerns. Based on the findings of the admission evaluation, a decision is made regarding whether or not the ingredient is admitted for monograph development.
In the USP, botanicals generally have three related monographs for the whole plant material, powdered plant material, and plant extract, as methods for identification vary based on plant form. In addition, many botanicals also have monographs for finished products (i.e. tablets and/or capsules). Establishment of the proper identity of a compendia article requires more than just meeting criteria for an identification test, and specifications in the monographs include identity, content/composition, purity, performance, and absence of contaminants (http://hmc.usp.org/). The monographs published by the USP contain titles and definitions that describe the specific part of the material being discussed, as different plant parts can have very different physiological effects. The monographs contain general chapters that describe different evaluations that need to be performed to accurately identify whether a material is similar enough to the material described in the monograph. Some of the botanical qualities that need to be identified include phytochemical, macroscopic, and microscopic features, as well as the minimum required amount of specific marker/biological compounds. Overall, the USP monographs serve to standardize botanical identity to allow for high quality, reproducible botanicals research.
The ultimate goal of enhancing rigor and reproducibility in botanicals research is to produce data that is of high enough quality to inform public health decision-making and the design of future studies. In order to address the concerns described above, evaluations need to be limited to well-characterized materials currently on the market, and new methods and practices need to be developed and embraced that may be better suited for studying complex mixtures.
IV. Regulation of Botanical Dietary Supplements
Botanical dietary supplements are regulated as a category of food per the stipulations of DSHEA (1994), which differs from the regulation of ingestible pharmaceutical, homeopathic, or medical products. As “food”, dietary supplements are not regulated based on therapeutic benefit and therefore a true risk/benefit analysis is not part of the existing regulatory paradigm. Regulations for botanical dietary supplements permit flexibility in product specifications (i.e. single manufacturers can establish their own specifications); therefore, the same botanical from different companies can have variable specifications. Pharmaceuticals on the other hand are considered therapeutic, which allows for risk/benefit evaluations. The composition of pharmaceutical products is very well-characterized, and, because they are generally synthetic products, there is little to no batch-to-batch variation. Botanical dietary supplements also differ from pharmaceuticals in that consumers choose to take them for a variety of reasons, including religious and cultural preferences and as non-prescription or over-the-counter methods for staying healthy. Of concern for regulators and safety assessors is that pharmaceutical products are often considered “new to nature” molecules, as there is no previous human exposure; whereas, consumers often perceive botanical dietary supplements as “safer” due to their natural origin and historic use as herbs, spices, and herbal medicines. Regulation of dietary supplements should ideally maintain a balance between accessibility and safety, while regulation of pharmaceuticals requires balance of accessibility, safety, and efficacy.
Regulation of botanical dietary supplements in the United States differs from that in other countries (Low et al., 2017) and differences in regulatory standards across countries can make maintaining quality and safety difficult. Over the years, various publications have reviewed and compared the regulatory guidelines for botanical products around the world (Alostad et al., 2018; Dobos et al., 2005; Fan et al., 2012; Low et al., 2017; Picking, 2017; WHO, 1998). The country-to-country differences begin with classification of botanical products. While botanicals are classified as either Traditional Chinese Medicines or natural medicinal products in China, they are classified as dietary supplements in the United States and natural health products in Canada (Picking, 2017). In Japan, botanical products are classified generally as food, either with or without health claims (Low et al., 2017). Globally, regulatory guidance for botanicals often depends on their intended use. For example, if they are being used as medicinal products in the European Union, then pre-market review of safety and efficacy are required (Low et al., 2017). Alternatively, in the United States, dietary supplements are prohibited from including label claims that they can be used to “diagnose, cure, mitigate, treat or prevent” illnesses (Picking, 2017). Other important differences include country-specific limits on contaminant levels (e.g., pyrrolizidine alkaloids, pesticides) and guidelines for executing and enforcing good manufacturing practices (Low et al., 2017; Picking, 2017).
Global differences in botanical regulation can also be viewed through the lens of restricted and banned substances. Fleischer et al., (2017) conducted a survey of restricted and banned botanicals in Taiwan, the United States, Germany, Australia, the United Kingdom, Israel, and Canada in an attempt to determine how these regulations affect the practice of traditional Chinese herbal medicine. Interestingly, they found that the United States had among the lowest number of banned botanical substances (Chinese Materia Medica), while Canada had the highest (9 and 98, respectively).
