Abstract
Attenuated Ca2+-activated Cl− secretion has previously been observed in the model of dextran sulfate sodium (DSS)-induced colitis. Prior studies have implicated dysfunctional muscarinic signaling from basolateral membranes as the potential perpetrator leading to decreased Ca2+-activated Cl− secretion. However, in our chronic model of DSS-colitis, cholinergic receptor muscarinic 3 (Chrm3) transcript (1.028 ± 0.12 vs. 1.029 ± 0.27, P > 0.05) and CHRM3 protein expression (1.021 ± 0.24 vs. 0.928 ± 0.09, P > 0.05) were unchanged. Therefore, we hypothesized that decreased carbachol (CCH)-stimulated Cl− secretion in DSS-induced colitis could be attributed to a loss of Ca2+-activated Cl− channels (CaCC) in apical membranes of colonic epithelium. To establish this chemically-induced colitis, Balb/C mice were exposed to 4% DSS for five alternating weeks to stimulate a more moderate, chronic colitis. Upon completion of the protocol, whole thickness sections of colon were mounted in an Ussing chamber under voltage-clamp conditions. DSS-induced colitis demonstrated a complete inhibition of basolateral administration of CCH-stimulated Cl− secretion that actually displayed a reversal in polarity (15.40 ± 2.22 μA/cm2 vs. −2.47 ± 0.25 μA/cm2). Western blotting of potential CaCCs, quantified by densitometric analysis, demonstrated no change in bestrophin-2 and cystic fibrosis transmembrane regulator, whereas anoctamin-1 [ANO1, transmembrane protein 16A (TMEM16A)] was significantly downregulated (1.001 ± 0.13 vs. 0.510 ± 0.12, P < 0.05). Our findings indicate that decreased expression of TMEM16A in DSS-induced colitis contributes to the decreased Ca2+-activated Cl− secretion in murine colon.
Keywords: Cl−; channels; colitis; colonic epithelium, muscarinic signaling; Ussing chamber
INTRODUCTION
Chloride secretion in secretory epithelium has been primarily subdivided into cAMP stimulated and Ca2+ activated through the use of different pharmacological agonists (9, 13). Aberrations in Cl− secretion have been observed in several pathological conditions, which include cystic fibrosis, Vibrio cholera infection, and inflammatory bowel disease (8, 30a, 37). Decreased Cl− secretion in ulcerative colitis (UC) or animal models of UC (dextran sulfate sodium, DSS) has been previously reported (10, 12). More specifically, Ca2+-activated Cl− secretion has been shown to be decreased in the DSS-induced colitis animal model (3, 16, 19). Previous studies focused on this phenomenon concluded the attenuation to be attributable to dysfunctional muscarinic signaling from the basolateral surface of colonic epithelium (1, 18). However, more recently it has been reported to be possible perturbations in ion channel function/expression in colonic epithelium (16).
Ca2+-activated Cl− channels (CaCCs) have been implicated to be involved in epithelial differentiation, salivary secretion, and mucociliary clearance (28, 30, 35). However, initial attempts to characterize CaCCs proved difficult, as potential candidates did not match the biophysical properties of the native protein (15). In the past decade a novel CaCC, anoctamin 1 [ANO1, transmembrane protein 16A (TMEM16A)], was identified by three separate groups (6, 34, 40). Young et al. (40) demonstrated that injected siRNA to Tmem16a significantly inhibited pilocarpine (muscarinic agonist)-stimulated salivary secretion in mice, while Caputo et al. (6) were able to achieve a similar knockdown of Ca2+-activated Cl− secretion with siRNA targeted to TMEM16A in primary human bronchial epithelial cells. Following these seminal discoveries, several groups utilizing a global gene knockout of Tmem16a demonstrated the absence of Ca2+-activated Cl− secretion in secretory epithelium isolated from trachea and colon (28, 30). However, the global deletion of the gene resulted in tracheal malacia and premature death of the animal (29). More recently, a tissue-specific knockout of Tmem16a was developed. Isolated distal colon from these mice demonstrated an absence of Ca2+-activated Cl− secretion following carbachol (CCH) administration (4).
