Abstract
Background
Prenatal folic acid supplementation is recommended to prevent birth defects. Some foods are fortified in the U.S. to ensure sufficient intake among reproductive-aged women. However, high prenatal folate exposure may be a risk factor for childhood atopic diseases. We investigated associations between prenatal folate and early childhood wheeze and atopic dermatitis in a U.S. cohort.
Methods
We studied 858 mother-child dyads, enrolled prenatally. Folate was measured in 2nd and 3rd trimester maternal plasma. Parents reported current wheeze (previous 12 months) and healthcare provider diagnosis of atopic dermatitis at 3 years. We examined associations using logistic regression, modeling folate continuously and dichotomously (< or ≥20 ng/ml), a level often considered supraphysiologic.
Results
Over half of women were African-American and on Medicaid. Median (interquartile range) folate levels were 22.6 (15.9–30.0) and 23.1 (16.1–30.0) ng/mL for 2nd and 3rd trimesters, respectively. Current wheeze and atopic dermatitis were reported for 20.4% and 26.8% of children, respectively. Second trimester folate as a continuous exposure was not significantly associated with outcomes. Decreased odds of current wheeze was observed in children born to mothers who had 2nd trimester folate ≥ 20 ng/mL (adjusted odds ratios = 0.67, 95% confidence interval = 0.46, 0.97) compared to children with maternal levels < 20 ng/mL. Third trimester folate was not associated with outcomes.
Conclusions
High plasma folate in mid-pregnancy was associated with decreased odds of current wheeze at age 3. Our findings do not support harmful effects of high prenatal folate levels on childhood atopic diseases in this setting.
Keywords: asthma, atopy, wheeze, atopic dermatitis, folic acid, folate, prenatal, child
Introduction
Wheezing and atopic dermatitis are common and associated with substantial morbidity in early childhood and often precede the onset of asthma later in childhood (1, 2). Genetics and host factors have been indicated in the complex etiology of atopic diseases, as have environmental and nutritional exposures (3). High prenatal folic acid exposure has been studied as a risk factor for childhood atopic diseases, due in part to its recognized capacity to epigenetically modify DNA (4). Some observational studies have suggested that higher folic acid during pregnancy may be associated with increased risk of allergic and respiratory phenotypes in offspring (5–10), while other studies found null (11–14) or inverse associations (13, 15).
Women of child-bearing age are advised to consume a daily supplement of 400 – 800 μg/day of folic acid to reduce the risk of infants born with neural tube defects (16). In the U.S., mandatory folic acid fortification of many enriched cereal and grain products has been implemented since 1998 to achieve sufficient intake, particularly among women who may have unplanned pregnancies (17).
To date, most studies of prenatal folate exposure and childhood atopic diseases have been conducted in countries without mandatory fortification, and many studies did not capture blood folate levels (6, 11, 12, 14). Relative to non-fortified populations, women in the U.S. may experience high levels of folate due to combined exposure to food fortification and prenatal supplementation. However, the relationship between prenatal folate exposure using biomarkers and childhood atopic disease has not been well characterized among U.S. women and children. Thus, we sought to examine the relationship of maternal prenatal folate status with child wheeze in the 3rd year of life and atopic dermatitis at age 3 years in a prenatal U.S. cohort of mother-child dyads.