The World Health Organization (WHO) established guidelines in 1998 to better delineate criteria for assessing safety, quality, and efficacy of botanical products (Liu and Salmon, 2010; WHO, 1998). Due to the many differences in regulation between countries, however, new strategies need to be developed to implement international standards for botanical dietary supplement quality and safety. Liu and Salmon (2010) reviewed the regulatory practices of the United States, Germany, and China, and determined that the biggest differences between the three countries were 1) education, 2) administrative environment, and 3) marketing authorizations. The authors recommended that the United States could benefit by establishing regulatory standards and systems similar to those of Europe and China, ultimately helping public health as a whole. Differences in international regulation of botanical dietary supplements are also extensively reviewed in Low et al (2017), who evaluated botanicals safety assessment criteria in the European Union (EU), US, Canada, Australia, New Zealand, India, Japan, and China. The authors concluded that more information on adverse outcomes from botanical dietary supplement exposure would be helpful in strengthening international regulatory decisions.
The FDA’s regulation of botanical dietary supplements benefits from cooperation and coordination from the different industries involved in the manufacturing of these supplements. The top priority of the FDA’s Office of Dietary Supplement Programs (ODSP) is to protect public health, which requires coordination of many different avenues including, but not limited to, reviewing premarket safety notifications surrounding new dietary ingredients (NDIs), conducting dietary supplement and ingredient-specific research, and monitoring reported adverse events and case studies. Some recent research is looking at the possibility of linking in vitro and in vivo results to specific human health endpoints such as cardiotoxicity and hepatotoxicity (Liu and Flynn, 2015; Liu and Santillo, 2016). The ODSP faces many challenges in regulating botanical dietary supplements due to the inherent complexity and variability of botanical-sourced ingredients, with the added challenge of intentional adulteration of supplements, a complex supply chain, a lack of a single standard that can be used to verify the integrity of each botanical sample, and failure of manufacturers to submit NDI notifications to the FDA. Manufacturers are generally required to submit 75-day premarket safety notifications for NDIs to the ODSP, and, as of March 2016, 610 unique NDI notifications had been filed since DSHEA was enacted. Required information for an NDI includes evidence of a history of safe use or other evidence of safety (i.e. clinical and/or animal testing), and the data should provide the basis for a conclusion that there is a reasonable expectation of safety under the proposed conditions of use.
When a supplement on the market is found to contain an NDI for which the required notification has not been submitted, the product is considered adulterated and the FDA can send warning letters to the companies selling the products. The FDA can also take additional enforcement action, such as injunctions or seizures, as needed. Examples of dietary ingredients that have been the subject of warning letters include AMP citrate (DMBA) and Acacia rigidula. A lack of a single standard that can be used to verify the identity, purity, and potency of each botanical presents a challenge in enforcement of the dietary supplement regulations, as not all botanical products that are derived from the same plant are standardized to the same marker compounds. The FDA is also responsible for enforcing cGMP compliance, which involves inspections of dietary supplement facilities. If non-compliance is detected, the dietary supplements manufactured at that facility are considered adulterated and the FDA can act, such as sending warning letters, enjoining, or seizing products as warranted.
The botanical dietary supplement industry also plays a significant role in regulation and safety, and a standard supplement safety paradigm for this industry includes: 1) ingredient selection, 2) manufacturing standards that comply with FDA cGMP regulations, and 3) mandatory post-market surveillance. Dietary supplement safety is a top priority and a legal requirement (21 U.S.C. 342) for any responsible industry, as consumers are the industry’s greatest asset. Therefore, the industry supports laws, regulations, and enforcement activities that help maintain consumer safety. Unfortunately, adulteration is a common problem in the manufacturing of these products. In addition to the food adulteration provisions, there are adulteration provisions that are specific to dietary supplements. A dietary supplement is considered adulterated if: 1) the supplement or a dietary ingredient in the supplement presents significant or unreasonable risk of illness or injury under conditions of use recommended in labeling, or under ordinary conditions of use, 2) the supplement contains an NDI for which there is inadequate information (e.g., no NDI notification) to provide reasonable assurance that such an ingredient does not present a significant or unreasonable risk of illness or injury, 3) the supplement poses an imminent hazard to public health or safety, 4) the supplement contains a dietary ingredient that renders it adulterated under section 402(a)(1) of the FD&C Act under the conditions of use recommended or suggested in the labeling of such dietary supplement, and 5) the supplement was made in a facility not compliant with cGMPs (DSHEA - 108 Stat. 4325, 1994).