Although TMEM16A has been identified as the primary mediator of Ca2+-activated Cl− secretion in colonic epithelium, it has not been studied in DSS-induced colitis. Thus, the goal of this study was to identify whether the previously described decreased Ca2+-activated Cl− secretion in colonic epithelium was attributable to decreased expression of TMEM16A in DSS-induced chronic colitis. Our results indicate that TMEM16A protein expression is downregulated in a chronic model of DSS-induced colitis and contributes to the observed loss in Ca2+-activated Cl− secretion.
MATERIALS AND METHODS
Animals.
Nonfasting male Balb/cAnNCrl mice (17 wk old; Charles River, NY) were provided normal chow for the entirety of the project. Prior to the beginning of the 10-wk project, mice (7 wk old) were randomly selected for either control or DSS groups. Balb/C mice selected for the DSS protocol were provided 4% DSS for 5 days (with 9 days of normal drinking water in between) for five alternating weeks, beginning with the first week. Animals utilized for the acute protocol were assigned to either control or DSS groups. DSS groups were administered 4.5% DSS in the drinking water for five (5) consecutive days followed by one day of normal drinking water. Both groups were then euthanized on the seventh day of the protocol. All animals used in the study were provided food and water ad libitum. All animals were anesthetized with intraperitoneal injection of Fatal Plus (Patterson Veterinary). The experimental protocols used in this study were approved by the West Virginia University Institutional Animal Care and Use Committee.
Histology and immunohistochemistry.
Distal colon segments were isolated from euthanized mice via bilateral pneumothorax. Isolated colonic segments were measured immediately upon removal with a constant temperature of 22–23°C. Distal colon segments were then rinsed with phosphate-buffered saline (PBS) to remove any residual content and debris. Colonic segments were then opened longitudinally along the mesenteric border. An approximately 3-mm piece of segment was then cut from the intact colon and immediately placed in 10% neutral-buffered formalin for tissue fixation. Fixed tissue was then given to the West Virginia University Translational Pathology Department for paraffin embedding and hematoxylin and eosin (H&E) staining. Prepared slides consisted of 5-μm-thick sections mounted on glass slides, followed by H&E staining for visualization of tissue architecture from the respective treatment groups. Immunohistochemistry was also performed on control and DSS tissue by the West Virginia University Translational Pathology Department to determine the localization of TMEM16A in whole thickness sections of mouse distal colon. Anti-TMEM16A antibody (Alomone, Jerusalem, Israel) was used at a dilution of 1:100. Sectioned tissue was incubated at 37°C for a period of 1 h in primary antibody. Following the initial step, tissue was incubated in goat anti-rabbit horseradish-peroxidase conjugated secondary antibody (1:1,000) for 1 h. Finally, a chromogenic substrate was added for visualization of immune-stained slides. All images were captured with an AxioImager (Carl Zeiss, Germany) on ×20 magnification.
Electrophysiology.
Distal colons were removed from euthanized mice via bilateral pneumothorax and rinsed with ice-cold PBS to clear residual contents. Segments were opened longitudinally along the mesenteric border to expose the epithelium. Full thickness tissue was then mounted on a pin slider with an oval aperture of 2.8 w × 11 l mm with a cross-sectional area of 0.30 cm2 and placed in an EasyMount Ussing chamber (Physiological Instruments, San Diego, CA) for electrophysiological study as previously described (26). Mounted tissue was bathed on both sides with Ringers’ solution (in mM: 115 NaCl, 25 NaHCO3, 2.4 K2HPO4, 0.4 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, and 10 glucose), while also being continuously voltage-clamped at 0 mV to abolish any developing electrochemical gradient. Short-circuit current (ISC) was continuously monitored with a 5-mV pulse applied to the tissue every 5 s to measure resistance (RTE; Ω). Transepithelial voltage (VTE) was calculated using Ohm’s law.