Methods
Study population
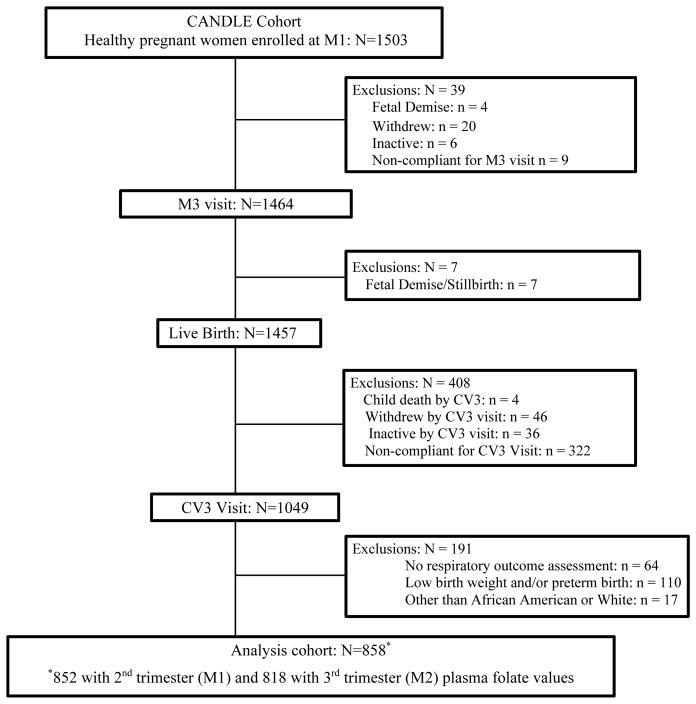
Participants were enrolled in the Conditions Affecting Neurocognitive Development in Early Childhood (CANDLE) study, a prospective prenatal cohort in Memphis, Tennessee. Briefly, 1503 healthy women, 16–40 years of age, with a singleton pregnancy, and intent to deliver at a study hospital were enrolled during 16–28 weeks estimated gestational age (EGA) from community-based obstetric practices between 2006 and 2011 (18, 19). The CANDLE study included 2nd and 3rd trimester and delivery visits during which we obtained demographics, psychosocial and medical histories, and biospecimens. Mother-child dyads were followed with in-person study visits annually. In total, there were 1457 live-births. Of these, 1049 dyads had a visit at age 3 years and 985 had a visit that included assessment of child respiratory and atopic disease (Fig 1). Low birth weight (LBW < 2500 g) and/or preterm (EGA < 37 weeks) (n=110) infants were excluded to study the relationship between prenatal folate levels and atopic diseases in children without potential preexisting lung disease. We restricted analyses to African-American and White women because women of other races were too few to study (n=17). The analytical sample included 858 dyads. The study was approved by the Institutional Review Boards of University of Tennessee Health Sciences Center (UTHSC) and Vanderbilt University.
Fig 1.
Flow chart of the mother-child dyads enrolled in the Conditions Affecting Neurocognitive Development in Early Childhood (CANDLE) study between 2006–2011 and the study cohort at child age 3 years
M1= maternal visit 1 (at 2nd trimester); M2= maternal visit 2 (at 3rd trimester);
M3= maternal visit 3 (at delivery); CV3= child visit at age 3 years
Maternal blood collection and laboratory measurements
Whole blood was collected at 2nd and 3rd trimester visits. Plasma was separated by centrifuging at 3000 pm for 10 minutes and stored at −70°C at UTHSC until analysis. Folate levels were assessed by a Lactobacillus casei microbiological assay at the University of Alabama at Birmingham (20). The minimum detection limit of the assay was 3 ng/mL, with 4.8 – 5.9 % intra-assay and 5.5 – 6.5% inter-assay variability for the laboratory assay controls.
Assessment of current wheeze and atopic dermatitis at age 3 years
We defined current wheeze as parental report of “wheezing or whistling in the chest at any time in the past” (21) and one or more episodes of wheezing within the past 12 months, which captured current wheeze in the 3rd year of life. Atopic dermatitis was based on affirmative response to the question, “has a doctor or a healthcare provider ever diagnosed your child with eczema or atopic dermatitis” administered during the 3rd year of life.
Statistical analysis
We report median and inter-quartile ranges (IQR) for continuous and frequencies and proportions for categorical measures. Maternal and child characteristics were compared by current wheeze and atopic dermatitis status using Kruskal-Wallis and chi-square tests for continuous and categorical variables.
Current wheeze and atopic dermatitis were modeled as binary outcomes. Folate concentration was included as continuous predictor (10 ng/mL change) in unadjusted and adjusted logistic regression analyses. A restricted cubic spline model with 3 knots was used to test the non-linear relationships between prenatal folate levels and the log-odds of current wheeze and atopic dermatitis. Folate was also analyzed as a dichotomous exposure: < or ≥ 20 ng/ml. Plasma folate levels > 20 ng/mL (~45 nmol/L) have been considered supraphysiologic, with some concern for harmful effects at this level in certain clinical populations (22, 23). Folate was also characterized by quartiles. We modeled 2nd and 3rd trimester folate levels separately for each approach.
