Abstract
Background
Previous work has shown that amygdala responsiveness to fearful expressions is inversely related to level of callous-unemotional (CU) traits (i.e. reduced guilt and empathy) in youth with conduct problems. However, some research has suggested that the relationship between pathophysiology and CU traits may be different in those youth with significant prior trauma exposure.
Methods
In experiment 1, 72 youth with varying levels of disruptive behavior and trauma exposure performed a gender discrimination task while viewing morphed fear expressions (0, 50, 100, 150 fear) and Blood Oxygenation Level Dependent responses were recorded. In experiment 2, 66 of these youth performed the Social Goals Task, which measures self-reports of the importance of specific social goals to the participant in provoking social situations.
Results
In experiment 1, a significant CU traits-by-trauma exposure interaction was observed within right amygdala; fear intensity-modulated amygdala responses negatively predicted CU traits for those youth with low levels of trauma but positively predicted CU traits for those with high levels of trauma. In experiment 2, a bootstrapped model revealed that the indirect effect of fear intensity amygdala response on social goal importance through CU traits is moderated by prior trauma exposure.
Conclusions
This study, while exploratory, indicates that the pathophysiology associated with CU traits differs in youth as a function of prior trauma exposure. These data suggest that prior trauma exposure should be considered when evaluating potential interventions for youth with high CU traits.
Keywords: Callous-unemotional traits, disruptive behavior, emotion, fear, trauma
Introduction
About 30–50% of all youth referred to mental health facilities present with disruptive behavior severe enough to warrant a disruptive behavior disorder (DBD) diagnosis [conduct disorder (CD), oppositional defiant disorder (ODD); Rappaport & Thomas, 2004]. Approximately 50% will later develop antisocial personality disorder (Robins & Price, 1991) and incur considerable costs to society (Scott et al. 2001). Youth with DBDs can be distinguished with respect to callous-unemotional (CU) traits (i.e. reduced guilt and empathy), which differentially relate to their aggression profile (McMahon et al. 2010), underlying neurobiology (Blair, 2013) and treatment response (Waschbusch et al. 2007; Haas et al. 2011; Masi et al. 2011; Blair, 2013; Manders et al. 2013; Frick et al. 2014). These findings led to the inclusion of a ‘with limited prosocial emotions’ specifier for CD in DSM 5 (Pardini et al. 2010; American Psychiatric Association, 2013).
Considerable data suggest that reduced amygdala responses to fearful expressions relate to increased CU traits (Marsh et al. 2008; Jones et al. 2009; Viding et al. 2012; White et al. 2012). However, there may be heterogeneity in the neurobiological underpinnings of CU traits. Data suggest that youth with DBDs and CU traits might differ according to their level of prior trauma exposure and resulting anxiety (Vaughn et al. 2009; Kimonis et al. 2011; Tatar II et al. 2014). For example, Kimonis et al. (2017) reported that aggressive youth with high CU traits and low trauma showed reduced augmentation of the startle reflex following visual threat primes. In contrast, aggressive youth with high CU traits and high prior trauma showed increased augmented startle reflexes. Reduced augmentation of the startle reflex is consistent with the compromised amygdala functioning thought to underpin CU traits (cf. Blair, 2013). In contrast, enhanced augmentation would suggest that those with high CU traits and prior maltreatment show enhanced amygdala responses to threat. Trauma exposure has been repeatedly shown to increase amygdala threat responsivity (McCrory et al. 2011; Tottenham et al. 2011; Bogdan et al. 2012) and consequently increases the augmentation of the startle reflex (Jovanovic et al. 2009). As such, the findings of Kimonis et al. (2017) and others (Vaughn et al. 2009; Kimonis et al. 2011, 2012; Tatar et al. 2014) suggest that only disruptive youth without significant prior maltreatment should show the typical inverse relationship between amygdala responsiveness and CU traits.
Disruptive youth with CU traits expect the outcomes of disruptive behavior to be more positive for themselves and care less about the negative consequences (Pardini et al. 2003; Pardini & Byrd, 2012). Specifically, on the Social Goals Task (Pardini, 2011), CU traits are associated with reduced ratings of the importance of positive social goals (i.e. reduced interest in avoiding conflict or reconciling with a provocative other) and increased ratings of the importance of negative social goals (i.e. dominating/forcing respect from or exacting revenge upon a provocator). The Blair model (2013) posits that amygdala responsiveness to others’ distress is important for socialization through its key role in forming associations between stimuli (i.e. representations of harmful acts) and reinforcement (i.e. the aversion induced by another’s distress). Impairment in this role of the amygdala (Blair, 2013) should result in an individual who considers avoiding conflict as less important but may be more willing to dominate/harm others.
We therefore had two goals in the current exploratory study: First, we examined how prior trauma moderates the relationship between amygdala responsivity to fearful expressions and CU traits. Participants performed a gender discrimination task while observing faces with differing fear intensity. We examined whether amygdala responsivity to fearful expressions interacted with trauma in predicting CU traits (Model 1 in Fig. 1). Specifically, we predicted that the typical inverse relationship between amygdala responsivity to fearful expressions and level of CU traits would be stronger in youth with low trauma exposure. Second, we sought to determine the extent to which the fear intensity amygdala response interacts with CU traits and prior trauma to predict social goal importance (SGI) (model 2 in Fig. 1). We predicted that SGI would be predicted by the indirect effect of the fear intensity amygdala response through CU traits and that this would be moderated by prior trauma exposure (model 2 in Fig. 1).
Fig. 1. Analysis outline.
