Abstract
Background
Late HIV diagnosis following severe co-morbidity remains common in resource limited settings. Neurodevelopmental recovery during antiretroviral therapy (ART) for late-diagnosed children is understudied. We determined 6-month neurodevelopmental trajectories in HIV-infected children initiating ART during hospitalization.
Methods
HIV-infected children initiated ART following HIV diagnosis during hospitalization in Kenya. The Malawi Developmental Assessment Tool (MDAT) was administered after clinical stabilization within 1-month and at 6-months post-ART initiation. Baseline versus 6-month Z-scores for each developmental domain were compared; cofactors for change in z-scores were evaluated using linear regression.
Results
Among 74 children, median age was 1.7 years (interquartile range, 0.8, 2.4) and median z-scores for gross motor, fine motor, social, and language domains were −1.34, −1.04, −0.53, and −0.95, respectively. At baseline, children with higher plasma viremia had lower social z-scores (P=0.008). Better nourished (weight-for-age z-score [WAZ] ≥-2) children had higher z-scores in all developmental domains (all P-values ≤0.05). After 6 months on ART (n=58), gross and fine motor z-scores improved significantly (mean change 0.39; P=0.007 and 0.43; P=0.001, respectively), but social and language did not. Children with better immune and growth response to ART had higher gains in gross motor (0.05 per unit-gain CD4%, P=0.04; 0.34 per unit-gain WAZ; P=0.006 and 0.44 per unit-gain height-for-age z-score (HAZ); P=0.005), social (0.37 per unit-gain WAZ; P=0.002) and language (0.25 per unit-gain HAZ; P=0.01).
Conclusions
Children had significant neurodevelopmental gains during 6-months of ART, and children with better growth and immune recovery had greater improvement. Prompt commencement of ART may improve neurodevelopment in addition to immunity and growth.
Keywords: child development, nutrition, Africa, Malawi Developmental Assessment Tool
INTRODUCTION
Globally, 1.8 million children are HIV-infected, most of whom reside in sub-Saharan Africa (1). With improved survival (2, 3) it is important to optimize long-term growth, development, health and quality of life. Early HIV diagnosis and treatment are essential for the best outcomes (4, 5). However, late HIV diagnosis remains common in sub-Saharan Africa (6–9). In 2015, UNAIDS estimated that only 49% of HIV-infected children were receiving antiretroviral therapy (ART) (1).
HIV-infected children have high risk of neurodevelopmental compromise. In historical birth cohorts of HIV-infected infants with either no or limited ART, 26–36% of children had motor or cognitive delay by age 24 months (10–12). In long-term treated pediatric cohorts, learning difficulties and low cognitive scores have been common (13–15). HIV-related cognitive impairment likely results from a local inflammatory cascade involving small molecules, cytokines and chemokines, that can damage neurons and is driven by low-level viral replication and recruitment of activated immune cells to the brain (16). Abnormalities in white matter microstructure and subcortical gray matter are linked to cognitive deficits in children and adolescents (17–20). Children with greater severity of HIV disease prior to ART have worse cognition compared with asymptomatic children (13, 21, 22).
For infants who start ART within the first few months of life, cognitive compromise may be minimal. Early-treated infants in South Africa had similar one-year neurodevelopmental ability as HIV-uninfected infants (5). The extent to which ART benefits neurodevelopment in older children is less clear. Few studies have prospectively followed children from ART initiation. School-aged Thai and Cambodian children did not have cognitive improvements during their first 3 years of ART (23), and similarly, South African children (median age, 5 years), did not have improved cognitive performance 6 months after initiating ART (24). However, in the Democratic Republic of Congo, children with median age 44 months had significant 1-year gains in both motor and cognitive scores after initiating ART (25).
We characterized neurodevelopmental trajectories and cofactors for change among HIV-infected children age <6 years and initiating ART after hospitalization in Kenya. We hypothesized that children with better response to ART would have higher neurodevelopmental gains during ART.
METHODS
Study Population
This study was nested within the Pediatric Urgent Start of Highly Active Antiretroviral Treatment (PUSH Study) randomized clinical trial (RCT) of urgent (ART at <48 hours) versus post-stabilization (ART at 7–14 days) ART (NCT02063880) (n=191) (26). Ethical approval was obtained from the University of Washington (UW) Institutional Review Board, the University of Nairobi/Kenyatta National Hospital (KNH) National Hospital Ethics and Research Committee, the Pharmacy and Poisons Board, and the Ministry of Health, Kenya. From 2013–2015, hospitalized HIV-infected children were identified at KNH and Mbagathi District Hospital in Nairobi and Jaramogi Oginga Odinga Teaching & Referral Hospital and Kisumu East District Hospital in Kisumu, Kenya. Routine HIV testing was conducted as described previously (9). Inclusion criteria for the parent RCT were: HIV-positive, <12 years of age, and ART naïve [other than antiretrovirals used for prevention of mother to child transmission (PMTCT)]. Children were excluded from the parent RCT if they had evidence of, or suspected, central nervous system infection. At enrollment and scheduled follow-up visits, children had a clinical exam and developmental and growth assessments. All children in both treatment arms, who were clinically stable, and who were within the age inclusion criteria for the Malawi Developmental Assessment Tool (MDAT) (age <5.5 years to accommodate the 6 month follow-up) were eligible to receive neurodevelopmental assessments. Weight, height and head circumference were determined using a calibrated weighing scale, a measuring rod and a non-elastic measuring tape. Blood specimens were collected at enrollment and week 24 for hemoglobin, plasma HIV RNA, and CD4 count and percentage.
