Abstract
The idea that gut-derived satiation signals influence food reward has recently gained traction, but this hypothesis is largely based on studies focused on neural circuitry, not the peripherally released signals. Here, we directly tested the hypothesis that intragastric (IG) nutrient infusion can suppress motivation for food. In a series of experiments, IG sucrose infusion (15 kcal) significantly and reliably reduced operant responding for a sucrose reward on a progressive ratio (PR) schedule. Moreover, food deprivation for 24 h before the test session did not prevent the suppressive effect of nutrients. The suppressive effect of IG sucrose on fixed ratio 5 (FR5) operant responding was also assessed as a comparison. The effect of IG nutrients to reduce motivation was not limited to sucrose; IG Ensure infusion (9.3 kcal) also significantly reduced PR operant responding for sucrose pellets. To verify that these effects were not secondary to the osmotic challenge of concentrated nutrients, we tested IG infusion of noncaloric saline solutions equiosmolar to 40% sucrose or Ensure and found no effect. Finally, we focused on glucagon-like peptide-1 (GLP-1) and cholecystokinin (CCK) as candidate mediators for the effect of IG nutrients. Pretreatment with exendin-9, a GLP-1 receptor antagonist, delivered intraperitoneally, significantly attenuated the ability of IG nutrients to suppress PR responding and breakpoint in males, but not in females, whereas pretreatment with devazepide, a CCKA receptor antagonist, failed to do so in both sexes. Together, these data support the idea that nutrient-induced satiation signals influence food reward and may implicate GLP-1 in this process.
Keywords: food intake, food reward, operant conditioning, satiation signals
INTRODUCTION
Feeding is influenced by diverse signals, including short-term satiation signals related to ongoing meals, long-term signals of stored energy, and reward-related factors such as motivation to obtain food or palatability of food. The presence of nutrients in the gastrointestinal tract stimulates the release of satiation signals, which accumulate and result in meal termination. Recently, the idea has gained traction that one of the mechanisms through which satiation signals cause meal termination is via an effect on food reward (27, 30, 42, 50). However, this hypothesis is largely based on studies targeting drug delivery and other manipulations to the brain as opposed to direct tests of the effects of gut nutrients or gastrointestinal signals themselves (13, 16, 29, 45). In apparent contrast with this hypothesis, a large body of work has focused on the ability of gut nutrients to serve as reinforcement and play a positive feedback role in maintaining ongoing ingestion (2, 39). The most extensive evidence for this comes from studies by Sclafani and colleagues, in which rodents are trained to drink flavored nonnutritive solutions paired with intragastric (IG) infusion of small volumes of nutrients (38–40). The effects of IG nutrients under these conditions manifest as appetition (rapid increases in ingestion during an ongoing meal) and conditioned acceptance of and preference for the nutrient-paired flavor in future meals (38, 39, 51). The phenomena of appetition and conditioned flavor acceptance and preference are clear evidence that gastrointestinal nutrients can be reinforcing, and at the same time there is no question that gastrointestinal nutrients can be satiating, particularly as a larger load of nutrients accumulates. We propose that physiological controls of feeding are sufficiently flexible for nutrients to have both of these effects; some sequelae of gastrointestinal nutrient detection reinforce continued ingestive behavior, whereas one of the roles of nutrient-induced satiation signals is to suppress motivation for food, facilitating meal termination.
Reliably, food restriction or deprivation increases motivation for food (6, 12, 14, 19–21, 25, 43), and it may seem reasonable to assume that supplying nutrients to the gastrointestinal tract must then do the opposite and suppress motivation. However, studies demonstrating appetition, as noted above, show that low “doses” of gut nutrients can in fact acutely enhance motivation (41, 46), and a direct test of the effect of larger loads has not yet been performed. Here, we address this gap in the literature. We operationally define “motivation” as performance on a progressive ratio (PR) operant responding task, specifically, the breakpoint. On a PR schedule, the subject must make an increasing number of operant responses to obtain each subsequent reinforcer, and the breakpoint (the highest completed ratio before the subject stops responding) is widely considered to serve as an indicator of motivation (19). The PR task is well suited to address our hypothesis, because this schedule of reinforcement typically results in a large number of operant responses emitted but only a small number of food reinforcers delivered. In the present studies, we manipulated satiation state by delivering large nutrient loads or a saline control load IG. If the food pellet reinforcers obtained were also a significant source of calories or volume, and if the number obtained varied across subjects and conditions, we would lose tight control over IG load during test sessions. Because rats typically obtain fewer than 10 reinforcer pellets in the PR session, and we used 45-mg sucrose pellets in these studies, the additional nutrient (0.18 kcal per pellet) and volume contribution from reinforcers earned, and any change in this number across conditions, is extremely small.
Previous studies attempting to manipulate satiation state to determine whether satiation reduces PR performance have suggested that it does not reduce motivation. However, substantial confounding factors in these experiments have made it unclear whether this is a sound conclusion. In one such study, Ferguson and Paule (11) asked whether the timing of daily scheduled food availability relative to a PR session, in which the animal works for a rodent chow reinforcer, would influence motivated behavior. In this study, the prefeeding interval was taken as a manipulation of satiation status at the time of the PR test session, because chronically food-restricted and schedule-fed rats readily consume their entire daily food allotment (9–12 g of chow) when food is presented, presumably inducing satiation. Under these experimental conditions, there was no effect of prefeeding interval on PR responding when varied from 15 min to 6 h before the PR session, and the authors concluded that satiation status does not play a role in motivation for food. However, it is important to consider that in chronically food-restricted rats satiation induced by this prefed meal would not necessarily be comparable to that which would occur in ad libitum-fed rats. Considerable evidence shows that fasting reduces sensitivity to satiation signals, resulting in the intake of larger meals (28, 47, 48); so fasting may also preclude an effect of satiation on motivation. Moreover, the authors discussed the possibility that those subjects were so extensively trained that their operant performance might have become habitual as opposed to goal-directed behavior (1), making these data difficult to interpret. Given these issues, a more direct test is warranted of the hypothesis that satiation suppresses motivation for food.
In the present studies, we used IG nutrient infusion to induce gut satiation signaling immediately before operant responding test sessions. The IG infusion approach is superior, because it allows complete experimenter control of infusion timing and volume, eliminates any possible effect of orosensory stimulation, and permits the inclusion of a nonnutrient control infusion as a comparison condition. Importantly, our experiments were designed to deliver nutrient loads only rarely to each subject, and subjects had no way to associate environmental or orosensory flavor cues with nutrient load, because any such cues were identical across saline IG infusion conditions. In contrast to the conditioned flavor preference paradigm mentioned above, we were not asking whether experience with IG nutrient loads would affect behavior through learned associations. These studies were instead designed to address the acute effects of large nutrient loads that would be expected to stimulate significant satiation signaling.
We first used the PR operant responding task to determine the effect of an IG infusion of sucrose on motivation to obtain sucrose pellet reinforcement in ad libitum-fed and 24-h-fasted states. For comparison, we examined the effects of IG sucrose infusion on fixed ratio (FR) responding for sucrose pellet reinforcement, which requires effort to obtain reinforcement, but is not a clear test of motivation because the effort required does not change during the test session. Sucrose was initially selected as the IG stimulus to match the nutrient composition of the reinforcer pellets, but we then determined whether a mixed-macronutrient IG infusion (Ensure) would have a similar effect. In a separate study, we sought to replicate the effect of IG nutrients on motivation for food in females and to further examine whether this effect varied across the estrus cycle. As a control for the possibility that osmotic load has adverse effects that suppress motivation, we also examined the effects of IG infusions of hypertonic saline on PR responding. Finally, we investigated whether the effects of IG Ensure infusion on motivation for food reinforcement are indeed mediated by gastrointestinal satiation signals. Specifically, we assessed the role of endogenous glucagon-like peptide-1 (GLP-1) and cholecystokinin (CCK). Both GLP-1 and CCK are released from the gastrointestinal tract in response to nutrient presence in the gut and have been demonstrated to be important contributors to the control of food intake (8, 31). Mounting evidence suggests that GLP-1 in the brain can influence food reward, but the role of CCK in reward has been less well defined (17, 44). Together, our studies address the fundamental question whether nutrient-induced satiation signals suppress motivation to obtain food. Our results support the hypothesis that they can, and highlight a potential role for GLP-1 in this effect in males.