Covariates were selected a priori based on the literature and plausible associations with primary exposure and outcome (6–8). Multivariable analyses were adjusted for maternal age at enrollment, self-reported race (African-American/White), education (less than high-school/high-school or beyond), prenatal smoking (yes/no), asthma (yes/no), pre-pregnancy body mass index (BMI, Kg/m2), 2nd trimester vitamin D levels, parity (none/ ≥1), delivery route (vaginal/caesarean), and child sex and birth weight. Because there was 12% missing data on maternal report of breastfeeding, we conducted sensitivity analysis on the subset that included breastfeeding (yes/no) as a potential covariate. For the subset that completed a 2nd trimester Food Frequency Questionnaire (FFQ), intake of other nutrients important for DNA methylation (vitamin B6, B12, choline, methionine and betaine) were estimated (18) and were tested as potential confounders in multivariable models using a 10% change in estimate criteria.
We conducted sensitivity analyses including dyads with infants born pre-term and/or LBW in the models of continuous and dichotomized folate measures with child current wheeze and adjusted for birth weight and EGA along with other covariates. Additionally, we conducted sensitivity analyses including insurance status (Medicaid vs private/employee/other) in the multivariable models. We tested for potential interaction between prenatal folate levels and child outcomes by maternal asthma, prenatal smoking, and vitamin D levels in separate covariate-adjusted models. Prenatal vitamin D status was tested in adjusted models independently by race (African-American and White) and additionally adjusted for birth season. All statistical tests were considered significant at p< 0.05. Statistical analyses were conducted using STATA 12.0 (STATA Corp, College Station, TX).
Results
Of the 858 dyads 63.4% of mothers were African-American and, more than half had less than high-school education (53.9%) and on Medicaid (53.7%). About 11% reported a history of asthma. Infants were born at median 39.2 EGA weeks and weighed a median 3324 grams (Table 1). Compared to those included in our analysis, the other term, non-low birth weight dyads who were lost to follow-up were more likely to be African-American (66.1%), have less than high-school education (66.7%), be on Medicaid (67.2%), and be single (48.3% vs. 40.4%), and have lower prenatal folate. However, maternal asthma, delivery route, breastfeeding, child sex and EGA did not differ between the two groups (Table S1).
Table 1.
Maternal and child characteristics by current wheeze and atopic dermatitis at age 3 years in the CANDLE Study
| Characteristics | Wheeze in the 3rd year of life | Atopic dermatitis | All1 n=858 |
||
|---|---|---|---|---|---|
|
| |||||
| Yes n=174 |
No n=675 |
Yes n=231 |
No n=626 |
||
|
| |||||
| Maternal characteristics | |||||
|
| |||||
| Age, median (IQR), y | 26 (21 – 30) | 27 (22 – 31)* | 26 (22 – 30) | 27 (22 – 31) | 27 (22 – 31) |
|
| |||||
| Race, % (n) | |||||
| African American | 69.8 (120) | 61.7 (414) | 76.7 (175) | 58.4 (364) | 63.4 (540) |
| White | 30.2 (52) | 38.3 (257) | 23.2 (53) | 41.6 (259)** | 36.6 (312) |
|
| |||||
| Education, % (n) | |||||
| Less than high school | 59.9 (103) | 52.2 (350) | 56.1 (128) | 53.3 (332) | 53.9 (462) |
| High school or more | 40.1 (69) | 47.8 (321) | 43.9 (100) | 46.7 (291) | 46.1 (396) |
|
| |||||
| Insurance type, % (n) | |||||