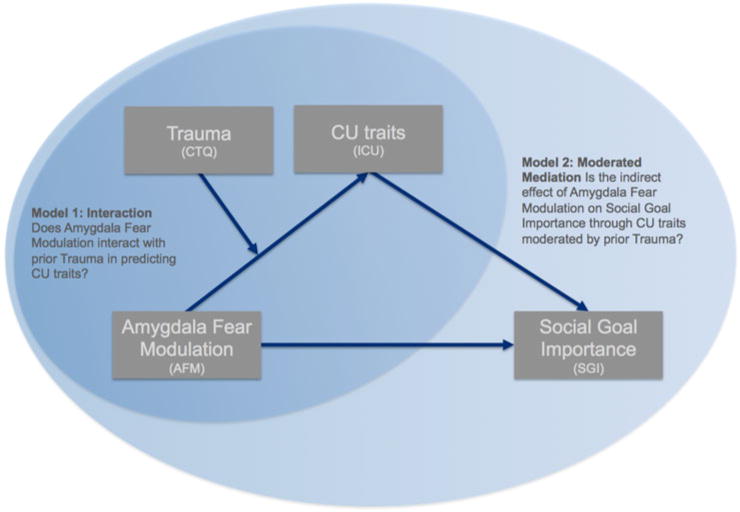
The figure summarizes the main analysis strategy for the current paper. In the first analysis (model 1), we test a moderation model. Specifically, we test whether fear responsivity in the amygdala interacts with prior trauma to predict CU traits. We then extend this model in the second analysis (model 2). In this model, we examined how fear intensity-modulated amygdala responses, trauma, and CU traits influence the importance of social goals. That is, we examined whether the indirect effect of fear intensity modulation in the amygdala on Social Goal Importance through CU traits was moderated by prior trauma.
Materials and methods
The current study involved two experiments. During experiment 1, participants completed the functional Magnetic Resonance Imaging (fMRI) Morphed Faces Task (Marsh et al. 2008). During experiment 2, participants completed the Social Goals Task (adapted from Lochman et al. 1993; Pardini, 2011).
Experiment 1: Morphed faces fMRI task
Participants
Eighty-two youth, aged 10–18 years, completed experiment 1. Ten youth were excluded from analysis (missing trauma questionnaires (4), scanner technical difficulties (4), excessive motion [2; 46 and 53 volumes above the motion threshold (group mean = 1.4 ± 2.6) and 0.76 and 0.60 mm average motion per volume (group mean = 0.097 mm ± 0.113 mm)].
Of the 72 remaining youth, 47 received a DBD diagnosis (CD or ODD; n = 33) or ADHD (n = 14) and 25 were without psychopathology [typically developing (TD); n = 25]; see Table 1. According to the Childhood Trauma Questionnaire (CTQ, see below), 13 participants reported ‘Severe to Extreme’, 7 ‘Moderate to Severe’, 16 ‘Low to Moderate’ and 36 ‘None or Minimal’ trauma on at least one of the subscales. Youth were drawn from the Boys Town Family Home Program (n = 21) and the greater Omaha area (n = 51). Youth recruited from the Family Home Program had been referred for behavioral and mental health problems. Participants from the community were recruited through flyers and were both youth with psychopathology (n = 26) and TD youth (n = 25). Clinical characterization was done through psychiatric interviews by licensed and board-certified psychiatrists with the participants and their parents, to adhere closely to common clinical practice. Of note, semi-structural interviews preformed by a clinical psychologist were not conducted. The Boys Town National Research Hospital institutional review board approved this study. A doctoral level researcher or a member of the clinical research team obtained written informed consent and assent. In all cases, youth had the right to decline participation at any time before or during the study. With respect to community participants, informed consent was obtained from the youths’ parents/legal guardians at the beginning of the on-site screening. At this time, the consent document was reviewed in detail and the parents/legal guardians had the opportunity to have their questions answered before being asked to sign the consent form. After that, informed assent was obtained from the youth themselves. This procedure differed slightly for youth recruited from the Boys Town campus. Consent documents were sent to parents/legal guardians via postal mail and discussed with them by phone. Assent was obtained from the youth in a separate session, but only after the written consent forms had been returned.
Table 1.
Demographic and assessment information. Information is provided across all participants and for three meaningful subgroups in order to provide further information on the make-up of the sample
| Gender | % Male | TDa (n = 25) 68% |
– | ADHDa (n = 14) 79% |
– | DBD (±ADHD)a (n = 33) 82% |
– | Total (n = 72) 76% |
– | χ2 0.460 |
|---|---|---|---|---|---|---|---|---|---|---|
| Handedness | % Right | 92% | _ | 86% | – | 94% | – | 92% | – | 0.645 |
| Race | % Black or African American | 4% | – | 0% | – | 9% | – | 6% | – | 0.725 |
| % White | 84% | – | 86% | – | 79% | – | 82% | – | ||
| % More than one race | 8% | – | 7% | – | 9% | – | 8% | – | ||
| % American Indian | 4% | – | 0% | – | 0% | – | 1% | – | ||
| % Unknown | 0% | – | 7% | – | 3% | – | 3% | – | ||
| Stimulants | 0% | – | 86% | – | 55% | – | 41% | – | <0.001 | |
| Antipsychotics | 0% | – | 0% | – | 30% | – | 14% | – | 0.001 | |
| Antidepressants | 0% | – | 0% | – | 24% | – | 11% | – | 0.005 |
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | One-way ANOVA p (post-hoc) |
|
|---|---|---|---|---|---|---|---|---|---|
| Age | 13.80 | 2.06 | 13.36 | 2.10 | 13.94 | 2.41 | 13.78 | 2.22 | 0.716 |
| IQ | 111.00 | 13.56 | 102.93 | 13.46 | 101.55 | 10.87 | 105.10 | 12.95 | 0.016 (a) |
| CBCL ODD | 52.72 | 4.40 | 55.71 | 6.59 | 69.09 | 9.63 | 60.81 | 10.78 | <0.001 (b) |
| CBCL CD | 52.12 | 4.01 | 54.93 | 6.98 | 72.30 | 11.41 | 61.92 | 12.91 | <0.001 (b) |
| CBCL PTSD | 51.04 | 1.79 | 58.36 | 6.54 | 69.61 | 9.80 | 60.97 | 11.09 | <0.001 (c) |
| CTQ | 30.76 | 6.55 | 37.29 | 10.87 | 41.70 | 14.06 | 37.04 | 12.21 | 0.002 (d) |
| ICUb | 18.84 | 7.16 | 28.93 | 6.41 | 40.88 | 9.10 | 30.90 | 12.67 | <0.001 (c) |
a, TD>DBD; b, TD<DBD;ADHD<DBD; c, TD<ADHD<DBD; d, TD<DBD.
