Abstract
Propensity to relapse, even following long periods of abstinence, is a key feature in substance use disorders. Relapse and relapse‐like behaviors are known to be induced, in part, by re‐exposure to drug‐associated cues. Yet, while many critical nodes in the neural circuitry contributing to relapse have been identified and studied, a full description of the networks driving reinstatement of drug‐seeking behaviors is lacking. One area that may provide further insight to the mechanisms of relapse is the habenula complex, an epithalamic region composed of lateral and medial (MHb) substructures, each with unique cell and target populations. Although well conserved across vertebrate species, the functions of the MHb are not well understood. Recent research has demonstrated that the MHb regulates nicotine aversion and withdrawal. However, it remains undetermined whether MHb function is limited to nicotine and aversive stimuli or if MHb circuit regulates responses to other drugs of abuse. Advances in circuit‐level manipulations now allow for cell‐type and temporally specific manipulations during behavior, specifically in spatially restrictive brain regions, such as the MHb. In this study, we focus on the response of the MHb to reinstatement of cocaine‐associated behavior, demonstrating that cocaine‐primed reinstatement of conditioned place preference engages habenula circuitry. Using chemogenetics, we demonstrate that MHb activity is sufficient to induce reinstatement behavior. Together, these data identify the MHb as a key hub in the circuitry underlying reinstatement and may serve as a target for regulating relapse‐like behaviors.
Keywords: acetylcholine, cocaine, conditioned place preference, DREADDs, medial habenula, reinstatement
Introduction
The medial habenula (MHb) is an epithalamic subregion composed of a substance P enriched dorsal (dMHb) region and a dense cholinergic ventral (vMHb) region (Claudio Cuello et al. 1978). The dMHb and vMHb send strong projections to distinct subnuclei within the predominantly GABAergic interpeduncular nucleus (IPN), which, in turn, regulates serotonergic signaling from the raphe nuclei (Han et al. 2017; Quina et al. 2017). In contrast, the lateral habenula negatively regulates dopamine signaling primarily through projections to GABAergic interneurons within the rostral medial tegmental gray (Ji & Shepard 2007; Stamatakis & Stuber 2012). As a result, the habenular complex is frequently described as an “antireward” system, responsible for anxiety‐like, aversion and fear behaviors. Recent work has shed light on how the MHb may regulate these behaviors, suggesting that the MHb has a more complex role in regulating fear, learned helplessness and adaptive behaviors (Lee et al. 2010; Zhang et al. 2016). More specifically, the MHb may play a larger role in adaptive behaviors, where mounting evidence has demonstrated that loss of vMHb function leads to deficits in fear extinction and escape behaviors. Nevertheless, while the circuitry and cellular composition of the MHb has been well‐characterized, the endogenous functions of the MHb remain relatively unknown and have recently become the focus of addiction neuroscience (Qin & Luo 2009; Aizawa et al. 2012; Lima et al. 2017).
Due to the density of cholinergic neurons and the expression of unique nicotinic acetylcholine receptors (including the β4 and α5 subunits) in the vMHb, much of the addiction field has focused on nicotine‐associated behaviors (Salas et al. 2009; Fowler, Lu Qun 2011; Fowler & Kenny 2012; Velasquez et al. 2014; Tuesta et al. 2017). The cholinergic population of the vMHb has been shown to be necessary for nicotine self‐administration, withdrawal and the aversive properties of nicotine (Fowler & Lu Qun 2011; Frahm et al. 2011; Antolin‐Fontes et al. 2014). While it is clear that the vMHb has a key role in regulating nicotine response and behavior, few studies have extensively evaluated how the vMHb regulates the response to other drugs of abuse. Recent work, however, implicates the cholinergic population of the MHb in regulating self‐administration and reinstatement behavior of other psychostimulants, including cocaine and methamphetamine (Glick et al. 2006; Hussain et al. 2008; McCallum et al. 2012). Yet, the mechanisms by which the MHb may modulate the behaviors associated with these various psychostimulants remain unknown. The goal of this study is to investigate how the MHb may regulate relapse‐like behavior and adaptive response to cocaine. Here, we use a cell‐type specific chemogenetic approach to causally link vMHb activity to changes in cocaine‐associated behaviors.
Materials and methods
Animals: Eight‐week‐old male wild‐type C57BL/6J mice were purchased from the Jackson Laboratory. ChAT‐IRES‐CreCre/Cre mice were purchased from the Jackson Laboratory (stock no. 006410). Male and female heterozygous ChAT‐IRES‐Cre mice (ChAT‐Cre) were bred and maintained in 12‐hour light/dark cycle with food and water provided ad libitum. All experiments were conducted according to the National Institutes of Health guideline for animal care and use. Experiments were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Stereotaxic surgeries: Two to five‐month‐old heterozygous ChAT‐Cre mice received 0.5‐μL bilateral infusions to the MHb (M/L, ±0.35 mm; A/P, −1.5 mm; D/V; −3.0) of AAV2.8‐hSyn‐DIO‐HM3D‐Gq‐mCherry (2.2 × 1012 vg/ml) or AAV2.8‐hSyn‐DIO‐mCherry (5.3 × 1012 vg/ml). Viruses were infused at a rate of 6 μL/h by using a 30 gauge Neuros Hamilton syringe (product #65459‐01) mounted to either a Harvard Apparatus Nanomite Syringe Pump (product #MA1 70‐2217) or Leica Biosystems Nanoinjector Motorized f/Stereotaxics (product #39462901) (Lopez et al. 2015). All infusions used the Leica Microsystems Angle Two Stereotaxic System. For behavioral experiments, the animals were allowed to recover for a minimum of 7 days before handling. For electrophysiological recordings, the animals were allowed to recover for a minimum of 4 weeks before recording.
Chemogenetic viruses: All viruses were purchased from UNC Vector Core (HM3D Lot: AV4979bc, 2013, mCherry Lot: AV4981CD, 2014) or AddGene (HM3D Lot: v4330, 2016). Viral cDNA was extracted with proteinase K in 1 percent SDS/10‐mM Tris‐HCl in water, and viral purity of chemogenetic constructs was confirmed via Sanger Sequencing (Genewiz) and Endpoint PCR. Universal Amplification Forward Primer: 5′‐gccacccttggtcaccttcag‐3′, Universal Amplification Reverse Primer: 5′‐gccatacgggaagcaatagca‐3′. Universal Sequencing Primer: 5′‐cgatctcgaactcgtggccgt‐3′ (Supporting Information 1A & 1B).