METHODS
Subjects.
Naïve male (250–300 g at arrival) and female (175–200 g at arrival) Wistar rats (Harlan, Indianapolis, IN) were individually housed in plastic cages in a temperature-controlled vivarium on a 12:12-h light-dark cycle. Rats had ad libitum access to distilled water and pelleted rat chow (no. 5001; Purina, St. Louis, MO) except where otherwise noted. All subjects were handled daily and habituated to experimental procedures before experiment onset. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (32).
Surgery.
Under 2–4% isoflurane delivered at a rate of 1 l/min, rats were implanted with IG catheters according to a technique adapted from Davis and Campbell (9). Briefly, a 3-cm midline incision was made through the skin and muscle of the abdominal wall. A small incision was made in the fundus of the stomach, and the IG catheter, a 30-cm length of Silastic tubing (Dow Corning, Midland, MI; 0.76 mm inner diameter, 1.65 mm outer diameter) was inserted. The IG catheter was secured in the stomach wall using a purse stitch (4-0 nonabsorbable silk suture; Ethicon, Somerville, NJ), and reinforced with a 5 × 5 mm square of Bard surgical mesh placed on the serosal surface (Davol, Warwick, RI). The distal end of the catheter was routed through the subcutaneous tissue and exteriorized through a 1-cm incision on the dorsal surface between the shoulder blades. A 10-mm 20-G backmount infusion port (Plastics One, Roanoke, VA) was connected to the distal end of the catheter and sutured to the intrascapular muscle to promote adhesion. IG catheters were flushed daily with 1 ml of 0.9% saline to maintain patency. Body weight and food intake were monitored while rats recovered for at least 7 days before experimental procedures began.
Apparatus.
All behavioral testing took place in Habitest operant chambers enclosed in sound- and light-controlled boxes (Coulbourn Instruments, Holliston, MA). Each chamber was fitted with two response levers and an infusion line. Presses on the active lever were reinforced, and presses on the inactive lever were not reinforced. A 5-s timeout followed each reinforcement, during which the reinforcement pellet was delivered and consumed.
The IG infusion lines consisted of polyethylene (PE) tubing (VWR, Radnor, PA) protected by a stainless steel spring. The PE tubing was connected to an infusion swivel (Instech Solomon, Plymouth Meeting, PA) mounted on a counterbalanced lever at the top of the chamber. This allowed the rats to move freely around the chamber while the infusion line was connected to the back-mounted IG catheter port. All infusions were 10 ml in volume and occurred at a rate of 1 ml/min, chosen to approximate rats’ average rate of ingestion. During operant responding sessions, a house light was continuously illuminated, and a cue light was illuminated above the active lever when it was possible to earn a reinforcer. Behavioral data were recorded by Graphic State 4 software (Coulbourn Instruments, Holliston, MA) and stored on the computer for later analysis.
Training procedure.
Rats were trained to lever press for 45-mg sucrose pellets (TestDiet, Richmond, IN) during the midlight phase, with food removed from their home cages 1–2 h before each session. To habituate rats to the experimental procedure that would be used for IG infusions, subjects were placed in the inactive operant chamber for 15-min before the operant sessions began. To signal experiment onset, the light-attenuating chamber doors were closed, and the house and cue lights were illuminated. Initially, rats were trained in 2-h sessions to receive a sucrose pellet on a fixed ratio one (FR1) schedule, where each response on the active lever resulted in the delivery of one sucrose pellet. Once responding was stable (less than 10% day-to-day variation), rats were switched to a 1-h FR3 schedule. When rats showed stable responding, they were moved to a 1-h FR5 schedule. Rats going into experiments utilizing a PR schedule were then placed on the PR. We used a PR schedule that followed the algorithm of Richardson and Roberts (35): 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, etc., lever presses for reinforcement. PR sessions ended when the rat failed to complete the current ratio run within 10 min, with a maximum session duration of 1 h. At that point, house and cue lights turned off, and all manipulanda became inactive. After the operant session, rats were returned to their home cages.
Experiment 1a: effect of IG sucrose on PR responding.
Naïve male rats (n = 11) implanted with IG catheters were trained to lever press for 45-mg sucrose pellets as described above. Once PR responding stabilized (within rat day-to-day variation being ±1 reinforcers earned for 3 consecutive training days), rats were habituated to IG infusion procedures over several sessions. First, they were simply connected to the infusion line, and then in subsequent sessions, rats received a 10-ml infusion of 0.9% saline beginning 15 min before the start of the operant test session. On experiment days, rats received an infusion of either 10 ml of 0.9% saline or 40% sucrose solution (1.5 kcal/ml, 15 kcal total). PR sessions began 5 min after the IG infusion was completed. After the PR session, rats were returned to their home cages, and body weight and overnight chow intake were monitored. All rats received both conditions in counterbalanced order, with treatments separated by at least 48 h. Between testing days, rats continued to have daily PR training sessions with no IG infusions. This experiment was repeated a second time in the same group.
Experiment 1b: effect of IG sucrose on PR responding in fasted rats.
The same male rats (n = 11) and procedures used in Experiment 1a were also used in this study. Three weeks after the completion of Experiment 1a, rats were food deprived during the 24-h period preceding the experimental PR test session. All rats received IG saline and IG 40% sucrose (1.5 kcal/ml) conditions in counterbalanced order, with treatments separated by 5 days to allow complete recovery from the food deprivation. Overnight chow intake was not recorded in this experiment, but body weight was monitored daily. Rats continued to have daily PR training sessions on nontesting days. Rats were tested only once per IG treatment under food-deprived conditions.
Experiment 2: effect of IG sucrose on FR5 operant responding.
Naïve male rats (n = 10) implanted with IG catheters were trained to lever press for 45-mg sucrose pellets on FR1, FR3, and then FR5 schedules, as described above. Once FR5 responding stabilized, rats were habituated to IG infusion. Habituation and experimental infusion procedures were conducted as described in Experiment 1a, with the FR5 session beginning 5 min after the IG infusion of either 10 ml of 0.9% saline or 40% sucrose (1.5 kcal/ml) solution ended. After the FR5 session, rats were returned to their home cages. All rats received both conditions in counterbalanced order, with treatments separated by 48 h. Between testing days, rats had daily FR5 training sessions, and body weight was monitored.
Experiment 3a: effect of IG Ensure on PR responding.
Male Wistar rats (n = 12) implanted with IG catheters were trained to lever press for 45-mg sucrose pellets as described above. After 10 PR training sessions, rats showed stable responding and were then habituated to the IG infusion procedure for an additional seven sessions. On experiment days, rats received a 10-ml IG infusion of either 0.9% saline or Original Chocolate Ensure (0.93 kcal/ml, 9.3 kcal total; Abbott Laboratories, Abbott Park, IL). PR sessions began 5 min after the conclusion of IG infusion. After the session concluded, rats were returned to their home cages, and body weight and overnight chow intake were monitored. Rats continued to have daily PR training sessions on nonexperiment days and received both IG conditions in counterbalanced order separated by 48 h.
Experiment 3b: effect of estrus cycle stage on the response to IG Ensure in intact cycling females.