| Medicare/ Medicaid | 59.9 (103) | 51.7 (347) | 59.2 (135) | 51.5 (321) | 53.7 (461) |
| Private/Employee | 40.1 (69) | 48.3 (324) | 40.8 (93) | 48.5 (302)* | 46.3 (397) |
|
| |||||
| Married/Living with a partner, % (n) | 50.0 (87) | 62.1 (419)** | 55.4 (128) | 61.0 (382) | 59.6 (511) |
|
| |||||
| Nulliparous, % (n) | 39.1 (68) | 41.6 (281) | 40.7 (94) | 40.9 (256) | 40.8 (350) |
|
| |||||
| Asthma, % (n) | 12.9 (22) | 11.0 (73) | 13.6 (31) | 10.5 (65) | 11.3 (96) |
|
| |||||
| Smoking during pregnancy, % (n) | 7.0 (12) | 8.8 (59) | 7.5 (17) | 8.8 (55) | 8.4 (72) |
|
| |||||
| Cesarean delivery, % (n) | 42.8 (74) | 35.4 (238) | 39.3 (90) | 35.9 (224) | 36.9 (315) |
|
| |||||
| Pre-pregnancy BMI, median (IQR), (kg/m2)2 | 26.4 (22.7 – 34.4) | 25.8 (22.5 – 31.2) | 26.6 (23.2 – 33.2) | 25.7 (22.3 – 31.2) | 25.8 (22.6 – 32.0) |
|
| |||||
| Plasma folate levels, median (IQR), ng/ml3 | |||||
| 2nd trimester; n=852 | 20.2 (14.4 – 28.8) | 23.1 (16.4 – 30.1) | 22.4 (14.7 – 28.9) | 22.6 (16.3 – 30.2) | 22.6 (15.9 – 30.0) |
| 3rd trimester; n=818 | 22.4 (14.8 – 28.8) | 23.2 (16.6 – 30.7) | 21.5 (14.3 – 29.4) | 23.8 (17.3 – 30.2)** | 23.1 (16.1 – 30.0) |
|
| |||||
| Used supplement containing folic acid at 2nd trimester, %(n); n=758 | 95.5 (142) | 93.4 (570) | 94.5 (190) | 95.3 (530) | 95.1 (721) |
|
| |||||
| 2nd trimester folate intake4, median (IQR); n=714 | |||||
| Food folate (μg/d) | 291 (207 – 394) | 281 (207 – 381) | 319 (231 – 420) | 268 (201 369)** | 284 (207 – 383) |
| Total folic acid (μg/d)5 | 733 (665 – 835) | 740 (674 – 858) | 744 (671 – 863) | 739 (673 – 844) | 739 (673 – 848) |
| Total DFE (μg/d)6 | 1576 (1359 –1787) | 1580 (1355 – 1827) | 1607 (1384 – 1844) | 1561 (1354 – 1797) | 1576 (1355 –1813) |
|
| |||||
| 2nd trimester plasma vitamin D level, median (IQR), ng/ml; n=848 | 22.3 (15.9 – 27.0) | 22.3 (16.2 – 27.4) | 21.3 (15.6 – 27.1) | 22.6 (16.3 – 27.4) | 22.1 (16.1 – 27.3) |
|
| |||||
| Breastfeeding7, % (n); n=750 | 46.4 (70) | 55.2 (327) | 45.8 (92) | 56.6 (310)* | 53.7 (405) |
|
| |||||
| Child characteristics | |||||
|
| |||||
| Gender, female, % (n) | 43.0 (74) | 52.6 (353)** | 50.9 (116) | 50.6 (315) | 50.6 (431) |
|
| |||||
| Gestational age, median (IQR), weeks | 39.2 (38.6 – 40.0) | 39.2 (38.6 – 40.0) | 39.2 (38.5 – 40) | 39.2 (38.6 – 40.0) | 39.2 (38.6 – 40.0) |
|
| |||||
| Birth weight, median (IQR), g | 3307 (3057 – 3677) | 3330 (3074 – 3625) | 3270 (3017 – 3500) | 3360 (3091–3363)** | 3324 (3070 – 3629) |
Includes dyads with information on at least one outcome; in total, 849 and 857 dyads had information on current wheeze and atopic dermatitis, respectively; Numbers do not always add up to 858 because of varied number of missing data for each variable
BMI indicates Body Mass Index; calculated from self-reported pre-pregnancy height and weight
1 ng/mL = ~2.3 nmol/L
Captured using a food-frequency questionnaire
total folic acid includes folic acid in fortified foods and supplements
DFE indicates Dietary Folate Equivalent; total DFE calculated as food folate + 1.7 x folic acid from fortified foods and supplement
Reported at 4 weeks postpartum.
P< 0.05
P< 0.01
Approximately 20% of children (174/849) had current wheeze in the 3rd year of life and 27% (231/857) had atopic dermatitis. Children with current wheeze were more likely to be males (57%, p< 0.01); for atopic dermatitis there were no differences by sex. Children with atopic dermatitis were more likely to have mothers who were African-American or on Medicaid than those without (Table 1).