Per 2011 Kenyan guidelines, children >3 years or >10 kilograms (kg) received abacavir (ABC) plus lamivudine (3TC) plus nevirapine (NVP) or efavirenz (EFV). Children <3 years or <10kg received ABC plus 3TC plus NVP or ritonavir-boosted lopinavir (LPV/r) if previously exposed to NVP as part of PMTCT (27). Starting September 2014, all children <3 years were started on LPV/r-based regimens regardless of NVP exposure, in accordance with national guideline changes (28).
Developmental Assessments
Assessments were performed using the MDAT, which has been validated and demonstrated to have good sensitivity and specificity in rural and urban African settings (29). A standardized script for each item was developed, which was translated to Kiswahili and back-translated to ensure accuracy prior to study start. Clinicians administered items in either English or Kiswahili depending on caregiver and child preference. Assessments were performed following clinical stabilization, at either 2 or 4 weeks post-ART (baseline) and at 24 weeks (6 months) post-ART initiation. The MDAT assesses gross motor, fine motor, social, and language domains, with 34 pass/fail items in each. Cognitive skills are assessed within the language and fine motor domains. Most gross and fine motor and language items must be directly observed, and some may be caregiver reported. All social items may be caregiver reported. Items were administered until a child had 6 consecutive passes and 6 consecutive fails. Domain specific raw scores were calculated using the number of passes, assuming passes for items preceding the 6 consecutive passes. Raw scores were converted to z-scores using norm data for healthy children in rural and urban Malawi (n=1445) and collected by MG (29).
Laboratory Testing
Laboratory assays were performed at the University of Nairobi and Centers for Disease Control and Prevention/Kenya Medical Research Institute (CDC/KEMRI) in Kisumu. HIV RNA was quantified using the Abbot Real-time HIV-1 Assay (Abbott Molecular Inc., Des Plaines, IL). CD4+ T cell count and percentage were determined using a FACSCount (Nairobi) and a FACSCalibur (Kisumu).
Statistical Analysis
Differences in baseline characteristics for children with and without baseline developmental data were compared using Wilcoxon rank sum and chi-square tests for dichotomous and continuous measures, respectively. Z-scores for weight-for-age (WAZ), height-for-age (HAZ), weight-for-height (WHZ) and head-circumference-for-age (HCZ) were calculated using the WHO child growth standard reference population (30). Underweight, stunting, wasting and microcephaly were defined as z-score <-2 for WAZ, HAZ, WHZ and HCZ, respectively. Dichotomized variables were used to evaluate severe immunosuppression (CD4% <15%), pre-ART viremia (HIV RNA >106 copies/mL [c/mL]), and post-ART lack of virologic suppression (HIV RNA >103 c/mL) (31). Cofactors for baseline developmental z-scores were evaluated using univariate and multivariate linear regression models. Primary cofactors of interest were HIV disease parameters (CD4% and plasma HIV RNA level) and nutritional status (WAZ and HAZ). Sociodemographic characteristics and potential confounders (listed below) were also evaluated. Baseline and 6-month developmental z-scores were compared using paired t-tests. Cofactors for the magnitude of change in developmental z-scores from baseline to 6-months were evaluated using univariate and multivariate linear regression models adjusting for baseline developmental z-scores. Primary cofactors of interest were baseline HIV, growth parameters, and 6-month change in CD4%, viral suppression (plasma HIV RNA <103 c/mL), and change in WAZ and HAZ. Sociodemographics (child age and sex, caregiver age, marital status, and education, and household rent, people per room and one-room house), pre-term birth (self-report), and receipt of PMTCT were also evaluated. Final models for the magnitude of change outcomes were adjusted for baseline developmental z-score, child sex and caregiver years of education. These variables were selected based on associations in the univariate models at significance P<0.1. Variables that were collinear with cofactors of interest were not included in multivariate analyses. Sensitivity analyses excluding children with history of pre-term birth were also evaluated and results were generally similar except where noted. All analyses were performed using Stata SE version 13.1 (Stata Corp., College Station, Texas, USA). Study data were collected and managed using REDCap electronic data capture tools hosted at the UW Institute of Translational Health Sciences (32).
