Abstract
The concept that the gut microbiota plays a broadly important role in health and disease in general, and metabolic health in particular, is now well established. However, many of the underlying mechanisms remain poorly understood while approaches to reliably manipulate the microbiota to promote health have not yet been clearly defined. Nonetheless, progress in these areas is steadily accelerating. Herein, we review select areas of progress that have been made in the last year that should hasten the era in which the microbiota can be therapeutically manipulated to promote metabolic health.
Keywords: inflammation, metabolic disorders
INTRODUCTION
The mammalian intestine harbors an interconnected community of bacteria, fungus, yeast, and virus, collectivity referred to as gut microbiota. The observation that animals lacking a microbiota (germfree animals) can live and breed in this state indicates that this complex community of microorganism is not absolutely required for host survival. Nonetheless, the intestinal microbiota plays a key role in determining host health, in part, by impacting the immune system and metabolism (9, 27, 42, 45). Pioneer studies by Turnbaugh et al. have brought to light the central impact of the microbiota on host metabolism, including the observation that obesity is associated with microbiota alterations that are sufficient to transfer metabolic abnormalities when transplanted to germfree recipient animals (46). Such observations hastened a new era in microbiota research that helped elucidate the role of the microbiota in development, health, and disease. In this review, we will highlight some the most recent and important studies in microbiota research, particularly related to metabolism.
HOW TO IMPACT MICROBIOTA AND METABOLISM? DIET, DIET, AND DIET
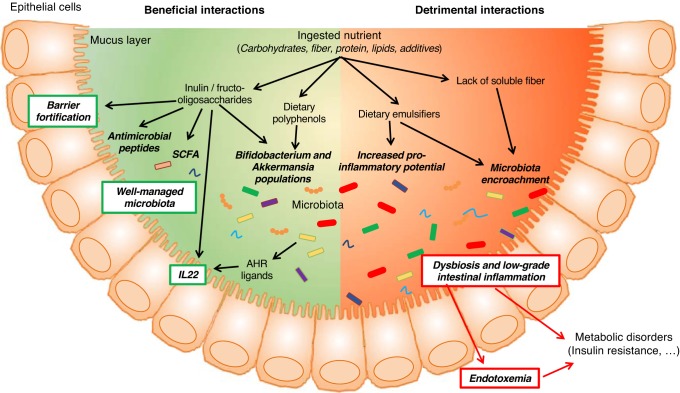
Although numerous factors determine microbiota composition, especially one’s early environment, a central role for diet has been recently appreciated. Moreover, considering that one cannot change their past early environmental exposures, diet is increasingly recognized as a modulatable parameter by which one can influence microbiota and, subsequently, impact metabolism (Fig. 1). In particular, recent studies have highlighted the importance of select molecule types that one eats, when one eats it, and the context in which they are eaten in impacting host-microbiota interactions and, consequently, host health. One dramatic example of this concept is the observation that switching mice from a regular chow diet to a high-fat diet has drastic and very rapid impact on the microbiota. Such altered microbiota was able to further promote metabolic alterations when transplanted to germfree animals, in part by driving low-grade inflammation in the new host (10). During the last years, many studies have further highlighted the role played by diet and dietary habits on metabolism, through microbiota-dependent mechanisms.
Fig. 1.
Overview of the recent findings regarding diet impacts on the microbiota with consequences on host metabolism. AHR, aryl hydrocarbon receptor; SCFA, short-chain fatty acid; IL-22, interleukin-22.
Among dietary factors impacting the microbiota, fermentable fibers are playing a particularly important role. Dietary fiber can be broadly categorized as soluble, which is readily fermentable by bacteria in the gastrointestinal tract of mice and humans, or insoluble, for example, cellulose, which is resistant to breakdown by mice and humans but can be fermented by ruminant animals with more extensive gastrointestinal tracts. However, such broad classification of fiber types is not sufficient to explain fiber impacts on microbiota and host phenotype, since recent studies demonstrated that specific carbohydrate structures of fiber and fiber size contribute to their effects as well (43, 44, 52).