Median (IQR) 2nd and 3rd trimester folate levels were 22.6 (15.9 – 30.0) ng/mL (n= 852) and 23.1 (16.1 – 30.0) ng/mL (n= 818), respectively (Table 1). There was a modest correlation (spearman rho= 0.5; p< 0.001) between 2nd and 3rd trimester folate levels. Among women for whom FFQs were available (n= 758), 95% reported consuming folic acid supplements during 2nd trimester (Table 1). Women with higher folate (≥ 20 ng/mL) were more likely to be older, White, more educated, have higher vitamin D levels, and were more likely to breastfeed than those with lower levels (< 20 ng/mL) (Table S2).
The relative odds of current wheeze in the 3rd year of life was approximately one-third lower in children born to mothers with 2nd trimester folate levels ≥ 20 ng/mL versus < 20 ng/mL [adjusted OR (aOR) = 0.67, 95% CI = 0.46, 0.97]. We did not detect a statistically significant association with current wheeze when 2nd trimester levels were modeled continuously in unadjusted [OR: 0.91, 95% CI = 0.80, 1.05] or adjusted logistic regression models [adjusted OR (aOR) = 0.97, 95% CI = 0.84, 1.12] (Table 2). We did not observe significant associations or a dose response relationship when prenatal folate was tested as quartiles (Table S3).
Table 2.
Association between prenatal folate status and child wheeze at age 3 years
| Current wheeze in the 3rd year of life | ||
|---|---|---|
|
| ||
| Unadjusted OR [95% CI] | Adjusted OR [95% CI]1 | |
| 2nd trimester (n= 843) | ||
| Maternal plasma folate level (10 ng/ml)2 | 0.91 [0.80, 1.05] | 0.97 [0.84, 1.12] |
|
| ||
| 2nd trimester | ||
| Maternal plasma folate level | ||
| < 20 ng/ml (n= 335) | 1.00 (ref) | 1.00 (ref) |
| ≥ 20 ng/ml3 (n= 508) | 0.61 [0.43, 0.85]** | 0.67 [0.46, 0.97]* |
|
| ||
| 3rd trimester (n= 809) | ||
| Maternal plasma folate level (10 ng/ml)2 | 0.91 [0.78, 1.05] | 1.01 [0.86, 1.18] |
|
| ||
| 3rd trimester | ||
| Maternal plasma folate level | ||
| < 20 ng/ml (n= 293) | 1.00 (ref) | 1.00 (ref) |
| ≥ 20 ng/ml3 (n= 516) | 0.73 [0.51, 1.04] | 0.91 [0.62, 1.34] |
Models adjusted for maternal age, race, education, parity, smoking during pregnancy, asthma history, pre-pregnancy BMI, 2nd trimester plasma vitamin D level, delivery route, and child sex and birth weight
Plasma folate concentrations entered as a continuous measure; expressed as 10 ng/ml increment
Considered as higher than physiologically relevant level of plasma folate
OR and 95% CI indicate Odds Ratio and 95% Confidence Interval
1 ng/mL = ~2.3 nmol/L
P< 0.05
P< 0.01
The tests for non-linear relationships between prenatal folate levels and child outcomes were also non-significant. We did not detect a statistically significant relationship between 3rd trimester folate and current wheeze in dichotomous (aOR = 0.91, 95% CI = 0.62, 1.34) or continuous models (aOR = 1.01, 95% CI = 0.86, 1.18) (Table 2). Study results did not appreciably change when breastfeeding was included as a covariate (data not shown). Associations between maternal folate levels and child wheeze did not differ by maternal asthma (p-interaction=0.2), maternal smoking (p-interaction=0.9), or vitamin D status (African-American: p-interaction=0.3; White: p-interaction=0.7).
Maternal 2nd trimester folate levels were not associated with child atopic dermatitis (Table 3). In unadjusted analyses, we observed decreased relative odds of atopic dermatitis with a 10 ng/mL increase in maternal 3rd trimester folate levels and with 3rd trimester folate levels ≥ 20 ng/mL. However, these differences did not reach statistical significance in multivariable models (aOR = 0.87 [95% CI = 0.75, 1.01] for continuous; aOR = 0.84 [95% CI = 0.59, 1.18] for dichotomous folate).