TD, typically developing; DBD, disruptive behavior disorder; ADHD, attention deficit hyperactivity disorder; IQ, intelligence quotient; CBCL, child behavior checklist; ODD, oppositional defiant disorder; CD, conduct disorder; PTSD, post-traumatic stress disorder; CTQ, childhood trauma questionnaire; ICU, inventory of callous-unemotional traits; S.D., standard deviation.
Based on psychiatric evaluation.
Range 8–64.
IQ was assessed with the Wechsler Abbreviated Scale of Intelligence – 2nd edition (WASI-II; Wechsler, 2011). Parents completed the child behavior checklist (CBCL; Achenbach, 2009) to index internalizing and externalizing behavior, including levels of Anxiety and post-traumatic stress disorder (PTSD). The CBCL has been found to be reliable (Achenbach, 2009) and effective in both identifying clinical disorders and in quantifying the severity of psychopathology in children and adolescents (Achenbach et al. 2003). CU traits were assessed by both youth and parent/guardian report using the Inventory of callous-unemotional traits (ICU; Frick, 2004). Following previous work, we used the highest total score from either informant (Kamphaus & Frick, 2005). The youth completed the CTQ (Bernstein et al. 1994), a self-report screening inventory of the history of abuse and neglect. The youth was asked to answer each stem based on ‘When I was growing up’.
Participants were excluded if IQ was below 80 or had medical illnesses that required the use of any medication that may have psychotropic effects, such as beta-blockers or steroids. However, medications provided for psychiatric disorders (specifically anti-psychotic, stimulant or mood stabilizing medications) were not exclusory. Youth diagnosed with active psychosis, pervasive developmental disorders, Tourette’s syndrome and neurologic disorder (including seizures) or suffering from claustrophobia were also excluded. Youth with mood and anxiety disorders were included given the high comorbidity with DBDs (e.g. Angold et al. 1999; Loeber et al. 2000; Greene et al. 2002; Nock et al. 2007).
Zero-order correlations revealed that ICU scores were significantly related to IQ (r = −0.292, p = 0.013), but not age (r = 0.186, p = 0.118), gender (r = 0.006, p = 0.958) or average motion per TR (r = 0.006, p = 0.954). CTQ was significantly related to IQ (r = −0.366, p = 0.002), but not age (r = 0.020, p = 0.867), gender (r = −0.055, p = 0.647) or average motion per TR (r = −0.116, p = 0.330). A multi-colinearity analysis shows a correlation of r = 0.396 between the ICU and CTQ and good tolerance (ICU = 0.837; CTQ = 0.747) and variance inflation factors (ICU = 1.195, CTQ = 1.339) for both the ICU and CTQ (Myers, 1990; Menard, 1995).
Experimental design
The task, adapted from Marsh and colleagues (Marsh et al. 2008), involved participants performing gender discriminations on images of neutral or intensity morphed fearful expressions (50/100/150%) during two 3 min runs, totaling ∼6 min (see online Supplementary 1 for further details).
Image acquisition
Participants were scanned using a 1.5 T Toshiba Vantage Titan scanner. Sixty-three functional images per run (two runs) were taken with a gradient echo planar imaging (EPI) sequence [repetition time = 3000 ms; echo time = 45 ms; 64 × 64 matrix; 83° flip angle; 25 cm field of view, 32 axial slices (thickness, 3 mm; 1 mm spacing; in-plane resolution, 3.91 × 3.91 mm2)]. A high-resolution anatomical scan was obtained [three-dimensional (3D) spoiled gradient recalled acquisition in a steady state; repetition time = 12 ms; echo time = 5 ms; 256 mm field of view; 20° flip angle; 78 axial slices; thickness = 2 mm; 256 × 256 matrix] in register with the EPI data.
Image processing and data analysis
Data were analyzed using Analysis of Functional Neuroimages (AFNI; Cox, 1996). The first five volumes in each scan series were discarded. Motion correction was performed by registering all volumes in the EPI datasets to the minimal outlier volume. Participants’ anatomical scans were registered to the Talairach and Tournoux atlas (Talairach & Tournoux, 1988) using nonlinear registration and the TT_N27 template. The EPI data were then registered to the Talairach anatomical scan within AFNI (Talairach space).
The EPI datasets were spatially smoothed (isotropic 6 mm kernel) and normalized by dividing the signal intensity of a voxel at each time-point by the mean signal intensity of that voxel for each run and multiplying the result by 100. The resultant regression coefficients represent a percentage of signal change from the mean.
Following this, one indicator regressor was generated for face onsets. One additional regressor was created by parametrically modulating the first indicator regressor by fear intensity. The values for each fear intensity level were: 0 = neutral, 1 = 50% fear, 2 = 100% fear, 3 = 150% fear. This second regressor was our regressor of interest for the current paper, as it captures variation in the Blood Oxygenation Level Dependent (BOLD) response as a function of fear intensity. A positive beta for this regressor indicates a positive relationship between BOLD response and fear intensity, a negative beta indicates decreasing BOLD responses with increasing fear intensity; i.e. this regressor is the ‘fear intensity-modulated BOLD response’. Every volume and its predecessor on which motion exceeded 1 mm (Euclidean Norm) was censored. All regressors were created by convolving the train of stimulus events with a gamma variate hemodynamic response function. Linear regression modeling was performed using the two regressors described above plus regressors to model a first-order baseline drift function. This produced a regression coefficient and associated t statistic for each voxel and regressor.