Fermentable fibers are thought to nourish a diverse microbial community, and the importance of fermentable fiber can be readily appreciated by examining the consequences of its absence in mice diets. For example, Martens and colleagues observed that lack of fiber resulted in microbiota consuming the mucus layer, which made the intestine more prone to attack by invasive pathogens (19), whereas we reported that lack of fermentable fibers results in greatly reduced epithelial cell proliferation that leads to a thin mucosa in which bacteria readily encroach (13, 35). Such promotion of low-grade inflammation was responsible for a significant portion of metabolic syndrome induced by high-fat diet, which is generally devoid of fermentable fiber. These publications add to the established body of work that previously demonstrated the beneficial impact of fiber supplementation on microbiota and metabolism. Notably, work from the laboratories of Delzenne and Cani demonstrated that dietary supplementation with soluble fibers, such as inulin or fructooligosaccharides (an inulin-type fructan), is sufficient to improve high-fat diet-induced diabetes through mechanisms involving fortification of the intestinal barrier by modulating tight junction protein expression and epithelium proliferation, together with prevention of endotoxemia and increased expression of antimicrobial peptides (7, 8, 21, 22, 52). Moreover, inulin supplementation was reported to increase the number of colonic goblet cells that associate with an increase in mucus thickness (30), wherein such effects associated with beneficial alteration in microbiota composition characterized by increases in the Bifidobacterium and Akkermansia genus (20–22).
In accord with these notions, recent papers by ourselves and Bäckhed and colleagues reported that supplementing high-fat diet with the fermentable fiber inulin, but not the insoluble fiber cellulose, resulted in a microbiota-dependent fortification of the mucosa that prevented microbiota encroachment and protected against high-fat diet-induced metabolic syndrome (13, 35, 40, 53). Backhed’s group found that the key change in microbiota composition conferred by inulin enrichment was a large increase in the relative abundance of Bifidobacteria, which are known to be prodigious producers of short-chain fatty acids (SCFA) (40). However, interestingly, our recent study found that such protective effects of fermentable fiber were not largely mediated by SCFA generation but rather by a previously unidentified mechanism involving induction of interleukin-22 (IL-22), whose levels were depleted by lack of fiber and restored by inulin in a microbiota-dependent manner (53).
Besides fiber content and composition, other nutrients are also directly impacting the intestinal microbiota, such as dietary lipids, with, for example, the previous observation that lard and fish oil are differentially impacting the intestinal microbiota with subsequent consequences on the host metabolism (6). Most recently, it was observed that enrichment of diet with butter, but not olive oil, detrimentally impacts metabolism, which correlated with alterations of the intestinal microbiota composition, thus suggesting distinct impacts of saturated and polyunsaturated fats on these parameters (38).
Another recent finding highlighting the importance of diet on microbiota and metabolism is the finding that intermittent fasting, an effective and natural strategy for weight control, stimulates the development of beige fat and dramatically improves obesity, insulin resistance, and hepatic steatosis (33). The authors found that every-other-day fasting led to alterations in microbiota composition that subsequently plays a central role on metabolism improvement, in that an antibiotic regiment prevented fasting’s beneficial effects, whereas microbiota transplantation from fasted mice was sufficient to activate beiging of adipose tissue and improve metabolic homeostasis. Hence, not only is diet content important, but meal frequency can play a role in determining microbiota composition, which can influence metabolic disorders.