Whole cell recording: Coronal slices (250–300 μm) through the thalamus were cut on a vibratome and transferred into ACSF containing the following (in mM): 124 NaCl, 3 KCl, 1.25 KH2PO4, 1.5 MgSO4, 26 NaHCO3, 2.5 CaCl2 and 10 dextrose. Recordings were made in a submerged chamber superfused with carbogen‐saturated ACSF at a speed of 2–3 ml/min, 32°C. Labeled neurons of the medial habenular nuclei were identified by using an upright fluorescence microscope. Loose cell attached recordings (110–250 MΩ) were achieved from identified fluorescent cells by using infrared differential interference contrast (Choi et al. 2016). Recordings (Axopatch 200A amplifier) were made with 5–7 MΩ pipettes filled with 0.9 percent NaCl. Data were analyzed with pClamp (Molecular Device) and Minianalysis (Synaptosoft).
Conditioned place preference (CPP)‐reinstatement paradigm: Cocaine‐induced CPP was performed as previously described (Mueller & Stewart 2000; Malvaez et al. 2013; Calipari et al. 2016; White et al. 2016). Briefly, the animals were handled for 2 minutes for 3 consecutive days. Following handling, the mice were conditioned over four consecutive days, receiving either cocaine‐HCl (10 mg/kg, IP; Sigma) or 0.9 percent saline, in a context‐dependent manner (counter‐balanced, unbiased). Following conditioning, the animals were allowed to freely explore, in a drug‐free state, the complete chamber to assess preference, established as the difference between time spent in the cocaine‐paired chamber and the saline‐paired chamber, in seconds. To extinguish preference, the animals were repeatedly reintroduced to the chamber daily until criteria met (defined by at least two consecutive days of preference score not statistically different from 0). Once extinguished, the animals were reinstated with either cocaine‐HCl (5 mg/kg, IP; Sigma) or 0.9 percent saline and allowed to freely explore the complete chamber. For HM3D experiments, the animals received either 3‐mg/kg CNO (0.3‐mg/ml CNO, 0.5 percent DMSO, 0.9 percent saline, IP) or vehicle (0.5 percent DMSO, 0.9 percent saline) 40 minutes prior to being reinstated with 0.9 percent saline. The animals were sorted post hoc into reinstatement groups following extinction to ensure reinstatement groups equally acquired and extinguished preference. Behavior was assessed and analyzed by using Ethovision XT 11.5.
Fluorescent in situ hybridization and fluorescent immunohistochemistry: Forty‐five minutes following the reinstatement session, animals were euthanized and their brains flash frozen in dry ice‐chilled isopentane. Twenty micrometer coronal sections were collected using a Leica CM 1850 cryostat at −20°C. For hybridization, a fluorescent oligodeoxynucleotide against cFos was used in an adapted hybridization protocol to quantitatively analyze gene expression (Wang et al. 2012). Briefly, tissue was fixed in 4 percent PFA and blocked in 0.5 percent Triton X‐100 in PBS. The tissue was then hybridized with a fluorescent cFos‐specific probe (100 nM) in hybridization buffer (4X SSC, 4 percent salmon sperm DNA, 0.5‐mM EDTA, 25 percent formamide in ddH2O). Following hybridization, the tissue was washed in 2X SSC, incubated in DAPI [1:15000] in 0.1‐M PBS and subsequently coverslipped by using VectaShield Mounting Medium (product #H‐1000). For immunohistochemistry, slices were fixed in 4 percent PFA for 10 minutes, washed in 0.1‐M PBS and permeated in 0.1 percent Triton X‐100 in 0.1‐M PBS. The slices were then blocked in blocking serum (8 percent NGS, 0.3 percent Triton X‐100, in PBS; 1 hour) and incubated at 4°C overnight in primary solution (2 percent NGS, 0.3 percent Triton X‐100; anti‐AcH4K8 [1:1000], Cell Signaling #2594S; anti‐DsRed [1:1000], Clontech #1408015). The slices were then incubated in secondary solution (2 percent NGS, 0.3 percent Triton X‐100; AcH4K8, Alexa Fluor goat anti‐rabbit 488; DsRed, Alexa Fluor goat anti‐rabbit 555; in PBS). The tissue was imaged by using Olympus Slide Scanner VSBX61. Fluorescence was quantified by using imagej. Briefly, background signal was collected from a soma‐free region and subtracted from MHb signal. All values were normalized to saline‐reinstated controls.
Data analysis: All data were analyzed and graphed by using graphpad prism 7.02.
Results
Drug‐primed reinstatement of cocaine‐induced CPP engages the MHb
To determine if the MHb is engaged during cocaine‐primed reinstatement of cocaine‐induced CPP, wild‐type C57BL/6J mice were conditioned, extinguished and reinstated with either saline or cocaine by using a previously described counter‐balanced protocol (Fig. 1a) (Malvaez et al. 2011; White et al. 2016). Both saline‐reinstated and cocaine‐reinstated animals equally acquired and extinguished a conditioned preference (Fig. 1b & c). Twenty‐four hours following final extinction session, the animals were primed with either saline or cocaine‐HCl immediately prior to the reinstatement test. As predicted, cocaine‐primed animals exhibited a significant increase in preference score compared with saline‐primed animals during the cocaine‐primed reinstatement test (Fig. 1d). There was no significant difference in locomotion during posttest and a trend for an increase in locomotion in cocaine‐primed animals during reinstatement (Supporting Information 2A & 2B). Additionally, in cocaine‐reinstated animals, we found a significant increase in cFos expression (Fig. 1e & f) and an increase in acetylated H4K8 (Fig. 1g & h) in the MHb. We have observed that increased H4K8Ac at the cFos promoter is associated with increased expression of cFos in a previous study (Malvaez et al. 2013). The alterations in cFos expression within the MHb are not induced acutely following a single acquisition of cocaine‐paired CPP (Fig. 1i & k) or a single injection of cocaine in the home cage (Fig. 1j & l). These data suggest that the MHb is specifically engaged during cocaine‐primed reinstatement of a cocaine‐induced CPP.
Figure 1.