RESULTS
Cohort Characteristics at Baseline
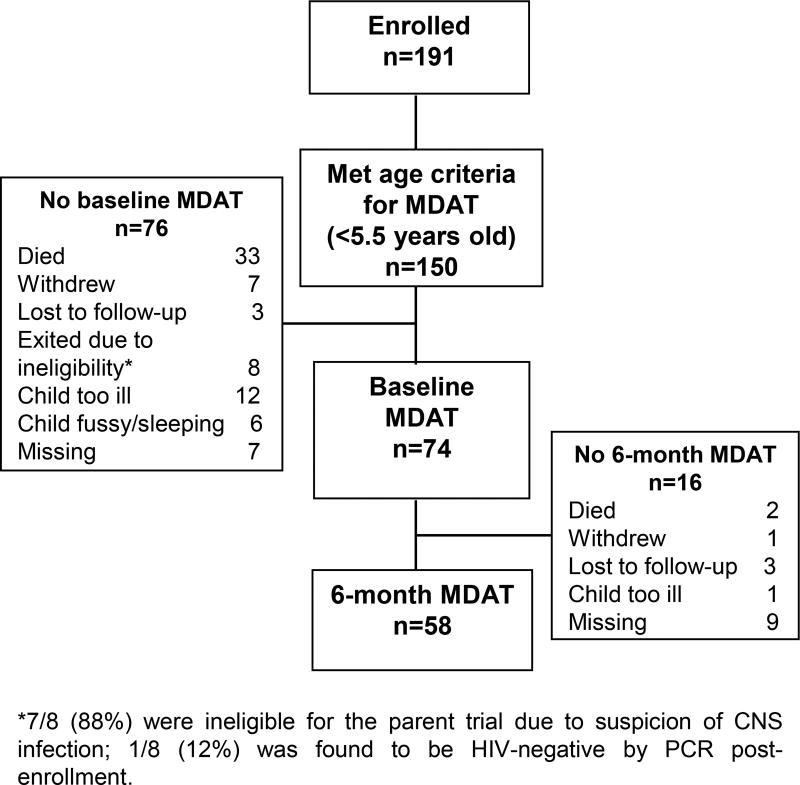
Among 74 children <5.5 years of age with baseline MDAT data (Figure 1), the median enrollment age was 1.7 years (25th, 75th percentiles, 0.8, 2.4) (Table 1). Nineteen percent of children had received PMTCT. Median CD4% was 18% (25th, 75th percentiles, 11, 23). Most (61.6%) children had a WHO Stage diagnosis of 3 or 4. Most (91.9%) had at least mild anemia (hemoglobin <11 g/dL) and 23.0% had severe anemia (hemoglobin <7.0 g/dL) (33). Most were underweight (WAZ <-2; 54.1%) or stunted (HAZ <-2; 58.1%). Nearly all children (93.2%) were cared for by their biological mother, and all had breastfed. Nine percent of caregivers reported having pre-term birth. Children who did not have an MDAT assessment (most often due to death or being too ill; Figure 1), were more likely to be underweight (77.0% vs 54.1%, P=0.003), younger (medians, 1.3 vs 1.7, P=0.05), immunosuppressed (CD4% medians, 13.4 vs 17.8, P=0.04), WHO stage 3 or 4 (78.7% vs 61.6%, P=0.02) and from the Nairobi catchment site (60.8% vs 39.5% P=0.009).
Figure 1.
Flow chart depicting study participants with baseline and follow-up developmental assessments.
Table 1.
Baseline characteristics of children with baseline developmental assessment (n=74)
| Baseline characteristics* | Median (25th, 75th percentiles) or n (%) |
|---|---|
| Child characteristics | |
| Age (years) | 1.7 (0.8, 2.4) |
| Female | 34 (46) |
| Pre-term birth | 7 (9) |
| Ever breastfed | 73 (100) |
| Received PMTCT† | 13 (19) |
| WAZ | −2.14 (−3.93, −1.04) |
| Underweight (WAZ <−2) | 40 (54) |
| HAZ | −2.34 (−3.49, −1.18) |
| Stunting (HAZ <−2) | 43 (58) |
| WHZ | −1.55 (−3.38, −0.09) |
| Wasting (WHZ <−2) | 31 (42) |
| HCZ | −0.53 (−1.49, 0.50) |
| Microcephaly (HCZ <−2) | 9 (13) |
| CD4 T cell percentage | 18 (11, 23) |
| CD4 T cell percentage <15% | 28 (38) |
| WHO Stage 3 or 4 | 45 (62) |
| Blood hemoglobin (g/dL) | 8.7 (7.5, 9.8) |
| Plasma HIV RNA (log10 c/ml) | 5.6 (5.0, 6.2) |
| Child ART initiation characteristics | |
| Randomized to ART at <48 hours | 31 (42) |
| Initial ART Regimen: | |
| ABC, 3TC, EFV | 15 (20) |
| ABC, 3TC, LPV/r | 38 (51) |
| ABC, 3TC, NVP | 21 (28) |
| Time from ART initiation to developmental assessment (days) | 28 (27, 28) |
| Caregiver or household characteristics | |
| Age (years) | 27 (24, 31) |
| Biological mother | 69 (93) |
| Married | 46 (62) |
| Education (years) | 8 (7, 11) |
| One-room house | 28 (38) |
| Number of people per room | 3 (2, 4) |
| Residing in Kisumu catchment | 45 (60.8) |
n ≥70 for all variables except received PMTCT, n=69.