TRANSLATING ANIMAL STUDIES TO HUMAN OBSERVATIONS
Many pioneering studies on microbiota and its impact on metabolism were performed in rodents. Although such studies provide a tractable platform for mechanistic studies, they may not be reflective of the situation in humans. Hence, a variety of approaches have been employed to begin to bridge this gap. We previously hypothesized that emulsifiers, which are added to most processed foods to aid texture and extend shelf life, could play a role in the post- to mid-20th-century increase in incidence of metabolic syndrome and inflammatory bowel disease. Indeed, we demonstrated that emulsifiers induced a chronic intestinal inflammation that promotes development of chronic colitis in susceptible mice and metabolic alterations in wild-type mice (11). Specifically, by treating mice with two commonly used emulsifiers, namely polysorbate 80 (P80) and carboxymethylcellulose (CMC), we observed changes in species composition of the gut microbiota and in its proinflammatory potential. This altered microbiota had enhanced capacity to infiltrate the dense mucus layer that lines the intestine, which is normally devoid of bacteria. Moreover, the alterations of the microbiota were characterized by an increased expression of bacterial inflammatory molecules, such as flagellin and lipopolysaccharide, which can in turn activate the expression of proinflammatory genes by the immune system. Such functional changes of the microbiota triggered, in wild-type animals, low-grade intestinal inflammation and metabolic syndrome, characterized by increased adiposity and hyperglycemia. To better understand the underlying mechanism, and to start understanding the impact that emulsifiers might have on human microbiota, we recently collaborated with T. Van de Wiele, from Ghent University (Ghent, Belgium), who has developed a state-of-the-art in vitro microbiota model, namely the mucosal-simulated human intestinal microbiota ecosystem (M-SHIME), a dynamic model that simulates the lumen- and mucus-associated human intestinal microbial ecosystem (24, 48, 49). Using this in vitro microbiota model, we demonstrated that both P80 and CMC acted directly on a single human microbiota to alter microbiota composition and/or gene expression (measured by a metatranscriptomic approach), demonstrating that microbiota is a direct target of these commonly used food additives (15). Importantly, when transferred to germfree recipient animals, emulsifier-treated M-SHIME microbiotas induced low-grade intestinal inflammation that associated with metabolic abnormalities in the context of a high-fat diet feeding (15). Hence, these results demonstrate a novel paradigm of deconstructing host-microbiota interactions and indicate that the microbiota can be directly impacted by these commonly used food additives in a manner that subsequently drives intestinal inflammation. Moreover, demonstrating that those compounds can act on human microbiota was an important step toward the ultimate goal of deciphering the impact they may have on human metabolism.
Although interventional clinical trials will ultimately be needed to determine the impact of emulsifiers on host-microbiota interactions in humans, observational studies have afforded us a more general means of determining if functional alterations in human microbiota-host interactions correlated with metabolic disease in humans. Numerous host mechanisms are present to protect the intestine from the potentially harmful microbiota. Among them, mucoid structures that coat the epithelium play an essential role in keeping the intestinal microbiota at a safe distance from host cells. We and others have previously demonstrated that altered microbiota associated with altered metabolism are characterized by the ability to penetrate the normally impermeable mucus layer (11, 12, 29, 53). More recently, by using confocal microscopy to measure bacterial-epithelial distance, we reported that microbiota encroachment is a feature of insulin resistance-associated dysglycemia in humans (14). Such observations, although correlative and not informative of the impact of diet per se, suggest that microbiota encroachment may play a causative role in metabolic health in humans and open avenue to understand how alterations in microbiota can promote metabolic disease.
DAWN OF MUCOSAL MICROBIOLOGY
Similarly to the term “mucosal immunology,” which was created to reflect the appreciation that the study of the immune system at mucosal surfaces would require new techniques and paradigms, it now appears important to consider a “mucosal microbiology” field when studying the intestinal microbiota and its impact on health and diseases. Indeed, the vast majority of microbiota studies, including ours, are conducted by 16S/DNA/RNA sequencing of fecal material, representing the low-hanging fruit of this community, and it is now well appreciated that this community differs from the mucosal bacterial community (5, 16, 23), with intraluminal oxygen gradient being suspected to shape mucus-associated and fecal microbiota (1). Moreover, accumulating data demonstrate that the microbiota subset penetrating the mucus layer, or even associated with the mucosa, is playing a central role in host disturbance (11, 36, 51). This is, for example, highlighted with the observation that immunoglobulin A-coated bacteria, and hence bacteria that have been, at some point, recognized by the intestinal immune system, represent a microbiota subpopulation particularly potent in driving intestinal inflammation. Altogether, this suggests that particular effort should be made on this specific community, since it may lead to the identification of instigators of metabolic alterations, and specific targeting such members of the community may reveal particularly efficient to prevent and/or treat such complex diseases. Interestingly, alterations in mucus-associated microbiota precede the onset of intestinal inflammation in the Mdr1a−/− mouse model, and a recent publication in Science reports that biofilms at the surface of the colonic epithelium are involved in colon carcinogenesis (18), further supporting the important role that this microbiota subpopulation may play in inflammatory diseases, either acute or chronic (25).