Table 3.
Prenatal folate status and atopic dermatitis at 3 years of age
| Atopic dermatitis at age 3 years | ||
|---|---|---|
|
| ||
| Unadjusted OR [95% CI] | Adjusted OR [95% CI]1 | |
| 2nd trimester (n= 851) | ||
| Maternal plasma folate level (10 ng/ml)2 | 0.96 [0.85, 1.09] | 1.04 [0.92, 1.19] |
|
| ||
| 2nd trimester | ||
| Maternal plasma folate level | ||
| < 20 ng/ml (n= 339) | 1.00 (ref) | 1.00 (ref) |
| ≥ 20 ng/ml3 (n= 512) | 0.92 [0.67, 1.26] | 1.19 [0.85, 1.68] |
|
| ||
| 3rd trimester (n= 817) | ||
| Maternal plasma folate level (10 ng/ml)2 | 0.82 [0.97, 0.99]** | 0.87 [0.75, 1.01] |
|
| ||
| 3rd trimester | ||
| Maternal plasma folate level | ||
| < 20 ng/ml (n= 297) | 1.00 (ref) | 1.00 (ref) |
| ≥ 20 ng/ml3 (n= 520) | 0.71 [0.52, 0.98]* | 0.84 [0.59, 1.18] |
Models adjusted for maternal age, race, education, parity, smoking during pregnancy, asthma history, pre-pregnancy BMI, 2nd trimester plasma vitamin D level, delivery route, and child sex and birth weight
Plasma folate concentrations entered as a continuous measure; expressed as 10 ng/ml increment
Considered as higher than physiologically relevant level of plasma folate
OR and 95% CI indicate Odds Ratio and 95% Confidence Interval
1 ng/mL = ~2.3 nmol/L
P< 0.05
P< 0.01
Results were not significantly different when dyads with infants born pre-term and/or LBW were included in models (Table S4). Additionally, sensitivity analyses that included insurance status or intakes of methyl donors (vitamin B6, B12, choline, methionine and betaine) did not appreciably change the results (data not shown).
Discussion
We investigated the association between prenatal plasma folate and early childhood wheeze and atopic dermatitis, two childhood conditions that commonly precede asthma. In our cohort, over half of the women had high prenatal folate levels (above 20 ng/mL). This is in contrast with other studies with much lower prenatal folate levels (5, 7, 8, 13). Despite concerns that high folate exposure may be associated with adverse health effects, we observed that these higher 2nd trimester folate levels were associated with a modest decrease in relative odds of child wheeze in the 3rd year of life. While we cannot rule out unmeasured confounding, this association was robust to adjustment from multiple potential confounders, suggesting the protective association with folate is not driven by factors such as higher socio-economic status among the highly-exposed. Notably, we did not observe a similar association using 3rd trimester measurements.
Our finding of a decreased risk of current wheeze in the third year of life among children with higher maternal plasma folate (≥ 20 ng/mL) is in line with findings from a nested case-control study using the KOALA cohort in the Netherlands (13). A dose-related decreased odds of asthma at 6–7 years was found with higher maternal 3rd trimester erythrocyte folate levels (13). In contrast, the Norwegian Mother and Child Cohort Study (MoBa), reported an increased risk of asthma at 3 years in children born to mothers in the highest plasma folate levels (> 17.8 nmol/L or ~ 8 ng/mL) relative to children with mothers in the lowest levels (folate < 5.5 nmol/L or ~ 2.5 ng/mL) (8). A recent MoBa study reported a modest but increased risk of asthma at age 7 years with higher maternal total folate intake (5). However, 2nd trimester maternal plasma folate levels (median 8.7 nmol/L or ~3.8 ng/mL) were not associated with asthma in a sub-set of 2681children (5).
Folic acid supplement use or prescription fills have been associated with increased risk of child wheeze or asthma in populations with (6) and without mandatory folic acid fortification (9–12). The contrasting results between studies of self-reported prenatal folate intake or supplement use and those measuring circulating folate levels could be due to potential challenges in accurately capturing usual intake or differences in timing of the exposure. Furthermore, other lifestyle factors, genetic polymorphisms, gut microbiome or other nutrients could affect folate absorption, metabolism, blood folate status, and subsequent associations with child outcomes (24).