Provided to mother only (n=2), provided to infant only (n=4), provided to both (n=7)
Baseline Developmental Functioning
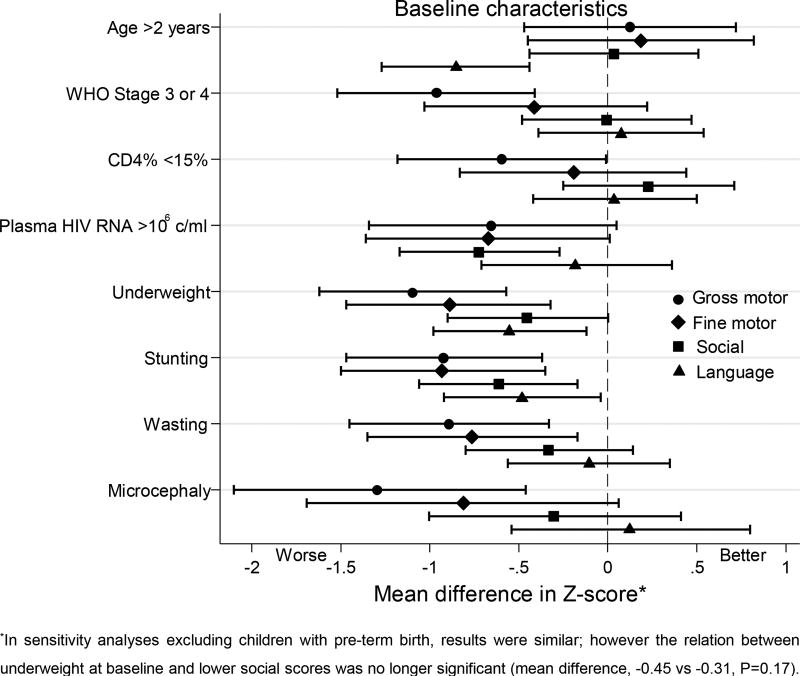
At baseline, median developmental z-scores for gross motor, fine motor, social and language domains were −1.34, −1.04, −0.53, and −0.95, respectively. Children with poorer HIV disease severity indicators and nutritional status had lower baseline developmental scores (Figure 2 and Table, Supplemental Digital Content 1). Children with higher plasma HIV RNA log10 had lower social scores (regression coefficient (RC) or mean decrease per unit HIV RNA increase, −0.34, P=0.008), with similar trends for gross and fine motor (P=0.07 and P=0.06). Children who were underweight or who had stunting had lower scores in all domains (all P values ≤0.05), and results were similar in models adjusted for baseline CD4% and plasma virus level. Children with microcephaly had significantly lower gross motor scores (mean difference, −1.29; P=0.003). Children whose caregivers reported pre-term birth had lower developing functioning in gross motor, fine motor and social domains (mean differences, −1.06, P=0.03; −1.08, P=0.03; and −1.12, P=0.004, respectively).
Figure 2.
Forest plot summarizing cofactors for baseline developmental functioning (n=74). Linear regression coefficients (95% confidence intervals) are denoted by black markers (horizontal black lines and whiskers). Circle, diamond, square and triangle markers represent gross motor, fine motor, social, and language domains, respectively.
Developmental Functioning at 6 Months Post- ART Initiation
Developmental assessments were available for 58 children at 6-months post-ART initiation (Figure 1). After 6 months of ART, gross motor (mean change, 0.39; standard deviation (SD), 1.01; P=0.007) and fine motor (mean change, 0.43; SD, 0.92; P=0.001) z-scores improved significantly, while social and language did not (mean changes, 0.11; SD, 1.06; P=0.4 and −0.20; SD, 0.65; P=0.03, respectively) (Table 2).
Table 2.
Mean baseline and 6-month developmental z-scores (N=58)
| Baseline | 6-month | |||
|---|---|---|---|---|
| N | Mean (SD) | Mean (SD) | P* | |
| Gross motor | 54 | −1.05 (1.36) | −0.66 (1.38) | 0.007 |
| Fine motor | 53 | −1.03 (1.31) | −0.60 (1.15) | 0.001 |
| Social | 57 | −0.49 (1.08) | −0.38 (1.02) | 0.4 |
| Language | 53 | −0.89 (0.99) | −1.09 (1.03) | 0.03 |
Paired t-test.