In addition, while most efforts made to date in this area relate to the intestinal microbiota focused on the bacterial community, it will soon need to be extended to other classes of microbes, since emerging results continue to demonstrate an important role for fungi, viruses, and bacteriophages in inflammatory diseases (26, 34, 41).
THERAPEUTIC MANIPULATION OF THE MICROBIOTA
Whereas much progress has been made in understanding the myriad of factors that can disturb the intestinal microbiota and promote metabolic disturbances, an elusive goal remains to develop effective means to restore a health-promoting microbiota, yet there has been recent progress in this area. For example, it was demonstrated that metabolic alterations are associated with a reduced proportion of A. muciniphila within the microbiota in both animals and a subset of obese patients (17, 20, 39). Importantly, Cani and colleagues showed that exogenous administration of A. muciniphila to mice is sufficient to prevent the development of obesity and associated complications. Most recently, this group demonstrated that A. muciniphila retains its therapeutic efficacy when grown on a synthetic medium compatible with human administration (37). Furthermore, pasteurization of A. muciniphila enhanced its benefits on metabolism, and the outer membrane protein Amuc_1100 of A. muciniphila was found to improve gut barrier and metabolism, partly recapitulating the beneficial effects of the whole bacterium (37). Such discoveries are important steps toward the development of therapeutic approaches for human metabolic disorders.
Although such studies directly administered a bacterium associated with metabolic health, other groups recently demonstrated that the microbiota can be modulated through diet extract, such as polyphenol-rich cranberry extract. Previously, such polyphenol-rich cranberry extract was found to be sufficient to reduce high fat-associated weight gain, visceral obesity and liver triglyceride accumulation and to improve insulin sensitivity (3). Interestingly, cranberry extract treatment was recently observed to associate with increase in Akkermansia spp bacteria. These results highlight that such purified extract has the ability to beneficially reshape the intestinal microbiota, leading to protection against metabolic health (3, 4). However, whether such compounds have the power to reverse already established metabolic abnormalities was unclear and was only recently investigated. Although cranberry extract cannot reverse weight gain or fat accumulation in high-fat diet-fed animals, it is now reported sufficient to improve glucose tolerance and to fully reverse hepatic steatosis, with mechanism involving microbiota composition modulation (2). Similar findings were recently observed for cinnamon and grape pomace, further highlighting the important role that polyphenols may have in beneficially impacting host metabolism through modulation of the microbiota (50).
Other therapeutic strategies being developed to restore a healthy host/microbiota include the recent finding by Lamas and collaborators. In their study, the authors first reported that the deletion of Card9 gene, a central component of the innate antifungal immune response, renders mice more prone to chemically induced colitis by dextran sulfate sodium (31). Investigating the mechanism beyond such observation, the authors demonstrated that Card9 knockout mice (Card9−/−) display alteration of immune-related signaling pathways in the colon, with a strong decrease in IL-22 production. Using fecal microbial transplantation, they demonstrated that transfer of colitic-associated microbiota from Card9−/−-susceptible hosts were sufficient to transfer colitis susceptibility and IL-22 cytokine production impairment to germ-free wild-type (Card9 sufficient) recipients. Importantly, the colitic-associated microbiota of Card9−/− mice are characterized by the absence of bacteria metabolizing tryptophan in indole derivatives, ligands for the aryl hydrocarbon receptor (AHR) that can drive local production of IL-22 by innate lymphoid cells and T cells (32). Hence, as therapeutic strategy, the authors postulated that altering the intestinal microbiota in genetically susceptible host so as to increase its ability to generate AHR ligands could protect from intestinal inflammation. Importantly, supplementation with three commensal Lactobacilllus strains with high tryptophan-metabolic activities was sufficient to restore intestinal IL-22 production and to reverse the colitis susceptibility observed in susceptible Card9−/− mice (31). Interestingly, in nongenetically susceptible mice, increasing the generation of AHR ligand by dietary supplementation of tryptophan also protects against chemically induced colitis (28). Such data demonstrate that the identification of specific mechanisms beyond intestinal inflammation susceptibility allow new therapeutic strategies.