We did not find statistically significant associations between 2nd or 3rd trimester folate and child atopic dermatitis. Our results are consistent with those of an Australian Birth Cohort Study which reported that 3rd trimester serum folate levels were not associated with infant atopic dermatitis (25). In contrast, the Generation R study found slightly increased atopic dermatitis at age 4 in Dutch children born to mothers with 1st trimester plasma folate > 16.2 nmol/L (~7 ng/mL) (7), and higher mid-pregnancy serum folate (>9.5 ng/mL) was associated with decreased atopic dermatitis at 2 years in a South Korean cohort (15).
The mechanisms behind potential effects of folate on child wheeze are not clear. Potentially, folate may act as an antioxidant (26) or may reduce inflammation via altering the immune system through epigenetic changes (27). Wheezing is not always related to atopy, and different immune mechanisms may be involved in atopic and non-atopic wheezing (28), which may explain somewhat differential associations observed (7, 13, 28).
There are limitations to consider. We measured plasma folate which reflects short-term folate status (~3 weeks). However, levels were assessed at defined time points and the 2nd and 3rd trimester folate levels were significantly correlated, indicating relatively stable folate status across pregnancy. Although we used parental report to characterize outcomes (29), previous studies have found good agreement between the parent report and physician diagnosis of atopic diseases (7). Moreover, we captured wheeze in the 3rd year of life as opposed to wheezing that occurred in the first two years; it is therefore less likely to be attributed to viral bronchiolitis. While we adjusted for maternal asthma, this did not include other atopic diseases, however maternal asthma history is one of the strongest predictors of childhood asthma (30). Loss to follow-up is another limitation, however included participants were similar to original cohort in that they were predominantly African-American, low-income dyads with high maternal prenatal folate levels. Our study sample remains an understudied population with wide generalizability
In summary, in this prospective U.S. cohort of dyads, more than half were exposed to 20 ng/mL or greater folate during pregnancy. High plasma folate levels in the 2nd trimester was associated with a decreased relative odds of child wheeze in the 3rd year of life. Concerns of potential harmful effects of high maternal gestational folate levels on early childhood wheeze were not supported in this setting.
Supplementary Material
Acknowledgments
We acknowledge the contributions of study research staffs and families that are enrolled in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood study.
References
- 1.Hovland V, Riiser A, Mowinckel P, Carlsen KH, Lodrup Carlsen KC. The significance of early recurrent wheeze for asthma outcomes in late childhood. Eur Respir J. 2013;41:838–45. doi: 10.1183/09031936.00071512. [DOI] [PubMed] [Google Scholar]
- 2.van der Hulst AE, Klip H, Brand PL. Risk of developing asthma in young children with atopic eczema: a systematic review. J Allergy Clin Immunol. 2007;120:565–9. doi: 10.1016/j.jaci.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Campbell DE, Boyle RJ, Thornton CA, Prescott SL. Mechanisms of allergic disease - environmental and genetic determinants for the development of allergy. Clin Exp Allergy. 2015;45:844–58. doi: 10.1111/cea.12531. [DOI] [PubMed] [Google Scholar]
- 4.Crider KS, Yang TP, Berry RJ, Bailey LB. Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate’s role. Adv Nutr. 2012;3:21–38. doi: 10.3945/an.111.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parr CL, Magnus MC, Karlstad O, et al. Maternal Folate Intake During Pregnancy and Childhood Asthma in a Population Based Cohort. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201604-0788OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veeranki SP, Gebretsadik T, Mitchel EF, et al. Maternal Folic Acid Supplementation During Pregnancy and Early Childhood Asthma. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiefte-de Jong JC, Timmermans S, Jaddoe VW, et al. High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr. 2012;142:731–8. doi: 10.3945/jn.111.154948. [DOI] [PubMed] [Google Scholar]