A linear regression analysis was conducted on a bilateral amygdala region of interest (ROI) using AFNI’s 3dttest++, given our hypotheses with respect to this region. This linear regression analysis was performed using our first level fear intensity-modulated BOLD response beta coefficients as dependent variable and ICU, CTQ, and the interaction between ICU and CTQ as predictor variables. The amygdala ROIs were created using a review on facial expression processing (Fusar-Poli et al. 2009). Nine mm spheres (diameter) were drawn around the coordinates with the maximum activation likelihood estimation for emotional face processing in the amygdala (xyz = −20; −6; −12 and 18; −6; −14), which created two spheres of 123 voxels each. These spheres covered large parts of superficial, laterobasal, and a small portion of centromedial amygdala (Amunts et al. 2005; Eickhoff et al. 2005). The spheres were intersected with a group mask based on the template to which all scans were registered resulting in a right amygdala ROI of 100 and a left amygdala ROI of 96 voxels. The main interaction in the amygdala, on which this paper reports, contained data from 90.2% of the sample.
Correction for multiple comparisons
Multiple comparison correction was conducted within AFNI’s 3dttest++ non-parametric approach to cluster size simulation (Cox et al. 2017a, b), giving excellent false discovery rate control. The -Clustsim option uses the residuals to simulate approximately 10 000 null 3D results. 3dClustSim is then run with those results to generate cluster-threshold tables. Given our a priori hypotheses with respect to the amygdala, we performed a small volume correction on this region. For our small volume correction analysis, we applied an initial uncorrected p value of 0.05 and for our whole brain analysis we applied an initial uncorrected p value of 0.005. We then used the cluster-threshold tables to determine the analysis-specific cluster extent threshold to obtain a map-wise false-positive probability of p < 0.05 (20 voxels within the ROIs and 58 within the gray matter). In addition, we reported effect sizes with each analysis in line with recent recommendations (Chen et al. 2017).
Unpacking interaction effects
To unpack interaction effects, we extracted the average beta coefficients for the fear intensity-modulated BOLD response in regions with significant effects for each participant. We then used the PROCESS macro for SPSS (Model 1, Hayes, 2013) to examine how fear intensity modulation interacts with prior trauma to predict CU traits (Fig. 1, model 1). PROCESS provides beta estimates of each independent variable and moderator variable as well as their interaction through bootstrapping (10 000 iterations). PROCESS reports bias-corrected 95% confidence intervals as indicators of significance. Of note, during the second-level processing of the MR data, we used the fear intensity-modulated BOLD response as the outcome variable, whereas CU traits was the outcome variable in our follow-up moderation analysis. This switch was made to adhere more closely to our theoretical model, where reduced amygdala responsiveness to others’ distress increases the risk for CU traits and antisocial behavior due to an indifference to causing others’ harm (Blair, 2005). Given that our data are correlational in nature, however, the MR analysis will identify those regions for which the moderation model is significant.
Experiment 2: Social goals task
Participants
Sixty-six of the youth from experiment 1 also completed experiment 2. A logistic regression with attrition as the dependent variable and fear intensity-modulated BOLD response (from the region showing an interaction effect in Fig. 2), ICU score and CTQ scores as independent variables revealed that none of these variables were related to attrition (all p > 0.16).
Fig. 2. Does amygdala fear modulation interact with prior trauma in predicting CU traits?
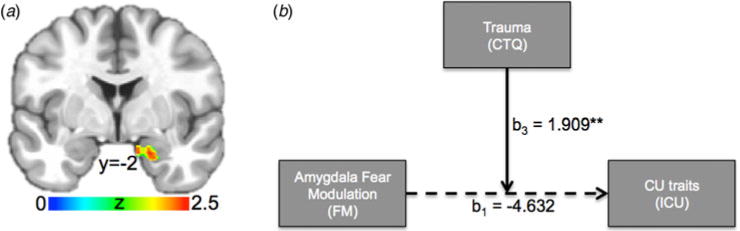
(a) The fear intensity-modulated BOLD response in right amygdala (29 voxels, Z = 2.53, Cohen’s d = 0.30, xyz = 19.2; 2.5–18.0) showed an interaction between ICU and CTQ (small volume corrected). (b) The moderation model (i.e. model 1) tested using the PROCESS macro for SPSS (Hayes, 2013). Numbers reflect beta estimates. ** = p < 0.01. Solid arrows reflect statistically significant effects; i.e. the main effect of amygdala fear modulation on ICU score is not significant. However, the effect of amygdala fear modulation on CU traits is significantly moderated by prior trauma.
Experimental design
In the Social Goals Task (adapted from Lochman et al. 1993; Pardini, 2011), youth read five short vignettes involving minor social conflicts (e.g. ‘You are throwing balls in the schoolyard with your classmates. One kid never throws the ball to you’). Youth rated, via mouse click, the importance of several social goals on a four-point scale (1 = ‘not important’ to 4 = ‘very important’). Five goals were assessed: (i) avoiding conflict (‘avoid problems with him/her’), (ii) dominance (‘let the guy/girl know who is in charge or who’s boss’), (iii) revenge (‘get back at him/her’) (iv) forced respect (‘make him/her show you some respect’), and (v) reconciliation (‘Work things out with him/her so you could possibly be friends’); see online Supplementary 2 for questionnaire layout/vignettes.
Data analysis
Participant’s responses across social goals for each vignette were summed to generate an aggregate Social Goal Importance (SGI) score according to the following formula:
A higher SGI score reflects a participant’s stronger rating of the importance of negative social goals relative to positive social goals for social conflict situations.
We extracted each participant’s average parameter estimates for the fear intensity-modulated amygdala response (i.e. within the cluster that showed an interaction between ICU and CTQ in experiment 1). We then used the PROCESS macro for SPSS (Hayes, 2013) to examine how SGI depends on the effect of fear intensity amygdala modulation (FM), prior trauma (CTQ) and ICU scores. More precisely, we examined whether the indirect effect of fear intensity amygdala modulation on SGI through CU traits was moderated by prior trauma (Fig. 1, model 2). Thus, we started with the linear regression model for predicted ICU scores:
| (1) |
and the linear regression model for predicted SGI:
| (2) |
We then expressed SGI in terms of fear intensity-modulated BOLD responses and prior trauma, by substituting (1) into (2) to produce the following equation:
| (3) |
Model 2 (see Fig. 1) was estimated using the PROCESS macro (model 7, Hayes, 2013). In order to visualize the indirect effect, we then used beta estimates provided by the PROCESS macro and the linear regression model for SGI (Eqn. 3) to plot trend lines for the association between SGI and fear intensity-modulated amygdala responses at low, medium and high levels of prior trauma.