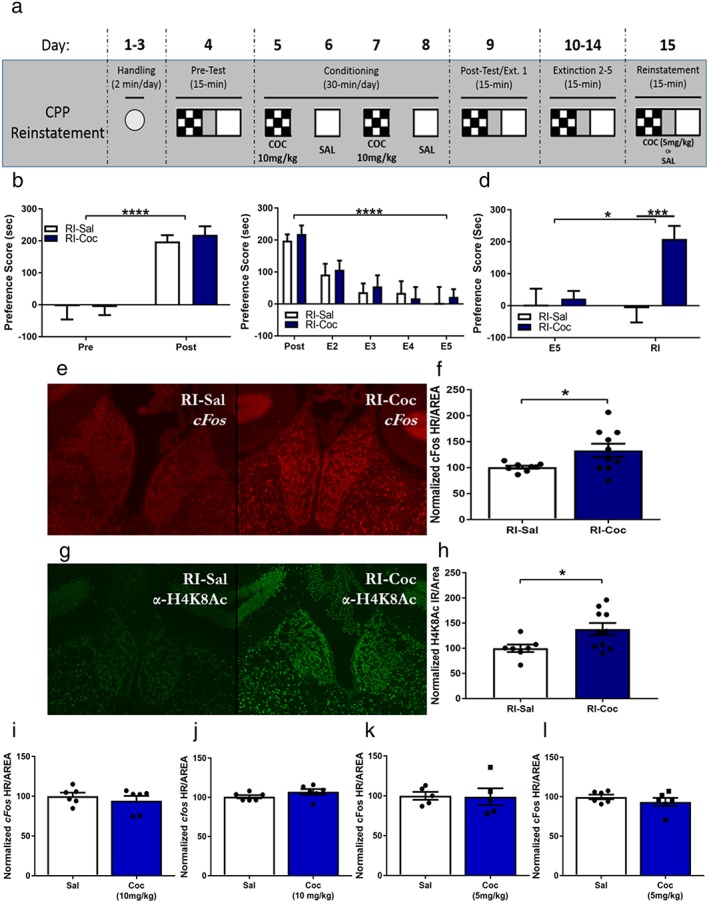
The medial habenula (MHb) is engaged by cocaine‐primed reinstatement of conditioned place preference (CPP). (a) Cocaine‐induced CPP cocaine‐primed reinstatement paradigm. (b) Wild‐type mice acquire cocaine‐induced CPP (2‐way ANOVA, main effect of conditioning: F 1,18 = 56.15, p < 0.0001), with no differences between saline‐primed (n = 9) and cocaine‐primed animals (n = 11) (2‐way ANOVA, no main effect of reinstatement priming: F 1,18 = 0.0958, p = 0.7604). (c) Conditioned place preference can be extinguished with repeated drug‐free exposures to conditioning apparatus (2‐way ANOVA, main effect of extinction: F 4,72 = 17.99, p < 0.0001); saline‐primed and cocaine‐primed animals extinguish equally (no main effect of reinstatement priming, F 1,18 = 0.1222, p = 0.7308). (d) Cocaine‐primed animals significantly reinstate previously extinguished cocaine‐induced CPP compared with saline‐primed controls (2‐way ANOVA, main effect of cocaine priming: F 1,18 = 7.359, p = 0.0143; main effect of reinstatement session: F 1,18 = 5.814, p = 0.0268; effect of Interaction F 1,18 = 7.431, p = 0.0139). Sidak's post‐hoc analysis indicates that cocaine‐primed animals have a significantly increased preference during reinstatement test compared with final extinction session (t 15 = 4.204, p = 0.0015) and reinstatement of saline‐primed controls (t 30 = 3.829, p = 0.0025). (e) Representative images of FISH against cFos in the MHb of animal reinstated with (left) saline and reinstated with (right) 5‐mg/kg cocaine‐HCl. (f) Cocaine‐reinstated animals (n = 10) show a significant increase in cFos hybridization reactivity in the MHb compared with saline‐primed (n = 8) controls (Welch's corrected 2‐tailed t‐test t 9.922 = 2.467, p = 0.0334). (g) Representative images of IHC against H4K8Ac in the MHb of (left) saline‐reinstated animal and (right) cocaine‐HCl reinstated animal. (h) Cocaine‐reinstated animals (n = 10) show a significant increase in H4K8Ac immunoreactivity in the MHb compared with saline‐primed (n = 7) controls (2‐tailed t‐test t 15 = 2.407, p = 0.0294). (i) A single cocaine (n = 6, 10 mg/kg) CPP conditioning session does not alter cFos expression in the MHb compared with saline‐paired controls (n = 6, equal w/v) (t 10 = 0.7891, p = 0.4484). (j) An acute dose of cocaine (n = 6, 10 mg/kg) alone does not alter cFos expression in the MHb compared with saline‐injected controls (n = 6, equal w/v) (t 10 = 1.5, p = 0.1645). (k) A single cocaine (n = 5, 5 mg/kg) CPP conditioning session does not alter cFos expression in the MHb compared with saline‐paired controls (n = 5, equal w/v) (t 8 = 0.09397, p = 0.9274). (l) An acute dose of cocaine (n = 6, 5 mg/kg) alone does not alter cFos expression in the MHb compared with saline‐injected controls (n = 6, equal w/v) (t 10 = 1.061, p = 0.3137) (*p ≤ 0.05, ***p ≤ 0.001)
Chemogenetic activation of the cholinergic MHb population reinstates place preference
To establish a causal link between MHb activity and reinstatement of cocaine‐induced CPP, we employed a chemogenetic approach to selectively activate the MHb cell populations during reinstatement. DIO‐HM3D(Gq)‐mCherry was infused bilaterally in the MHb of ChAT‐IRES‐CreCre/+ knock‐in mice (called ChAT‐Cre mice). ChAT‐IRES‐Cre animals have been shown to effectively limit expression of Cre‐dependent (DIO) constructs within cholinergic cell populations, particularly in the MHb (Fig. 2a) (Harris et al. 2014). First, we examined whether engagement of Gq‐coupled signaling increases firing of MHb ChAT expressing neurons. DIO‐HM3D was injected bilaterally in the MHb of ChAT‐Cre mice and recordings made with loose seal (110–250 MΩ) clamps from labeled neurons in coronal slices through the MHb prepared from mice injected at least 4 weeks previously (Choi et al. 2016). Recording was continued until a stable, 10‐minute baseline rate (0.5 to 9.0 Hz) of spontaneous spiking was collected. CNO (10 μM) was then infused for an additional 10 minutes. The agonist increased firing frequency within 1–2 minutes of application in all cells examined (Fig. 2b–d, representative trace Fig. 2e). The mean increase in rate was 110.2 ± 39.5 percent (Fig. 2b & c), with the largest percent effects occurring in cells with an initially low spiking rate (Fig. 2d), demonstrating that HM3D‐mCherry expressing MHb ChAT neurons can be activated chemogenetically, in a CNO‐dependent manner.
Figure 2.