Hence, an important step to consider for future studies, especially related to translating animal observations to humans, is the identification of specific bacteria able to disturb or to restore host metabolism, as well as the underlying mechanism, with those findings being central for therapeutic considerations. Indeed, more studies are now trying to mechanistically decipher how an altered microbiota can impact metabolism, allowing the identification of specific instigators of such complex disease. For example, it was recently identified that Ralstonia pickettii, a bacterium with increased abundance in obese subjects with diabetes, is sufficient to exacerbate glucose intolerance in high-fat diet-fed animals (47). Those data importantly demonstrate that, whereas global community composition is important, some single bacterium may play an important role in driving metabolic abnormalities and may specifically target for metabolic improvement.
CONCLUSIONS AND FUTURE DIRECTIONS
As outlined herein, progress continues to be made in deciphering mechanisms by which the microbiota impact metabolism. The last year was especially rich in progress toward understanding the role of diet, which, as a modifiable factor, likely holds the key to exploit our increasing understanding of the microbiota to prevent disease and improve health. Although much work needs to be done, some of the pieces are now in play with combinations of nutrients and bacteria that will work in concert to support each other and synergistically promote intestinal and metabolic health. It should be an exciting year.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-099071 (A. T. Gewirtz) and DK-083890 (A. T. Gewirtz). B. Chassaing is a recipient of the Career Development Award from the Crohn’s and Colitis Foundation and an Innovator Award from the Kenneth Rainin Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.B., A.T.G., and B.C. edited and revised manuscript; A.B., A.T.G., and B.C. approved final version of manuscript; A.T.G. and B.C. drafted manuscript.
REFERENCES
- 1.Albenberg L, Esipova TV, Judge CP, Bittinger K, Chen J, Laughlin A, Grunberg S, Baldassano RN, Lewis JD, Li H, Thom SR, Bushman FD, Vinogradov SA, Wu GD. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147: 1055–1063, 2014. doi: 10.1053/j.gastro.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anhê FF, Nachbar RT, Varin TV, Vilela V, Dudonné S, Pilon G, Fournier M, Lecours MA, Desjardins Y, Roy D, Levy E, Marette A. A polyphenol-rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab 6: 1563–1573, 2017. doi: 10.1016/j.molmet.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64: 872–883, 2015. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 4.Anhê FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D, Marette A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr Obes Rep 4: 389–400, 2015. doi: 10.1007/s13679-015-0172-9. [DOI] [PubMed] [Google Scholar]
- 5.Burrough ER, Arruda BL, Plummer PJ. Comparison of the Luminal and Mucosa-Associated Microbiota in the Colon of Pigs with and without Swine Dysentery. Front Vet Sci 4: 139, 2017. doi: 10.3389/fvets.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab 22: 658–668, 2015. doi: 10.1016/j.cmet.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 50: 2374–2383, 2007. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 8.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103, 2009. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28: 203–209, 2015. [PMC free article] [PubMed] [Google Scholar]
- 10.Chassaing B, Gewirtz AT. Has provoking microbiota aggression driven the obesity epidemic? BioEssays 38: 122–128, 2016. doi: 10.1002/bies.201500116. [DOI] [PubMed] [Google Scholar]
- 11.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–96, 2015. doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 147: 1363–1377, 2014. doi: 10.1053/j.gastro.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chassaing B, Miles-Brown J, Pellizzon M, Ulman E, Ricci M, Zhang L, Patterson AD, Vijay-Kumar M, Gewirtz AT. Lack of soluble fiber drives diet-induced adiposity in mice. Am J Physiol Gastrointest Liver Physiol 309: G528–G541, 2015. doi: 10.1152/ajpgi.00172.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 4: 205–221, 2017. doi: 10.1016/j.jcmgh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chassaing B, Van de Wiele T, De Bodt J, Marzorati M, Gewirtz AT. