Abstract
Wilhelm His (1831–1904) provided lasting insights into the development of the central and peripheral nervous system using innovative technologies such as the microtome, which he invented. 150 years after his resurrection of the classical germ layer theory of Wolff, von Baer and Remak, his description of the developmental origin of cranial and spinal ganglia from a distinct cell population, now known as the neural crest, has stood the test of time and more recently sparked tremendous advances regarding the molecular development of these important cells. In addition to his 1868 treatise on ‘Zwischenstrang’ (now neural crest), his work on the development of the human hindbrain published in 1890 provided novel ideas that more than 100 years later form the basis for penetrating molecular investigations of the regionalization of the hindbrain neural tube and of the migration and differentiation of its constituent neuron populations. In the first part of this review we briefly summarize the major discoveries of Wilhelm His and his impact on the field of embryology. In the second part we relate His´ observations to current knowledge about the molecular underpinnings of hindbrain development and evolution. We conclude with the proposition, present already in rudimentary form in the writings of His, that a primordial spinal cord-like organization has been molecularly supplemented to generate hindbrain ‘neomorphs’ such as the cerebellum and the auditory and vestibular nuclei and their associated afferents and sensory organs.
Introduction
Wilhelm His Sr. (1831–1904) combined, in an unprecedented way, technical innovation [he invented the microtome for thin sections (His 1870a)], detailed anatomical insights [his major histological study of brain and body development (His 1880) is among the most cited papers from this era], and comprehensive intellectual assessment of the implications of these insights [the latter is best exemplified by his exceptional lecture on the importance of embryology for biology in general (His 1870b)] and by the introduction of a revised anatomical nomenclature, which he spearheaded (His 1895). While his son, Wilhelm His Jr., became famous for identifying the His bundle in the heart, Wilhelm His Sr. will forever be connected with the development of the ‘Zwischenstrang’ [known now as the neural crest (His 1868)], and of the rhombencephalon (‘Rautenhirn’ in German), and the definition of distinct parts of the brain anlage in cross section [(floor plate, basal plate, alar plate, roof plate, and delimiting sulcus limitans of His (His 1890b; His 1890a)]. His careful work allowed him also to recognize longitudinal subdivisions of the rhombencephalon that were only much later confirmed as molecularly distinct entities, such as the the midbrain-hindbrain region (Fritzsch and Glover 2006) and the isthmus (His 1890a; His 1895) now known to be characterized by a unique pattern of transcription factor expression (Harada et al. 2015; Watson et al. 2017b).
His also developed substantial insight into the intimate relationship between embryonic development and evolution. He was instrumental in retaining information collected by his nephew, Friedrich Miescher, about the latter’s discovery of DNA [referred to as ‘nuclein’ by Miescher in a letter to His in 1869 (His 1897)]. His rejected Haeckel’s simplified scheme of embryology (ontogeny recapitulates phylogeny), preferring the more sophisticated insights of von Baer (Von Baer 1828) that were inspired by the earlier work of Wolff (Wolff 1768). The conceptual framework of Wolff/von Baer highlighted the notion that general characteristics of a phylum develop in an embryo before detailed species-specific characteristics. His studied under Remak in Berlin, and it was Remak who provided him with an appreciation of the work of Wolff and von Baer (Remak 1855). Remak also presented His with divergent opinions of Reichert’s view (Reichert 1837). Reichert rejected the germ-layer theory of Wolff whereas Remak and his embraced it.
Citing the developmental fate of gill slits in bony fish, amphibians and amniotes, His (His 1868; His 1870b) underscored the value of the von Baer/Wolff principle: pharyngeal slits can be transient embryological features in amniotes or permanent organs for breathing in bony fish or larval openings in amphibians without directly attached gills. Using this example, His hinted toward hidden developmental patterns highly conserved during phylogeny, with modifications leading to divergent adult structures and functions. This view was in line with Darwin’s ideas (first presented publicly in 1858) and contrasted sharply with ideas on the inheritance of acquired characters. Unfortunately, possibly due to their negative view of Haeckel’s postulate that development simply recapitulates evolution, neither His nor Miescher fully appreciated Haeckel’s insight that the nuclei carry information from one generation to the next (Haeckel 1866). This underappreciation delayed an understanding of the importance of ‘nuclein’ (later renamed nucleic acid) for the ‘inheritance of developmental principles‘ (now understood as the vertical transfer of genetic information) (Dahm 2008).
It is important to realize the influence His had on contemporaries, exercised through his technical advancements and by maintaining continuity with earlier insights into the germ layer theory of embryonic development (His 1868; His 1870b). His conclusions regarding the derivatives of the ‘Zwischenstrang’ proved to be mostly accurate (trigeminal, glossopharyngeal, vagus, ciliary ganglia) but in certain cases not (he concluded that the ear anlage aligned with the ‘Zwischenstrang’ and was thus derived from it). Most notably, he pointed out early on that the geniculate ganglion of cranial nerve VII does not derive from the ‘Zwischenstrang’, thus indicating a different developmental origin for what he referred to as ‘visceral ganglia‘. It was only later that the true origins of such ganglia as well as of the ear anlage were identified as placodal (Von Kupffer 1891). A more detailed account of the development and history of neural crest and placodes has recently been provided and the reader is referred to this review for more details on the subject (O’Neill et al. 2012).
Our review will concentrate on the insights provided by His on the human hindbrain with an emphasis on its longitudinal regionalization and on the rhombic lip (‘Rautenlippe’). His defined specific longitudinal domains based on external and internal features, and identified the rhombic lip as the source of migratory cell populations giving rise to major dorsal and ventral nuclei including the pontine nuclei (‘Brueckenkerne‘) and the superior and inferior olives, as well as the origin of cochlear and vestibular nuclei. We will complement His’ account with examples of current molecular understanding of the development of these unique hindbrain features, cast in the context of an elaboration of a basal spinal cord-like organization. Our review extends an excellent previous review on the rhombic lip (Ray and Dymecki 2009) by providing this evolutionary context and by adding more recent molecular insights.
Overview of His’ observations on the hindbrain of human embryos
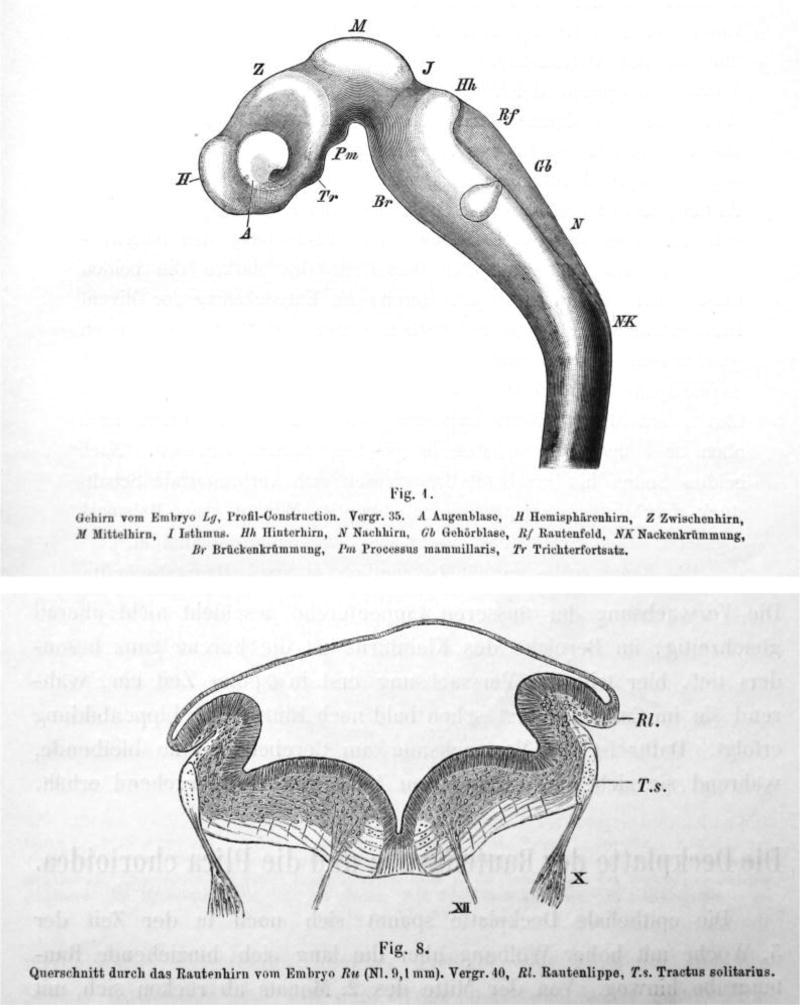
His was a member of the ‘Entwicklungsmechanik’ school, believing in a mechanical explanation of the development of brain structure. A major concern for him was to obtain a mechanistic understanding of changes in the shape and form of the brain by identifying and formally describing the underlying mechanical forces. His noted that the brain of amniotes, and in particular of humans, in contrast to the brains of fish and batrachians (an older taxonomic term for salamanders and frogs), undergoes significant shape changes that open up the 4th ventricle and generate the flexures of the pons, midbrain, and the hindbrain/spinal cord transition. His basic working hypothesis was that the neural tube behaves like a rubber tube cut (or weakened) dorsally and subsequently compressed ventrally to create these features. His description of the roof of the 4th ventricle and its shape, the appearance of the caudal part being similar to that of a quill pen (‘calamus scriptorius’), showed clearly his perception of the shape changes as consequences of external forces (Figure. 1). To His, the lateral recesses of the 4th ventricle were formed by the force of the pons pushing against the medulla oblongata in conjunction with a block to lateral expansion more caudally provided by the developing ear anlagen. Thus, the form of the ‘Rautenhirn’ emerged: a tube fully closed at the isthmus and spinal cord but with an increasingly wider gap in between, stretching the thin, overlying roof plate.
Fig 1.
(Showing His, 1890 Fig.1) Brain of embryo Lg, profile-construction, Mag 35, a, eye vesicle, H, forebrain hemisphere, Z, diencephalon; M, mesencephalon; I, isthmus; Hh, pons; N, medulla oblongata; Gb, ear vesicle; Rf, rhombencephalon; NK, spinal flexure; Br, pontine flexure; Pm, mammillary bodies; Tr; pituitary. Note the shaded area below RF that shows the choroid plexus (not labeled as such by His).