Manufacturers of botanical dietary supplements are under numerous legal obligations to ensure products are not adulterated. Industry believes that some of the legal obligations can be challenging and counterproductive to manufacturing products. For example, when it comes to rules regarding contaminants, specifically pesticides, botanical dietary supplement makers are at a disadvantage to other regulated industries that work with botanicals. There is currently a zero-tolerance policy for pesticides in all foods imported from outside the United States, including botanical dietary supplements, which, while beneficial in theory, actually acts as an inhibitor to efficient and consistent manufacturing. Due to the zero-tolerance policy, many botanical products (that are of the highest quality standards and would be allowed on the market in the European Union) are immediately rejected at U.S. ports of entry due to the presence of any level of pesticide residue; the levels of quantitation (LOQs) can be extremely low for some of the residues and may not represent a safety concern. The USP has proposed that the U.S. Environmental Protection Agency (EPA) should set tolerances for pesticides on imported botanicals so that high quality and safe raw material is not rejected immediately, as the current zero-tolerance standards are generally unattainable and outside of the control of cultivators, sellers, and manufacturers (USP Botanical Dietary Supplements Herbal Medicines Expert Committee, 2016).
One of the goals of the botanicals industry is to provide high quality products to consumers, and many manufacturers take the initiative to get USP (http://www.usp.org/verification-services/program-participants) or NSF (http://info.nsf.org/Certified/Dietary/) certification. The botanicals industry can be consulted to provide an important perspective on manufacturing, production, and context of a botanical relative to those evaluated in safety and/or toxicity studies.
Through collaborations with research agencies such as the NTP, the botanical dietary supplement industry has been able to recommend specific botanicals for toxicity testing. As such, this can lead to better information that can be provided to consumers for them to use when making personal choice decisions. An example of where these collaborations have aided in informing public safety is that of Aloe vera. The NTP evaluated the toxicology and carcinogenicity of non-decolorized whole leaf Aloe vera extract following oral exposure; this is important to clarify because this form of Aloe vera products is not recommended for internal use by regulatory agencies. Products for internal use are filtered or “decolorized”, which removes the latex of the plant where the aloin is found. Despite the recommendations against internal use, however, Aloe vera is still consumed orally as an herbal remedy for systemic conditions (NTP, 2013a). A two-year oral exposure rodent study was performed on the non-decolorized whole leaf Aloe vera extract, and the NTP found increased incidences of tumors in the large intestine of both rats and mice. The International Agency for Research on Cancer (IARC) supported the NTP’s findings, the California Office of Environmental Health Hazard Assessment listed this particular form of Aloe vera as carcinogenic, and the ultimate result was verification that non-decolorized Aloe vera should not be recommended for internal use. Collaborations such as these can help consumers make more informed decisions about botanicals purchases and improve their health choices as a whole.
V. Conclusions
All stakeholders (industry, government, the public) involved in the research, manufacturing, regulation, and consumption of botanical dietary supplements have a common goal of high quality and safe products in the marketplace. To date, the safety data on botanicals is limited and there are numerous difficulties in interpreting existing data due to the variability in and complexity of the botanical products being used. The NCCIH is working to implement “best practices” for clinical evaluations of the safety and efficacy of botanical dietary supplements with a goal to produce high quality, reproducible research that can better inform public health; this research helps consumers weigh the costs and benefits of using botanical dietary supplements. The FDA functions to regulate botanical dietary supplements through GMP and post-market surveillance along with NDI requirements. The botanicals industry is a rapidly growing industry that faces many challenges in ensuring quality botanical products in the marketplace. The goal of the NTP Botanicals Workshop (2016) was to bring different perspectives together to find common ground and make progress in ensuring the safety of botanical dietary supplements. The outcomes and discussion from the workshop are highlighted in this manuscript as well as in companion manuscripts (Catlin et al., submitted; Roberts et al., submitted; Waidyanatha et al., submitted) that focus on key topics in botanical research.