- 8.Haberg SE, London SJ, Nafstad P, et al. Maternal folate levels in pregnancy and asthma in children at age 3 years. J Allergy Clin Immunol. 2011;127:262–4. 64e1. doi: 10.1016/j.jaci.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–4. doi: 10.1136/adc.2008.142448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitrow MJ, Moore VM, Rumbold AR, Davies MJ. Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol. 2009;170:1486–93. doi: 10.1093/aje/kwp315. [DOI] [PubMed] [Google Scholar]
- 11.Bekkers MB, Elstgeest LE, Scholtens S, et al. Maternal use of folic acid supplements during pregnancy, and childhood respiratory health and atopy. Eur Respir J. 2012;39:1468–74. doi: 10.1183/09031936.00094511. [DOI] [PubMed] [Google Scholar]
- 12.Martinussen MP, Risnes KR, Jacobsen GW, Bracken MB. Folic acid supplementation in early pregnancy and asthma in children aged 6 years. Am J Obstet Gynecol. 2012;206:72e1–7. doi: 10.1016/j.ajog.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magdelijns FJ, Mommers M, Penders J, Smits L, Thijs C. Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics. 2011;128:e135–44. doi: 10.1542/peds.2010-1690. [DOI] [PubMed] [Google Scholar]
- 14.Granell R, Heron J, Lewis S, Davey Smith G, Sterne JA, Henderson J. The association between mother and child MTHFR C677T polymorphisms, dietary folate intake and childhood atopy in a population-based, longitudinal birth cohort. Clin Exp Allergy. 2008;38:320–8. doi: 10.1111/j.1365-2222.2007.02902.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Jeong KS, Ha EH, et al. Relationship between prenatal and postnatal exposures to folate and risks of allergic and respiratory diseases in early childhood. Pediatr Pulmonol. 2015;50:155–63. doi: 10.1002/ppul.23025. [DOI] [PubMed] [Google Scholar]
- 16.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317:183–89. doi: 10.1001/jama.2016.19438. [DOI] [PubMed] [Google Scholar]
- 17.Food additives permitted for direct addition to food for human consumption; folic acid (folacin) Vol. 61. US Food and Drug Administration; 1996. pp. 8798–807. [PubMed] [Google Scholar]
- 18.Völgyi E, Carroll K, Hare M, et al. Dietary Patterns in Pregnancy and Effects on Nutrient Intake in the Mid-South: The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) Study. Nutrients. 2013;5:1511–30. doi: 10.3390/nu5051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tylavsky FA, Kocak M, Murphy LE, et al. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients. 2015;7:9918–30. doi: 10.3390/nu7125499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piyathilake CJ, Macaluso M, Hine RJ, Richards EW, Krumdieck CL. Local and systemic effects of cigarette smoking on folate and vitamin B-12. Am J Clin Nutr. 1994;60:559–66. doi: 10.1093/ajcn/60.4.559. [DOI] [PubMed] [Google Scholar]
- 21.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 22.Piyathilake CJ, Macaluso M, Alvarez RD, Bell WC, Heimburger DC, Partridge EE. Lower risk of cervical intraepithelial neoplasia in women with high plasma folate and sufficient vitamin B12 in the post-folic acid fortification era. Cancer Prev Res (Phila) 2009;2:658–64. doi: 10.1158/1940-6207.CAPR-08-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–33. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Litonjua A. Asthma, allergy, and responses to methyl donor supplements and nutrients. J Allergy Clin Immunol. 2014;133:1246–54. doi: 10.1016/j.jaci.2013.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunstan JA, West C, McCarthy S, et al. The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy. 2012;67:50–7. doi: 10.1111/j.1398-9995.2011.02714.x. [DOI] [PubMed] [Google Scholar]
- 26.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radic Biol Med. 2001;30:1390–9. doi: 10.1016/s0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 27.Dhur A, Galan P, Hercberg S. Folate status and the immune system. Prog Food Nutr Sci. 1991;15:43–60. [PubMed] [Google Scholar]
- 28.Turkeli A, Yilmaz O, Taneli F, et al. IL-5, IL-8 and MMP -9 levels in exhaled breath condensate of atopic and nonatopic asthmatic children. Respir Med. 2015;109:680–8. doi: 10.1016/j.rmed.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Cane RS, Ranganathan SC, McKenzie SA. What do parents of wheezy children understand by “wheeze”? Arch Dis Child. 2000;82:327–32. doi: 10.1136/adc.82.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158:176–81. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.