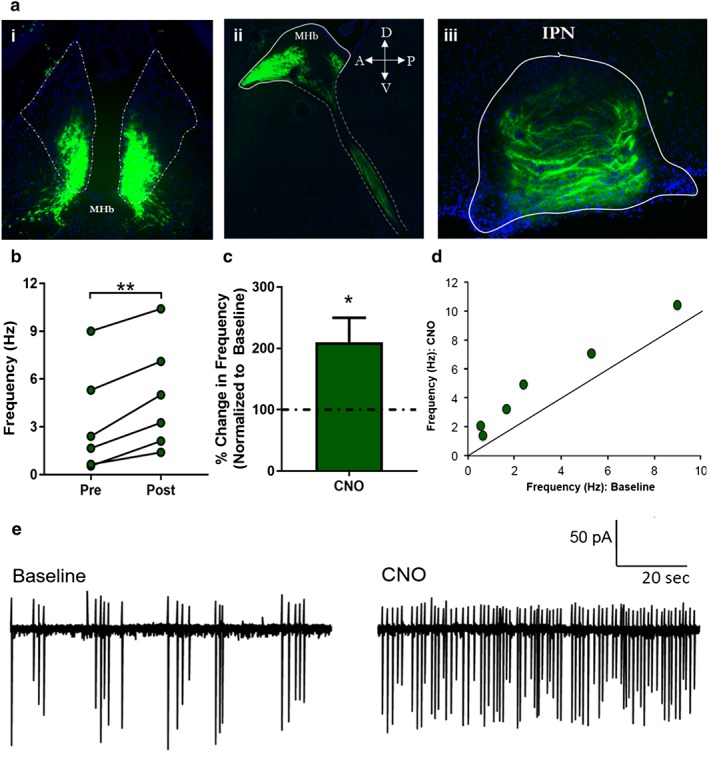
Combinatorial approach using Cre‐dependent DREADD in Cre‐driver line. (a) Immunohistochemistry against DIO‐HM3D(Gq)‐mCherry: (i) coronal and (ii) sagittal sections show limited expression of mCherry‐tagged HM3D DREADD to MHbChAT population. (iii) Coronal section of interpeduncular nucleus (IPN) shows expression of HM3D DREADD in axon terminals in the IPN, known to receive dense innervation from the cholinergic population of the vMHb. (b) Mean firing rate during baseline and following CNO (10 μM) infusion. HM3D‐mCherry expressing MHb neurons (n = 6) showed a 110.2 ± 39.5 percent increase in firing rate following CNO application (paired t‐test, t 5 = 6.574, p = 0.0012). (c) When normalized to baseline, HM3D‐mCherry expressing MHb neurons showed a significant increase in percent change in firing rate (t 5 = 2.787, p = 0.0386). (d) Frequency of spiking for HM3D‐mCherry expressing MHb cells during infusion, as a function of baseline values. (e) Loose seal recordings from representative fluorescent MHb neuron prior to baseline and 2 minutes following CNO (10 μM) infusion (*p ≤ 0.05, **p ≤ 0.01)
To determine if chemogenetic activation of MHb ChAT expressing neurons is sufficient for reinstatement of cocaine‐induced CPP, we infused DIO‐HM3D bilaterally into the MHb of ChAT‐Cre mice. HM3D‐infused ChAT‐Cre animals underwent CPP conditioning and extinction as previously described (Fig. 3a). HM3D‐infused animals both acquired (Fig. 3b) and extinguished (Fig. 3c) a cocaine‐induced CPP, with no differences between CNO‐reinstated and Veh‐reinstated animals. To test whether activity within MHb ChAT expressing neurons is sufficient to drive reinstatement of a cocaine‐induced CPP, we administered CNO or vehicle 40 minutes prior to a saline‐primed reinstatement session. We found that CNO‐primed animals had a significantly increased preference score compared with Veh‐primed controls during the reinstatement test (Fig. 3d). We did not observe significant changes in locomotion during posttest or CNO‐primed reinstatement (Supporting Information 2C & 2D). Additionally, we did not find Sex‐dependent effects on CPP acquisition between male mice (n = 8) and female mice (n = 9) (2‐way ANOVA, no main effect of sex, F 1,15 = 0.5065, p = 0.4876; no sex‐conditioning interaction, F 1,15 = 1.172, p = 0.2961) nor sex‐dependent effects on extinction (no main effect of sex, F 1,15 = 0.01755, p = 0.8964). Although there was a sex‐extinction interaction (F 5,75 = 2.734, p = 0.0253), Sidak's post‐hoc analysis does not indicate any significant differences between sexes across extinction trials. Lastly, our initial analyses did not find a sex‐dependent effect on CNO‐induced reinstatement between male mice (n = 6) and female mice (n = 3) (no sex‐reinstatement interaction, F 1,7 = 3.242, p = 0.1148; no main effect of sex, F 1,7 = 0.5496, p = 0.4836) (data not shown). Thus, chemogenetic activation of MHb ChAT expressing neurons artificially induces reinstatement behavior in previously cocaine‐conditioned animals, even in the absence of cocaine, demonstrating that the cholinergic signaling in the MHb mediates behavioral effects of cocaine‐primed reinstatement.
Figure 3.