Supplementary Material
The use of botanical dietary supplements is widespread in the United States
High quality botanicals research requires accurate characterization of products
The complexity and variability of these supplements present many challenges
Both manufacturers and regulators are responsible for the safety of these products
Addressing the challenges in botanical safety is an important public health goal
Acknowledgments
We would like to thank Dr. Michelle Hooth and Dr. Kembra Howdeshell for their review of this manuscript. This work was supported in part by the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman A, Anyangwe N, Carlacci L, Casper S, Danam RP, Enongene E, Erives G, Fabricant D, Gudi R, Hilmas CJ, Hines F, Howard P, Levy D, Lin Y, Moore RJ, Pfeiler E, Thurmond TS, Turujman S, Walker NJ. The Safety and Regulation of Natural Products Used as Foods and Food Ingredients. Toxicol Sci. 2011;123:333–348. doi: 10.1093/toxsci/kfr198. [DOI] [PubMed] [Google Scholar]
- Al-Amier H, Nasr KA, Lueck L, Gardner ZE, Craker LE. Phytochemical Variation in Black Cohosh Populations. In: Khan IA, editor. Proceedings of the IVth International Conference on Quality and Safety Issues Related to Botanicals: University, MS, USA, August 15 - 18, 2005. ISHS; Leuvin, Belgium: 2006. pp. 95–100. [Google Scholar]
- Alostad AH, Steinke DT, Schafheutle EI. International Comparison of Five Herbal Medicine Registration Systems to Inform Regulation Development: United Kingdom, Germany, United States of America, United Arab Emirates and Kingdom of Bahrain. Pharm Med. 2018;32:39–49. doi: 10.1007/s40290-018-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azwanida NN. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Medicinal & Aromatic Plants. 2015;4:196–202. [Google Scholar]
- Barnes PM, Bloom B, Nahin RL. National Health Statistics Report. 12. National Center for Health Statistics; Hyattsville, MD: 2008. Complementary and alternative medicine use among adults and children: United States, 2007. [PubMed] [Google Scholar]
- Binns SE, Arnason JT, Baum BR. Phytochemical variation within populations of Echinacea angustifolia (Asteraceae) Biochem Syst Ecol. 2002;30:837–854. [Google Scholar]
- Catlin RN, Collins B, Auerbach SS, Ferguson SS, Harnly J, Gennings C, Waidyanatha S, Khan I, LeVanseler K, Rice G, Smith-Roe S, MacGregor J, Witt KL, Rider CV. How Similar is Similar Enough? A Sufficient Similarity Case Study with Ginkgo biloba extract. Food Chem Toxicol. doi: 10.1016/j.fct.2018.05.013. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Li YQ, Rana J, Persons K, Hyun C, Shen S, Mulder T. Qualitative categorization of supplement grade Ginkgo biloba leaf extracts for authenticity. J Funct Foods. 2011;3:107–114. [Google Scholar]
- Chou MW, Fu PP. Formation of DHP-derived DNA adducts in vivo from dietary supplements and Chinese herbal plant extracts containing carcinogenic pyrrolizidine alkaloids. Toxicol Ind Health. 2006;22:321–327. doi: 10.1177/0748233706071765. [DOI] [PubMed] [Google Scholar]
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. National Health Statistics Reports. 2015;10:1–16. [PMC free article] [PubMed] [Google Scholar]
- Cowan RS, Fay MF. Challenges in the DNA Barcoding of Plant Material. In: Sucher NJ, Hennell JR, Carles MC, editors. Plant DNA Fingerprinting and Barcoding. Springer; 2012. [DOI] [PubMed] [Google Scholar]
- Davidson JRT, Gadde KM, Fairbank JA, Krishnan RR, Califf RM, Binanay C, Parker CB, Pugh N, Hartwell TD, Vitiello B, Ritz L, Severe J, Cole JO, de Battista C, Doraiswamy PM, Feighner JP, Keck P, Kelsey J, Lin KM, Londborg PD, Nemeroff CB, Schatzberg AF, Sheehan DV, Srivastava RK, Taylor L, Trivedi MH, Weisler RH, G, H.D.T.S Effect of Hypericum perforatum (St John’s wort) in major depressive disorder - A randomized controlled trial. Jama-J Am Med Assoc. 2002;287:1807–1814. doi: 10.1001/jama.287.14.1807. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD, Ginkgo Evaluation of Memory Study, I Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008;300:2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami N, Mishra AD. Phytochemical variation: How to resolve the quality controversies of herbal medicinal products? J Herb Med. 2015;5:118–127. [Google Scholar]