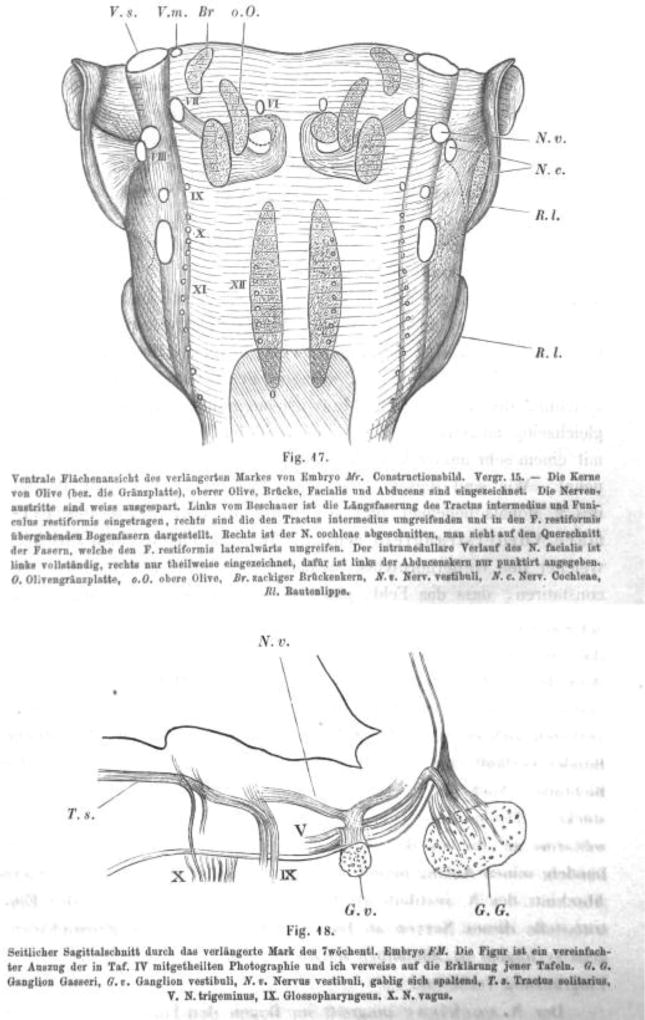
His noted that in transverse sections through the rhombencephalon of the human embryo the extremes of the alar plates, near their junctions with the roof plate, were set off by a longitudinal pial sulcus, forming what he called the ‘Rautenlippen‘, or rhombic lips (Figure 2). Much of his subsequent work focused on neuron populations that he believed were derived from the rhombic lips. His realized, based purely on histological evidence, that a number of hindbrain neuron populations were generated through tangential migrations of neuroblasts delaminating from the rhombic lips. His was the first to correctly identify these migratory cells as they traverse the first tract laid down in the rhombencephalon, the solitary tract (Figure 2). He also clearly noted the early formation of the internal facial genu and the fiber bundles looping around the abducens motor nucleus (Figure 3) now known to arise through the differential migration of facial branchial motor neuron populations in some vertebrates (Glasco et al. 2012; Yang et al. 2014). His also noted the developmental progression of the spinal dorsal root ganglion afferents and their eventual overlap with the trigeminal afferents (Figure 4), and postulated this as an indication of an evolutionary relationship between the spinal cord and hindbrain. Lacking physiological methods, he did not, however, realize the functional implications of this overlap as a link between similar sensory modalities in the spinal and hindbrain alar plates.
Fig. 2.
(Showing His, 1890 Fig. 8) Coronal section through the rhombencephalon of embryo Bm, Nl 0.1 mm, Mag 40, Rl rhombic lip; Ts, solitary tract.
Fig 3.
(Showing His, 1890; Fig. 17) Ventral view of the rhombencephalon of embryo Mr, image of a reconstruction, Ma 15 – The olivary nuclei (or pons), superior olive, pons, facial, abducens are shown). Nerve roots are shown in white. The left shows the intermediate tract and the restiform body, the right shows the arcuate fibers surrounding the intermediate and pass to the restiform body. The right cochlear nucleus is removed to show the fibers that surround laterally the restiform body. The intramedullary course of the facial nerve is completely shown on the left, but only partially on the right, but the abducens is only outlined by a dotted line. G, pons, oO superior olive, Br, dentate potine nucleus; Nv, vestibular nerve; Nc, cochlear nerve; Rl, rhombic lip.
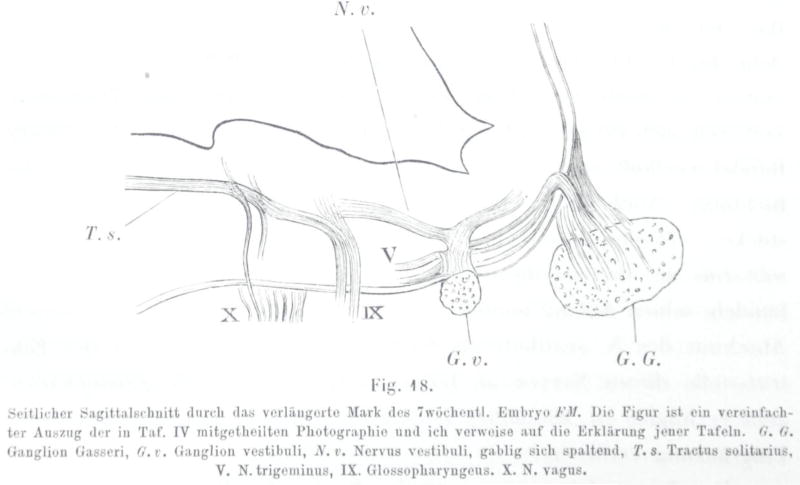
Fig. 4.
(Showing His, 1890; Fig. 18) Parasagittal section of the rhombencephalon of the 7 week old embryo FM. This Fig is a simplified excerpt of the photograph in plate IV and I refer to this plate for explanations. Gg, Gasserian ganglion, Gv vestibular ganglion, Nv, vestibular nerve, bifurcating; Ts, solitary tract; V, descending tract of V; IX Glossopharyngeus; X, vagus.
Following his early insights into the distinct origins of cranial and spinal sensory ganglia, His provided a detailed reconstruction of a ventral view of the human embryonic brainstem that highlighted all nerve fiber roots and many of their associated nuclei. His also drew some of the nuclei he postulated to be derived from the rhombic lip, such as the pontine and superior olivary nuclei (‘Brueckenkern’, ‘obere Olive’; Figure 4). The development of the auditory centers was of particular interest to him, as well as to his son (His Jr 1889). However, His Sr. did not consider the different auditory and vestibular subnuclei and relied on the more sophisticated work of Ramon y Cajal for insights into the central projections of auditory and vestibular afferents as they relate to these subnuclei and to the cerebellum. Although His noted that the above-mentioned nuclei, as well as the rhombic lip and the choroid plexus of the fourth ventricle, are features unique to the hindbrain, he did not speculate about their evolutionary significance. Later work by the American school of functional neuroanatomists identified some of these structures as mammalian or amniote novelties, and Herrick (Herrick 1948) referred to them as ‘neomorphs’ of the hindbrain. Below we will expand on this basic insight, rooted in Wilhelm His’ observations, and explore how these ‘neomorphic’ hindbrain nuclei may have formed as an elaboration of a basic spinal cord organization. We will put these neomorphic transformations into the context of the distinctive neuromeric and longitudinal organization of the hindbrain (Bermingham et al. 2001; Mishima et al. 2009; Ray and Dymecki 2009; Millen et al. 2014; Hernandez-Miranda et al. 2016; Fritzsch and Elliott 2017).
His’ definition of longitudinal hindbrain regions, with an emphasis on the sensory alar plate
His explicitly defined distinct longitudinal regions of the brainstem, in caudal to rostral sequence (Figure 1): a) the ‘Schaltstueck’ (an intermediate segment between the spinal cord proper and the tip of the calamus scriptorius where the rhombic lips start to diverge); b) the calamus scriptorius region containing the gracile and cuneate nuclei; c) the medulla oblongata (‘Nachhirn’); d) the pons (‘Bruecke’ or ‘Hinterhirn’) and e) the isthmus. Throughout this region, the roof plate (‘Deckplatte’) in his description is either a thin epithelial sheet that elaborates the choroid plexus of the fourth ventricle or contains parenchymal nervous tissue (within the isthmus and ‘Schaltstueck’ regions). His noted that the alar plate (‘Fluegelplatte’) and basal plate (‘Grundplatte’) develop into various partially identifiable nuclei. The left and right basal plates are connected by the diminutive midline floor plate (‘Bodenplatte’), essentially following the nomenclature of His’ teacher, Remak.
His noted that the cranial nerves entering the alar plate at different longitudinal levels formed distinct fascicles of sensory axons that in some cases were longitudinally continuous. His identified, correctly, that one of these fascicles, composed of the trigeminal, facial, glossopharyngeal and vagal nerve afferents, overlapped with the fascicle composed of ascending spinal afferents (Figure 3). He thus perceived the hindbrain alar plate as an elaboration of a basic spinal cord alar plate organization. However, due to the lack of suitable tracing techniques, he was not able to identify the vestibular, auditory and solitary nuclei and associated tracts as elements unique to the hindbrain. It remains unclear through his writing whether His was aware of the posthumously published work of Deiters that clearly showed the lateral vestibular nucleus, restiform body and cochlear nuclei at the entrance of cranial nerve VIII (Deiters 1865).
Thus, His described the hindbrain as being composed of longitudinal domains of different character, each containing specific sets of afferent inputs terminating in more or less defined central nuclei, with other nuclei established at specific sites along the longitudinal axis by migratory neuroblasts derived from the rhombic lip. He hinted at relationships linking afferent populations entering at different longitudinal levels including at spinal levels. Later work, mainly by the American school of neuroanatomists, placed the hindbrain sensory nuclei in their scheme of functional longitudinal columns following the ideas of Gaskell (Gaskell 1886). In this scheme, the solitary tract and nucleus were placed in the ‘special visceral’ afferent column and the vestibular and auditory nuclei in the ‘special somatic’ afferent column. Initially the use of visceral and somatic in this regard were meant to imply some degree of continuity with the general visceral and general somatic columns of the spinal cord. Herrick only later considered these as ‘neomorphs’ of the hindbrain and accorded them status as ‘special’ columns restricted to the rhombencephalon (Herrick 1948).