Naïve female Wistar rats (n = 16) implanted with IG catheters were trained to lever press for 45-mg sucrose pellets as described above. After six PR training sessions, rats showed stable responding and were then habituated to the IG infusion procedure for an additional seven sessions. On experiment days, rats received a 10-ml IG infusion of either 0.9% saline or Original Chocolate Ensure (0.93 kcal/ml). To assess whether the response to IG nutrients varies across the estrus cycle, IG saline and IG Ensure infusions were conducted on both diestrus and estrus cycle stages for each rat, making for this experiment a total of four conditions. PR sessions began 5 min after the conclusion of IG infusion. After the session concluded, rats were returned to their home cages. Body weight, cycle stage, and overnight chow intake were monitored daily. Estrus cycle stage was identified using vaginal cytology. Vaginal smears were taken daily during the first half of the light phase, before operant sessions. Samples were obtained by inserting a cotton swab moistened with 0.9% saline into the vagina and transferred to an untreated microscope slide for examination. Cycle stage was determined using standard criteria (5), and cycle stage labels were assigned to the 24-h period beginning at the preceding dark onset and spanning the light phase during which sampling and operant sessions occurred. Rats continued to have daily PR training sessions on nonexperiment days and received all four conditions in counterbalanced order separated by at least 48 h.
Experiment 4: effect of osmotic challenge on PR responding.
To verify that the response to IG nutrient infusion was not due to the osmotic challenge of the IG nutrient conditions, we infused saline solutions that were osmotically matched to 40% sucrose (1.1686 Osm, 3.415% saline) and Original Chocolate Ensure (0.50 Osm, 1.461% saline) before the PR task. Male Wistar rats (n = 7) implanted with IG catheters were trained to lever press for 45-mg sucrose pellets as described above. On experiment days, rats received a 10-ml IG infusion of 0.9% saline, 1.461% saline, or 3.415% saline. PR sessions began 5 min after the conclusion of IG infusion. After the session concluded, rats were returned to their home cages, and body weight, overnight chow, and overnight water intake were monitored. Rats continued to have daily PR training sessions on nonexperiment days and received all three IG conditions in counterbalanced order separated by 48 h. Following the completion of this osmolarity experiment, we tested the effect of 10 ml of IG Ensure, as described above, to confirm that we had observed nutrient-induced reduction in PR responding in this group of rats.
Experiment 5a: effect of GLP-1R and CCKAR blockade on the response to IG Ensure: drugs and injections.
The GLP-1R antagonist exendin-9 (Ex9; California Peptide Research, Napa, CA) was dissolved in sterile 0.9% saline. Ex9 was administered intraperitoneally (ip) at a dose of 100 μg/kg. The CCKA receptor (CCKAR) antagonist devazepide (DVZ; Tocris, Minneapolis, MN) was dissolved in a solution of 90% sterile 0.9% saline, 5% Tween 80, and 5% DMSO. DVZ was administered ip at a dose of 0.5 mg/kg. These doses were chosen based on preliminary data yielding no significant effect of drug treatment on PR performance variables when combined with IG saline infusion (data not shown). An injection volume of 1 ml/kg was used for all treatments.
A subset of rats from Experiment 3a was used in this study, following the completion of Experiment 3a. Two rats from this group were not used in this study due to the loss of patency of IG catheters. The remaining rats received five operant sessions after ip saline injection, to habituate them to the ip injection procedure. Another two rats were not used for the experiment due to increased day-to-day variability in their operant responding during these ip injection habituation sessions, leaving a total of eight subjects for the experiment. On experiment days, rats received an ip injection of vehicle (90% saline, 5% Tween 80, 5% DMSO), Ex9 (100 μg/kg), or DVZ (0.5 mg/kg). Ten minutes later, rats received an IG infusion of either 10 ml of saline or Original Chocolate Ensure (0.93 kcal/ml). The PR test session began 5 min after the IG infusion ended. After each session, rats were returned to their home cage, and body weight and overnight chow intake were monitored. Rats received all six conditions in counterbalanced order, separated by 48 h. Between testing days, rats continued to receive PR training sessions.
Experiment 5b: effect of GLP-1R and CCKAR blockade on the response to IG Ensure during estrus in intact cycling females.
A subset of rats from Experiment 3b was used in this study, following the completion of Experiment 3b. One rat from this group was not used in this study due to the development of recurring seizures, and three were removed due to irregular or discontinued cycling. The remaining rats received an additional four operant sessions preceded by ip saline injection, to habituate them to the ip injection procedure. Another three rats were not used for the experiment due to increased day-to-day variability in their operant responding during these ip injection habituation sessions, leaving a total of nine subjects for the experiment.
Because we observed a trend toward a greater effect of IG nutrient infusion on PR performance during estrus in Experiment 3b, we chose to test the effect of GLP-1R and CCKAR blockade on the response to IG Ensure during estrus. On experiment days, rats received an ip injection of vehicle (90% saline, 5% Tween 80, 5% DMSO), Ex9 (100 μg/kg), or DVZ (0.5 mg/kg). Ten minutes later, rats received an IG infusion of either 10 ml of saline or Original Chocolate Ensure (0.93 kcal/ml). The PR test session began 5 min after the IG infusion ended. After each session, rats were returned to their home cage, and body weight, cycle stage, and overnight chow intake were monitored the following day. Cycle stage was assessed and assigned as described above in Experiment 3b. Rats received all six conditions in counterbalanced order, separated by at least 48 h. Between testing days, rats continued to receive PR training sessions.
Statistical analysis.
For all variables, data are reported as means ± SE. For Experiment 1a, in which the conditions were replicated, we present the data as a mean of the two runs for each condition, except for overnight chow intake, where we collected data in only the second repetition of the experiment. For Experiment 1a, changes in operant responding variables were assessed via repeated-measures two-way ANOVA, with IG nutrient and replication as factors; overnight chow intake from the second repetition was analyzed using paired-samples Student's t-test. Data from Experiments 1b, 2, and 3a were analyzed via paired-samples Student's t-test to evaluate the effect of IG nutrient infusion relative to IG saline infusion. For Experiment 3b, data were analyzed using repeated-measures two-way ANOVA, with IG nutrient and estrus cycle stage as factors. For Experiment 3b, one subject was excluded from analysis of overnight chow intake due to food hoarding behavior that prevented accurate measurement. For Experiment 4, changes in operant responding variables were assessed via repeated-measures ANOVA, with IG saline concentration as the factor. For Experiments 5a and 5b, changes in operant responding variables were evaluated via repeated-measures two-way ANOVA, with IG nutrient and ip drug as factors. Holm-Bonferroni tests were conducted as planned pairwise comparisons. P < 0.05 was taken to be significant.
RESULTS
Experiment 1a: effect of IG sucrose on PR operant responding.
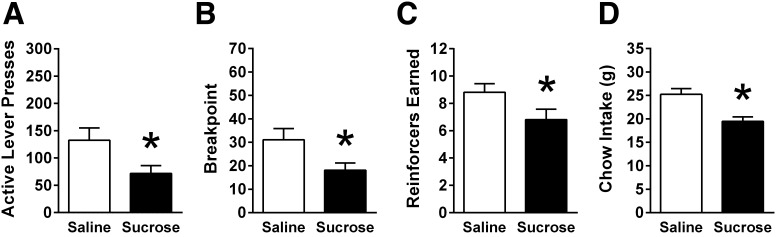
IG sucrose infusion reduced operant responding for sucrose reinforcement on a PR schedule. IG sucrose infusion reduced active lever presses for sucrose reinforcement [F(1,10) = 15.188, P < 0.01; Fig. 1A]. Breakpoint, the highest completed ratio requirement, was also significantly decreased by IG infusion of sucrose [F(1,10) = 13.33, P < 0.01; Fig. 1B]. Total number of reinforcers obtained was significantly decreased by IG sucrose [F(1,10) = 16.526, P < 0.01; Fig. 1C]. There were no main effects of replication and no IG nutrient × replication interactions on any of the PR variables. Overnight chow intake following the second repetition of PR test sessions was also significantly decreased by IG sucrose infusion [t (10) = 7.97, P < 0.01; Fig. 1D] (chow intake data were not obtained for the first round of this experiment).
Fig. 1.