- Dicke M, van Loon JJ, Soler R. Chemical complexity of volatiles from plants induced by multiple attack. Nat Chem Biol. 2009;5:317–324. doi: 10.1038/nchembio.169. [DOI] [PubMed] [Google Scholar]
- Dobos GJ, Tan L, Cohen MH, McIntyre M, Bauer R, Li X, Bensoussan A. Are national quality standards for traditional Chinese herbal medicine sufficient? Current governmental regulations for traditional Chinese herbal medicine in certain Western countries and China as the Eastern origin country. Complement Ther Med. 2005;13:183–190. doi: 10.1016/j.ctim.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dudareva N, Klempien A, Muhlemann JK, Kaplan I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013;198:16–32. doi: 10.1111/nph.12145. [DOI] [PubMed] [Google Scholar]
- EFSA. Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA Journal. 2012;10:2663. [Google Scholar]
- Enioutina EY, Salis ER, Job KM, Gubarev MI, Krepkova LV, Sherwin CM. Herbal Medicines: challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expert Rev Clin Pharmacol. 2017;10:327–338. doi: 10.1080/17512433.2017.1268917. [DOI] [PubMed] [Google Scholar]
- Fabricant D, Hilmas CJ. NPA White Paper: DNA Barcoding for Botanical Authentication, Natural Products Association 2015 [Google Scholar]
- Fan TP, Deal G, Koo HL, Rees D, Sun H, Chen S, Dou JH, Makarov VG, Pozharitskaya ON, Shikov AN, Kim YS, Huang YT, Chang YS, Jia W, Dias A, Wong VC, Chan K. Future development of global regulations of Chinese herbal products. Journal of ethnopharmacology. 2012;140:568–586. doi: 10.1016/j.jep.2012.02.029. [DOI] [PubMed] [Google Scholar]
- Fleischer T, Su YC, Lin SJ. How do government regulations influence the ability to practice Chinese herbal medicine in western countries. Journal of ethnopharmacology. 2017;196:104–109. doi: 10.1016/j.jep.2016.11.047. [DOI] [PubMed] [Google Scholar]
- Frei-Kleiner S, Schaffner W, Rahlfs VW, Bodmer C, Birkhauser M. Cimicifuga racemosa dried ethanolic extract in menopausal disorders: a double-blind placebo-controlled clinical trial. Maturitas. 2005;51:397–404. doi: 10.1016/j.maturitas.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Fu PP, Chou MW, Xia Q, Yang YC, Yan J, Doerge DR, Chan PC. Genotoxic pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides - Mechanisms leading to DNA adduct formation and tumorigenicity. J Environ Sci Heal C. 2001;19:353–385. [Google Scholar]
- Galasso S, Pacifico S, Kretschmer N, Pan SP, Marcianci S, Piccolella S, Monaco P, Bauer R. Influence of seasonal variation on Thymus longicaulis C. Presl chemical composition and its antioxidant and anti-inflammatory properties. Phytochemistry. 2014;107:80–90. doi: 10.1016/j.phytochem.2014.08.015. [DOI] [PubMed] [Google Scholar]
- Garg V, Dhar VJ, Sharma A, Dutt R. Facts about standardization of herbal medicine: a review. Journal of Chinese Integrative Medicine. 2012;10:1077–1083. doi: 10.3736/jcim20121002. [DOI] [PubMed] [Google Scholar]
- Geller SE, Shulman LP, van Breemen RB, Banuvar S, Zhou Y, Epstein G, Hedayat S, Nikolic D, Krause EC, Piersen CE, Bolton JL, Pauli GF, Farnsworth NR. Safety and efficacy of black cohosh and red clover for the management of vasomotor symptoms: a randomized controlled trial. Menopause. 2009;16:1156–1166. doi: 10.1097/gme.0b013e3181ace49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C, Kaplan F, Kopka J, Selbig J, Hincha DK. Metabolomics of temperature stress. Physiol Plantarum. 2008;132:220–235. doi: 10.1111/j.1399-3054.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- Hopp C. Past and Future Research at National Center for Complementary and Integrative Health with Respect to Botanicals. HerbalGram. 2015:44–51. [Google Scholar]