Current molecular understanding of the longitudinal regionalization of the hindbrain
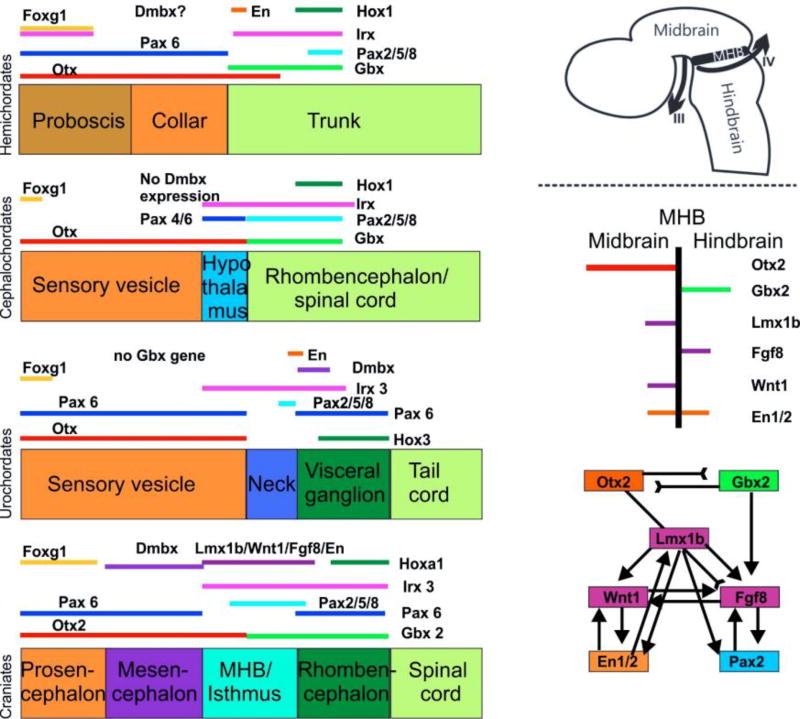
Whereas the definition of the rostrocaudal extent of the hindbrain is possible based on visible external morphological features in craniate vertebrates (sulcus isthmus and calamus scriptorius, and in mammals the pyramidal decussation), such landmarks are less obvious in chordate outgroups (lancelets and tunicates). Nevertheless, specific sequences of gene expression (Figure 5) have been linked to the development of some of these anatomical features, thus providing a means to assess phylogenetic relationships [see (Puelles and Ferran 2012) for an example of genoarchitectural mapping in the mouse brain]. This is particularly true for the midbrain/hindbrain boundary (MHB) and adjacent isthmus (Fritzsch and Glover 2006; Albuixech-Crespo et al. 2017). It has been clarified that adjacent, sequential and partially overlapping expression of a set of genes (Irx expression regulates expression of Otx and Gbx) initiates the formation of the MHB in craniates. Expression boundaries of Otx/Gbx regulate the differentiation of cell types within the isthmic and posterior midbrain compartments in a series of increasingly sophisticated interactions (Figure 5). For example, Lmx1b (Mishima et al. 2009; Millen et al. 2014) is needed to upregulate Fgf8 (Guo et al. 2007) as well as to stabilize the expression of Wnt1 (McMahon and Bradley 1990) which in turn is needed to maintain the expression of Fgf8 (Lee et al. 1997) which initiates the proliferation of specific neuronal progenitors such as those of the oculomotor and trochlear motor neurons (Fritzsch et al. 1995). This set of molecular interactions is only partly conserved in chordates (Fritzsch and Glover 2006; Albuixech-Crespo et al. 2017) and other deuterostomes (Figure 5). Neither the MHB as such can be identified in craniate outgroups nor do motor neurons form in the topographically equivalent area (Fritzsch 1996), supporting the notion that these motor neurons are craniate novelties [a notion that is also supported by their unique dependence on Phox2a, which evidently arose in vertebrate craniates through duplication of an ancestral Phox2 gene (Pattyn et al. 1997; Fritzsch et al. 2017)].
Fig. 5.
The evolution of gene expression at the midbrain–hindbrain boundary (MHB) is shown for deuterostomes. The MHB of vertebrates exhibits abutting domains of Otx2 and Gbx2 expression (d–g). This stabilizes the expression of Fgf8 (g), which in turn stabilizes the expression of Wnt1 and engrailed (En1). Mutation of Otx2, Gbx2, Fgf8, or Wnt1 eliminates the MHB. Pax2/5/8 are also expressed at the MHB, whereas the expression of Dmbx occurs immediately rostral to the MHB in the midbrain to later expand into the hindbrain and spinal cord (d). Note the partial overlap of Pax2/5/8 with the caudal expression of Otx2 and the rostral expression of Gbx2 (d). Hemichordates (a) have overlapping expression of Gbx, Otx, Irx and En in the rostral trunk. Pax6 abuts Gbx2 whereas Pax2/5/8 overlaps with the caudal expression of Gbx2. Outgroup data suggest that coelenterates have a Dmbx ortholog, thus raising the possibility that hemichordates (a) also have a Dmbx gene. Cephalochordates (b) have no Dmbx expression in the ‘brain’. The Otx expression domain abuts the Gbx expression domain, as in vertebrates. However, Gbx overlaps with Pax2/5/8 and most of Irx3. Urochordates (c) have no Gbx gene but have a Pax2/5/8 and Pax6 configuration comparable to vertebrates. Dmbx overlaps with the caudal end of the Irx3 expression whereas Dmbx expression is rostral to Irx3 in vertebrates. Together, these data show that certain gene expression domains are topographically conserved (Foxg1, Hox, Otx), whereas others show varying degrees of overlap. It is conceivable that the evolution of nested expression domains of transcription factors is causally related to the evolution of specific neuronal features such as the evolution of oculomotor and trochlear motoneurons (d, e) around the MHB. Experimental work has demonstrated that the development of these motor centers depends on the formation of the MHB. Modified after (Fritzsch and Glover 2006; Guo et al. 2007; Mishima et al. 2009; Fritzsch et al. 2015; Albuixech-Crespo et al. 2017))
At its caudal end, the transition of the hindbrain into the spinal cord is less clear since a sharp external morphological boundary is difficult to define unambiguously in a way that encompasses all vertebrate taxa. In lampreys there is a distinct internal transition from the hindbrain branchial motor neurons to the spinal somatic motor neurons (Fritzsch and Northcutt 1993), but this transition becomes obscured in jawed vertebrates due to what has been interpreted as a shift of the somatic hypoglossal neurons from the spinal cord to the hindbrain (Higashiyama et al. 2016). As a consequence, nearly nothing is known about the molecular underpinnings of the hindbrain/spinal cord boundary, although the expression domains of caudally expressed Hox genes (Tomás-Roca et al. 2016) and of key genes specific respectively to hindbrain branchial and parasympathetic visceral motor neurons (exemplified by Phox2b) and to spinal somatic and sympathetic visceral motor neurons [exemplified by Olig2 (Fritzsch et al. 2017)] afford a potential avenue by which to discover them. It has also been argued that the pyramidal decussation marks the hindbrain/spinal cord transition (Tomás-Roca et al. 2016), but this feature, present only in vertebrates with a well-developed motor cortex, provides little help in assessing phylogenetic relationships. Moreover, neither the pyramidal decussation nor the associated expression boundary of the caudally expressed Hoxb8 gene provide a sharp delineation between hindbrain and spinal neuron populations [although the possibility remains open that the expression boundaries of other Hox8 paralogues may do so (Tomás-Roca et al. 2016)].
Between these two extremes lie His’ calamus scriptorius region (containing the gracile and cuneate nuclei), ‘Nachhirn’ (medulla oblongata), and ‘Hinterhirn’ (pons), which he evidently distinguished in large part based on the shape and appearance of the 4th ventricle. Although His, having worked on chicken embryos, had almost certainly seen rhombomeres himself, he did not include these morphological features in his description of human hindbrain regions [rhombomeres are also visible in the human embryo; see (Atwell and Danchakoff 1930)]. We now know that the overt segmentation of the pontine and upper medullary (in some accounts termed “retropontine”) regions into rhombomeres, and the differential specification of their constituent neuron populations, is driven by a complex interaction of signaling systems and transcription factors in which retinoic acid, specific fibroblast growth factors (Fgf8a/b) and Hox genes play pivotal roles [reviewed in (Glover et al. 2006; Parker and Krumlauf 2017)]. A similar mechanism, albeit without the elements that instate physical segmentation, is likely to underly the specification of the cryptic pseudorhombomeres which continue in sequence from the last true rhombomere (rhombomere 6) to pseudorhombomere 11 (Cambronero and Puelles 2000; Tomás-Roca et al. 2016). Based on comparison of His’ drawings and contemporary studies of overt and cryptic hindbrain segmentation, the first rhombomere lies within or in close proximity to what His described as the isthmus, rhombomeres 2–4 correspond to the Hinterhirn, rhombomeres 5 and 6 to the upper part of the Nachhirn, and pseudorhombomeres 7–11 correspond to the lower part of the Nachhirn and the calamus scriptorius region. A recent study indicates that, in its modern definition, the isthmus is a domain separate and immediately rostral to the first rhombomere, and specified by the expression of Fgf8 (Watson et al. 2017b).
The Hinterhirn, or pons, is of particular interest, since recent work has shown that its traditional definition, based on the outward ventral bulge associated with the basilar pontine nuclei, represents only a superficial feature rather than a core division of the neural tube (Puelles and Ferran 2012; Nieuwenhuys and Puelles 2016). This is particularly misleading in humans, where the basilar pontine nuclei, as the main relay for information from the cortex to the cerebellum, are so well developed that they completely dominate the ventral surface of the upper hindbrain. For decades this has been assumed to indicate that the hindbrain parenchyma deep to the basilar pons constitutes a separate division of the hindbrain, but this is an illusion. Recent studies have shown that the pontine nuclei originate from the rhombic lips through a well-orchestrated migration, with the neurons eventually settling on the ventral surface of the hindbrain several rhombomeres more rostral (see the section on His’ postulated neuroblast migrations below). Moreover, supernumerary pontine nuclei, generated through genetic manipulation of migratory mechanisms, can settle at ectopic ventral sites (Di Meglio et al. 2013), a clear indication that the pontine nuclei do not define a specific domain of the underlying neural tube (Watson et al. 2017a).