Tissues were continuously maintained at 37°C and gassed with 5% CO2-95% O2 (pH 7.4). Mounted tissue was incubated for 30 min with 5 μM indomethacin and 1 μM tetrodotoxin to inhibit prostaglandin synthesis and potentially activated enteric neurons, respectively. Following equilibration, 10 μM amiloride was added apically to inhibit minor electrogenic Na+ absorption (ENaC) in the distal colon. Upon reaching a steady state, tissue was administered 100 μM CCH basolaterally to activate Gαq muscarinic receptors to indirectly activate apical Ca2+-activated Cl− secretion via intracellular increases in Ca2+ (11, 22). Fifteen minutes following CCH addition, 10 μM forskolin (FSK, an adenylate cyclase inhibitor that increases intracellular cAMP) was basolaterally added to assess tissue viability. All representative traces and presented group data are designated as positive deflections indicating apical anion secretion/cation absorption or negative deflections representative of cation secretion/anion absorption.
Colonic epithelial cell isolation.
Distal colons were isolated from euthanized mice via bilateral pneumothorax and immediately rinsed with ice-cold PBS to clear luminal contents. The distal colon was then opened longitudinally along the mesenteric border. Tissue was then cut into ~2-mm-long pieces and submerged in 40 ml of ice-cold PBS with 5 mM EDTA in a 50-ml Falcon tube. The pieces of tissue in PBS-EDTA were then incubated at 37°C with gentle rocking for 30 min. Following this incubation, colonic tissue was vigorously shaken to disperse colonic crypts and surface epithelium in solution. Supernatant was then loaded into 1.5 ml of microcentrifuge tubes and spun at 1 × 103 g for 5 min to pellet suspended cells. Isolated epithelial cells were used in downstream applications, such as RT-qPCR and immunoblotting.
RNA isolation and RT-qPCR analyses.
Isolated colonic epithelium were resuspended in QIAzol (Qiagen, Valencia, Ca). Total-RNA was isolated using the MicroRNEasy kit, per manufacturer’s instructions (Qiagen). Concentrated RNA was immediately quantified using the Nanodrop 1000 (Thermo, Waltham, MA). RNA was then stored at −80°C until further application. Real-time PCR was carried out using a one-step kit that included 10 min of reverse transcription of total-RNA (50 ng/well) followed immediately by quantitative PCR utilizing a dsDNA-binding dye (New England Biolabs, Ipswich, MA). Custom primers (400 nM/primer/well) designed using the NCBI primer-blast application were utilized to amplify specific targets (Table 1). Determination of differential expression was achieved using the 2−ΔΔCt method (21). β-Actin threshold cycles for each sample were obtained and then subtracted from threshold cycles of transcripts of interest to obtain a ΔCt value. The ΔCt value from control samples was then subtracted from DSS-colitis samples to obtain a ΔΔCt value. Fold changes of transcripts of interest were then calculated as 2-ΔΔCt. This method was utilized for comparison of all quantitative PCR values presented within the text and figures.
Table 1.
Custom qPCR primers for determination of transcript abundance
| Gene ID (Ref Seq) | Primers (Forward and Reverse) | Product Length, bp | Melting Temp, °C |
|---|---|---|---|
|
Tmem16a NM_178642.5 |
F: TCTGTGTTTATGGCCCTCTGG R: TGACAGCTTCCTCCTCCTCC |
115 | F: 59.72 R: 60.62 |
|
Best2 NM_001130194.1 |
F: CCGCCTATCGCTTCTTACTGG R: GATGCACCACCAGAGTCACG |
132 | F: 60.60 R: 61.02 |
|
Cftr NM_021050.2 |
F: ATTTCACGCTCCACAGAGGC R: CCATTAACGGGGTTGTTTTTAAGC |
118 | F: 60.67 R: 59.31 |
|
Chrm3 NM_033269.4 |
F: CTGCCAGATATGACCAGCAATGG R: TCACTTGGTCAGAACGCAGC |
141 | F: 61.61 R: 60.88 |
|
Itpr3 NM_080553.3 |
F: ACATTGTGTCCCTGTACGCC R: CACTTTGAAGAGGCAATCTCGG |
143 | F: 60.32 R: 59.58 |
All primers were designed utilizing the NCBI primer-blast application with the indicated reference sequence (Ref Seq). Best2, bestrophin-2; Chrm3, cholinergic receptor muscarinic 3; F, forward; Itpr3, inositol trisphosphate receptor; R, reverse; Temp, temperature.