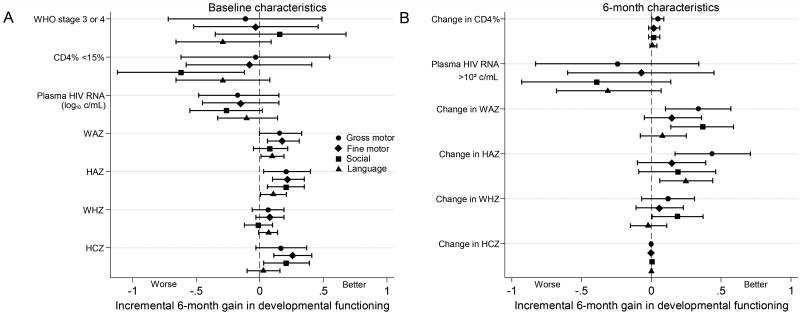
Children with better baseline immune and nutritional status (weight, height and head circumference) had greater improvements in developmental functioning after 6 months on ART (Figure 3 and Table, Supplemental Digital Content 2). In multivariate analysis adjusting for baseline developmental z-scores, child sex and caregiver years of education, children with baseline CD4% <15% had lower gains in social scores (mean difference in gain, −0.62; P=0.02), (Figure 3 and Table, Supplemental Digital Content 2). Children beginning ART with higher baseline stature had greater gains in gross and fine motor, social and language. For each one-unit increase in baseline HAZ, there was a 0.21 mean gain in gross motor z-score (P=0.02), with similar results for other domains. Children with larger baseline head circumference had greater gains in fine motor (mean gain 0.26 per unit increase in HCZ; P=0.001) and social (mean gain 0.21; P=0.02). Older children had higher gains in social z-scores (mean gain, 0.22 per year increased age; P=0.03).
Figure 3.
Forest plot summarizing cofactors for 6-month change in developmental z-scores for baseline cofactors (A) and 6-month cofactors (B) adjusting for baseline developmental z-scores, child sex, and caregiver years of education (n=58). Analyses of change variables (CD4%, WAZ, HAZ, WHZ, and HCZ) are also adjusted by their baseline counterpart. Linear regression coefficients (95% confidence intervals) are denoted by black markers (horizontal black lines and whiskers). Circle, diamond, square and triangle markers represent gross motor, fine motor, social, and language domains, respectively.
During ART, children with higher gains in weight, height, and CD4% had greater improvement in gross motor (mean gain, 0.34 per gain in WAZ; P=0.006, 0.44 per gain in HAZ; P=0.002, and 0.05 per gain in CD4%; P=0.04), social (mean gain, 0.37 per gain in WAZ; P=0.002) and language (mean gain, 0.25 per gain in HAZ; P=0.01), (Figure 3 and Table, Supplemental Digital Content 2).
DISCUSSION
Among newly diagnosed HIV-infected children age <5.5 years, we found significant gains in gross and fine motor domains, but not in social or language during 6 months on ART. Children who were better nourished, had lower viremia and better immune status at baseline started with higher baseline developmental z-scores. During ART, children with taller baseline stature, and better immune and growth recovery while on ART had greater developmental improvements.
During the first 6 months on ART, children in our study had substantial gains (approximately +0.4 SDs) in gross and fine motor, whereas social and language did not improve. These gains in motor function may reflect reduced virus or inflammation within the brain or improved overall health or both. A previous study among similarly aged (median, 44 months) African children with late HIV diagnosis, found substantial motor and cognitive improvements after initiating ART (25). However, South African children initiating ART at mean age 60 months and with severe immunosuppression had no neurodevelopmental improvement during 6 months on ART (24). In another study, South African infants (mean age 5 months) had limited improvement, with no gain in language or motor and trend for improved cognition during 6 months of ART (34). Our finding of significant improvement following ART in an older cohort suggests residual neuroplasticity in response to viral suppression and immune recovery and underscores the role of prompt ART to prevent cognitive decline.
The first 2 years are critical for brain development (35). Several mechanisms, including irreversible HIV-related neuronal damage, HIV brain reservoirs refractory to ART, and undernutrition may limit neurodevelopmental benefits of ART. In our study, children with advanced HIV had poorer developmental scores at presentation. Other studies have also shown that children with HIV disease progression had greater memory and learning deficits (36), psychiatric (22) and cognitive impairment (13, 21, 22, 37) cortical atrophy, and white matter microstructural changes indicative of neuronal and myelin injury (17, 18, 38).
We found that children with poor nutritional status had significantly lower baseline MDAT scores. Undernutrition is a major risk factor for poor neurodevelopment (39). Children in this study had substantial undernutrition compared with the general population in Kenya, in whom underweight, stunting and wasting were 11.0%, 26.0% and 4.0%, respectively, in 2014 (40). Undernutrition in this cohort may have reflected combined food insecurity, and the metabolic cost of HIV and acute co-morbidities.
Given that late perinatal HIV diagnosis continues to be common in sub-Saharan Africa (6–9), it is important to identify cofactors of better neurodevelopmental recovery in this context. In US cohorts, children with viral suppression prior to age 5 had higher school-age IQ scores (41). We found that children with better pre-ART immune and nutritional status, particularly as indicated by height, and those with better immune and growth responses to ART had higher developmental gains. These findings suggest that nutritional support in newly diagnosed HIV-infected children might benefit their developmental trajectories.
Even under optimized circumstances, successful ART may not prevent neurodevelopmental impairment in HIV-infected children. In adults, evidence of neuronal damage can persist even in the context of effective viral suppression (42). Among very early treated infants, a subset still manifested brain abnormalities by age 32 months (43). During 6 months on ART, we did not find improvements in either social or language functioning, and z-scores remained approximately 0.5 SDs below the norm for both domains. Importantly, lack of improvement in these domains may precede long-term deficits that compromise behavior, functioning, school achievement and employment. School aged HIV-infected children often have deficits in processing speed, working memory, visual-spatial processing, attention, language and executive functioning (37, 44–48).