In ad libitum-fed male rats (n = 11) responding on a progressive ratio (PR) schedule of reinforcement, intragastric (IG) sucrose infusion (15 kcal) significantly reduced (A) active lever presses, (B) breakpoint, and (C) number of reinforcers earned. D: IG sucrose significantly suppressed overnight chow intake. Data for A–C are means ± SE of two replicate experiments, whereas chow intake data for D is from the second replicate alone. PR data were analyzed using repeated-measures two-way ANOVA (IG nutrient × replication), and chow intake from the second repetition was analyzed using paired-samples t-test. *P < 0.05 relative to saline.
Experiment 1b: effect of IG sucrose on PR operant responding in fasted rats.
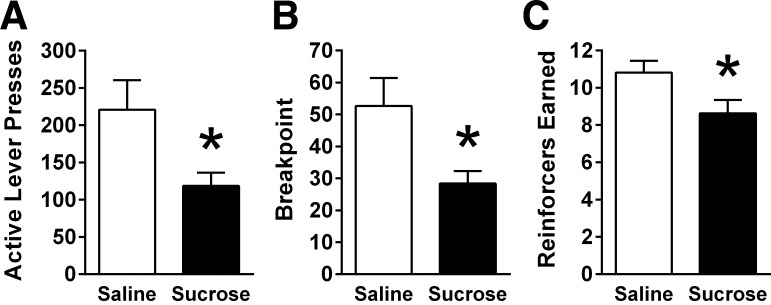
Following overnight food deprivation, IG sucrose infusion significantly decreased the number of active lever presses emitted for sucrose reinforcement [t (10) = 2.99, P < 0.01; Fig. 2A]. There was also a significant reduction in breakpoint [t (10) = 3.24, P < 0.01; Fig. 2B], as well as the number of reinforcers obtained [t (10) = 3.73, P < 0.01; Fig. 2C]. Overnight chow intake data were not obtained for this experiment.
Fig. 2.
In overnight-fasted male rats (n = 11) responding on a PR schedule of reinforcement, IG sucrose infusion (15 kcal) significantly reduced (A) active lever presses, (B) breakpoint, and (C) number of reinforcers earned. Data are means ± SE. Data were analyzed using paired-samples t-test. *P < 0.05 relative to saline.
Experiment 2: effect of IG sucrose on FR5 operant responding.
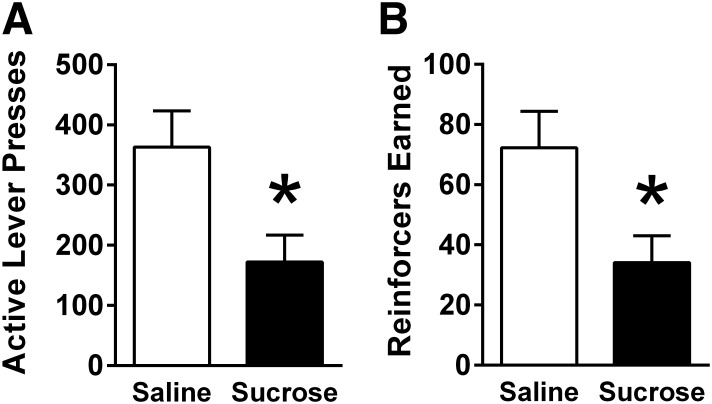
IG sucrose infusion had a significant effect on operant responding for sucrose reinforcement on an FR5 schedule. Presses on the active lever and sucrose reinforcers obtained were both decreased by IG sucrose infusion [t’s (9) = 3.78, P’s < 0.01; Fig. 3].
Fig. 3.
In male rats (n = 10) responding on a fixed ratio 5 (FR5) schedule of reinforcement, IG sucrose infusion (15 kcal) significantly reduced (A) active lever presses and (B) number of reinforcers earned. Data are means ± SE. Data were analyzed using paired-samples t-test. *P < 0.05 relative to saline.
Experiment 3a: effect of IG Ensure on PR operant responding.
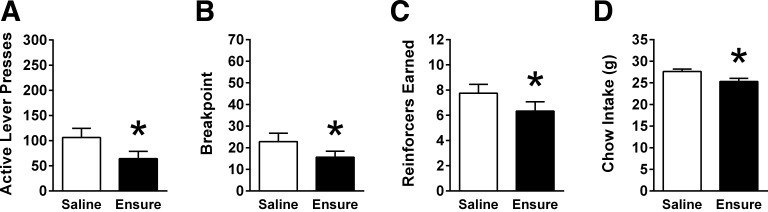
IG infusion of Ensure suppressed operant responding for sucrose reinforcement. IG Ensure reduced presses on the active lever [t (11) = 3.66, P < 0.01; Fig. 4A]. Breakpoint was also significantly reduced by IG Ensure [t (11) = 3.39, P < 0.01; Fig. 4B], as well as the number of sucrose reinforcers obtained [t (11) = 3.96, P < 0.01; Fig. 4C]. Overnight chow intake on test days was significantly decreased by IG infusion of Ensure [t (11) = 4.45, P < 0.01; Fig. 4D].
Fig. 4.
In male rats (n = 12) responding on a PR schedule of reinforcement, IG Ensure infusion (9.3 kcal) significantly reduced (A) active lever presses, (B) breakpoint, and (C) number of reinforcers earned. D: IG Ensure significantly suppressed overnight chow intake. Data are means ± SE. Data were analyzed using paired-samples t-test. *P < 0.05 relative to saline.
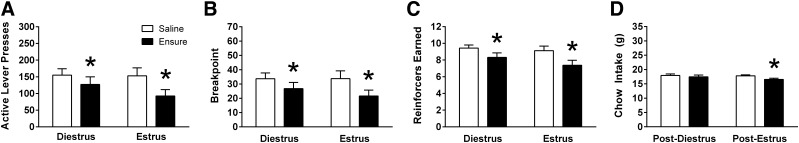
Experiment 3b: effect of estrus cycle stage on the response to IG Ensure in intact cycling females.
IG infusion of Ensure suppressed operant responding for sucrose reinforcement during both estrus and diestrus stages of the ovarian cycle. IG Ensure significantly reduced presses on the active lever [F(1,15) = 22.09, P < 0.001]. There was also a tendency for estrus cycle stage to influence active lever presses [F(1,15) = 4.49, P = 0.051], as well as a trend toward a cycle stage x IG nutrient interaction [F(1,15) = 3.072, P = 0.100], but these did not reach significance. Breakpoint was significantly reduced by IG Ensure [F(1,15) = 18.11, P < 0.001], and ANOVA also revealed main effects of both IG Ensure [F(1,15) = 18.41, P < 0.001] and cycle stage [F(1,15) = 5.282, P < 0.05] on the number of sucrose reinforcers obtained, but no interaction.
Planned comparisons revealed that PR responding following IG saline infusion was no different when tested during diestrus and estrus (Fig. 5, A–C). Notably, IG infusion of Ensure significantly reduced active lever presses, breakpoint, and reinforcers obtained relative to IG saline during both diestrus and estrus (P’s < 0.02; Fig. 5, A–C). Moreover, for each of these variables, PR responding was significantly lower following IG Ensure during estrus compared with IG Ensure during diestrus (P’s < 0.02).
Fig. 5.
In female rats (n = 16) responding on a PR schedule of reinforcement, IG Ensure infusion (9.3 kcal) significantly reduced (A) active lever presses, (B) breakpoint, and (C) number of reinforcers earned during both diestrus and estrus phases of the ovarian cycle. D: IG Ensure significantly suppressed overnight chow intake only following the PR test sessions conducted during estrus. Data are means ± SE. Data were analyzed using repeated-measures two-way ANOVA (IG nutrient × cycle stage), and Holm-Bonferroni tests were conducted as planned comparisons. *P < 0.05 relative to saline.
ANOVA for overnight chow intake revealed a trend toward a main effect of IG Ensure [F(1,14) = 2.988, P = 0.105]. Planned comparisons showed that IG Ensure significantly reduced chow intake relative to IG saline following estrus test sessions (P < 0.01; Fig. 5D).