Current understanding of the developmental and evolutionary origins of sensory afferent inputs to the hindbrain alar plate
Understanding the developmental and evolutionary origins of sensory nuclei unique to the hindbrain is tightly linked to the developmental and evolutionary origin of their afferents. While His noted that the geniculate ganglion did not originate from his ‘Zwischenstrang’, he was not able to identify its unique placodal origin (Von Kupffer 1891; O’Neill et al. 2012). Indeed, at the time the dual origin of cranial ganglia from placodes and neural crest was clarified, the organizational scheme of hindbrain sensory nuclei as a continuation of spinal organization, albeit with some idiosyncrasies (Gaskell 1886), was already deeply rooted and was not adapted to these new insights. We know now that ventral epibranchial placode-derived afferents terminate in the ‘special visceral column’ (taste bud afferents of cranial nerves VII, IX, X to the solitary tract) and dorsolateral otic placode-derived afferents provide the sole sensory input to the ‘special somatic column’ consisting of the vestibular/auditory nuclei. Thus, the evolutionary origin of Herrick’s neomorphic hindbrain nuclei is linked to the emergence of cranial placodes. There is growing evidence that placodal precursor cells may be present in the lancelet and ascidians based on the presence of peripheral cells that express specific miR and bHLH genes and that project axons into the central nervous system (Fritzsch 1996; Pierce et al. 2008; Candiani et al. 2011; Jahan et al. 2012; Tang et al. 2013; Rigon et al. 2017; Schlosser 2017). But the particulars about how such basal chordate sensory cells evolved into the hair cells and sensory neurons of vertebrate cranial placodes remains to be elucidated (Fritzsch and Elliott 2017).
It is interesting to note that epibranchial (geniculate, petrosal, nodosal) placode-derived ganglia are the first to develop and uniquely depend on Neurog2, whereas all neural crest- and otic placode-derived ganglia develop later and depend on Neurog1 (Fode et al. 1998; Ma et al. 1998; Ma et al. 2000). If the sequence of afferent development reflects evolution, epibranchial placode-derived sensory neurons evolved before neural crest-derived and otic placode-derived sensory neurons and, in sequence, ear, mechanosensory and electrosensory placode-derived ganglia (O’Neill et al. 2012; Chagnaud et al. 2017). If this in turn predicts the evolution of their central targets, that sequence would be branchial motor neurons and the solitary nucleus before somatic motor neurons and the vestibular and auditory nuclei. We have argued earlier on the basis of phylogenetic relationships that branchial motor neurons evolved before true somatic motor neurons [with peripheral axon projections (Fritzsch et al. 2017)]. Evidence for potential co-evolution of a system of efferents, afferents and central targets comes from the finding that branchial motor neurons, epibranchial placodes and their projections to the solitary nucleus and tract all depend critically on the expression of Phox2b (Dubreuil et al. 2002; Häming et al. 2011; Hernandez-Miranda et al. 2016), and thus likely arose together with the appearance of this gene in the chordate lineage. For aquatic animals that breathe and filter feed with the gills, a developmental mechanism whereby a single transcription factor drives the formation of branchial motor neurons that innervate gill-associated muscles and of a reflex arc composed of gill-associated chemosensors and their central sensory targets provides an efficient evolutionary solution.
His and his contemporaries were keenly aware that the vestibular and auditory afferents entered the brain as distinct nerves adjacent to the facial nerve (Figure 4) as this was already drawn by Deiters (Deiters 1865). Later comparative work demonstrated in addition the existence, in primary aquatic vertebrates, of mechanosensory lateral line organs and electroceptive ampullary organs that derive from a series of dorsolateral neurogenic placodes (O’Neill et al. 2012). None of these latter structures were known to His. Due to technical difficulties in differentially tracing the afferents of these organs relative to auditory and vestibular afferents, the overall area of termination of vestibular, auditory, lateral line and electroceptive sensory afferents was dubbed the ‘octavo-lateral area’, assuming some degree of afferent overlap between lateral line and inner ear afferents (Herrick 1948). Later work using modern tract tracing techniques has shown that the octavo-lateral afferent central projections develop after neural crest-derived trigeminal afferents and project in a spatiotemporal sequence from the most ventral (auditory afferents, terminating just above the trigeminal afferents) to the most dorsal (electroceptive ampullary organ afferents), in the same sequence as the development of the peripheral sensory organs (Fritzsch et al. 2005). Thus, these afferent populations segregate centrally in a way that reflects their temporal formation, raising the question of whether they in fact exhibit an active and selective targeting of specific central neuron populations. More recent data indicate a surprising degree of autonomy in auditory afferent projections that develop in the absence of any peripheral or central target (generated by knockout of Atoh1 (Elliott et al. 2017)). Similarly, taste bud afferents terminate correctly in the area of the solitary tract even when the formation of the solitary nucleus has been disrupted (Qian et al. 2001; Dauger et al. 2003).
The autonomy of ingrowing afferents suggests that the developmental formation of epibranchial and dorsolateral placode-derived sensory neurons and afferent projections precedes the formation of distinct target nuclei in the hindbrain, much as the formation of specific areas of neuropil precedes formation of distinct nuclei in the visual system (Herrick 1948; Rettig et al. 1981; Morona et al. 2017). This accords somewhat with the neurobiotactic idea of Ariens-Kappers (Kappers et al. 1936) that central neurons migrate into areas of their major sensory input and thus generate sensory ‘nuclei’. The idea that afferents generally ‘attract’ neurons to migrate to them was later dismissed by showing that motor neuron migration occurs in the absence of afferents and is driven by identified molecules related to the Wnt signaling and other pathways (Yang et al. 2014). Unfortunately, most second order sensory neurons within hindbrain nuclei die in the absence of an afferent input (Levi-montalcini 1949; Rubel and Fritzsch 2002; Elliott et al. 2015) rendering it nearly impossible to assess whether their migration is independent of afferents.
We make here a short aside on an issue of anatomical terminology. Based on the above, we would argue that the use of “general” and “special” columns, introduced by Herrick decades ago with a clear but incorrect notion of evolutionary sequentiallity, should be replaced by terms that reflect the developmental source of their afferents. Thus, the general somatic and general visceral columns should be renamed the “neural crest somatic” and “neural crest visceral” columns, respectively, and the “special somatic” and “special visceral” columns should be renamed the “dorsolateral placodal” and “epibranchial” columns. This would eliminate the now obscure semantic origins of “general” versus “special” senses and their unfortunate connotations of a sequence of evolutionary events that apparently did not happen and is certainly not recapitulated during development.
Molecular fate-mapping of dorsal sensory nuclei derived from the rhombic lip and adjacent alar plate regions
As noted above, the definition of the rhombic lip was originally based on morphological features discerned by His in cross sections of the hindbrain of the human embryo. Since these features are not as obvious in other species, a definitive morphological delineation of the rhombic lip has not been possible in experimental studies. Rather, an operational definition has been used based on the expression of transcription factors by neural progenitors in the ventricular zone at the extreme of the alar plate (Figure 6). In particular, the early domain of expression of Wnt1 has been taken to delineate the rhombic lip (Nichols and Bruce 2006; Ray and Dymecki 2009), and the expression of other transcription factors within this Wnt1 domain defines subpopulations of rhombic lip progenitors (Ray and Dymecki 2009). This is not a consensus definition, since the rhombic lip of rhombomere 1, which gives rise to the external granule layer of the cerebellum, is considered by some to be restricted to the Atoh1/Math1 progenitor domain (Wang et al. 2005; Chizhikov et al. 2010). A full validation of progenitor domain-based definitions of the rhombic lip as they relate to His’ original would require the same assessment of transcription factor expression in the human embryo, which has not been performed. Nevertheless, genetic fate mapping of the progenitor subdomains in the mouse embryo has shown that they give rise to multiple neuron types determined by His to derive from the original Rautenlippe. In addition to the ventral migratory neuron populations that will be considered in the following section, these include neurons of the cerebellum, which originate from the r1 rhombic lip, and those of the auditory, vestibular, lateral line and electroreceptive nuclei, which originate from the rhombic lip in more caudal rhombomeres. We focus on the latter sensory nuclei, as these are most relevant to our topic. We note that the development and evolution of the cerebellum, a part of the hindbrain which varies volumetrically in craniates from being nearly absent in hagfish to being by far the largest part of the brain in certain electroreceptive bony fish, is a significant feature of hindbrain development (Millen et al. 2014) and evolution (Butts et al. 2014) but will not be covered here [for information about the developmental origins of the cerebellum, see (Wang et al. 2005; Hagan and Zervas 2012; Hoshino 2012)].
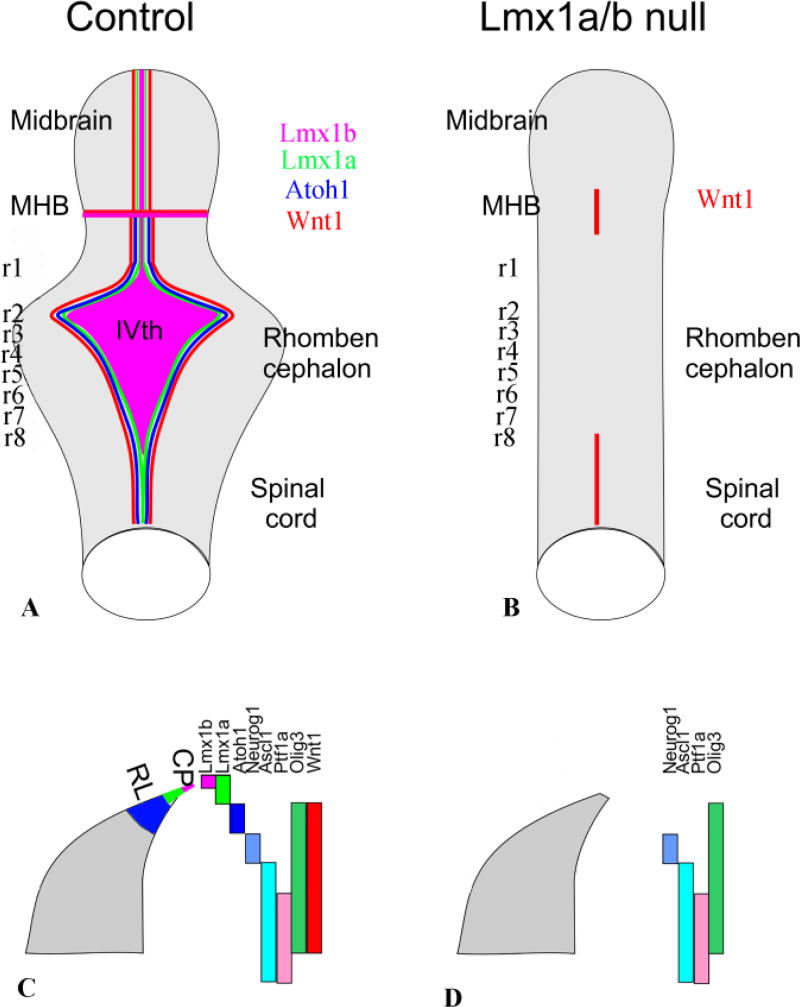
Fig. 6.