Results
Experiment 1: morphed faces fMRI task
Behavioral results
On average, participants made 3.86 errors (S.D. = 4.91) and missed 2.14 responses (S.D. = 3.06). Average response time for correct trials was 909.30 ms (S.D. = 147.10). Number of errors, missed responses and response time correlated negatively with age (rs = −0.380, −0.371, −0.484, p = 0.001, respectively), but not ICU, CTQ, IQ or gender (p > 0.05).
Model 1: Does amygdala fear modulation interact with prior trauma in predicting CU traits?
First, a linear regression model (AFNI’s 3dttest++) was conducted on the fear intensity beta coefficients, using ICU, CTQ, and the interaction between ICU and CTQ as predictor variables. Notably, there was an interaction between ICU and CTQ in right amygdala on the fear intensity-modulated BOLD response (29 voxels, Z = 2.53, Cohen’s d = 0.30, xyz = 19.2;2.5–18.0, see Fig. 2a). ICU or CTQ alone did not associate with the amygdala’s fear intensity-modulated BOLD response.
We extracted the average beta-coefficients for the fear intensity-modulated BOLD response in this region for each participant and used the PROCESS macro for SPSS (Hayes, 2013) to examine how right amygdala fear intensity modulation interacts with prior trauma to predict CU traits (Fig. 2b). PROCESS provides beta estimates of each independent variable and moderator variable, as well as their interaction through bootstrapping (see online Supplementary Fig. S1c for linear regression equation for predicted ICU scores). This revealed a significant interaction between right amygdala fear intensity modulation and CTQ (see online Supplementary Fig. S1D: b3, p = 0.0012) on ICU. The Johnson–Neyman Technique in the PROCESS macro indicates points of transition in significance (i.e. significant to not significant and not significant to significant). This shows that higher fear intensity-modulated amygdala responses predict lower ICU scores for relatively low levels of trauma (CTQ ≤ 32) and higher fear intensity-modulated amygdala responses predict higher ICU scores for relatively high levels of trauma (CTQ ⩾ 52). Fear intensity-modulated amygdala responses were not significantly correlated with ICU scores at medium levels of trauma (32<CTQ<52); see Fig. 3. There was also a significant main effect of CTQ on ICU [β(b2) = 0.467; p < 0.001]; higher levels of prior trauma are associated with higher ICU scores. There was no main effect of right amygdala fear intensity modulation on ICU [β(b1) = 0.467; p = 0.465].
Fig. 3. Trend lines and associated confidence intervals for the conditional effect of the amygdalar fear intensity-modulated BOLD response on ICU at values of CTQ.
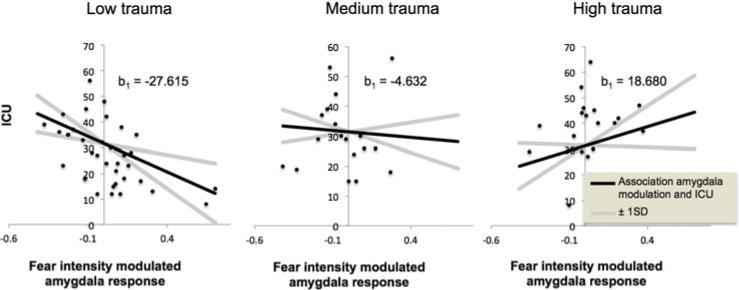
[using beta estimates provided by PROCESS at the following values for CTQ: low (−1 SD CTQ score), medium (mean CTQ score), and high (+ 1 S.D. CTQ score)].
fMRI results within whole brain
We repeated our linear regression model, using AFNI’s 3dttest++, for the whole brain. There were no brain regions for which the fear intensity-modulated BOLD response was associated with the ICU, the CTQ or the interaction between the ICU and the CTQ. There was, however, a main effect of modulation by fear intensity within a large region involving bilateral middle occipital/fusiform cortex (left: 533 voxels, z = 5.55, Cohen’s d = 0.65, xyz = −22.5; −67.5; −9.5; right: 336 voxels, z = 4.44, Cohen’s d = 0.52, xyz = 31.5; −64.5; −12.5).
Follow-up analyses
A series of analyses examined whether the current results might be due to participant outliers, diagnostic groups and/or confounds. However, the interaction result within the amygdala was not due to these variables (for details, see online Supplementary 3 and 4). In addition, the results were comparable whether total CTQ score or Neglect/Abuse sub-scores were used (see online Supplementary 5). Using the level of Anxiety or PTSD symptomatology, rather than CTQ score, revealed that only PTSD symptomatology significantly moderates the relationship of CU traits with amygdala fear responsiveness (see online Supplementary 6).
Experiment 2: Social goals task
Model 2: Is the indirect effect of fear intensity modulation in the amygdala on SGI through CU traits moderated by prior trauma?
We first extracted each participant’s parameter estimates for the fear intensity-modulated amygdala response (the interaction cluster in experiment 1). Then we examined (PROCESS model 7, Hayes, 2013) how the effect of fear intensity-modulated amygdala responses on SGI is influenced by trauma and ICU scores. Specifically, we examined whether the indirect effect of fear intensity modulation in the amygdala on SGI through CU traits was moderated by trauma (Fig. 4a). To this end, we expressed SGI as a function of fear intensity-modulated amygdala responses and trauma (see ‘Methods’ section). Model estimates are summarized in online supplementary Fig. S2C. The bootstrapped model revealed a significant moderated mediation: The indirect effect of fear intensity modulation in the amygdala on SGI through CU traits is moderated by prior trauma (Index of moderated mediation = −0.0455; S.E. = 0.0254). The conditional indirect effect of fear intensity-modulated responses on SGI at values of CTQ is listed in online supplementary Fig. S2D. This shows that there is only a significant negative association between SGI and fear intensity-modulated amygdala responses at low levels of prior trauma (CTQ = 25, but not at medium or high levels of trauma); see online Supplementary 7.