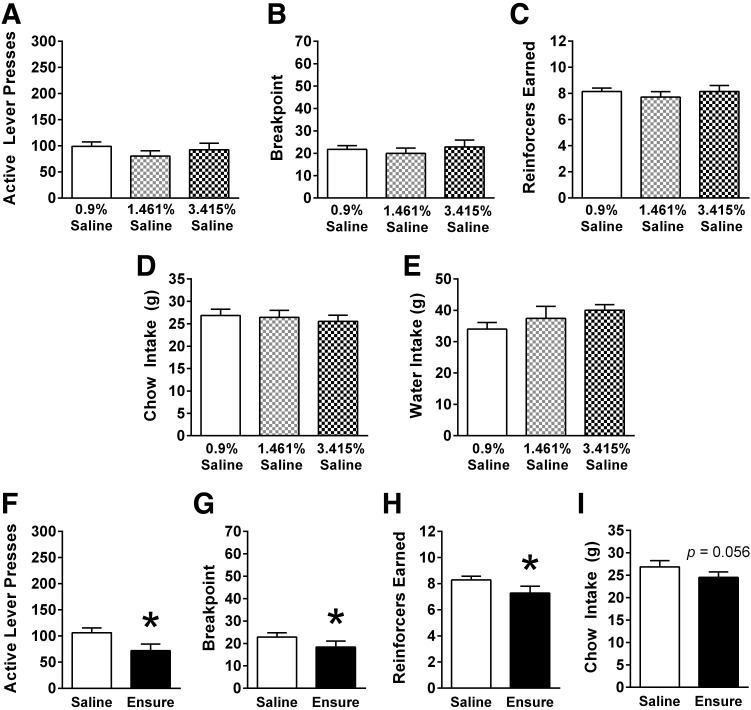
Experiment 4: effect of hypertonic saline osmotic challenge on PR responding.
IG infusions of saline equiosmolar to 40% sucrose and Ensure nutrient solutions had no effects on active lever presses, breakpoint, pellets received, or overnight chow and water intake following the PR session (Fig. 6, A–E).
Fig. 6.
In male rats (n = 7) responding on a PR schedule of reinforcement, a 10 ml IG infusion of hypertonic 1.461% saline or 3.415% saline (of equal osmolarity to Original Chocolate Ensure or 40% sucrose, respectively) did not alter (A) active lever presses, (B) breakpoint, or (C) number of reinforcers earned during the PR test, nor did it alter (D) overnight chow or (E) overnight water intake relative to IG 0.9% saline. Importantly, these same rats responded to IG Ensure infusion (9.3 kcal) with reduced (F) active lever presses, (G) breakpoint, and (H) reinforcers earned, and (I) a trend toward reduced overnight chow intake relative to IG 0.9% saline. Data are means ± SE. Data for A–E were analyzed using repeated-measures ANOVA, and Holm-Bonferroni tests were conducted as planned comparisons, whereas data for F–I were analyzed using paired-samples t-test. *P < 0.05 relative to 0.9% saline.
As expected, IG infusion of Ensure did suppress PR responding in this group of rats relative to IG 0.9% saline. IG Ensure reduced presses on the active lever [t (6) = 5.36, P < 0.001], breakpoint [t (6) = 2.82, P < 0.02], and the number of sucrose reinforcers obtained [t (6) = 3.24, P < 0.01; Fig. 6, F–H]. Overnight chow intake on test days tended to be reduced following IG infusion of Ensure [t (6) = 1.864, P = 0.056; Fig. 6I).
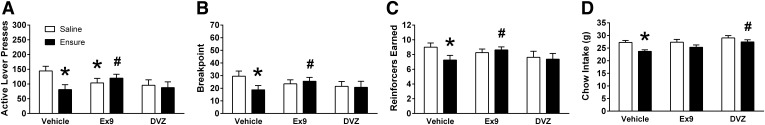
Experiment 5a: effect of peripheral Ex9 and DVZ on the response to IG Ensure.
As in the other studies, IG nutrient infusion suppressed operant responding for sucrose reinforcement, evident here in a significant main effect of IG Ensure on active lever presses [F(1,7) = 7.89, P < 0.05] and a trend toward a main effect on breakpoint [F(1,7) = 3.74, P = 0.094] and number of reinforcers obtained [F(1,7) = 4.12, P = 0.08]. Drug treatment did influence the ability of IG Ensure to suppress responding, with a significant IG Ensure × ip drug interaction for active lever presses [F(2,14) = 6.15, P < 0.05], a trend toward an interaction for breakpoint [F(2,14) = 3.701, P = 0.051], and a significant interaction for number of reinforcers obtained [F(2,14) = 4.963, P < 0.05].
Planned comparisons revealed that, after ip vehicle injection, IG Ensure significantly suppressed active lever presses, breakpoint, and reinforcers obtained (P’s < 0.01; Fig. 7, A–C). After ip Ex9, IG Ensure infusion no longer suppressed lever presses, breakpoint, or reinforcers earned relative to IG saline. By each of these measures, responding after ip Ex9/IG Ensure was, in fact, significantly higher than after ip vehicle/IG Ensure (P’s < 0.01). IG Ensure was also ineffective relative to IG saline after ip DVZ treatment (Fig. 7, A–C). However, in contrast to the results obtained with Ex9, responding after ip DVZ/IG Ensure did not differ from ip vehicle/IG Ensure for any variables measured in the PR test. This may have been due to a surprising effect of the drug on baseline responding under IG saline conditions. Both ip Ex9 and DVZ before IG saline infusion unexpectedly reduced active lever presses relative to ip saline injection; however, the difference was only statistically significant for ip Ex9 (P < 0.05) after correction for multiple comparisons (Fig. 7A). In the case of ip Ex9, this reduction in active lever presses was not sufficient to reduce breakpoint or reinforcers earned (Fig. 7, B and C). DVZ did strongly tend to reduce both breakpoint and reinforcers earned in the IG saline condition, but these differences failed to reach significance after correction for multiple comparisons.
Fig. 7.
In male rats (n = 8) responding on a PR schedule of reinforcement, pretreatment with glucagon-like peptide-1 receptor (GLP-1R) antagonist exendin-9 (Ex9; 100 μg/kg), but not CCKA receptor (CCKAR) antagonist devazepide (DVZ; 0.5 mg/kg), attenuated the effect of IG Ensure infusion (9.3 kcal) on (A) active lever presses, (B) breakpoint, and (C) number of reinforcers earned. D: conversely, pretreatment with DVZ, but not Ex9, attenuated the effect of IG Ensure infusion on overnight chow intake. Data are means ± SE. Data were analyzed using repeated-measures two-way ANOVA (IG nutrient × ip drug), and Holm-Bonferroni tests were conducted as planned comparisons. *P < 0.05 relative to ip vehicle/IG saline and #P < 0.05 relative to ip vehicle/IG Ensure.
ANOVA for overnight chow intake revealed main effects of both IG Ensure [F(1,7) = 25.78, P < 0.01] and ip drug treatment [F(2,14) = 8.847, P < 0.01], but no interaction. Planned comparisons showed that, following ip vehicle, IG Ensure significantly reduced chow intake relative to IG saline (P < 0.01), but not Ex9 or DVZ (Fig. 7D). There was a trend for DVZ to increase overnight chow intake following IG saline infusion, but this failed to reach significance (P = 0.052; Fig. 7D). DVZ treatment attenuated the effect of IG Ensure to reduce chow intake, as intake following ip DVZ/ IG Ensure was significantly greater than ip vehicle/IG Ensure (P < 0.01). However, the effect of Ex9 to attenuate the anorexic response to IG Ensure was a non-significant trend (P = 0.06 for ip vehicle/IG Ensure vs. ip Ex9/IG Ensure).
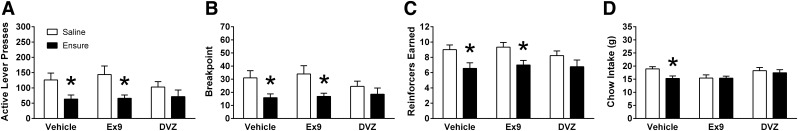
Experiment 5b: effect of GLP-1R and CCKAR blockade on the response to IG Ensure during estrus in intact cycling females.