Loss of Lmx1a/b results in absence of the typical rhombencephalic form with a IVth ventricle covered by a choroid plexus. It also eliminates the midbrain-hindbrain boundary and thus anterior hindbrain/midbrain structures such as oculomotor and trochlear motneurons and the substantia nigra. The cross sections of the alar plate show the partially overlapping expression of different factors in the alar plate that regulate development of subpopulations in rhombomere 7. Note that the expression domains of bHLH genes in r1–5 are only partially clarified. Note also that loss of Lmx1a/b eliminates expression of Atoh1 and Wnt1 throughout most of the hindbrain. Modified after (Mishima et al. 2009; Ray and Dymecki 2009)
Genetic fate mapping in the mouse has shown that the different auditory nuclei derive collectively from the rhombic lip of r2–r5 and from non-rhombic lip progenitor domains of r3–5 (Farago et al. 2006). This is the region between the entry points of cranial nerves V and IX, as correctly depicted by Wilhelm His (see Figure 4). Many of the rhombic lip-derived neurons in the auditory nuclei originate from the Atoh1/Math1 progenitor subdomain (Wang et al. 2005; Farago et al. 2006; Fritzsch et al. 2006), which contributes heavily to the parvocellular populations of the anteroventral and posteroventral cochlear nuclei (AVCN, PVCN), but only meagerly to the largely magnocellular dorsal cochlear nucleus (DCN). The remaining portions of the auditory nuclei receive rhombic lip contributions from the Ptf1a progenitor subdomain (Yamada et al. 2007; Fujiyama et al. 2009; Di Bonito and Studer 2017). Although the auditory nuclei in the chicken are also known to derive from dorsal progenitors (Tan and Le Douarin 1991; Cramer et al. 2000), it is not known to what extent these are restricted to the rhombic lip. Moreover, there are striking differences in the rostrocaudal origins of auditory nuclei in the mouse (rhombomeres 2–5) and chicken (rhombomere 3 to pseudorhombomere 8), suggesting that functionally comparable nuclei in the two species are not developmentally homologous but may have arisen through convergent evolution (Fritzsch 2003; Farago et al. 2006).
The different vestibular nuclei and their constituent projection neuron subpopulations have been shown to derive from specific rhombomeres in the mouse, chicken, fish and frog (Díaz et al. 1998; Glover 2000; Pasqualetti et al. 2007; Bonito et al. 2013; Straka et al. 2014; Chagnaud et al. 2017), in a pattern that features certain elements that are highly conserved and others that are phylogenetically variable. Although their origins from specific dorsoventral domains has not yet been described systematically, (Fritzsch et al. 2006; Ray and Dymecki 2009) have reported a transient expression of Neurog1 in caudal vestibular nuclei. Ongoing studies, however, have shown that only some vestibular projection neuron populations derive from the Neurog1 domain, whereas others derive from nonrhombic lip progenitor domains (J. C. Glover and S. Dymecki, unpublished).
The origins of lateral line and electroreceptive nuclei in aquatic vertebrates are poorly understood. A comprehensive assessment of their segmental and rhombic lip/alar plate origins as they relate to those of the auditory and vestibular nuclei will be necessary to fully understood the evolutionary relationships among these nuclei across primary aquatic (Chagnaud et al. 2017) and amniote (Fritzsch and Elliott 2017) vertebrate taxa.
Neuroblast migrations from the rhombic lip to ventral nuclei postulated by His have been confirmed using modern techniques
One of the most impressive conclusions reached by Wilhelm His Sr. was that he could trace tangential migratory streams of neuroblasts that left the rhombic lip, traversed the solitary nuclei and tract and formed arcuate cell streams that populated the superior and inferior olive as well as the pontine nuclei. Later work, first using chicken-quail transplantation (Tan and Le Douarin 1991), then lineage tracing using viral (Hemond and Glover 1993) and eventually more sophisticated transgenic techniques (reviewed in (Ray and Dymecki 2009; Kratochwil et al. 2017), confirmed and extended these findings. It is noteworthy that it took over 100 years and the invention of several new technologies to not only confirm but go beyond the details of rhombic lip contribution to various nuclei. In addition, His only distinguished between caudal (medullary) and rostral (pontine) rhombic lip as migratory origins. More recent studies have taken advantage of the longitudinal and dorsoventral expression patterns of specific genes to generate intersectional genetic fate-mapping approaches to pinpoint specifically from which rhombomere and which dorsoventral progenitor subpopulation certain nuclei derive, adding the Hox gene expression and neuromeric model to the overall scheme developed by Wilhelm His Sr (Nieuwenhuys and Puelles 2016; Parker and Krumlauf 2017). For example, studies utilizing a variety of transgenic mouse lines has demonstrated that the neurons that make up the basilar pontine nuclei originate from the rhombic lip of rhombomere 6 and pseudorhombomeres 7–8 and migrate in a curved, tangential path (the “anterior extramural stream”) to settle on the ventral surface of rhombomeres 3 and 4 (Wang et al. 2005; Nichols and Bruce 2006)(Watson et al. 2017a). The superior olive, an auditory relay center in the ventral brainstem was claimed to derives from the rhombic lip of rhombomere 6 and pseudorhombomeres 7–8 (Farago et al. 2006) or rhombomeres 4 and 5 (Bonito et al. 2013; Di Bonito et al. 2013; Tomás-Roca et al. 2016). Other rhombic lip-derived migratory neuron populations that His did not discern include the reticular tegmental nucleus, which has an origin similar to that of the basilar pontine nuclei, and the lateral reticular nucleus and external cuneate nucleus, which originate from the rhombic lip of rhombomere 6 and pseudorhombomeres 7–8 (Ray and Dymecki 2009; Martinez-de-la-Torre et al. 2017). Additional populations that originate from the rhombic lip are paralemniscal cell populations at prepontine levels (Martinez-de-la-Torre et al. 2017).
Lmx1a and Lmx1b specify the roof plate and choroid plexus, and secondarily regulate the specification of adjacent rhombic lip progenitors
We already noted above the role played by Lmx1b in defining the MHB boundary via induction of Fgf8 and stabilization of Wnt1/Fgf8 expression (Fritzsch and Glover 2006; Guo et al. 2007; Mishima et al. 2009). In addition to defining the MHB, Lmx1b is coexpressed with the related LIM-homeodomain transcription factor, Lmx1a, in the dorsally most extreme progenitor domain of the hindbrain, which gives rise to the roof plate of the 4th ventricle and its later derivative, the choroid plexus (Mishima et al. 2009). The Lmx1a/b expressing 4th ventricle roof plate functions as a signaling center that regulates the development of the adjacent progenitor domain through secretion of Bmps, which induce the expression of Atoh1/Math1 (Chizhikov et al. 2006). Atoh1/Math1 is a master regulatory bHLH gene (Bermingham et al. 2001; Wang et al. 2005) whose expression is essential for the development of cerebellar glutamatergic neurons and neurons of the auditory nuclei (Fritzsch et al. 2006; Ray and Dymecki 2009), and presumably other neurons that derive from the Atoh1/Math1 progenitor domain.
Genetic fate mapping has shown that Lmx1a plays a non-redundant role in segregating the roof plate lineage from adjacent cerebellar progenitors in rhombomere 1 (Chizhikov et al. 2010). The roof plate of the 4th ventricle appears essentially normal in Lmx1b-null embryos, and is not severely compromised in early Lmx1a (dreher) mutant embryos, although its growth is reduced in older dreher mutant embryos. By contrast, the roof plate of the 4th ventricle fails to develop in Lmx1a/b double mutants, indicating that the two Lmx1 genes are necessary for and play redundant roles in the induction of this structure (Mishima et al. 2009). Consistent with the loss of the roof plate and its Bmp signaling function, Atoh1/Math1 is not expressed in the rhombic lip of Lmx1a/b double mutants (Figure 6). Wnt1 expression is also largely lost, indicating that Lmx1a/b stabilize the expression of Wnt genes here as Lmx1b does at the MHB (Mishima et al. 2009; Ray and Dymecki 2009; Hernandez-Miranda et al. 2016). Morphological and immunohistochemical analyses reveal a spinal cord-like morphology of the Lmx1a/b double mutant hindbrain, with an almost complete lack of the cerebellum (Mishima et al. 2009). Other dorsal hindbrain neuron populations were not specifically investigated.
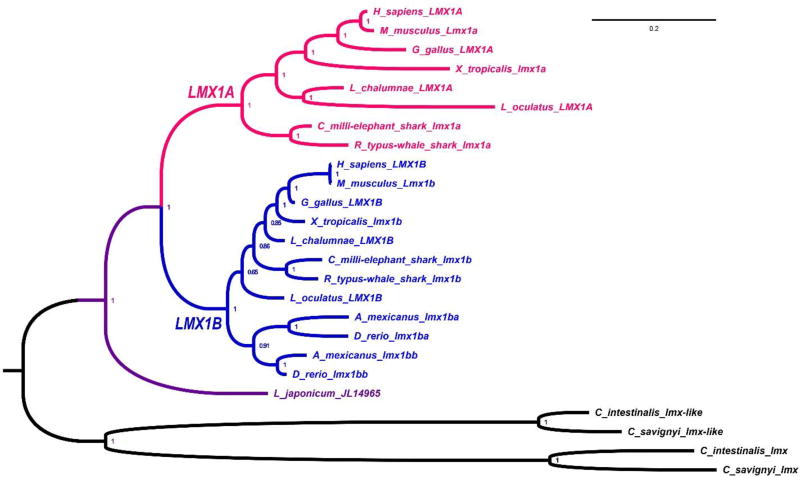
Obviously, Lmx1b and Lmx1a, in addition to being required for roof plate specification, could contribute more generally to the evolution of the hindbrain, as they regulate key aspects of midbrain-hindbrain boundary formation as well as rhombic lip formation through roof plate specification and signaling. It is noteworthy in this context that Lmx1b is apparently the ancestral gene, also found in other phyla, whereas Lmx1a has only been identified in chordates (Figure 7). It is interesting that ascidians, which lack Gbx genes (a major component of MHB formation, Figure 5), have multiplied Lmx1 genes into two distinct groups of paralogs (Figure 7). It will be important to characterize the expression of these ascidian genes to better understand how the evolution of the protein coding sequence correlates with the evolution of regulatory elements that drive Lmx1a/b expression.
Fig. 7. Phylogenetic analysis of the chordate LMX1 protein family.
Vertebrate LMX1A and LMX1B subclades are depicted in red and blue, respectively, and the predicted LMX1 peptide from the Japanese lamprey (L. japonicum JL14965) is in purple (REF #1). Non-vertebrate, chordate out-group sequences (black) consist of a pair of duplicated LMX1 genes, lmx-like and lmx, which are present in the genomes of each of the two ascidians Ciona intestinalis and Ciona savignyi. Node values represent posterior probabilities after phylogenetic analysis via Bayesian inference.