Fig. 4. Is the indirect effect of fear intensity modulation in the amygdala on Social Goal Importance (SGI) through CU traits moderated by prior trauma?
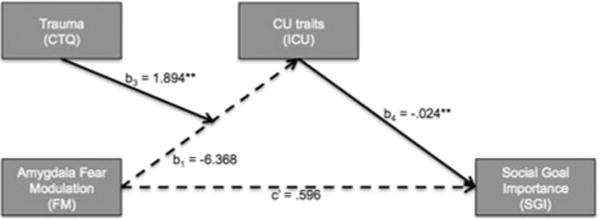
(a) Moderated mediation model tested using the PROCESS macro for SPSS (Hayes, 2013). Numbers reflect beta estimates. ** = p < 0.01. Solid arrows reflect statistically significant effects. (b) The linear regression model for ICU scores (i) was used to express SGI (ii) in terms of fear intensity-modulated BOLD responses and prior trauma (iii).
Discussion
This study examined: (i) how prior trauma moderates the relationship between amygdala fearful expression responsivity and CU traits and (ii) the association between amygdala fear responsiveness and youth’s social goals importance as a function of CU traits and trauma. There were two main findings. First, fear intensity-modulated right amygdala responses interacted with prior trauma to predict CU traits; fear intensity-modulated amygdala responses negatively predicted CU traits for youth with low trauma, whereas fear intensity-modulated amygdala responses positively predicted CU traits for youth with high trauma. Second, a moderated mediation analysis on The Social Goals Task data showed that the indirect effect of the fear intensity amygdala response on SGI through CU traits is moderated by trauma.
The principal goal of the current paper was to determine how the pathophysiology associated with CU traits differs as a function of trauma exposure. Previous work had reported that aggressive youth with high CU traits but low trauma show reduced augmentation of the startle reflex following visual threat primes, but that those aggressive youth with high CU traits and trauma showed increased augmented startle reflexes (Kimonis et al. 2017). The current study sought to extend these previous findings to the neurobiological level. In line with predictions based on earlier behavioral work (Vaughn et al. 2009; Kimonis et al. 2011, 2012, 2017; Tatar II et al. 2014), the typical inverse relationship between amygdala responsiveness to fearful expressions and CU traits (Marsh et al. 2008; Jones et al. 2009; Viding et al. 2012; White et al. 2012) was only seen in youth with low levels of prior trauma. Youth with high levels of prior trauma displayed a positive relationship between amygdala responsiveness to fearful expressions and CU traits.
Blair has posited (e.g. 2005) that reduced amygdala responsiveness to others’ distress increases the risk for CU traits and antisocial behavior due to an indifference to causing others’ harm. Indeed, work has shown that amygdala fearful expression responsiveness mediates the positive relationship between CU traits and instrumental aggression (Lozier et al. 2014), reinforcing the Blair model that it is the underlying neurobiology which is important in determining subsequent behavior. Previous work revealed that youth with high CU traits rate negative social goals as more (dominance, revenge, forced respect) and positive social goals (avoiding conflict, reconciliation; Pardini, 2011) as less important following provocations. Within the Blair model, this relationship between CU traits and SGI reflects the effect of the fear intensity amygdala response. However, if, as indicated by the results of experiment 1, trauma exposure modulates the relationship between amygdala responding and CU traits, then the effect of fear intensity amygdala response on SGI through CU traits should be moderated by prior trauma exposure. This prediction was confirmed by experiment 2. Only youth with low levels of trauma showed a negative association between fear-modulated amygdala responses and increased ratings of the importance of negative relative to positive social goals following provocation.
It has been argued that trauma exposure might foster the development of CU traits (Porter, 1996). In line with this, prior trauma predicts CU traits in delinquent boys (Krischer & Sevecke, 2008) and abused children (≤ age 11) show increased CU traits compared with matched controls as adults (Weiler & Widom, 1996). It has been hypothesized that CU traits develop through dissociation (Porter, 1996), a process of emotional overmodulation (Lanius et al. 2011). Here, however, greater CU traits were not associated with reduced but increased amygdala responsiveness to fearful expressions in high trauma youth, a finding inconsistent with increased emotion regulation. Moreover, a previous work has found no indications of increased dissociative symptoms in externalizing youth with high prior trauma exposure (Poythress et al. 2006; Tatar II et al. 2014). Thus, it appears that individuals can present with elevated CU traits that relate to strikingly different underlying pathophysiologies. Previously, it has been shown that youth diagnosed with CD can show very different underlying pathologies and can be distinguished behaviorally via their level of CU traits (e.g. Pardini et al. 2003; Blair, 2004, 2013; Frick, 2006; Frick et al. 2014). The current data imply that different pathologies can be also found within those disruptive youths with elevated CU traits and that this relates to prior maltreatment. Of course, important questions remain, regarding what this means for their manifestation of CU traits and how specifically trauma might produce this manifestation. Perhaps the CU traits of those with high prior trauma represent a learned behavioral ‘mask’ of their emotional lability? It will be important to determine behavioral indicators of the differences between these groups akin to the differences observed here in endorsed motivations for aggression. In this respect, it is also noteworthy that PTSD symptomatology moderates the relationship of CU traits with amygdala fear responsiveness. Future studies should parse out whether exposure and/or subsequent symptomatology are important in predicting CU traits.