As in the other studies, IG infusion of Ensure suppressed operant responding for sucrose reinforcement, evident here in a significant main effect of IG Ensure on active lever presses [F(1,8) = 14.21, P < 01], breakpoint [F(1,8) = 12.46, P = 0.01], and number of reinforcers obtained [F(1,8) = 21.85, P = 0.01]. Drug treatment weakly influenced the ability of IG Ensure to suppress responding, with a trend toward an interaction for breakpoint [F(2,16) = 2.87, P = 0.086], but no interactions for active lever presses or number of reinforcers earned.
Planned comparisons revealed that, after ip vehicle injection, IG Ensure significantly suppressed active lever presses, breakpoint, and reinforcers obtained (P’s < 0.01; Fig. 8, A–C). After ip Ex9, IG Ensure infusion continued to suppress active lever presses, breakpoint, or reinforcers earned relative to IG saline (P’s < 0.01; Fig. 8, A–C). After ip DVZ, IG Ensure failed to significantly reduce presses on the active lever, breakpoint, and reinforcers obtained relative to IG saline (Fig. 8, A–C). However, responding after ip DVZ/IG Ensure did not differ from ip vehicle/IG Ensure for any variables measured in the PR test.
Fig. 8.
In female rats (n = 9) responding on a PR schedule of reinforcement, pretreatment with GLP-1R antagonist Ex9 (100 μg/kg) or CCKAR antagonist DVZ (0.5 mg/kg) failed to significantly attenuate the effect of IG Ensure infusion (9.3 kcal) on (A) active lever presses, (B) breakpoint, and (C) number of reinforcers earned when tested during estrus. D: conversely, pretreatment with either DVZ or Ex9 tended to attenuate the effect of IG Ensure infusion on overnight chow intake. Data are means ± SE. Data were analyzed using repeated-measures two-way ANOVA (IG nutrient × ip drug), and Holm-Bonferroni tests were conducted as planned comparisons. *P < 0.05 relative to respective ip condition with IG saline infusion.
ANOVA for overnight chow intake revealed a main effect of IG Ensure [F(1,8) = 30.68, P < 0.05], but no effect of ip drug treatment and no interaction. Planned comparisons showed that, following ip vehicle, IG Ensure significantly reduced chow intake relative to IG saline (P < 0.01), but IG Ensure did not suppress overnight chow intake after ip Ex9 or DVZ (Fig. 8D). DVZ treatment had a tendency to attenuate the suppressive effect of IG Ensure on chow intake, but this trend toward increased intake following ip DVZ/ IG Ensure compared with ip vehicle/IG Ensure failed to reach significance (P = 0.071). ip Ex9 treatment tended to reduce overnight chow intake after IG saline relative to ip vehicle (P < 0.017), but this difference was not significant when corrected for multiple comparisons, and there was also no difference in overnight chow intake between the ip vehicle/IG Ensure and ip Ex9/IG Ensure conditions.
DISCUSSION
We demonstrate here, using a PR operant responding task, that IG nutrient load can suppress motivation for a food reward. Gastrointestinal nutrient infusion reduces subsequent food intake, and our results suggest that this may be mediated, in part, through a decrease in motivation to obtain food. It was already well established that acute or chronic food restriction and deprivation could strongly promote motivation for food reward (6, 12, 14, 19–21, 25, 43), and a great deal of previous research on gut nutrients and reward has focused on situations in which nutrients are reinforcing, as in appetition and conditioned flavor preference paradigms (2, 38–40, 46). It has been largely assumed that bigger, satiating nutrient loads can suppress motivation for food, but the present study is the first to our knowledge to directly test this hypothesis. Using a PR responding task to assess motivation, we found a reliable effect of IG nutrient infusion to reduce motivation for food in both male and female rats. We also established that the effects of nutrient load are not likely due to osmotic challenge. Finally, our results suggest that GLP-1 released in response to IG nutrients may play a role in this effect in males, although this requires further investigation to confirm.
In our first study, we observed that, in ad libitum-fed male rats, IG sucrose infusion reduced motivation for food in the PR task, as measured by a significant decrease in active lever presses, breakpoint, and reinforcers earned relative to saline. The effect was reliable, replicated in the same group, and then observed repeatedly with different subjects under varying conditions in the other studies presented here. Although the effect of IG nutrient infusion is modest in size, at least under our test conditions, this consistency increases confidence in the finding. To confirm that the suppressive effect of IG nutrient infusion on motivation for food reward is not due to an adverse osmotic effect of the concentrated nutrient loads, we tested whether infusion of saline solutions of equal osmolarity to the 40% sucrose or Ensure infusion conditions utilized in our experiments would influence PR performance. We observed no effect of IG infusion of 1.461% and 3.415% saline on PR responding for a sucrose reinforcer relative to 0.9% saline, the control infusion used in all experiments. This result suggests that the osmotic challenge induced by IG infusion of an equiosmotic, but noncaloric, solution is not sufficient to reduce motivation for sucrose reinforcement. Importantly, these same rats did respond to IG Ensure infusion with a reduction in PR performance. Together, these findings support the idea that it is indeed the caloric content of the nutrient load that reduces motivation to obtain food.
We also evaluated whether IG nutrient infusion would suppress PR responding in overnight-fasted subjects that are highly motivated to obtain a food reinforcer. As expected, PR responding was elevated when rats were food deprived for 24 h before the test session relative to typical behavior of nondeprived rats. Notably, fasted rats continued to respond to IG sucrose with a significant reduction in active lever presses, breakpoint, and reinforcers earned relative to IG saline. Although previous studies concluded that satiation does not influence motivation, one interpretive concern was that they were conducted in food-restricted or -deprived subjects (11). We speculated that the negative energy balance induced by chronic food restriction might preclude an effect of satiation signaling on motivation. Here, we demonstrate that, to the contrary, food deprivation does not alter the effect of IG nutrients to reduce motivation for food. Previous work shows that fasting reduces sensitivity to satiation signals, making meal sizes larger (28, 47, 48). Our data show that this ability of fasting to modulate sensitivity to satiation signals does not eliminate the effect of nutrients to reduce motivation even from the increased baseline. This finding raises the possibility that fasting may differentially influence the behavioral mechanisms by which satiation signals reduce food intake.
Nutrient infusion robustly suppresses subsequent food intake, as evidenced by the effect of IG nutrient infusions on ad libitum overnight home cage chow intake. However, feeding in this situation occurs much later relative to IG infusion and likely involves other factors in addition to motivation. For comparison with our PR data, we examined the effect of IG sucrose infusion on operant responding for sucrose pellets on an FR5 schedule. The FR5 task is ideal for comparison with our PR data because both tasks required that animals be trained to perform behavior in the operant chamber to earn sucrose pellet reinforcement and are conducted at the same time of the day and the same time relative to IG infusion. The FR5 task requires effort to obtain the food reward, but because the effort required does not change during the test session, it is not a test of motivation alone. Due to this difference in paradigm, rats obtain far more sucrose pellet reinforcers under both saline and nutrient load conditions on the FR5 schedule than on the PR schedule. In the FR5 test situation, we found that IG infusion of sucrose significantly decreased the number of active lever presses, and thus sucrose pellets earned, and this effect was larger in magnitude than what we observed in the PR paradigm, where motivation is more isolated. The larger effect in the FR5 paradigm is likely due to the contribution of other factors in addition to any effect on motivation. Rats’ behavior in the FR5 experiment under IG sucrose conditions also provides useful information for interpretation of the nutrient load effect observed in the PR experiments. It could be suggested that the reason the 10-ml IG nutrient infusion reduces responding is that there is a threshold for volume in the gastrointestinal tract, and the rats will not consume beyond that point. The finding that rats earned and consumed an average of 34 pellets in the FR5 test session after IG sucrose infusion demonstrates that under these infusion conditions, they are indeed capable of consuming substantially more than the seven to nine pellets typically earned in the PR sessions following nutrient infusions. Therefore, the suppression of PR responding and breakpoint cannot be due to a limit on gastrointestinal capacity but rather reflects a change in motivation for the sucrose pellets.