In our cohort, mean baseline developmental z-scores were ~0.5–1 SD below a Malawian norm and though improved significantly in some domains, remained below average after 6 months of ART. Our findings are similar to a South African cohort (mean age 5 months) whose developmental scores remained significantly lower than HIV-exposed uninfected infants after 6 months of ART (34). In randomized trials, psychosocial stimulation improved neurodevelopment in HIV-infected children on ART in Uganda (mean age 3.8 years) and in South Africa (mean age 18 months) (49, 50). Further development and scale-up of interventions to augment ART are needed to optimize child outcomes in perinatally HIV-infected children.
To our knowledge, our study is the first to use the MDAT, a publicly accessible tool that was culturally adapted for use in Africa, for serial developmental assessment of HIV infected children (29). The MDAT can be administered in 20–60 minutes and may be a useful tool for screening and monitoring HIV-infected infants in HIV-care settings. Unlike the Denver Developmental Screening Test, the MDAT provides a score that can be used to compare to local norms, and monitor for improvements or declines over time. Additional studies are needed to determine the utility of the MDAT for scaled-up assessment of child developmental outcomes in HIV care settings.
Strengths of this study are the sample size, prospective design, use of a detailed developmental assessment, and inclusion of HIV-infected children newly initiating ART. Children were HIV diagnosed in hospital, which unfortunately remains common (6–8). This study used a detailed developmental assessment, which allowed quantitative group comparisons, and may have greater potential for scale-up than methods requiring more intensive training and resources.
Limitations include the relatively short follow-up, and high attrition prior to first developmental assessment. In addition, we lacked detailed information regarding likely key determinants of early childhood development including history of obstetric or birth complications, duration of breastfeeding, and the home caregiving environment. History of pre-term birth was limited due to self-report and lack of ultrasound in this setting. The MDAT is less widely validated than other assessments, such as the Bayley Scales of Infant and Toddler Development, which has been used in several other studies of infant neurodevelopment in HIV-infected children (10–12, 14, 15, 34). We did not evaluate developmental trajectories in an age-matched HIV-unexposed Kenyan cohort. Lack of change in language and social z-scores may have been due to low sensitivity in these domains or subtle differences in language, culture or environmental and psychosocial contexts, given that this analysis relied on Malawian norm data. The MDAT is currently being used in other African countries (personal communication, M. Gladstone); however, validation studies for use of MDAT in these settings have not yet been published.
In conclusion, HIV-infected children with late diagnosis had improved gross and fine motor functioning during the first 6 months on ART, no improvement in social or language functioning and overall, scores remained below African norms. Immune and nutritional status were correlated with developmental improvement, suggesting that optimizing nutrition and ART may improve neurodevelopmental benefits. Residual deficits underscore the need for further interventions to optimize neurodevelopmental outcomes in late-diagnosed HIV-infected children.
Supplementary Material
Acknowledgments
We thank the PUSH study participants and their families, the PUSH administrative, clinical, and data teams and the CDC-Kemri laboratory for their dedication and support. We thank the Data Safety and Monitoring Board for their thoughtful oversight: Drs Michael Hughes (chair), Shahin Lockman, James McIntyre, Lynne Mofenson, Philippa Musoke, Denise Russo and Sarah Walker. We thank the Kizazi Mother Infant Working Group, the UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) and Kenya Research and Training Center (KRTC) for their thoughtful input during development of this manuscript.
Funding This work was supported by the Eunice Kennedy Shrive National Institute of Child Health and Human Development (NICHD) [R01 HD023412 to G.J.-S.]. Field site and biostatistic support were provided by the University of Washington International and Biometrics Cores of the Center for AIDS Research (CFAR), an National Institutes of Health (NIH) funded program [P30 AI027757], supported by the following NIH Institutes and Centers: National Institute of Allergy and Infectious Diseases (NIAID); National Cancer Institute; National Institute of Mental Health; National Institute on Drug Abuse; NICHD; National Heart, Lung, and Blood Institute; and National Center for Complementary and Alternative Medicine. S.B.-N. was supported by CFAR, 2 R01 HD023412, and the National Institute of Neurological Disorders and Stroke [K01 NS080637]. G.J.-S. was supported by the NIH [K24 HD054314]. L.A.G. was supported by [5P30AI027757-28S1]. L.M.C was a supported by NICHD [K12 HD000850], and the American Pediatric Society and American Academy of Pediatrics. REDCap at the Institute of Translational Health Science (ITHS) was supported by National Center for Research Resources/NIH [UL1 RR025014].
Footnotes
None of the authors have conflicts of interest to disclose.