Several caveats should be considered with respect to the current data: First, within the sample, IQ correlated with level of CU traits and age correlated with response times. In addition, youth differed on prescribed medication. To account for these and other potential confounds, we conducted our analysis with these variables included as covariates. This analysis revealed that the fear intensity-modulated BOLD response in the right amygdala maintained an interaction between ICU and CTQ. Second, the results might be due to the presence of outliers. A Mahalanobis Distance analysis revealed four outliers. The fear-modulated amygdala response maintained an interaction between ICU and CTQ after exclusion of these four participants. Third, the results might differ as a function of diagnostic (i.e. TD, ADHD or DBD) or placement group (i.e. residential or community). However, the relationship between CU traits and trauma did not differ as a function of diagnostic or placement group. Fourth, the results might be form-of-prior-trauma specific (McLaughlin et al. 2014). Exploratory analyses suggested that the interactions between ICU and abuse or neglect scores on the fear intensity-modulated BOLD response were very similar to using total CTQ scores. However, this may reflect the high association between level of abuse and neglect (r = 0.44, p < 0.001). Fifth, prior trauma exposure in the current study was measured using child self-report (Bernstein et al. 1994), following the strategy taken in much previous work on the impact of trauma exposure (e.g. Grant et al. 2011; Bogdan et al. 2012; Dannlowski et al. 2012; Garrett et al. 2012). However, it is possible that the use of a single trauma measure introduces response biases, though it is unclear why such putative biases would drive the current results. Sixth, clinical characterization was done through psychiatric interviews by licensed and board-certified psychiatrists with the participants and their parents. We did not have data available from semi-structured clinical interviews. Seventh, given that moderation and moderated mediation models are correlational in nature, and study measures were collected at the same time, causal inference is not possible. Data should be viewed within the larger literature.
In conclusion, although the findings are exploratory and in need of replication, the current results suggest that it may be important to consider prior trauma exposure when evaluating a youth’s level of CU traits. The current results suggest that the optimal treatments for youth with DBDs and CU traits may differ according to level of trauma exposure. Interventions designed to increase responsiveness to distress cues may prove beneficial in youth with high CU traits but low levels or prior trauma (amygdala responsivity to fear within this group was negatively associated with negative goal importance). However, the current findings suggest that this strategy may not be beneficial for youth with high CU traits and high prior trauma exposure (given their already higher levels of amygdala responsiveness). In short, the current data suggest the potential importance of determining the pathophysiology underpinning a particular behavioral presentation rather than relying on the behavioral presentation alone. Focusing on the individual youth’s specific forms of underlying neurocognitive dysfunction may optimize intervention delivery.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank Ronald Copsey and Kimmy VanHorn for their help and support and Gebbe Meffert for support in figure design. Research was supported by the Intramural Research Program of the National Institute of Mental Health under award number 1-ZIA-MH002860-08 and by grant support from the National Institute of Mental Health, National Institutes of Health (1 K22 MH109558-01, Dr. Blair) and (1-K01-MH110643, Dr. White).
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718000156
Declaration of Interest. The authors declare no competing financial interests.
References
- Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory, and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families; 2009. [Google Scholar]
- Achenbach TM, Dumenci L, Rescorla LA. DSM-oriented and empirically based approaches to constructing scales from the same item pools. Journal of Clinical Child and Adolescent Psychology. 2003;32:328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology. 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry. 1999;40:57–87. doi: 10.1111/1469-7610.00424. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. The American Journal of Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Blair JR. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Consciousness and Cognition. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Blair JR. The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience. 2013;14:786–799. doi: 10.1038/nrn3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor iso/val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. American Journal of Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Taylor PA, Cox RW. Is the statistic value All We should care about in neuroimaging? NeuroImage. 2017;147:952–959. doi: 10.1016/j.neuroimage.2016.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. doi: S0010480996900142 [pii] [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI Clustering and False Positive Rates. arXiv:1702.04846 [q-bio, stat] 2017a doi: 10.1073/pnas.1614961114. Available at http://arxiv.org/abs/1702.04846 (Accessed: 21 September 2017) [DOI] [PMC free article] [PubMed]
- Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connectivity. 2017b;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. Mechanisms of Compromised Stress Resilience During Development and Aging. 2012;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Frick PJ. The Inventory of Callous-Unemotional Traits. 2004 doi: 10.1080/15374416.2018.1504297. [Unpublished Ratings Scale] [DOI] [PubMed] [Google Scholar]
- Frick PJ. Developmental pathways to conduct disorder. Child and Adolescent Psychiatric Clinics of North America. 2006;15:311–331, vii. doi: 10.1016/j.chc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin. 2014;140:1–57. doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry & Neuroscience. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depression and Anxiety. 2012;29:449–459. doi: 10.1002/da.21892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research. 2011;45:886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene RW, Biederman J, Zerwas S, Monuteaux MC, Goring JC, Faraone SV. Psychiatric comorbidity, family dysfunction, and social impairment in referred youth with oppositional defiant disorder. American Journal of Psychiatry. 2002;159:1214–1224. doi: 10.1176/appi.ajp.159.7.1214. Export Date 13 August 2013. [DOI] [PubMed] [Google Scholar]
- Haas SM, Waschbusch DA, Pelham WE, Jr, King S, Andrade BF, Carrey NJ. Treatment response in CP/ADHD children with callous/unemotional traits. Journal of Abnormal Child Psychology. 2011;39:541–552. doi: 10.1007/s10802-010-9480-4. [DOI] [PubMed] [Google Scholar]
- Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York: The Guilford Press; 2013. Available at http://www.guilford.com/books/Introduction-to-Mediation-Moderation-and-Conditional-Process-Analysis/Andrew-Hayes/9781609182304 (Accessed: 15 February 2017) [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depression and Anxiety. 2009;26:1018. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphaus RW, Frick PJ. Clinical Assessment of Child and Adolescent Personality and Behavior. 2nd. New York, NY, US: Springer Science+Business Media; 2005. [Google Scholar]