- Job KM, Kiang TKL, Constance JE, Sherwin CMT, Enioutina EY. Herbal medicines: challenges in the modern world. Part 4. Canada and United States. Expert Rev Clin Phar. 2016;9:1597–1609. doi: 10.1080/17512433.2016.1238762. [DOI] [PubMed] [Google Scholar]
- Jones CG, Firn RD. On the Evolution of Plant Secondary Chemical Diversity. Philos T Roy Soc B. 1991;333:273–280. [Google Scholar]
- Kaur T, Bhat R, Vyas D. Effect of contrasting climates on antioxidant and bioactive constituents in five medicinal herbs in Western Himalayas. J Mt Sci-Engl. 2016;13:484–492. [Google Scholar]
- Kellogg JJ, Graf TN, Paine MF, McCune JS, Kvalheim OM, Oberlies NH, Cech NB. Comparison of Metabolomics Approaches for Evaluating the Variability of Complex Botanical Preparations: Green Tea (Camellia sinensis) as a Case Study. J Nat Prod. 2017;80:1457–1466. doi: 10.1021/acs.jnatprod.6b01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak AJ, Hopp DC, Williamson JS, Betz JM, Sorkin BC. Approaches by the US National Institutes of Health to support rigorous scientific research on dietary supplements and natural products. Drug Test Anal. 2016;8:413–417. doi: 10.1002/dta.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT, 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K, Ramirez G, Mulrow CD, Pauls A, Weidenhammer W, Melchart D. St John’s wort for depression - An overview and meta-analysis of randomised clinical trials. Brit Med J. 1996;313:253–258. doi: 10.1136/bmj.313.7052.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FX, Salmon JW. Herbal Medicine Regulation in China, Germany, and the United States. Integrative Medicine. 2010;9:54–61. [Google Scholar]
- Liu Y, Lu F. Adulterated pharmaceutical chemicals in botanical dietary supplements: novel screening approaches. Reviews in Analytical Chemistry. 2017;36 online. [Google Scholar]
- Liu YT, Flynn TJ. CYP3A4 inhibition by Psoralea corylifolia and its major components in human recombinant enzyme, differentiated human hepatoma HuH-7 and HepaRG cells. Toxicology Reports. 2015;2:530–534. doi: 10.1016/j.toxrep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YT, Santillo MF. Cytochrome P450 2D6 and 3A4 enzyme inhibition by amine stimulants in dietary supplements. Drug Test Anal. 2016;8:307–310. doi: 10.1002/dta.1863. [DOI] [PubMed] [Google Scholar]
- Lobstein A, Rietschjako L, Haagberrurier M, Anton R. Seasonal-Variations of the Flavonoid Content from Ginkgo-Biloba Leaves. Planta Med. 1991;57:430–433. doi: 10.1055/s-2006-960142. [DOI] [PubMed] [Google Scholar]
- Low TY, Wong KO, Yap ALL, De Haan LHJ, Rietjens IMCM. The Regulatory Framework Across International Jurisdictions for Risks Associated with Consumption of Botanical Food Supplements. Compr Rev Food Sci F. 2017;16:821–834. doi: 10.1111/1541-4337.12289. [DOI] [PubMed] [Google Scholar]
- Marcus DM. Dietary supplements: What’s in a name? What’s in the bottle? Drug Test Anal. 2016;8:410–412. doi: 10.1002/dta.1855. [DOI] [PubMed] [Google Scholar]
- Mathon C, Edder P, Bieri S, Christen P. Survey of pyrrolizidine alkaloids in teas and herbal teas on the Swiss market using HPLC-MS/MS. Anal Bioanal Chem. 2014;406:7345–7354. doi: 10.1007/s00216-014-8142-8. [DOI] [PubMed] [Google Scholar]
- Mattoli L, Cangi F, Maidecchi A, Ghiara C, Ragazzi E, Tubaro M, Stella L, Tisato F, Traldi P. Metabolomic fingerprinting of plant extracts. J Mass Spectrom. 2006;41:1534–1545. doi: 10.1002/jms.1099. [DOI] [PubMed] [Google Scholar]
- Melito S, Petretto GL, Podani J, Foddai M, Maldini M, Chessa M, Pintore G. Altitude and climate influence Helichrysum italicum subsp microphyllum essential oils composition. Ind Crop Prod. 2016;80:242–250. [Google Scholar]
- Neuman MG, Cohen LB, Opris M, Nanau R, Jeong H. Hepatotoxicity of Pyrrolizidine Alkaloids. J Pharm Pharm Sci. 2015;18:825–843. doi: 10.18433/j3bg7j. [DOI] [PubMed] [Google Scholar]