We also report here that the effect of IG nutrient infusion to reduce operant responding for a sucrose reinforcer is not limited to sucrose; IG infusion of Ensure, a mixed nutrient solution, significantly reduced PR responding for a sucrose reward. This effect of IG Ensure establishes that the IG nutrient and reinforcer need not be identical for this effect to occur. Although Ensure does contain sugar, it is also composed of other nutrients such as fat and protein, and the total sugar content of the Ensure infusion was much lower than in our sucrose solution infusions. Despite these differences, we observed that the effect of IG Ensure to suppress PR responding was similar in magnitude to the effect of the sucrose infusion. This finding supports the idea that multiple satiation signals could be involved, as the complement of gastrointestinal signals generated by a purely carbohydrate load will differ from those generated by a mixed nutrient load.
It is well established that fluctuations in estrogens across the estrus cycle influence satiation signaling and thus food intake (3, 10). This effect is most evident during estrus, when estrogens modulate responsiveness to satiation signals, resulting in a potent reduction in food intake. Recently, it has been suggested that estrogens may also reduce motivation for food, with female rats showing significantly reduced PR performance during estrus relative to diestrus (34). Therefore, in a separate study, we sought to replicate the effect of IG nutrients on motivation for sucrose reinforcement in females and to further examine whether this effect of satiation on motivation varies across the estrus cycle. As in the other studies reported here, IG nutrient-induced satiation does reduce motivation for sucrose reinforcement in the PR task in females. There was a tendency for the effect of IG nutrient infusion to be greater when rats were tested during estrus compared with diestrus, but we failed to see a significant interaction between cycle stage and IG nutrient infusion on any measure of PR performance. Additionally, analysis of the IG saline conditions did not reveal a reduction in PR responding for a sucrose reward during estrus, a finding consistent with another recently published paper (26). Together, these data suggest that the known effect of estrogens to potentiate satiation signaling in cycling females may not be a major influence on the ability of IG nutrient infusion to reduce motivation for food, at least under the testing conditions utilized here.
To begin to elucidate the gut hormones that may be involved in IG nutrient-induced reduction in motivation for food, we focused on the GLP-1 and CCK systems. GLP-1 release is most potently stimulated by ingested fat and carbohydrate, whereas CCK release is generated in response to fat and protein (18, 23, 24, 36). The IG infusion of Ensure can be expected to stimulate the release of both GLP-1 and CCK from intestinal endocrine cells because of its complex macronutrient composition (59.5% carbohydrate, 24.3% fat, 16.2% protein). Previous studies demonstrate that blockade of the GLP-1R or CCKAR attenuates nutrient-induced satiation in tests of ad libitum feeding, demonstrating the endogenous contribution of these gut signals in the control of feeding (7, 33, 37, 49). Here, we took a similar approach to examine the contribution of endogenous, nutrient-stimulated GLP-1 and CCK in motivation for food in both male rats and cycling female rats in estrus.
Pretreatment with GLP-1R antagonist Ex9 significantly attenuated the effect of IG Ensure on PR responding for a sucrose reward in males. This finding suggests that endogenous peripheral GLP-1 activity is involved in the suppressive effect of IG nutrients on food reward. We do not discount the possible role of brain GLP-1R in motivation for food, but we interpret the results of our experiment as indicative of peripheral GLP-1 action for two reasons. First, due to the very short half-life of active GLP-1, it is unlikely that significant quantities of active GLP-1 released from the intestine circulate to the brain (22). Second, previous work showed that intracerebroventricular delivery of Ex9, to block brain GLP-1R, is not able to attenuate the intake-suppressive effects of ip GLP-1 administration (49). Thus, in the present study, ip Ex9 is most likely exerting its effect on peripheral GLP-1R. Although Ex9 treatment did significantly reduce active lever presses following IG saline control infusion in males, this decrease did not translate into a significant reduction in breakpoint, or sucrose pellets earned, which are the key measures of motivation in the PR task. Importantly, responding after ip Ex9/IG Ensure was significantly higher than after ip vehicle/IG Ensure for all variables in the PR task, demonstrating that the suppressive effect of Ensure was prevented by the Ex9 treatment. In contrast to what we observed in males, GLP-1R blockade did not attenuate nutrient-induced suppression of motivation for sucrose reinforcement in estrus females. Whether this divergent response across these experiments reflects a sex difference requires further investigation. The lack of complete reversal of the IG Ensure effect with Ex9 in males and lack of effect of Ex9 pretreatment in females suggests that endogenous peripheral GLP-1 may be one of several satiation signals that contribute to nutrient-induced suppression of motivation for food reward.
Pretreatment with DVZ, the CCKAR antagonist, failed to influence the effect of IG nutrients on motivation for sucrose reinforcement. In males in particular, there was a trend for DVZ to suppress all variables measured in the PR operant responding task when followed by the control IG saline infusion, although this did not reach significance when corrected for multiple comparisons. While our data from the separate male and female experiments show that following DVZ treatment IG Ensure failed to significantly reduce PR responding relative to IG saline, PR performance following ip DVZ/IG Ensure was no different than ip vehicle/IG Ensure, suggesting that the full suppressive effect of IG Ensure was still expressed after DVZ treatment. The nature of the observed trend toward an inhibitory effect of DVZ treatment on PR responding is unknown. IG saline infusion would not be expected to stimulate endogenous CCK release, and CCKAR blockade would be predicted to increase, rather than decrease, feeding-related behaviors, given CCK’s well-established function as a satiation signal. It is possible that basal endogenous CCK in fact increases motivation, contrary to expectations (4, 15). It is also possible that DVZ interferes with some other function required to perform the PR task, although we saw no evidence that DVZ interfered with motor function or rats’ mastery of the task (e.g., there was no alteration in inactive lever presses). Nevertheless, in both males and females, DVZ treatment did attenuate the nutrient-induced suppression in overnight chow intake, an expected effect of this drug based on previous studies examining the role of endogenous CCK in ad libitum feeding (7, 33, 37). This effect serves as a positive control, demonstrating that DVZ blocked CCKAR effectively.
Taken together, our data provide the first direct evidence that IG infusion of satiating nutrient loads can reduce motivation to obtain a food reward and highlight the GLP-1 system as a potential contributor to this effect. These findings provide support for the hypothesis that nutrient-induced satiation signals reduce subsequent food intake, in part by decreasing motivation for food. Future studies will be required to identify the specific peripheral and neural mechanisms underlying this effect.
GRANTS
This work was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK-095757) to D. L. Williams.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.B.M., G.C.L., and D.L.W. conceived and designed research; C.B.M., G.C.L., N.L., and S.J.T. performed experiments; C.B.M., G.C.L., and D.L.W. analyzed data; C.B.M., G.C.L., and D.L.W. interpreted results of experiments; C.B.M. prepared figures; C.B.M. and D.L.W. drafted manuscript; C.B.M., G.C.L., S.J.T., and D.L.W. edited and revised manuscript; C.B.M., G.C.L., N.L., S.J.T., and D.L.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Nelson Medina for assistance during portions of the behavioral experiments.
Current address for G. C. Loney: Department of Psychology, University at Buffalo, Buffalo, NY 14260.