- Kimonis ER, Fanti KA, Goulter N, Hall J. Affective startle potentiation differentiates primary and secondary variants of juvenile psychopathy. Development and Psychopathology. 2017;29:1149–1160. doi: 10.1017/S0954579416001206. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Cauffman E, Goldweber A, Skeem J. Primary and secondary variants of juvenile psychopathy differ in emotional processing. Development and Psychopathology. 2012;24:1091–1103. doi: 10.1017/S0954579412000557. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Skeem JL, Cauffman E, Dmitrieva J. Are secondary variants of juvenile psychopathy more reactively violent and less psychosocially mature than primary variants? Law and Human Behavior. 2011;35:381–391. doi: 10.1007/s10979-010-9243-3. [DOI] [PubMed] [Google Scholar]
- Krischer MK, Sevecke K. Early traumatization and psychopathy in female and male juvenile offenders. International Journal of Law and Psychiatry. 2008;31:253–262. doi: 10.1016/j.ijlp.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Bluhm RL, Frewen PA. How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatrica Scandinavica. 2011;124:331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- Lochman JE, Wayland KK, White KJ. Social goals: relationship to adolescent adjustment and to social problem solving. Journal of Abnormal Child Psychology. 1993;21:135–151. doi: 10.1007/BF00911312. [DOI] [PubMed] [Google Scholar]
- Loeber R, Burke JD, Lahey BB, Winters A, Zera M. Oppositional defiant and conduct disorder: a review of the past 10 years, part I. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39:1468–1484. doi: 10.1097/00004583-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, Van Meter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71:627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders WA, Deković M, Asscher JJ, Van Der Laan PH, Prins PJM. Psychopathy as predictor and moderator of multisystemic therapy outcomes among adolescents treated for antisocial behavior. Journal of Abnormal Child Psychology. 2013;41:1121–1132. doi: 10.1007/s10802-013-9749-5. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Masi G, Manfredi A, Milone A, Muratori P, Polidori L, Ruglioni L, et al. Predictors of nonresponse to psychosocial treatment in children and adolescents with disruptive behavior disorders. Journal of Child and Adolescent Psychopharmacology. 2011;21:51–55. doi: 10.1089/cap.2010.0039. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, et al. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience and Biobehavioral Reviews. 2014;47:578. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon RJ, Witkiewitz K, Kotler JS. Predictive validity of callous-unemotional traits measured in early adolescence with respect to multiple antisocial outcomes. Journal of Abnormal Psychology. 2010;119:752–763. doi: 10.1037/a0020796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard S. Sage university paper series on quantitative applications in the social sciences. 2nd. Thousand Oaks, CA: Sage; 1995. Applied Logistic Regression Analysis. (series no. 106). [Google Scholar]
- Myers R. Classical and Modern Regression with Applications. 2nd. Boston, MA: Duxbury; 1990. [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, Kessler RC. Lifetime prevalence, correlates, and persistence of oppositional defiant disorder: results from the national comorbidity survey replication. Journal of Child Psychology and Psychiatry. 2007;48:703–713. doi: 10.1111/j.1469-7610.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- Pardini DA. Perceptions of social conflicts among incarcerated adolescents with callous-unemotional traits: “you’re going to Pay. It’s going to hurt, but I don’t care. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2011;52:248. doi: 10.1111/j.1469-7610.2010.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Byrd AL. Perceptions of aggressive conflicts and others’ distress in children with callous-unemotional traits: “I’ll show you who’s boss, even if you suffer and I get in trouble. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2012;53:283–291. doi: 10.1111/j.1469-7610.2011.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Frick PJ, Moffitt TE. Building an evidence base for DSM-5 conceptualizations of oppositional defiant disorder and conduct disorder: introduction to the special section. Journal of Abnormal Psychology. 2010;119:683–688. doi: 10.1037/a0021441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardini DA, Lochman JE, Frick PJ. Callous/unemotional traits and social-cognitive processes in adjudicated youths. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:364–371. doi: 10.1097/00004583-200303000-00018. [DOI] [PubMed] [Google Scholar]
- Porter S. Without conscience or without active conscience? The etiology of psychopathy revisited. Aggression and Violent Behavior. 1996;1:179–189. doi: 10.1016/1359-1789(95)00010-0. [DOI] [Google Scholar]
- Poythress NG, Skeem JL, Lilienfeld SO. Associations among early abuse, dissociation, and psychopathy in an offender sample. Journal of Abnormal Psychology. 2006;115:288–297. doi: 10.1037/0021-843X.115.2.288. [DOI] [PubMed] [Google Scholar]
- Rappaport N, Thomas C. Recent research findings on aggressive and violent behavior in youth: implications for clinical assessment and intervention. The Journal of Adolescent Health. 2004;35:260–277. doi: 10.1016/j.jadohealth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Robins LN, Price RK. Adult disorders predicted by childhood conduct problems: results from the NIMH epidemiologic catchment area project. Psychiatry. 1991;54:116–132. doi: 10.1080/00332747.1991.11024540. [DOI] [PubMed] [Google Scholar]
- Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. British Medical Journal. 2001;323:191–194. doi: 10.1136/Bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxix Atlas of the Human Brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tatar II, JR, Cauffman E, Kimonis ER, Skeem JL. Victimization history and posttraumatic stress: an analysis of psychopathy variants in Male Juvenile offenders. Journal of Child & Adolescent Trauma. 2014;5:102–113. doi: 10.1080/19361521.2012.671794. [DOI] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Science. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn MG, Edens JF, Howard MO, Smith ST. An investigation of primary and secondary psychopathy in a statewide sample of incarcerated youth. Youth Violence and Juvenile Justice. 2009;7:172–188. [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, et al. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. American Journal of Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Waschbusch DA, Carrey NJ, Willoughby MT, King S, Andrade BF. Effects of methylphenidate and behavior modification on the social and academic behavior of children with disruptive behavior disorders: the moderating role of callous/unemotional traits. Journal of Clinical Child and Adolescent Psychology. 2007;36:629–644. doi: 10.1080/15374410701662766. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. 2nd. San Antonio, TX: NCS Pearson; 2011. [Google Scholar]
- Weiler BL, Widom CS. Psychopathy and violent behaviour in abused and neglected young adults. Criminal Behaviour and Mental Health. 1996;6:253–271. doi: 10.1002/cbm.99. [DOI] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, et al. Reduced amygdala response in youths With disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased Top-down attention to nonemotional features. American Journal of Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