- NTP. National Toxicology Program. Research Triangle Park, NC: 2013a. NTP TECHNICAL REPORT ON THE TOXICOLOGY AND CARCINOGENESIS STUDIES OF A NONDECOLORIZED WHOLE LEAF EXTRACT OF ALOE BARBADENSIS MILLER (ALOE VERA) IN F344/N RATS AND B6C3F1 MICE (DRINKING WATER STUDIES) [PubMed] [Google Scholar]
- NTP. NTP Technical Report on the Toxicology and Carcinogenesis Studies of Ginkgo biloba Extract (CAS No. 90045-36-6) in F344/N Rats and B6C3F1/N Mice (Gavage Studies) In: NTP, editor. Technical Report Series. NIEHS/NTP. Research Triangle Park, NC: 2013b. [PubMed] [Google Scholar]
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol-Chicago. 1998;55:1409–1415. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- Ong ES. Extraction methods and chemical standardization of botanicals and herbal preparations. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;812:23–33. doi: 10.1016/j.jchromb.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Pacifico S, Piccolella S, Galasso S, Fiorentino A, Kretschmer N, Pan SP, Bauer R, Monaco P. Influence of harvest season on chemical composition and bioactivity of wild rue plant hydroalcoholic extracts. Food Chem Toxicol. 2016;90:102–111. doi: 10.1016/j.fct.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Pferschy-Wenzig EM, Bauer R. The relevance of pharmacognosy in pharmacological research on herbal medicinal products. Epilepsy Behav. 2015;52:344–362. doi: 10.1016/j.yebeh.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Picking D. The Global Regulatory Framework for Medicinal Plants. Pharmacognosy: Fundamentals, Applications and Strategies. 2017:663–675. [Google Scholar]
- Pohlit AM, Rezende AR, Baldin ELL, Lopes NP, Neto VFD. Plant Extracts, Isolated Phytochemicals, and Plant-Derived Agents Which Are Lethal to Arthropod Vectors of Human Tropical Diseases - A Review. Planta Med. 2011;77:618–630. doi: 10.1055/s-0030-1270949. [DOI] [PubMed] [Google Scholar]
- Rider CV, Nyska A, Cora MC, Kissling GE, Smith C, Travlos GS, Hejtmancik MR, Fomby LM, Colleton CA, Ryan MJ, Kooistra L, Morrison JP, Chan PC. Toxicity and carcinogenicity studies of Ginkgo biloba extract in rat and mouse: liver, thyroid, and nose are targets. Toxicol Pathol. 2014;42:830–843. doi: 10.1177/0192623313501235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger G, Muller M, Guttenberger H, Bucar F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J Agr Food Chem. 2008;56:9080–9086. doi: 10.1021/jf801104e. [DOI] [PubMed] [Google Scholar]
- Roberts GK, Gardner D, Foster PM, Howard PC, Lui E, Walker L, van Breemen RB, Auerbach SS. Finding the Bad Actor: Challenges in Identifying Toxic Constituents in Botanical Dietary Supplements. Food Chem Toxicol. doi: 10.1016/j.fct.2018.12.026. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha T, Amaral JS, Oliveira MBPP. Adulteration of Dietary Supplements by the Illegal Addition of Synthetic Drugs: A Review. Compr Rev Food Sci F. 2016;15:43–62. doi: 10.1111/1541-4337.12173. [DOI] [PubMed] [Google Scholar]
- Roeder E. Medicinal-Plants in Europe Containing Pyrrolizidine Alkaloids. Pharmazie. 1995;50:83–98. [PubMed] [Google Scholar]
- Roeder E, Wiedenfeld H, Edgar JA. Pyrrolizidine alkaloids in medicinal plants from North America. Pharmazie. 2015;70:357–367. [PubMed] [Google Scholar]
- Schilter B, Andersson C, Anton R, Constable A, Kleiner J, O’Brien J, Renwick AG, Korver O, Smit F, Walker R. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem Toxicol. 2003;41:1625–1649. doi: 10.1016/s0278-6915(03)00221-7. [DOI] [PubMed] [Google Scholar]
- Schoop R, Klein P, Suter A, Johnston SL. Echinacea in the prevention of induced rhinovirus colds: A meta-analysis. Clin Ther. 2006;28:174–183. doi: 10.1016/j.clinthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Seaman JT. Pyrrolizidine Alkaloid Poisoning of Sheep in New-South-Wales. Aust Vet J. 1987;64:164–167. doi: 10.1111/j.1751-0813.1987.tb09674.x. [DOI] [PubMed] [Google Scholar]
- Simmler C, Chen SN, Anderson J, Lankin DC, Phansalkar R, Krause E, Dietz B, Bolton JL, Nikolic D, van Breeman RB, Pauli GF. Botanical Integrity: The Importance of the Integration of Chemical, Biological, and Botanical Analyses, and the Role of DNA Barcoding. HerbalGram. 2015;106:56–58. [PMC free article] [PubMed] [Google Scholar]