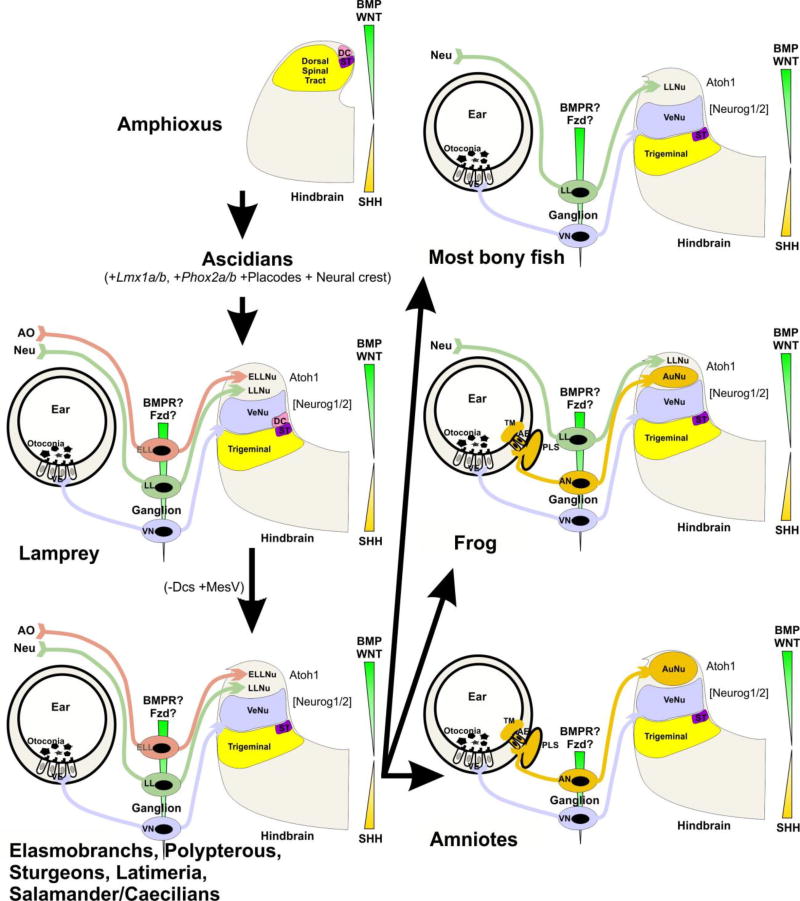
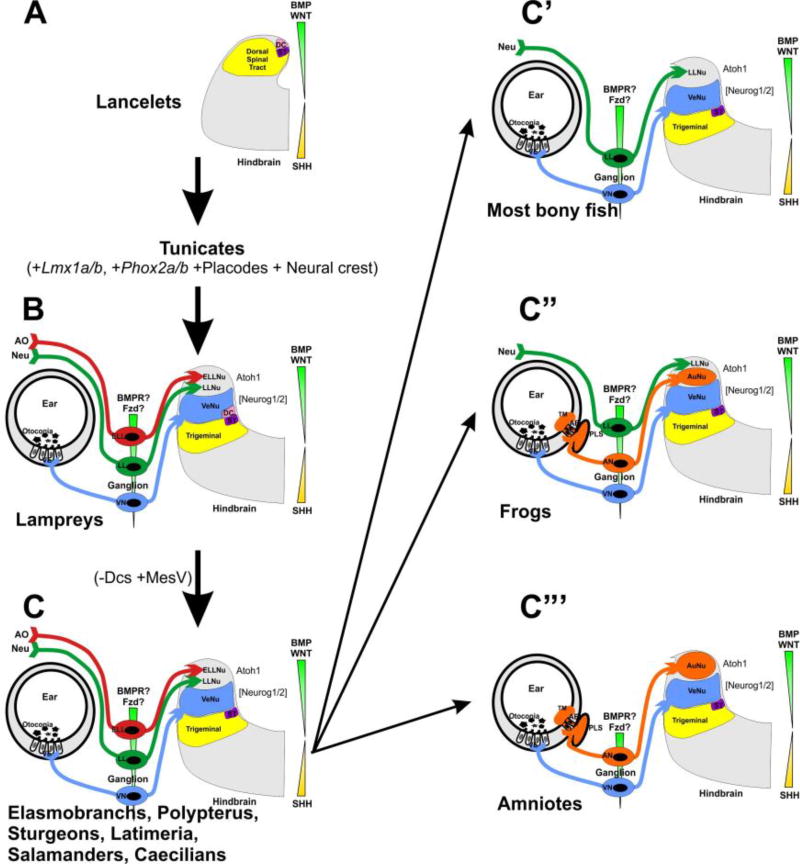
The near complete absence of cerebellum in Lmx1a/b double mutants (Mishima et al. 2009) clearly highlights the importance of Lmx1 genes for the evolution of the cerebellum (Pose-Méndez et al. 2016). Since Atoh1/Math1 is required for formation of cochlear nuclei (Elliott et al. 2017), cochlear nuclei are also expected to be affected in Lmx1a−/−;b−/− mutants and their evolution may depend on Lmx1 genes as well. The development of sensory nuclei associated with electroreceptive ampullary organs and mechanosensory lateral line organs seem also to depend on Atoh1/Math1, and thus may additionally depend on dorsal expression of Lmx1 genes (Figure 8).
Fig. 8. Proposed octavo-lateral evolution.
Evolution from the common ancestor of Amphioxus and Lamprey resulted in the eventual expansion of the alar plate to accommodate the vestibular, lateral line, and electroreceptive nuclei. Sharks, Latimeria, and salamanders have lost Dorsal cells (DC), but acquired the sensory MesV nucleus. Most bony fish have retained a lateral line projection, but have lost electroreception. Similarly, frogs have done the same, but have additionally gained auditory nuclei. Amniotes have lost both lateral line projections and electroreception, but have retained auditory nuclei. AO, ampullary organs; Neu, neuromasts; ST, solitary tract. Modified after (Fritzsch and Elliott 2017)
The degree to which Lmx1a/b regulate other rhombic lip progenitor subpopulations, in particular those in r2–8, has not been determined. How the expression of Lmx1a/b itself is controlled by emerging upstream regulators (Burzynski et al. 2013; Doucet-Beaupré et al. 2015) also remains to be elucidated, as does the evolution of the Lmx1a/b sequence (Figure 7). Multiple splice forms of Lmx1a have been described, and several proteins that regulate differential splicing are expressed in the hindbrain (Saito et al. 2016), suggesting that differential splicing of the Lmx1 genes may regulate Lmx1a/b-mediated signaling.
His was unaware of the diversity of dorsolateral placode-derived organs (O’Neill et al. 2012). He was therefore unaware of one of the most sophisticated evolutionary changes in the hindbrain: the loss of electroreception and the mechanosensory lateral line, found now only in primary aquatic species, and the overlapping gain of the auditory nuclei (Fritzsch et al. 2006; Fritzsch and Elliott 2017) (Figure 8). Numerous ideas have been proposed about how the loss of one sensory modality and the gain of another could occur during evolution. Assessing the differential effects of evolutionarily diverse Lmx1a/b sequences (Figure 7) in regulating the proliferation and differentiation of different rhombic lip progenitor subpopulations in multiple vertebrate lineages (Doucet-Beaupré et al. 2015) offers novel possibilities to explain the enlargement or reduction of rhombic lip derivatives such as the cerebellum and the auditory nuclei. Given the partial overlap and possible redundancy of Lmx1a/b-mediated signaling, knockin strategies of Lmx1b into the Lmx1a locus (and vice versa) will be needed to fully elucidate the non-overlapping function of the Lmx1a/b proteins. In parallel, more comparative, molecular information is needed about how placode-derived sensory afferents interact with their target hindbrain sensory nuclei. Work along these lines will provide a clearer picture of how the signaling role of Lmx1a/b and the emergence of new sensory neuron modalities have shaped the hindbrain. His could not foresee how his descriptions of the neural crest, different hindbrain regions, and the hindbrain rhombic lip and roof plate would one day lead to groundbreaking genetic experiments that promise to contribute profound molecular insights into hindbrain development and evolution.
Summary
In summary, Wilhelm His Sr. not only laid the foundations for understanding the neural crest as the origin of much of the peripheral sensory nervous system but also provided keen insights into the importance of the adjacent rhombic lip in the formation of central neuron populations novel to the hindbrain. His work thus contributes to an understanding of the evolutionary elaboration of a basic spinal cord organization into the more complex and functionally diverse hindbrain region. This elaboration is likely to have been driven by the appearance of epibranchial and dorsolateral placodes and their specialization to generate new sensory afferent types, and the consequent recruitment of neuronal progenitors in the rhombic lip and alar plate, potentially driven by the actions of Lmx1a/b, to provide novel central sensory nuclei as targets. Further investigation of the molecular underpinnings of both placode and rhombic lip development is a key objective for future studies. While some of the molecular basis for the variability in rhombic lip derivatives in different vertebrate lineages is emerging, more work on the complex interplay between Lmx1a and Lmx1b proteins and downstream effector genes such as Atoh1/Math1 is needed before we gain a full understanding of how the rhombic lip contributes to making the hindbrain unique.
Highlights.
The description of Wilhelm His Sr of the developing human hindbrain is reviewed and put in the context of modern insights.
Major contributions of the rhombic proposed by His have been confirmed and detailed with modern techniques.
The role of the choroid plexus, first indicated by His in the definition of the rhombic lip is molecularly highlighted using the recent data on the evolution and development of Lmx1a and b.
The morphological diversity of the vertebrate alar plate is considered in the context of placodal and rhombic lip evolution.