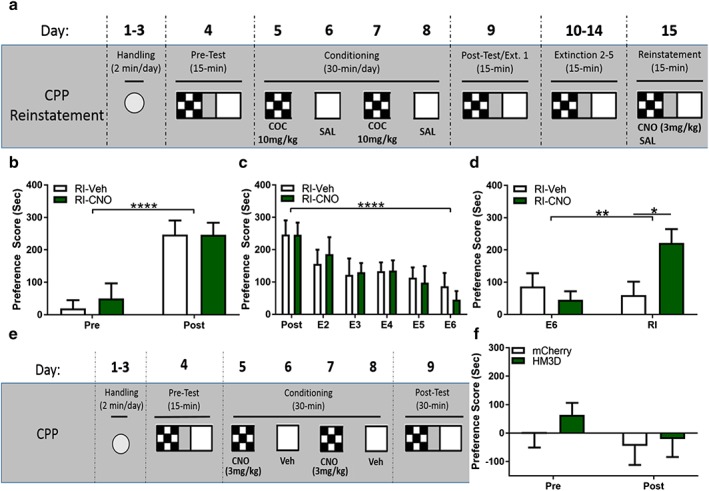
Chemogenetic activation of MHbChAT artificially induces reinstatement of conditioned place preference (CPP). (a) Cocaine‐induced CPP CNO‐primed reinstatement paradigm. (b) ChAT‐Cre HM3D infused mice acquire cocaine‐induced CPP (2‐way ANOVA, main effect of conditioning, F 1,15 = 37.52, p < 0.0001), with no differences between CNO‐primed (n = 9) and Veh‐primed (n = 8) animals (no main effect of CNO‐priming F 1,15 = 0.1159, p = 0.7383). (c) Conditioned place preference in HM3D‐infused animals is subsequently extinguished with repeated drug‐free exposures to chamber (2‐way ANOVA, main effect of extinction, F 5,75 = 9.002, p < 0.0001). Both CNO‐primed and Veh‐primed animals extinguish CPP equally (no main effect of CNO priming, F 1,15 = 0.0055152, p = 0.9437). (d) CNO‐primed animals significantly reinstate previously extinguished cocaine‐induced CPP (2‐way ANOVA, interaction between CNO priming and reinstatement session, F 1,15 = 16.02, p = 0.0012; main effect of Reinstatement session, F 1,15 = 8.721, p = 0.0099). Sidak's post‐hoc analysis reveals a significant increase in preference score during reinstatement session in CNO‐primed animals compared with final extinction session (t 15 = 5.07, p = 0.0003) and compared with reinstatement of Veh‐primed animals (t 30 = 2.988, p = 0.0111). (e) CNO‐induced CPP paradigm. (f) CNO alone is unable to establish a conditioned place preference or aversion in either mCherry‐infused (n = 6) or HM3D‐infused (n = 6) animals (no main effect of CNO conditioning, F 1,10 = 3.749, p = 0.0816; no main effect of DREADD, F 1,10 = 0.3974, p = 0.5426; no interaction, F 1,10 = 0.431, p = 0.5263). Although there may be a trend of CNO conditioning, Sidak's post‐hoc analysis shows no significant difference in change in preference score of mCherry‐infused animals (t 10 = 0.9049, p = 0.6240) or HM3D‐infused animals (t 10 = 1.833, p = 0.1839). (*p ≤ 0.05, **p ≤ 0.01, ****p ≤ 0.0001)
Activation of cholinergic MHb neurons does not induce CPP or CPA
We have demonstrated that MHb cholinergic neurons are sufficient for inducing reinstatement of cocaine‐induced CPP, mimicking the drug‐primed phenotype. However, how activity in the vMHb, specifically the cholinergic population, affects reward processing remains unclear. Thus, we tested if chemogenetic activation of the MHb ChAT expressing neurons alone is able to induce a conditioned preference or aversion. ChAT‐IRES‐Cre animals were infused bilaterally with either DIO‐mCherry or DIO‐HM3D in the MHb. The animals were subsequently handled and underwent an adapted CNO‐primed CPP paradigm where they received alternating conditioned pairings of Veh (0.5 percent DMSO, 0.9 percent saline; i.p.) or CNO (3 mg/kg CNO, 0.5 percent DMSO, 0.9 percent saline; i.p.) 40 minutes prior to exposure to conditioning chambers (Fig. 3e). Twenty‐four hours following final conditioning session, the animals were tested for preference for or aversion to CNO‐paired chamber, in a CNO‐free state. We found no effect of CNO‐conditioning in either DIO‐mCherry or DIO‐HM3D animals during posttest, and we found no effect on locomotion during posttest (Fig. 3f & Supporting Information 2E). These data suggest that activation of the MHb is unable to induce a conditioned preference or aversion.
CNO administration does not induce reinstatement behavior in DREADD‐free mice
Recent work has further characterized the pharmacological mechanism by which chemogenetic manipulations, specifically DREADDs, function. Others have demonstrated that CNO alone has no effect on reinstatement behaviors or psychostimulant self‐administration (Ferguson et al. 2011; Mahler et al. 2014; Scofield et al. 2015; Augur et al. 2016; Kerstetter et al. 2016). However, Gomez et al. implicate clozapine as the specific ligand to DREADDs and its back metabolism from CNO to engage DREADD‐mediated changes in cell function (2017). Due to the potential psychoactive effects of clozapine, we next tested if the chemogenetic‐induced reinstatement (Fig. 3d) and changes to MHbACh firing rate (Fig. 2b–d) were due to engagement of the expressed DREADD receptor or an off‐target effect of CNO exposure. We infused DIO‐mCherry in the MHb of ChAT‐Cre mice. DIO‐mCherry‐infused animals subsequently underwent CPP acquisition and extinction as previously described (Fig. 4a). mCherry‐infused animals both acquired (Fig. 4b) and extinguished (Fig. 4c) a cocaine‐induced CPP, with no differences between CNO‐reinstated and Veh‐reinstated animals. To test whether CNO is sufficient to drive reinstatement of a cocaine‐induced CPP in DREADD‐free animals, we administered CNO or vehicle 40 minutes prior to a saline‐primed reinstatement session. We found no difference between CNO‐primed and Veh‐primed controls during reinstatement test (Fig. 4d). We further demonstrate that CNO alone has no effect on firing rate of DREADD‐free MHb‐ChAT neurons. CNO (10 μM) was infused in MHb containing slices of DIO‐mCherry infused animals with no effect on firing rate in mCherry‐expressing MHb‐ChAT neurons (Fig. 4e & f), demonstrating that effects on firing and reinstatement behavior occur in a CNO/DREADD combinatorial fashion.
Figure 4.