- Simpraga M, Takabayashi J, Holopainen JK. Language of plants: Where is the word? J Integr Plant Biol. 2016;58:343–349. doi: 10.1111/jipb.12447. [DOI] [PubMed] [Google Scholar]
- Smith T, Kawa K, Eckl V, Morton C, Stredney R. Herbal Supplement Sales in US Increase 7.7% in 2016. HerbalGram. 2017;115:56–65. [Google Scholar]
- Stegelmeier BL, Edgar JA, Colegate SM, Gardner DR, Schoch TK, Coulombe RA, Molyneux RJ. Pyrrolizidine alkaloid plants, metabolism and toxicity. J Nat Toxins. 1999;8:95–116. [PubMed] [Google Scholar]
- Sullivan D, Crowley R. Development and validation of analytical methods for dietary supplements. Toxicology. 2006;221:28–34. doi: 10.1016/j.tox.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Tanko H, Carrier DJ, Duan L, Clausen E. Pre- and post-harvest processing of medicinal plants. Plant Genetic Resources. 2005;3:304–313. [Google Scholar]
- Tournas VH, Calo JR, Sapp C. Fungal profiles in various milk thistle botanicals from US retail. Int J Food Microbiol. 2013;164:87–91. doi: 10.1016/j.ijfoodmicro.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An evaluation of Echinacea angustifolia in experimental rhinovirus infections. New Engl J Med. 2005;353:341–348. doi: 10.1056/NEJMoa044441. [DOI] [PubMed] [Google Scholar]
- USP. The United States Pharmacopeial Convention. Rockville, MD: 2015. Dietary Supplements Compendium. [Google Scholar]
- USP Botanical Dietary Supplements Herbal Medicines Expert Committee. Stimuli to the Revision Process: Need for Clear Regulation of Pesticide Residue Limits for Articles of Botanical Origin. Pharmacopeial Forum. 2016;42:17. [Google Scholar]
- van Beek TA, Lelyveld GP. Concentration of Ginkgolides and Bilobalide in Ginkgo-Biloba Leaves in Relation to the Time of Year. Planta Med. 1992;58:413–416. doi: 10.1055/s-2006-961503. [DOI] [PubMed] [Google Scholar]
- van Breemen RB. Development of Safe and Effective Botanical Dietary Supplements. J Med Chem. 2015;58:8360–8372. doi: 10.1021/acs.jmedchem.5b00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidyanatha S, Ryan K, Roe AL, Jia W, Paine MF, Ferguson SS, Gurley BJ, Welch K, Chow MSS, DeVito M, Rider CV. Follow that Botanical: Challenges and Recommendations for Assessing Absorption, Distribution, Metabolism and Excretion of Botanical Dietary Supplements. Food Chem Toxicol. doi: 10.1016/j.fct.2018.08.062. submitted. [DOI] [PubMed] [Google Scholar]
- Wang JE. FDA Regulatory Requirements for Botanical INDs. Regulatory Focus. 2009:376–341. [Google Scholar]
- Wang ZH, Huang LF. Panax quinquefolius: An overview of the contaminants. Phytochem Lett. 2015;11:89–94. [Google Scholar]
- Weng JK. The evolutionary paths towards complexity: a metabolic perspective. New Phytol. 2014;201:1141–1149. doi: 10.1111/nph.12416. [DOI] [PubMed] [Google Scholar]
- WHO, P.o.T.M. Regulatory situation of herbal medicines : A worldwide review. Geneva: 1998. [Google Scholar]
- Wolsko PM, Solondz DK, Phillips RS, Schachter SC, Eisenberg DM. Lack of herbal supplement characterization in published randomized controlled trials. Am J Med. 2005;118:1087–1093. doi: 10.1016/j.amjmed.2005.01.076. [DOI] [PubMed] [Google Scholar]
- Xie SB, Zhou J. Harnessing Plant Biodiversity for the Discovery of Novel Anticancer Drugs Targeting Microtubules. Front Plant Sci. 2017;8 doi: 10.3389/fpls.2017.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XM, Kang H, Feng J, Yang YY, Tang KL, Zhu RX, Yang L, Wang ZT, Cao ZW. Identification of Toxic Pyrrolizidine Alkaloids and Their Common Hepatotoxicity Mechanism. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Tan M, Peng X, Luo Q. Standardization and Quality Control of Herbal Extracts and Products. In: Liu WJH, editor. Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies. John Wiley & Sons, Inc; Hoboken, NJ: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