Acknowledgments
This work was supported by grants form NIDCD (RO1 DC005590 to BF; R03 DC015333 to KE; R01 NS093009 to VC) and the Norwegian Research Council (JCG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuixech-Crespo B, López-Blanch L, Burguera D, Maeso I, Sánchez-Arrones L, et al. Molecular regionalization of the developing amphioxus neural tube challenges major partitions of the vertebrate brain. PLoS biology. 2017;15:e2001573. doi: 10.1371/journal.pbio.2001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwell WJ, Danchakoff V. A human embryo with seventeen pairs of somites. Carnegie Institution of Washington; 1930. [Google Scholar]
- Bermingham NA, Hassan BA, Wang VY, Fernandez M, Banfi S, et al. Proprioceptor pathway development is dependent on Math1. Neuron. 2001;30:411–422. doi: 10.1016/s0896-6273(01)00305-1. [DOI] [PubMed] [Google Scholar]
- Bonito M, Glover JC, Studer M. Hox genes and region-specific sensorimotor circuit formation in the hindbrain and spinal cord. Developmental Dynamics. 2013;242:1348–1368. doi: 10.1002/dvdy.24055. [DOI] [PubMed] [Google Scholar]
- Burzynski GM, Reed X, Maragh S, Matsui T, McCallion AS. Integration of genomic and functional approaches reveals enhancers at LMX1A and LMX1B. Molecular genetics and genomics. 2013;288:579–589. doi: 10.1007/s00438-013-0771-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts T, Green MJ, Wingate RJ. Development of the cerebellum: simple steps to make a ‘little brain’. Development. 2014;141:4031–4041. doi: 10.1242/dev.106559. [DOI] [PubMed] [Google Scholar]
- Cambronero F, Puelles L. Rostrocaudal nuclear relationships in the avian medulla oblongata: a fate map with quail chick chimeras. Journal of Comparative Neurology. 2000;427:522–545. doi: 10.1002/1096-9861(20001127)427:4<522::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Candiani S, Moronti L, Tonelli DDP, Garbarino G, Pestarino M. A study of neural-related microRNAs in the developing amphioxus. Evodevo. 2011;2:15. doi: 10.1186/2041-9139-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnaud BP, Engelmann J, Fritzsch B, Glover JC, Straka H. Sensing External and Self-Motion with Hair Cells: A Comparison of the Lateral Line and Vestibular Systems from a Developmental and Evolutionary Perspective. Brain, behavior and evolution. 2017;90:98–116. doi: 10.1159/000456646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Currle DS, Rose MF, Monuki ES, et al. The roof plate regulates cerebellar cell-type specification and proliferation. Development. 2006;133:2793–2804. doi: 10.1242/dev.02441. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Lindgren AG, Mishima Y, Roberts RW, Aldinger KA, et al. Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. Proceedings of the National Academy of Sciences. 2010;107:10725–10730. doi: 10.1073/pnas.0910786107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KS, Fraser SE, Rubel EW. Embryonic origins of auditory brain-stem nuclei in the chick hindbrain. Developmental biology. 2000;224:138–151. doi: 10.1006/dbio.2000.9779. [DOI] [PubMed] [Google Scholar]
- Dahm R. Discovering DNA: Friedrich Miescher and the early years of nucleic acid research. Human genetics. 2008;122:565–581. doi: 10.1007/s00439-007-0433-0. [DOI] [PubMed] [Google Scholar]
- Dauger S, Pattyn A, Lofaso F, Gaultier C, Goridis C, et al. Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development. 2003;130:6635–6642. doi: 10.1242/dev.00866. [DOI] [PubMed] [Google Scholar]
- Deiters O. Untersuchungen über Gehirn und Rückenmark des Menschen und der Säugethiere. F. Veiweg; 1865. [Google Scholar]
- Di Bonito M, Narita Y, Avallone B, Sequino L, Mancuso M, et al. Assembly of the auditory circuitry by a Hox genetic network in the mouse brainstem. PLoS genetics. 2013;9:e1003249. doi: 10.1371/journal.pgen.1003249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonito M, Studer M. Cellular and molecular underpinnings of neuronal assembly in the central auditory system during mouse development. Frontiers in neural circuits. 2017;11 doi: 10.3389/fncir.2017.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio T, Kratochwil CF, Vilain N, Loche A, Vitobello A, et al. Ezh2 orchestrates topographic migration and connectivity of mouse precerebellar neurons. Science. 2013;339:204–207. doi: 10.1126/science.1229326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz C, Puelles L, Marín F, Glover JC. The relationship between rhombomeres and vestibular neuron populations as assessed in quail–chicken chimeras. Developmental biology. 1998;202:14–28. doi: 10.1006/dbio.1998.8986. [DOI] [PubMed] [Google Scholar]
- Doucet-Beaupré H, Ang S-L, Lévesque M. Cell fate determination, neuronal maintenance and disease state: The emerging role of transcription factors Lmx1a and Lmx1b. FEBS letters. 2015;589:3727–3738. doi: 10.1016/j.febslet.2015.10.020. [DOI] [PubMed] [Google Scholar]
- Dubreuil V, Hirsch M-R, Jouve C, Brunet J-F, Goridis C. The role of Phox2b in synchronizing pan-neuronal and type-specific aspects of neurogenesis. Development. 2002;129:5241–5253. doi: 10.1242/dev.129.22.5241. [DOI] [PubMed] [Google Scholar]
- Elliott KL, Houston DW, DeCook R, Fritzsch B. Ear manipulations reveal a critical period for survival and dendritic development at the single-cell level in Mauthner neurons. Developmental neurobiology. 2015;75:1339–1351. doi: 10.1002/dneu.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott KL, Kersigo J, Pan N, Jahan I, Fritzsch B. Spiral Ganglion Neuron Projection Development to the Hindbrain in Mice Lacking Peripheral and/or Central Target Differentiation. Frontiers in neural circuits. 2017;11 doi: 10.3389/fncir.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, et al. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode–derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Similarities and differences in lancelet and craniate nervous systems. Israel Journal of Zoology. 1996;42:S147–S160. [Google Scholar]
- Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain research bulletin. 2003;60:423–433. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL. Gene, cell, and organ multiplication drives inner ear evolution. Developmental Biology. 2017;431:3–15. doi: 10.1016/j.ydbio.2017.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Elliott KL, Glover JC. Gaskell revisited: new insights into spinal autonomics necessitate a revised motor neuron nomenclature. Cell and Tissue Research. 2017:1–15. doi: 10.1007/s00441-017-2676-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Glover J. Evolution of the Deuterostome Central Nervous System: An Intercalation of Developmental Patterning Processes with Cellular Specification Processes-2.01 2006 [Google Scholar]
- Fritzsch B, Gregory D, Rosa-Molinar E. The development of the hindbrain afferent projections in the axolotl: evidence for timing as a specific mechanism of afferent fiber sorting. Zoology. 2005;108:297–306. doi: 10.1016/j.zool.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Jahan I, Pan N, Elliott KL. Evolving gene regulatory networks into cellular networks guiding adaptive behavior: an outline how single cells could have evolved into a centralized neurosensory system. Cell and tissue research. 2015;359:295–313. doi: 10.1007/s00441-014-2043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Nichols D, Echelard Y, McMahon A. Development of midbrain and anterior hindbrain ocular motoneurons in normal and Wnt-1 knockout mice. Developmental Neurobiology. 1995;27:457–469. doi: 10.1002/neu.480270403. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Northcutt G. Cranial and spinal nerve organization in amphioxus and lampreys: evidence for an ancestral craniate pattern. Cells Tissues Organs. 1993;148:96–109. doi: 10.1159/000147529. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Feng F, Matei V, Nichols D. The molecular and developmental basis of the evolution of the vertebrate auditory system. International Journal of Comparative Psychology. 2006;19 [Google Scholar]
- Fujiyama T, Yamada M, Terao M, Terashima T, Hioki H, et al. Inhibitory and excitatory subtypes of cochlear nucleus neurons are defined by distinct bHLH transcription factors, Ptf1a and Atoh1. Development. 2009;136:2049–2058. doi: 10.1242/dev.033480. [DOI] [PubMed] [Google Scholar]
- Gaskell WH. On the structure, distribution and function of the nerves which innervate the visceral and vascular systems. The Journal of physiology. 1886;7:1–80. doi: 10.1113/jphysiol.1886.sp000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasco DM, Sittaramane V, Bryant W, Fritzsch B, Sawant A, et al. The mouse Wnt/PCP protein Vangl2 is necessary for migration of facial branchiomotor neurons, and functions independently of Dishevelled. Developmental biology. 2012;369:211–222. doi: 10.1016/j.ydbio.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JC. Neuroepithelial ‘compartments’ and the specification of vestibular projections. Progress in brain research. 2000;124:3–21. doi: 10.1016/S0079-6123(00)24004-1. [DOI] [PubMed] [Google Scholar]
- Glover JC, Renaud JS, Rijli FM. Retinoic acid and hindbrain patterning. Developmental Neurobiology. 2006;66:705–725. doi: 10.1002/neu.20272. [DOI] [PubMed] [Google Scholar]
- Guo C, Qiu H-Y, Huang Y, Chen H, Yang R-Q, et al. Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development. 2007;134:317–325. doi: 10.1242/dev.02745. [DOI] [PubMed] [Google Scholar]
- Haeckel E. Generelle Morphologie der Organismen allgemeine Grundzuge der organischen Formen-Wissenschaft, mechanisch begrundet durch die von Charles Darwin reformirte Descendenz-Theorie von Ernst Haeckel: Allgemeine Entwickelungsgeschichte der Organismen kritische Grundzuge der mechanischen Wissenschaft von den entstehenden Formen der Organismen, begrundet durch die Descendenz-Theorie. Verlag von Georg Reimer; 1866. [Google Scholar]
- Hagan N, Zervas M. Wnt1 expression temporally allocates upper rhombic lip progenitors and defines their terminal cell fate in the cerebellum. Molecular and Cellular Neuroscience. 2012;49:217–229. doi: 10.1016/j.mcn.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häming D, Simoes-Costa M, Uy B, Valencia J, Sauka-Spengler T, et al. Expression of sympathetic nervous system genes in Lamprey suggests their recruitment for specification of a new vertebrate feature. PloS one. 2011;6:e26543. doi: 10.1371/journal.pone.0026543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Omi M, Sato T, Nakamura H. Pea3 determines the isthmus region at the downstream of gf -Ras-ERK signaling pathway. Development, growth & differentiation. 2015;57:657–666. doi: 10.1111/dgd.12254. [DOI] [PubMed] [Google Scholar]