REFERENCES
- 1.Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol Sect B 34, 2b: 77–98, 1982. doi: 10.1080/14640748208400878. [DOI] [Google Scholar]
- 2.de Araujo IE, Ferreira JG, Tellez LA, Ren X, Yeckel CW. The gut-brain dopamine axis: a regulatory system for caloric intake. Physiol Behav 106: 394–399, 2012. doi: 10.1016/j.physbeh.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 305: R1215–R1267, 2013. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babcock AM, Livosky M, Avery DD. Cholecystokinin and bombesin suppress operant responding for food reward. Pharmacol Biochem Behav 22: 893–895, 1985. doi: 10.1016/0091-3057(85)90543-X. [DOI] [PubMed] [Google Scholar]
- 5.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146: 1650–1673, 2005. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 6.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav 76: 353–364, 2002. doi: 10.1016/S0031-9384(02)00759-X. [DOI] [PubMed] [Google Scholar]
- 7.Covasa M, Ritter RC. Attenuated satiation response to intestinal nutrients in rats that do not express CCK-A receptors. Peptides 22: 1339–1348, 2001. doi: 10.1016/S0196-9781(01)00461-2. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis J, Campbell C. Chronic intrajugular, intraportal, gastric, and duodenal cannulae for the rat. In: Physiological Techniques in Behavioral Research (Singh D, Avery D, editors). Monterey, CA: Brooks Cole, 1975, p. 163–177. [Google Scholar]
- 10.Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav 104: 517–524, 2011. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferguson SA, Paule MG. Lack of effect of prefeeding on food-reinforced temporal response differentiation and progressive ratio responding. Behav Processes 34: 153–160, 1995. doi: 10.1016/0376-6357(94)00062-L. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SA, Paule MG. Progressive ratio performance varies with body weight in rats. Behav Processes 40: 177–182, 1997. doi: 10.1016/S0376-6357(97)00786-9. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes MF, Sharma S, Hryhorczuk C, Auguste S, Fulton S. Nutritional controls of food reward. Can J Diabetes 37: 260–268, 2013. doi: 10.1016/j.jcjd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Figlewicz DP, MacDonald Naleid A, Sipols AJ. Modulation of food reward by adiposity signals. Physiol Behav 91: 473–478, 2007. doi: 10.1016/j.physbeh.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajnal A, Acharya NK, Grigson PS, Covasa M, Twining RC. Obese OLETF rats exhibit increased operant performance for palatable sucrose solutions and differential sensitivity to D2 receptor antagonism. Am J Physiol Regul Integr Comp Physiol 293: R1846–R1854, 2007. doi: 10.1152/ajpregu.00461.2007. [DOI] [PubMed] [Google Scholar]
- 16.Hayes MR, Mietlicki-Baase EG, Kanoski SE, De Jonghe BC. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu Rev Nutr 34: 237–260, 2014. doi: 10.1146/annurev-nutr-071812-161201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayes MR, Schmidt HD. GLP-1 influences food and drug reward. Curr Opin Behav Sci 9: 66–70, 2016. doi: 10.1016/j.cobeha.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann C, Göke R, Richter G, Fehmann HC, Arnold R, Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 56: 117–126, 1995. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 19.Hodos W. Progressive ratio as a measure of reward strength. Science 134: 943–944, 1961. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 20.Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav 6: 387–392, 1963. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoebel BG. Inhibition and disinhibition of self-stimulation and feeding: hypothalamic control and postingestional factors. J Comp Physiol Psychol 66: 89–100, 1968. doi: 10.1037/h0025962. [DOI] [PubMed] [Google Scholar]
- 22.Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-IV inhibitors. Diabetologia 48: 612–615, 2005. doi: 10.1007/s00125-005-1705-7. [DOI] [PubMed] [Google Scholar]
- 23.Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci 40: 1074–1082, 1995. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 24.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest 75: 1144–1152, 1985. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockie SH, Andrews ZB. The hormonal signature of energy deficit: Increasing the value of food reward. Mol Metab 2: 329–336, 2013. doi: 10.1016/j.molmet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Ferreras L, Richard JE, Noble EE, Eerola K, Anderberg RH, Olandersson K, Taing L, Kanoski SE, Hayes MR, Skibicka KP. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Mol Psychiatry 23: 1157–1168, 2018. doi: 10.1038/mp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin JT, McKie S. Human brain responses to gastrointestinal nutrients and gut hormones. Curr Opin Pharmacol 31: 8–12, 2016. doi: 10.1016/j.coph.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 28.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141: 4442–4448, 2000. doi: 10.1210/endo.141.12.7815. [DOI] [PubMed] [Google Scholar]
- 29.Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC, Hayes MR. The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 305: E1367–E1374, 2013. doi: 10.1152/ajpendo.00413.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T, Spector AC, le Roux CW. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr 96: 467–473, 2012. doi: 10.3945/ajcn.112.036921. [DOI] [PubMed] [Google Scholar]
- 31.Moran TH, Dailey MJ. Intestinal feedback signaling and satiety. Physiol Behav 105: 77–81, 2011. doi: 10.1016/j.physbeh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Research Council Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: National Academies Press, 2011. [Google Scholar]
- 33.Reidelberger RD, Heimann D, Kelsey L, Hulce M, Roger D. Effects of peripheral CCK receptor blockade on feeding responses to duodenal nutrient infusions in rats. Am J Physiol Regul Integr Comp Physiol 284: R389–R398, 2003. doi: 10.1152/ajpregu.00529.2002. [DOI] [PubMed] [Google Scholar]
- 34.Richard JE, López-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP. Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 78: 193–202, 2017. doi: 10.1016/j.psyneuen.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11, 1996. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 36.Ritzel U, Fromme A, Ottleben M, Leonhardt U, Ramadori G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol 34: 18–21, 1997. doi: 10.1007/s005920050059. [DOI] [PubMed] [Google Scholar]
- 37.Savastano DM, Covasa M. Intestinal nutrients elicit satiation through concomitant activation of CCK(1) and 5-HT(3) receptors. Physiol Behav 92: 434–442, 2007. doi: 10.1016/j.physbeh.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Sclafani A. Post-ingestive positive controls of ingestive behavior. Appetite 36: 79–83, 2001. doi: 10.1006/appe.2000.0370. [DOI] [PubMed] [Google Scholar]
- 39.Sclafani A. Gut-brain nutrient signaling. Appetition vs. satiation. Appetite 71: 454–458, 2013. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav 82: 89–95, 2004. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 41.Sclafani A, Ackroff K. Nutrient-conditioned flavor preference and incentive value measured by progressive ratio licking in rats. Physiol Behav 88: 88–94, 2006. doi: 10.1016/j.physbeh.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Shin ACC, Zheng H, Pistell PJJ, Berthoud H-R. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes 35: 642–651, 2011. doi: 10.1038/ijo.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shizgal P, Fulton S, Woodside B. Brain reward circuitry and the regulation of energy balance. Int J Obes Relat Metab Disord 25, Suppl 5: S17–S21, 2001. doi: 10.1038/sj.ijo.0801906. [DOI] [PubMed] [Google Scholar]
- 44.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci 7: 181, 2013. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skibicka KP, Dickson SL. Enteroendocrine hormones - central effects on behavior. Curr Opin Pharmacol 13: 977–982, 2013. doi: 10.1016/j.coph.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Tellez LA, Ferreira JG, Medina S, Land BB, DiLeone RJ, de Araujo IE. Flavor-independent maintenance, extinction, and reinstatement of fat self-administration in mice. Biol Psychiatry 73: 851–859, 2013. doi: 10.1016/j.biopsych.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unniappan S, Kieffer TJ. Leptin extends the anorectic effects of chronic PYY(3–36) administration in ad libitum-fed rats. Am J Physiol Regul Integr Comp Physiol 295: R51–R58, 2008. doi: 10.1152/ajpregu.00234.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55: 3387–3393, 2006. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 49.Williams DL, Baskin DG, Schwartz MW. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687, 2009. doi: 10.1210/en.2008-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woods SC, Begg DP. Regulation of the motivation to eat. Curr Top Behav Neurosci. 27: 15–34, 2016. doi: 10.1007/7854_2015_381. [DOI] [PubMed] [Google Scholar]
- 51.Zukerman S, Ackroff K, Sclafani A. Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol 301: R1635–R1647, 2011. doi: 10.1152/ajpregu.00425.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]