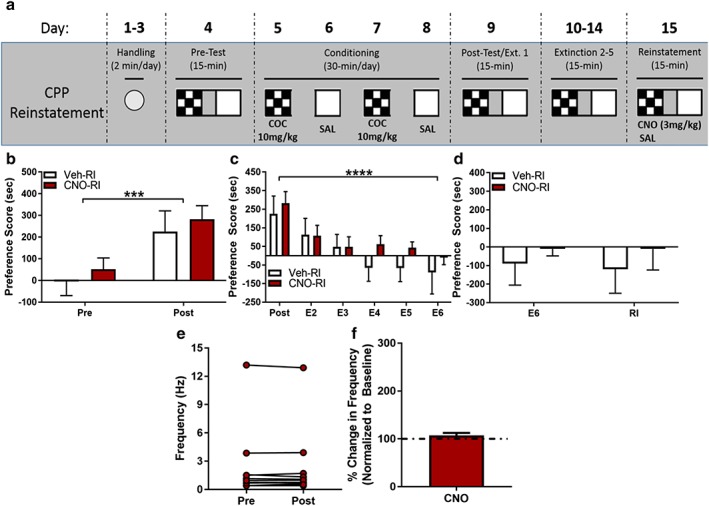
CNO priming does not induce reinstatement of conditioned place preference (CPP) in DREADD‐free animals. (a) Cocaine‐induced CPP CNO‐primed reinstatement paradigm. (b) ChAT‐Cre DIO‐mCherry‐infused mice acquire cocaine‐induced CPP (2‐way ANOVA, main effect of conditioning, F 1,9 = 30.56, p = 0.0004), with no differences between CNO‐primed (n = 6) and Veh‐primed (n = 5) animals (no effect main effect of CNO‐priming (F 1,9 = 0.4273, p = 0.5297). (c) Conditioned place preference in mCherry‐infused animals is subsequently extinguished with repeated drug‐free exposures to chamber (2‐way ANOVA, main effect of extinction, F 5,45 = 10.77, p < 0.0001). Both CNO‐primed and Veh‐primed animals extinguish CPP equally (no main effect of CNO‐priming, F 1,9 = 0.7064, p = 0.4224). (d) CNO‐primed animals show no significant reinstatement of previously extinguished cocaine‐induced CPP compared with Veh‐primed controls (2‐way ANOVA, no main effect of reinstatement, F 1,9 = 0.06347, p = 0.8068; no main effect of CNO priming, F 1,9 = 0.5076, p = 0.4942). (e) Mean firing rate during baseline and following CNO (10 μM) infusion. mCherry expressing MHb neurons (n = 11) show no significant change in firing rate following CNO application (paired t‐test, t 10 = 0.1149, p = 0.9108). (f) When normalized to baseline, mCherry expressing MHb neurons show no significant increase in percent change in firing rate (t 10 = 1.382, p = 0.1971). (***p ≤ 0.001, ****p ≤ 0.0001).
Discussion
In this study, we show that the MHb is specifically engaged by cocaine‐primed reinstatement (as measured by increases in cFos expression and H4K8Ac). The MHb did not appear to respond to either acute exposure to cocaine or during the consolidation phase of cocaine‐induced CPP. These data suggest that the MHb may be insensitive to early cocaine exposures but is specifically engaged during the CPP‐reinstatement process. In this study, we do not control for the specific drug history of these animals. More specifically, we do not definitively demonstrate that the MHb is engaged only during reinstatement and not reexposure to cocaine following forced withdrawal. However, using chemogenetics, we more causally linked activity within the MHb with changes in reinstatement behavior. DREADD‐mediated activation of MHb ChAT expressing neurons artificially induces reinstatement for a previous cocaine‐induced CPP, demonstrating that vMHb activity is sufficient for inducing reinstatement, even in the absence of cocaine.
We further demonstrate that chemogenetic activation of the vMHb is unable to induce a preference or aversion alone. Others found that modulating cholinergic signaling in nicotine‐naïve animals has no effect in mediating anxiety‐like behaviors, supporting the notion that neuroplastic adaptations within the vMHb pathway are altered and recruited by repeated exposure to drugs of abuse to drive reward‐associated and reward‐seeking behaviors. Conversely, previous work has shown that artificial activation of the dMHb is reinforcing and is able to induce intracranial self‐stimulation; meanwhile, optogenetic inhibition of the dMHb elicits a conditioned place aversion (Hsu et al. 2014). Together, these results highlight the unique roles of MHb subcircuits within the MHb‐IPN axis that should be further studied.
Although we selectively modulate cholinergic neurons of the MHb, this cell population has been previously shown to release glutamate and/or acetylcholine (Qin & Luo 2009; Ren et al. 2011; Aizawa et al. 2012). Therefore, it remains unclear which neurotransmitter system is responsible for driving the reinstatement phenotype. Recently, it has been demonstrated that MHb firing rate alters the neurotransmitter system used by the vMHb; tonic firing appears to engage glutamatergic transmission, while high‐rate phasic firing drives acetylcholine release (Ren et al. 2011). Given the significant increase in firing induced by CNO exposure in HM3D‐expressing MHb cells (Fig. 2d), it is likely that acetylcholine is being utilized in vivo to induce reinstatement behavior. Studies have linked vMHb‐IPN circuit activity with changes in median raphe nuclei and ventral tegmental function; downregulation of cholinergic signaling in the vMHb leads to a depressive‐like phenotype correlated with increase in serotonergic function from the median raphe, whereas increases in MHb signaling activate ventral tegmental dopaminergic neurons and suppress raphe serotonin (Han et al. 2017). It is possible that similar changes to cholinergic signaling within these pathways occur during cocaine‐primed reinstatement.
Previous work in the field has implicated the ChAT‐expressing MHb population in processing the aversive properties of nicotine and the negative affect throughout nicotine withdrawal (Salas et al. 2009; Velasquez et al. 2014). Work from Fowler and Kenny demonstrated that α5‐KO (α5‐expressing nAChRs are enriched in the vMHb‐IPN pathway) mice show escalations of nicotine self‐administration through a blunting of the aversive properties of nicotine and not an increase in the rewarding aspects of the drug (Fowler & Lu Qun 2011; Tuesta et al. 2011). Conversely, pharmacological antagonism of β4‐expressing nAChR (also enriched in the vMHb‐IPN pathway) inhibits the self‐administration of morphine and cocaine and blocks the formation of cocaine CPP (Glick et al. 2006; McCallum & Glick 2009; Khroyan et al. 2015). It is likely that a balance between α5 and β4 subunits is more influential in regulating drug response than a single subunit (Frahm et al. 2011; Harrington et al. 2015). Yet, how cocaine and nicotine exposure differentially affect MHb activity remains a key open question. Medial habenula activity may regulate the rewarding properties of other drugs of abuse, while encoding for the aversive properties of nicotine. Conversely, due to the population of nAChR in the MHb, but absence of known cocaine targets (dopamine transporter, serotonin transporter and norepinephrine transporter), it is possible that nicotine can directly modulate MHb activity, while other drugs of abuse, such as cocaine, engage the adaptive behaviors with which MHb function has been associated.
Recent work has brought into question the exclusivity of CNO as the ligand for DREADDs and demonstrated that rodents are capable of CNO to clozapine back metabolism (Gomez et al. 2017). While it has been shown that CNO is not significantly back metabolized to clozapine in mice, it is possible that the limited clozapine produced is sufficient to induce DREADD activity reported in Gomez et al., while being subthreshold for off‐target effects (Guettier et al. 2009). Moreover, while it has been demonstrated that systemic clozapine is able to induce changes in neural activity, these changes occur in response to significantly higher doses (35 mg/kg) than what could be back metabolized from our doses of CNO (3 mg/kg) (Werme et al. 2000). Within this current study, the inability of CNO alone to induce a conditioned preference/aversion (Fig. 3e), induce reinstatement (Fig. 4d) or induce changes in MHb firing rate (Fig. 4e & f) supports the idea that it is not CNO alone, or potential back metabolism to clozapine, that is mediating the observed effects during reinstatement.