- Hemond SG, Glover JC. Clonal patterns of cell proliferation, migration, and dispersal in the brainstem of the chicken embryo. Journal of Neuroscience. 1993;13:1387–1402. doi: 10.1523/JNEUROSCI.13-04-01387.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Miranda LR, Müller T, Birchmeier C. The dorsal spinal cord and hindbrain: From developmental mechanisms to functional circuits. Developmental biology. 2016 doi: 10.1016/j.ydbio.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Herrick CJ. The brain of the tiger salamander, Ambystoma tigrinum 1948 [Google Scholar]
- Higashiyama H, Hirasawa T, Oisi Y, Sugahara F, Hyodo S, et al. On the vagal cardiac nerves, with special reference to the early evolution of the head–trunk interface. Journal of morphology. 2016;277:1146–1158. doi: 10.1002/jmor.20563. [DOI] [PubMed] [Google Scholar]
- His W., Jr Zur Entwicklungsgeschichte des Acoustico-Facialis Gebietes beim Menschen. Arch. f. Anat. u. Physiol., supp. 1889:1. [Google Scholar]
- His W. Untersuchungen über die erste Anlage des Wirbelthierleibes: die erste Entwickelung des Hühnchens im Ei. FCW Vogel; 1868. [Google Scholar]
- His W. Beschreibung eines Mikrotoms. Archiv für mikroskopische Anatomie. 1870a;6:229–232. [Google Scholar]
- His W. Ueber die Bedeutung der Entwickelungsgeschichte für die Auffassung der organischen Natur. FCW Vogel; 1870b. [Google Scholar]
- His W. Anatomie menschlicher embryonen. FCW Vogel; 1880. [Google Scholar]
- His W. Die Entwickelung des menschlichen Rautenhirns vom Ende des ersten bis zum Beginn des dritten Monats. Abhandlungen der mathematisch-physischen Classe der koeniglich saechsischen Gesellschaft der Wissenschaftern. 1890a;17:4–74. [Google Scholar]
- His W. Histogenese und Zusammenhang der Nervenelemente 1890b [Google Scholar]
- His W. Die anatomische nomenclatur. Veit; 1895. [Google Scholar]
- His W. F. Miescher. Die histochemischen und physiologischen Arbeiten von Friedrich Miescher. 1897;1:5–32. [Google Scholar]
- Hoshino M. Neuronal subtype specification in the cerebellum and dorsal hindbrain. Development, growth & differentiation. 2012;54:317–326. doi: 10.1111/j.1440-169X.2012.01330.x. [DOI] [PubMed] [Google Scholar]
- Jahan I, Pan N, Kersigo J, Calisto LE, Morris KA, et al. Expression of Neurog1 instead of Atoh1 can partially rescue organ of Corti cell survival. PloS one. 2012;7:e30853. doi: 10.1371/journal.pone.0030853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers CA, Huber GC, Crosby EC. The Comparative Anatomy of the Nervous System of Vertebrates Including Man. The Journal of Nervous and Mental Disease. 1936;84:709–711. [Google Scholar]
- Kratochwil CF, Maheshwari U, Rijli FM. The Long Journey of Pontine Nuclei Neurons: From Rhombic Lip to Cortico-Ponto-Cerebellar Circuitry. Frontiers in neural circuits. 2017;11 doi: 10.3389/fncir.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Danielian PS, Fritzsch B, McMahon AP. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124:959–969. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- Levi-montalcini R. The development of the acoustico-vestibular centres in the chick embryo in the absence of the afferent root fibers and of descending fiber tracts. Journal of Comparative Neurology. 1949;91:209–241. doi: 10.1002/cne.900910204. [DOI] [PubMed] [Google Scholar]
- Ma Q, Anderson DJ, Fritzsch B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J Assoc Res Otolaryngol. 2000;1:129–143. doi: 10.1007/s101620010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Marín F, Puelles L. Morphological fate of rhombomeres in quail/chick chimeras: a segmental analysis of hindbrain nuclei. European Journal of Neuroscience. 1995;7:1714–1738. doi: 10.1111/j.1460-9568.1995.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Martine-de-la-Torre M, Lambertos A, Penafiel R, Puelles L. An exercise in brain genoarchitectonics: Analysis of AZIN2-Lacz expressing neuronal populations in the mouse hindbrain. Journal of neuroscience research. 2017 doi: 10.1002/jnr.24053. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Millen KJ, Steshina EY, Iskusnykh IY, Chizhikov VV. Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function. Proceedings of the National Academy of Sciences. 2014;111:E1777–E1786. doi: 10.1073/pnas.1315024111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima Y, Lindgren AG, Chizhikov VV, Johnson RL, Millen KJ. Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. Journal of Neuroscience. 2009;29:11377–11384. doi: 10.1523/JNEUROSCI.0969-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morona R, Ferran JL, Puelles L, González A. Gene expression analysis of developing cell groups in the pretectal region of Xenopus laevis. Journal of Comparative Neurology. 2017;525:715–752. doi: 10.1002/cne.24099. [DOI] [PubMed] [Google Scholar]
- Nichols DH, Bruce LL. Migratory routes and fates of cells transcribing the Wnt-1 gene in the murine hindbrain. Developmental dynamics. 2006;235:285–300. doi: 10.1002/dvdy.20611. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Puelles L. Towards a new neuromorphology. Springer; 2016. [Google Scholar]
- O’Neill P, Mak S-S, Fritzsch B, Ladher RK, Baker CV. The amniote paratympanic organ develops from a previously undiscovered sensory placode. Nature communications. 2012;3:1041. doi: 10.1038/ncomms2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HJ, Krumlauf R. Segmental arithmetic: summing up the Hox gene regulatory network for hindbrain development in chordates. Wiley Interdisciplinary Reviews: Developmental Biology. 2017;6 doi: 10.1002/wdev.286. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Díaz C, Renaud J-S, Rijli FM, Glover JC. Fate-mapping the mammalian hindbrain: segmental origins of vestibular projection neurons assessed using rhombomere-specific Hoxa2 enhancer elements in the mouse embryo. Journal of Neuroscience. 2007;27:9670–9681. doi: 10.1523/JNEUROSCI.2189-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet J-F. Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development. 1997;124:4065–4075. doi: 10.1242/dev.124.20.4065. [DOI] [PubMed] [Google Scholar]
- Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, et al. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evolution & development. 2008;10:106–113. doi: 10.1111/j.1525-142X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pose-Méndez S, Candal E, Mazan S, Rodríguez-Moldes I. Genoarchitecture of the rostral hindbrain of a shark: basis for understanding the emergence of the cerebellum at the agnathan–gnathostome transition. Brain Structure and Function. 2016;221:1321–1335. doi: 10.1007/s00429-014-0973-8. [DOI] [PubMed] [Google Scholar]
- Puelles L, Ferran JL. Concept of neural genoarchitecture and its genomic fundament. Frontiers in neuroanatomy. 2012;6 doi: 10.3389/fnana.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Fritzsch B, Shirasawa S, Chen C-L, Choi Y, et al. Formation of brainstem (nor) adrenergic centers and first-order relay visceral sensory neurons is dependent on homeodomain protein Rnx/Tlx3. Genes & development. 2001;15:2533–2545. doi: 10.1101/gad.921501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Dymecki SM. Rautenlippe Redux—toward a unified view of the precerebellar rhombic lip. Current opinion in cell biology. 2009;21:741–747. doi: 10.1016/j.ceb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert C. Über die Visceralbogen der Wirbelthiere im Allgemeinen und deren Metamorphosen bei den Vögeln und Säugethieren. Arch Anat Physiol Wiss Med. 1837;1837:120–220. [Google Scholar]
- Remak R. Untersuchungen über die Entwickelung der Wirbelthiere. G. Reimer; 1855. [Google Scholar]
- Rettig G, Fritzsch B, Himstedt W. Development of retinofugal neuropil areas in the brain of the alpine newt, Triturus alpestris. Anatomy and embryology. 1981;162:163–171. doi: 10.1007/BF00306488. [DOI] [PubMed] [Google Scholar]
- Rigon F, Gasparini F, Shimeld SM, Candiani S, Manni L. Developmental signature, synaptic connectivity and neurotransmission are conserved between vertebrate hair cells and tunicate coronal cells. Journal of Comparative Neurology. 2017 doi: 10.1002/cne.24382. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annual review of neuroscience. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Saito Y, Miranda-Rottmann S, Ruggiu M, Park CY, Fak JJ, et al. NOVA2-mediated RNA regulation is required for axonal pathfinding during development. Elife. 2016;5:e14371. doi: 10.7554/eLife.14371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser G. From so simple a beginning–what amphioxus can teach us about placode evolution. International Journal of Developmental Biology. 2017;61:633–648. doi: 10.1387/ijdb.170127gs. [DOI] [PubMed] [Google Scholar]
- Straka H, Fritzsch B, Glover JC. Connecting Ears to Eye Muscles: Evolution of a ‘Simple'Reflex Arc. Brain, behavior and evolution. 2014;3:162–175. doi: 10.1159/000357833. [DOI] [PubMed] [Google Scholar]
- Tan K, Le Douarin NM. Development of the nuclei and cell migration in the medulla oblongata. Anatomy and embryology. 1991;183:321–343. doi: 10.1007/BF00196834. [DOI] [PubMed] [Google Scholar]
- Tang WJ, Chen JS, Zeller RW. Transcriptional regulation of the peripheral nervous system in Ciona intestinalis. Developmental biology. 2013;378:183–193. doi: 10.1016/j.ydbio.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Tomás-Roca L, Corral-San-Miguel R, Aroca P, Puelles L, Marín F. Crypto-rhombomeres of the mouse medulla oblongata, defined by molecular and morphological features. Brain Structure and Function. 2016;221:815–838. doi: 10.1007/s00429-014-0938-y. [DOI] [PubMed] [Google Scholar]
- Von Baer KE. Über Entwickelungsgeschichte der Thiere: Beobachtung und Reflexion. Bornträger; 1828. [Google Scholar]
- Von Kupffer C. The development of the cranial nerves of vertebrates. Journal of Comparative Neurology. 1891;1:246–264. [Google Scholar]
- Wang VY, Rose MF, Zoghbi HY. Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron. 2005;48:31–43. doi: 10.1016/j.neuron.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Watson C, Kirkcaldie M, Puelles L. Developmental gene expression redefines the mammalian brain stem. In: Kaas J, editor. Evolution of the Nervous Systems. Elsevier; Oxford: 2017a. pp. 467–475. [Google Scholar]
- Watson C, Shimogori T, Puelles L. Mouse Fgf8-Cre-LacZ lineage analysis defines the territory of the postnatal mammalian isthmus. Journal of Comparative Neurology. 2017b doi: 10.1002/cne.24242. [DOI] [PubMed] [Google Scholar]
- Wolff C. De formatione intestinorum praecipue, turn et de amnio, aliisque partibus embryonis gallinacei nondum visis. Novi Commentarii Academiae Scientiarum Imperialis Petropolitanae. 1768;12:13. [Google Scholar]
- Yamada M, Terao M, Terashima T, Fujiyama T, Kawaguchi Y, et al. Origin of climbing fiber neurons and their developmental dependence on Ptf1a. Journal of Neuroscience. 2007;27:10924–10934. doi: 10.1523/JNEUROSCI.1423-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Bassuk AG, Stricker S, Fritzsch B. Prickle1 is necessary for the caudal migration of murine facial branchiomotor neurons. Cell and tissue research. 2014;357:549–561. doi: 10.1007/s00441-014-1925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]