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 66: 1414–1427, 2017. doi: 10.1136/gutjnl-2016-313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One 7: e39743, 2012. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, Kayser BD, Levenez F, Chilloux J, Hoyles L, Dumas ME, Rizkalla SW, Doré J, Cani PD, Clément K; MICRO-Obes Consortium . Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65: 426–436, 2016. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 18.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359: 592–597, 2018. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Nunez G, Martens EC. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167: 1339–1353, 2016. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, Possemiers S, Van Holle A, François P, de Vos WM, Delzenne NM, Schrenzel J, Cani PD. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60: 2775–2786, 2011. doi: 10.2337/db11-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everard A, Lazarevic V, Gaïa N, Johansson M, Ståhlman M, Backhed F, Delzenne NM, Schrenzel J, François P, Cani PD. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J 8: 2116–2130, 2014. doi: 10.1038/ismej.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galley JD, Yu Z, Kumar P, Dowd SE, Lyte M, Bailey MT. The structures of the colonic mucosa-associated and luminal microbial communities are distinct and differentially affected by a prolonged murine stressor. Gut Microbes 5: 748–760, 2014. doi: 10.4161/19490976.2014.972241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geirnaert A, Wang J, Tinck M, Steyaert A, Van den Abbeele P, Eeckhaut V, Vilchez-Vargas R, Falony G, Laukens D, De Vos M, Van Immerseel F, Raes J, Boon N, Van de Wiele T. Interindividual differences in response to treatment with butyrate-producing Butyricicoccus pullicaecorum 25-3T studied in an in vitro gut model. FEMS Microbiol Ecol 91: fiv054, 2015. doi: 10.1093/femsec/fiv054. [DOI] [PubMed] [Google Scholar]
- 25.Glymenaki M, Singh G, Brass A, Warhurst G, McBain AJ, Else KJ, Cruickshank SM. Compositional changes in the gut mucus microbiota precede the onset of colitis-induced inflammation. Inflamm Bowel Dis 23: 912–922, 2017. doi: 10.1097/MIB.0000000000001118. [DOI] [PubMed] [Google Scholar]
- 26.Heisel T, Montassier E, Johnson A, Al-Ghalith G, Lin YW, Wei LN, Knights D, Gale CA. High-fat diet changes fungal microbiomes and interkingdom relationships in the murine gut. MSphere 2: e00351-17, 2017. doi: 10.1128/mSphere.00351-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 535: 75–84, 2016. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 28.Islam J, Sato S, Watanabe K, Watanabe T, Ardiansyah, Hirahara K, Aoyama Y, Tomita S, Aso H, Komai M, Shirakawa H. Dietary tryptophan alleviates dextran sodium sulfate-induced colitis through aryl hydrocarbon receptor in mice. J Nutr Biochem 42: 43–50, 2017. doi: 10.1016/j.jnutbio.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63: 281–291, 2014. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleessen B, Hartmann L, Blaut M. Fructans in the diet cause alterations of intestinal mucosal architecture, released mucins and mucosa-associated bifidobacteria in gnotobiotic rats. Br J Nutr 89: 597–606, 2003. doi: 10.1079/BJN2002827. [DOI] [PubMed] [Google Scholar]
- 31.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22: 598–605, 2016. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, Colonna M. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13: 144–151, 2011. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Xie C, Lu S, Nichols RG, Tian Y, Li L, Patel D, Ma Y, Brocker CN, Yan T, Krausz KW, Xiang R, Gavrilova O, Patterson AD, Gonzalez FJ. Intermittent fasting promotes white adipose browning and decreases obesity by shaping the gut microbiota. Cell Metab 26: 672–685, 2017. doi: 10.1016/j.cmet.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mar Rodríguez M, Pérez D, Javier Chaves F, Esteve E, Marin-Garcia P, Xifra G, Vendrell J, Jové M, Pamplona R, Ricart W, Portero-Otin M, Chacón MR, Fernández Real JM. Obesity changes the human gut mycobiome. Sci Rep 5: 14600, 2015. doi: 10.1038/srep14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles JP, Zou J, Kumar MV, Pellizzon M, Ulman E, Ricci M, Gewirtz AT, Chassaing B. Supplementation of low- and high-fat diets with fermentable fiber exacerbates severity of DSS-induced acute colitis. Inflamm Bowel Dis 23: 1133–1143, 2017. doi: 10.1097/MIB.0000000000001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pédron T, Mulet C, Dauga C, Frangeul L, Chervaux C, Grompone G, Sansonetti PJ. A crypt-specific core microbiota resides in the mouse colon. MBio 3: e00116-12, 2012. doi: 10.1128/mBio.00116-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med 23: 107–113, 2017. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 38.Prieto I, Hidalgo M, Segarra AB, Martínez-Rodríguez AM, Cobo A, Ramírez M, Abriouel H, Gálvez A, Martínez-Cañamero M. Influence of a diet enriched with virgin olive oil or butter on mouse gut microbiota and its correlation to physiological and biochemical parameters related to metabolic syndrome. PLoS One 13: e0190368, 2018. doi: 10.1371/journal.pone.0190368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 5: 16643, 2015. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, Backhed F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 23: 27–40.e7, 2017. doi: 10.1016/j.chom.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman C, Cohen D, Liguori G, Bourrier A, Nion-Larmurier I, Cosnes J, Seksik P, Langella P, Skurnik D, Richard ML, Beaugerie L. Fungal microbiota dysbiosis in IBD. Gut 66: 1039–1048, 2017. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 535: 56–64, 2016. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suriano F, Bindels LB, Verspreet J, Courtin CM, Verbeke K, Cani PD, Neyrinck AM, Delzenne NM. Fat binding capacity and modulation of the gut microbiota both determine the effect of wheat bran fractions on adiposity. Sci Rep 7: 5621, 2017. doi: 10.1038/s41598-017-05698-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suriano F, Neyrinck AM, Verspreet J, Olivares M, Leclercq S, Van de Wiele T, Courtin CM, Cani PD, Bindels LB, Delzenne NM. Particle size determines the anti-inflammatory effect of wheat bran in a model of fructose over-consumption: Implication of the gut microbiota. J Funct Foods 41: 155–162, 2018. doi: 10.1016/j.jff.2017.12.035. [DOI] [Google Scholar]
- 45.Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature 535: 65–74, 2016. doi: 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- 46.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 47.Udayappan SD, Kovatcheva-Datchary P, Bakker GJ, Havik SR, Herrema H, Cani PD, Bouter KE, Belzer C, Witjes JJ, Vrieze A, de Sonnaville ESV, Chaplin A, van Raalte DH, Aalvink S, Dallinga-Thie GM, Heilig HG, Bergström G, van der Meij S, van Wagensveld BA, Hoekstra JB, Holleman F, Stroes ESG, Groen AK, Bäckhed F, de Vos WM, Nieuwdorp M. Intestinal Ralstonia pickettii augments glucose intolerance in obesity. PLoS One 12: e0181693, 2017. doi: 10.1371/journal.pone.0181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van den Abbeele P, Belzer C, Goossens M, Kleerebezem M, De Vos WM, Thas O, De Weirdt R, Kerckhof FM, Van de Wiele T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J 7: 949–961, 2013. doi: 10.1038/ismej.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van den Abbeele P, Roos S, Eeckhaut V, MacKenzie DA, Derde M, Verstraete W, Marzorati M, Possemiers S, Vanhoecke B, Van Immerseel F, Van de Wiele T. Incorporating a mucosal environment in a dynamic gut model results in a more representative colonization by lactobacilli. Microb Biotechnol 5: 106–115, 2012. doi: 10.1111/j.1751-7915.2011.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Hul M, Geurts L, Plovier H, Druart C, Everard A, Stahlman M, Rhimi M, Chira K, Teissedre PL, Delzenne NM, Maguin E, Guilbot A, Brochot A, Gerard P, Backhed F, Cani PD. Reduced obesity, diabetes and steatosis upon cinnamon and grape pomace are associated with changes in gut microbiota and markers of gut barrier. Am J Physiol Endocrinol Metab 314: E334–E352, 2017. doi: 10.1152/ajpendo.00107.2017. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Antonopoulos DA, Zhu X, Harrell L, Hanan I, Alverdy JC, Meyer F, Musch MW, Young VB, Chang EB. Laser capture microdissection and metagenomic analysis of intact mucosa-associated microbial communities of human colon. Appl Microbiol Biotechnol 88: 1333–1342, 2010. doi: 10.1007/s00253-010-2921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr 138: 439–442, 2008. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 53.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT, Gewirtz A. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 23: 41–53.e4, 2018. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]