References
- 1.UNAIDS. Children and HIV Fact Sheet. Geneva: UNAIDS; 2016. [Accessed August 23, 2016]. Availiable at: http://www.unaids.org/sites/default/files/media_asset/FactSheet_Children_en.pdf. [Google Scholar]
- 2.Fatti G, Bock P, Eley B, Mothibi E, Grimwood A. Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: an analysis in four provinces in South Africa, 2004–2009. J Acquir Immune Defic Syndr. 2011;58:e60–67. doi: 10.1097/QAI.0b013e3182303c7e. [DOI] [PubMed] [Google Scholar]
- 3.van Dijk JH, Sutcliffe CG, Munsanje B, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS One. 2011;6:e19006. doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. The New England journal of medicine. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS (London, England) 2012;26:1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leyenaar JK, Novosad PM, Ferrer KT, et al. Early clinical outcomes in children enrolled in human immunodeficiency virus infection care and treatment in lesotho. Pediatr Infect Dis J. 2010;29:340–345. doi: 10.1097/INF.0b013e3181bf8ecb. [DOI] [PubMed] [Google Scholar]
- 7.Wamalwa DC, Obimbo EM, Farquhar C, et al. Predictors of mortality in HIV-1 infected children on antiretroviral therapy in Kenya: a prospective cohort. BMC Pediatr. 2010;10:33. doi: 10.1186/1471-2431-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner A, Slyker J, Langat A, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr. 2015;15:10. doi: 10.1186/s12887-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Njuguna IN, Wagner AD, Cranmer LM, et al. Hospitalized Children Reveal Health Systems Gaps in the Mother-Child HIV Care Cascade in Kenya. AIDS Patient Care STDS. 2016;30:119–124. doi: 10.1089/apc.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase C, Ware J, Hittelman J, et al. Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Women and Infants Transmission Study Group. Pediatrics. 2000;106:E25. doi: 10.1542/peds.106.2.e25. [DOI] [PubMed] [Google Scholar]
- 11.Gay CL, Armstrong FD, Cohen D, et al. The effects of HIV on cognitive and motor development in children born to HIV-seropositive women with no reported drug use: birth to 24 months. Pediatrics. 1995;96:1078–1082. [PubMed] [Google Scholar]
- 12.Drotar D, Olness K, Wiznitzer M, et al. Neurodevelopmental outcomes of Ugandan infants with human immunodeficiency virus type 1 infection. Pediatrics. 1997;100:E5. doi: 10.1542/peds.100.1.e5. [DOI] [PubMed] [Google Scholar]
- 13.Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol. 2014;24:316–331. doi: 10.1002/rmv.1793. [DOI] [PubMed] [Google Scholar]
- 14.Nozyce ML, Lee SS, Wiznia A, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 15.Jeremy RJ, Kim S, Nozyce M, et al. Neuropsychological functioning and viral load in stable antiretroviral therapy-experienced HIV-infected children. Pediatrics. 2005;115:380–387. doi: 10.1542/peds.2004-1108. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nature reviews. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 17.Hoare J, Fouche JP, Phillips N, et al. White matter micro-structural changes in ART-naive and ART-treated children and adolescents infected with HIV in South Africa. AIDS. 2015;29:1793–1801. doi: 10.1097/QAD.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 18.Uban KA, Herting MM, Williams PL, et al. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS. 2015;29:1035–1044. doi: 10.1097/QAD.0000000000000648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis-de Los Angeles CP, Alpert KI, Williams PL, et al. Deformed Subcortical Structures Are Related to Past HIV Disease Severity in Youth With Perinatally Acquired HIV Infection. J Pediatric Infect Dis Soc. 2016;5:S6–S14. doi: 10.1093/jpids/piw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoare J, Fouche JP, Spottiswoode B, et al. A diffusion tensor imaging and neurocognitive study of HIV-positive children who are HAART-naïve "slow progressors". J Neurovirol. 2012;18:205–212. doi: 10.1007/s13365-012-0099-9. [DOI] [PubMed] [Google Scholar]
- 21.Smith R, Chernoff M, Williams PL, et al. Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J. 2012;31:592–598. doi: 10.1097/INF.0b013e318253844b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood SM, Shah SS, Steenhoff AP, Rutstein RM. The impact of AIDS diagnoses on long-term neurocognitive and psychiatric outcomes of surviving adolescents with perinatally acquired HIV. AIDS. 2009;23:1859–1865. doi: 10.1097/QAD.0b013e32832d924f. [DOI] [PubMed] [Google Scholar]
- 23.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected Children older than 1 year of age randomized to early versus deferred antiretroviral therapy: the PREDICT neurodevelopmental study. The Pediatric infectious disease journal. 2013;32:501–508. doi: 10.1097/INF.0b013e31827fb19d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith L, Adnams C, Eley B. Neurological and neurocognitive function of HIV-infected children commenced on antiretroviral therapy. South African Journal of Child Health. 2008;2 [Google Scholar]
- 25.Van Rie A, Dow A, Mupuala A, Stewart P. Neurodevelopmental trajectory of HIV-infected children accessing care in Kinshasa, Democratic Republic of Congo. Journal of acquired immune deficiency syndromes (1999) 2009;52:636–642. doi: 10.1097/QAI.0b013e3181b32646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Njuguna I, Cranmer LM, Otieno V, et al. Urgent Versus Post-Stabilization Antiretroviral Treatment (ART) in Hospitalized Children: A Randomized Controlled Trial. Lancet HIV. doi: 10.1016/S2352-3018(17)30167-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National AIDS and STI Control Programme, Ministry of Health, Republic of Kenya. Guidelines for antiretroviral thearapy in Kenya. [Accessed August 23, 2016];Program. (4). 2011 Availiable at: http://www.nascop.or.ke/index.php/care-treatment-downloads/
- 28.National AIDS and STI Control Programme, Ministry of Health, Republic of Kenya. [Accessed August 23, 2016];Guidelines on Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: A rapid advice. 2014 Availiable at: https://aidsfree.usaid.gov/sites/default/files/tx_kenya_2014.pdf.