How the MHb is recruited to regulate cocaine‐reinstatement behaviors remains unclear, especially considering that the main targets of cocaine action (dopamine transporter, norepinephrine transporter and serotonin transporter) are largely absent within the MHb. Recent work characterized the downstream targets of the MHb‐IPN pathway and show that serotonergic raphe nuclei receive dense innervation from vMHb‐innervated IPN neurons (Quina et al. 2017). The MHb also densely expresses the 5‐HT3A, ‐4, ‐5A and ‐5B receptors, suggesting that it receives serotonergic inputs, potentially from the same raphe‐serotonergic population receiving innervation from the IPN (Filip et al. 2004; Ichikawa et al. 2005; Wagner et al. 2014). It is possible that these inputs may be directly, but differentially, modulated by cocaine and nicotine.
The current study demonstrates a specific function of the vMHb in the reinstatement of cocaine‐induced CPP and adds to the growing body of work implicating the MHb in substance use disorders. The most relevant study with regard to cocaine is from James et al. (2011), which demonstrated changes in MHb activity during cue‐primed reinstatement of cocaine self‐administration (James et al. 2011). Medial habenula activity was indirectly measured by using Fos protein expression (as in our study) from rats following reinstatement. In support of this approach (measuring Fos expression), James et al. (2011) found high‐reinstating animals exhibited higher Fos expression in the MHb as compared with low‐reinstating animals. There remain numerous key open questions regarding the role of the MHb in reinstatement. How are specific molecular mechanisms engaged within the MHb (such as the increases in H4K8Ac shown in Fig. 1g & h), and how do they contribute to drug‐associated behaviors? As the MHb is a key regulator of withdrawal and relapse‐like behaviors, do long‐lasting molecular adaptations (such as epigenetic modifications) within the MHb confer the persistence and resilience of drug‐associated memories and drug‐seeking behavior?
Supporting information
Data S1. Supporting info item
Acknowledgements
This work was supported by the National Institute on Drug Abuse (DA025922 to M.A.W. and DA041838 to A.J.L.), the National Institute of Mental Health (MH101491 to M.A.W. and G.L., and support to A.J.L.), the National Institute of Neurological Disorders and Stroke (NS085709 to G.L. and NS105217 to A.J.L.) and National Institute of General Medical Sciences Minority Biomedical Research Support Initiative for Maximizing Student Development (MBRS‐IMSD) (GM055246 to A.J.L.). Special thanks to Dr Christie D. Fowler of UC Irvine for providing critical input on project. Special thanks to Dr John Guzowski of UC Irvine for use of Olympus Slide Scanner VSBX61.
Authors Contribution
AJL, GL and MAW were responsible for the study concept and design; AJL, AOW, JLK, ME, PH, RC, OC and YA performed the behavioral experiments; AJL and YJ conducted the electrophysiological experiments; AJL and DPM conducted the molecular experiments; AJL and YJ analyzed the data; AJL drafted the manuscript; MAW and GL provided critical revision of the manuscript.
López, A. J. , Jia, Y. , White, A. O. , Kwapis, J. L. , Espinoza, M. , Hwang, P. , Campbell, R. , Alaghband, Y. , Chitnis, O. , Matheos, D. P. , Lynch, G. , and Wood, M. A. (2019) Medial habenula cholinergic signaling regulates cocaine‐associated relapse‐like behavior. Addiction Biology, 24: 403–413. 10.1111/adb.12605.
References
- Aizawa H, Kobayashi M, Tanaka S, Fukai T, Okamoto H (2012) Molecular characterization of the subnuclei in rat habenula. J Comp Neurol 520:4051–4066. [DOI] [PubMed] [Google Scholar]
- Antolin‐Fontes B, Ables JL, Görlich A, Ibañez‐Tallon I (2014) The habenulo‐interpeduncular pathway in nicotine aversion and withdrawal. Neuropharmacology 96(Pt B):213–222 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augur IF, Wyckoff AR, Aston‐Jones G, Kalivas PW, Peters J (2016) Chemogenetic activation of an extinction neural circuit reduces cue‐induced reinstatement of cocaine seeking. J Neurosci 36:10174–10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ (2016) In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci 113:2726–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Lee Y, Lee C, Hong S, Lee S (2016) Optogenetic activation of septal GABAergic afferents entrains neuronal firing in the medial habenula. Nat Publ Gr 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio Cuello A, Emson PC, Paxinos G, Jessell T (1978) Substance P containing and cholinergic projections from the habenula. Brain Res 149:413–429. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y, Roth BL, Neumaier JF (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip M, Bubar M, Cunningham K (2004) Contribution of serotonin (5‐hydroxytryptamine; 5‐HT) 5‐HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J Pharmacol 310:1246–1254. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ (2012) Habenular signaling in nicotine reinforcement. Neuropsychopharmacology 37:306–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Qun JPMMJ, KPJ (2011) Habenular Alpha5 nACh receptor signalling controls nicotine intake. Nature 471:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm S, Ślimak MA, Ferrarese L, Santos‐Torres J, Antolin‐Fontes B, Auer S, Filkin S, Pons S, Fontaine JF, Tsetlin V, Maskos U, Ibañez‐Tallon I (2011) Aversion to nicotine is regulated by the balanced activity of β4 and α5 nicotinic receptor subunits in the medial habenula. Neuron 70:522–535. [DOI] [PubMed] [Google Scholar]
- Glick SD, Ramirez RL, Livi JM, Maisonneuve IM (2006) 18‐Methoxycoronaridine acts in the medial habenula and/or interpeduncular nucleus to decrease morphine self‐administration in rats. Eur J Pharmacol 537:94–98. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa‐shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M (2017) Chemogenetics revealed: dreadd occupancy and activation via converted clozapine. Science 507:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier J‐M, Gautam D, Scarselli M, de Azua IR, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, Roth BL, Wess J (2009) A chemical‐genetic approach to study G protein regulation of cell function in vivo. Proc Natl Acad Sci 106:19197–19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yang SH, Kim JY, Mo S, Yang E, Song KM, Ham B‐J, Mechawar N, Turecki G, Lee HW, Kim H (2017) Down‐regulation of cholinergic signaling in the habenula induces anhedonia‐like behavior. Sci Rep 7:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, Viñals X, Herrera‐Solís A, Flores A, Morel C, Tolu S, Faure P, Maldonado R, Maskos U & Robledo P (2015) Role of β4* nicotinic acetylcholine receptors in the habenulo‐interpeduncular pathway in nicotine reinforcement in mice. NeuropsychopharmacolEPUB ahead:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, Smith KA, Dang C, Sunkin S, Bernard A, Oh SW, Madisen L, Zeng H (2014) Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits 8:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain RJ, Taraschenko OD, Glick SD (2008) Effects of nicotine, methamphetamine and cocaine on extracellular levels of acetylcholine in the interpeduncular nucleus of rats. Neurosci Lett 440:270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa M, Okamura‐Oho Y, Okunishi R, Kanamori M, Suzuki H, Ritani A, Nitta H, Eguchi N, Urade Y, Hayashizaki Y (2005) Expression analysis of genes responsible for serotonin signaling in the brain. Neurobiol Dis 19:378–385. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV (2011) Propensity to ‘relapse’ following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199:235–242. [DOI] [PubMed] [Google Scholar]