- 29.Gladstone M, Lancaster GA, Umar E, et al. The Malawi Developmental Assessment Tool (MDAT): the creation, validation, and reliability of a tool to assess child development in rural African settings. PLoS Med. 2010;7:e1000273. doi: 10.1371/journal.pmed.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. [Accessed August 2009];The WHO child growth standards, 2006. 2006 Available at: http://www.who.int/childgrowth/en/
- 31.Mofenson LM, Korelitz J, Meyer WA, 3rd, et al. The relationship between serum human immunodeficiency virus type 1 (HIV-1) RNA level, CD4 lymphocyte percent, and long-term mortality risk in HIV-1-infected children. National Institute of Child Health and Human Development Intravenous Immunoglobulin Clinical Trial Study Group. J Infect Dis. 1997;175:1029–1038. doi: 10.1086/516441. [DOI] [PubMed] [Google Scholar]
- 32.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. [Accessed August 23, 2016];Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011 Availiable at: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
- 34.Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26:497–504. doi: 10.1080/09540121.2013.841828. [DOI] [PubMed] [Google Scholar]
- 35.Tau GZ, Peterson BS. Normal development of brain circuits. Neuropsychopharmacology. 2010;35:147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nichols SL, Chernoff MC, Malee K, et al. Learning and Memory in Children and Adolescents With Perinatal HIV Infection and Perinatal HIV Exposure. Pediatr Infect Dis J. 2016;35:649–654. doi: 10.1097/INF.0000000000001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruel TD, Boivin MJ, Boal HE, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54:1001–1009. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brouwers P, Tudor-Williams G, DeCarli C, et al. Relation between stage of disease and neurobehavioral measures in children with symptomatic HIV disease. AIDS. 1995;9:713–720. doi: 10.1097/00002030-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Engle PL, Black MM, Behrman JR, et al. Strategies to avoid the loss of developmental potential in more than 200 million children in the developing world. Lancet. 2007;369:229–242. doi: 10.1016/S0140-6736(07)60112-3. [DOI] [PubMed] [Google Scholar]
- 40.Kenya National Bureau of Statistics. [Accessed August 23, 2016];Kenya Demographic and Health Survey 2008–09. 2010 Availiable at: http://apps.who.int/medicinedocs/documents/s17116e/s17116e.pdf.
- 41.Crowell CS, Huo Y, Tassiopoulos K, et al. Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS. 2015;29:295–304. doi: 10.1097/QAD.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simioni S, Cavassini M, Annoni JM, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 43.Ackermann C, Andronikou S, Laughton B, et al. White Matter Signal Abnormalities in Children with suspected HIV-Related Neurologic Disease on Early Combination Antiretroviral Therapy. Pediatr Infect Dis J. 2014 doi: 10.1097/INF.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koekkoek S, de Sonneville LM, Wolfs TF, Licht R, Geelen SP. Neurocognitive function profile in HIV-infected school-age children. Eur J Paediatr Neurol. 2008;12:290–297. doi: 10.1016/j.ejpn.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 46.Wolters PL, Brouwers P, Civitello L, Moss H. Receptive and expressive language function of children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. AIDS. 1997;11:1135–1144. doi: 10.1097/00002030-199709000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Coplan J, Contello KA, Cunningham CK, et al. Early language development in children exposed to or infected with human immunodeficiency virus. Pediatrics. 1998;102:e8. doi: 10.1542/peds.102.1.e8. [DOI] [PubMed] [Google Scholar]
- 48.Phillips N, Amos T, Kuo C, et al. HIV-Associated Cognitive Impairment in Perinatally Infected Children: A Meta-analysis. Pediatrics. 2016;138:e20160893. doi: 10.1542/peds.2016-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boivin MJ, Bangirana P, Nakasujja N, et al. A year-long caregiver training program improves cognition in preschool Ugandan children with human immunodeficiency virus. J Pediatr. 2013;163:1409–1416. e1401–1405. doi: 10.1016/j.jpeds.2013.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Potterton J, Stewart A, Cooper P, Becker P. The effect of a basic home stimulation programme on the development of young children infected with HIV. Dev Med Child Neurol. 2010;52:547–551. doi: 10.1111/j.1469-8749.2009.03534.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.