- Ji H, Shepard PD (2007) Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor‐mediated mechanism. J Neurosci 27:6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Wunsch AM, Nakata KG, Donckels E, Neumaier JF, Ferguson SM (2016) Corticostriatal afferents modulate responsiveness to psychostimulant drugs and drug‐associated stimuli. Neuropsychopharmacology 41:1128–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan TV, Yasuda D, Toll L, Polgar WE, Zaveri NT (2015) High affinity a3b4 nicotinic acetylcholine receptor ligands AT‐1001 and AT‐1012 attenuate cocaine‐induced conditioned place preference and behavioral sensitization in mice. Biochem Pharmacol 97:531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Mathuru AS, Teh C, Kibat C, Korzh V, Penney TB, Jesuthasan S (2010) The habenula prevents helpless behavior in larval zebrafish. Curr Biol 20:2211–2216. [DOI] [PubMed] [Google Scholar]
- Lima LB, Bueno D, Leite F, Souza S, Gonçalves L, Furigo IC, Donato J, Metzger M (2017) Afferent and efferent connections of the interpeduncular nucleus with special reference to circuits involving the habenula and raphe nuclei. J Comp Neurol 525:2411–2442. [DOI] [PubMed] [Google Scholar]
- Lopez AJ, Kramar E, Matheos DP, Kwapis JL, White AO, Vogel‐Ciernia A, Wood M (2015) Promoter specific effects of DREADD modulation on synaptic plasticity and hippocampal learning. J Neurosci 36:3588–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston‐Jones G (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, McQuown SC, G a R, Astarabadi M, Jacques V, Carreiro S, Rusche JR, M a W (2013) HDAC3‐selective inhibitor enhances extinction of cocaine‐seeking behavior in a persistent manner. Proc Natl Acad Sci U S A 110:2647–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Mhillaj E, Matheos DP, Palmery M, M a W (2011) CBP in the nucleus accumbens regulates cocaine‐induced histone acetylation and is critical for cocaine‐associated behaviors. J Neurosci 31:16941–16948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Cowe MA, Lewis SW, Glick SD (2012) alpha3beta4 nicotinic acetylcholine receptors in the medial habenula modulate the mesolimbic dopaminergic response to acute nicotine in vivo. Neuropharmacology 63:434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Glick SD (2009) 18‐Methoxycoronaridine blocks acquisition but enhances reinstatement of a cocaine place preference. Neurosci Lett 458:57–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Stewart J (2000) Cocaine‐induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res 115:39–47. [DOI] [PubMed] [Google Scholar]
- Qin C, Luo M (2009) Neurochemical phenotypes of the afferent and efferent projections of the mouse medial habenula. Neuroscience 161:827–837. [DOI] [PubMed] [Google Scholar]
- Quina LA, Harris J, Zeng H, Turner EE (2017) Specific connections of the interpeduncular subnuclei reveal distinct components of the habenulopeduncular pathway. J Comp Neurol 525:2632–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M (2011) Habenula ‘cholinergic’ neurons co‐release glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron 69:445–452. [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M (2009) Nicotinic receptors in the habenulo‐interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci 29:3014–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Boger HA, Smith RJ, Li H, Haydon PG, Kalivas PW (2015) Gq‐DREADD selectively initiates glial glutamate release and inhibits cue‐induced cocaine seeking. Biol Psychiatry 78:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD (2012) Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci 15:1105–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Chen Z, Duncan A, Fowler CD, Ishikawa M, Lee BR, Liu X‐A, Lu Q, Cameron M, Hayes MR, Kamenecka TM, Pletcher M, Kenny PJ (2017) GLP‐1 acts on habenular avoidance circuits to control nicotine intake. Nat Neurosci 20:708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ (2011) Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self‐administration behavior. Biochem Pharmacol 82:984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez KM, Molfese DL, Salas R (2014) The role of the habenula in drug addiction. Front Hum Neurosci 8:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner F, French L, Veh RW (2014) Transcriptomic‐anatomic analysis of the mouse habenula uncovers a high molecular heterogeneity among neurons in the lateral complex, while gene expression in the medial complex largely obeys subnuclear boundaries. Brain Struct Funct . [DOI] [PubMed] [Google Scholar]
- Wang DO, Matsuno H, Ikeda S, Nakamura A, Yanagisawa H, Hayashi Y, Okamoto A (2012) A quick and simple FISH protocol with hybridization‐sensitive fluorescent linear oligodeoxynucleotide probes. RNA 18:166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werme M, Ringholm A, Olson L, Brene S (2000) Differential patterns of induction of NGFI‐B, Nor1 and c‐fos mRNAs in striatal subregions by haloperidol and clozapine. Brain Res 863:112–119. [DOI] [PubMed] [Google Scholar]
- White AO, Kramár EA, López AJ, Kwapis JL, Doan J, Saldana D, Davatolhagh MF, Alaghband Y, Blurton‐Jones M, Matheos DP, Wood MA (2016) BDNF rescues BAF53b‐dependent synaptic plasticity and cocaine‐associated memory in the nucleus accumbens. Nat Commun 7:11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y‐W a, Wang SD, Wang S, Morton G, Zariwala H a, de la Iglesia HO, Turner EE (2014) Role of the dorsal medial habenula in the regulation of voluntary activity, motor function, hedonic state, and primary reinforcement. J Neurosci 34:11366–11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Tan L, Ren Y, Liang J, Lin R, Feng Q, Zhou J, Hu F, Ren J, Wei C, Yu T, Zhuang Y, Bettler B, Wang F, Luo M (2016) Presynaptic excitation via GABAB receptors in habenula cholinergic neurons regulates fear memory expression. Cell 166:716–728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting info item