Protein isolation and Western blotting.
Pelleted colonic epithelium were resuspended in 350 μl of RIPA (radioimmunoprecipitation assay) buffer containing full protease inhibitor cocktail (Complete, Mini, Sigma-Aldrich, St. Louis, MO). Tissue was homogenized using glass homogenizers with a final sonication step to fully liberate transmembrane proteins. Protein concentration for each individual sample was determined using the Pierce BCA protein quantification kit (Thermo). Samples were then immediately frozen at −80°C and maintained there until further use. On the day of immunoblotting, protein samples were removed from the −80°C freezer and allowed to thaw on ice. Samples were diluted to 1 μg/μl with the addition of 4× Laemmli buffer (Bio-Rad, Hercules, CA) and remaining volume with diH2O. Following preparation, protein samples were warmed at 37°C in a water bath for 15 min and then loaded at 20 μg/well onto handcast Tris-glycine gels (SureCast, Invitrogen, Waltham, MA). Running conditions consisted of 85 V until passing the stacking buffer at which point the gel was completed at 135 V. Gels were transferred to polyvinylidene difluoride (PVDF) membranes for 90 min at a constant 20 V. Upon completion, PVDF membranes were blocked in 5% nonfat dry milk (Kroger, Cincinnati, OH) in Tris-buffered saline with 0.1% Tween 20 (TBST) for a period of 1 h at room temperature. PVDF membranes were then incubated in primary antibody: TMEM16A 1:500, CFTR 1:200, bestrophin-2 (BEST2) 1:500, muscarinic 3 (M3) receptor 1:200 (Alomone Laboratories), inositol (1,4,5)-trisphosphate (IP3) receptor 1:500, β-actin 1:1,000 (Cell Signaling, Danvers, MA) overnight at 4°C. In the morning, membranes were washed 5× in TBST for 5 min and then placed in goat anti-rabbit horseradish-peroxidase conjugated secondary antibody (1:10,000 dilution) for 2 h at room temperature. Membranes were washed 5× in TBST and imaged on the G-Box (Syngene, Cambridge, UK). All target proteins were quantified using ImageJ software (National Institutes of Health, Bethesda, MD) with normalization to β-actin.
Statistics.
All presented results represent means ± SE from five different animals, unless otherwise denoted. Statistical tests that were performed on the presented data sets were either unpaired Student’s t-test or one-way ANOVA with Tukey post hoc analysis using GraphPad Prism 6.0 (San Diego, CA). P < 0.05 was used for determination of significance.
Stock solutions.
DSS (MP Biomedicals, Burlington, MA) was at 4% in the drinking water. Ussing chamber stock solutions were 5 mM indomethacin (Indo, dissolved in ethanol, Sigma-Aldrich), 1 mM tetrodotoxin (dissolved in acetic acid; Tocris Pharmaceuticals, Minneapolis, MN), 10 mM amiloride (dissolved in DMSO, Sigma-Aldrich), 100 mM CCH (dissolved in deionized water, Sigma-Aldrich), 10 mM forskolin (FSK, dissolved in DMSO, Tocris Pharmaceuticals), and 3 μM ionomycin (IONO, dissolved in DMSO, Tocris Pharmaceuticals). All stock solutions were diluted 1:1,000 in the chamber to obtain [μM].
RESULTS
Induction of chronic DSS-colitis in Balb/c mice.
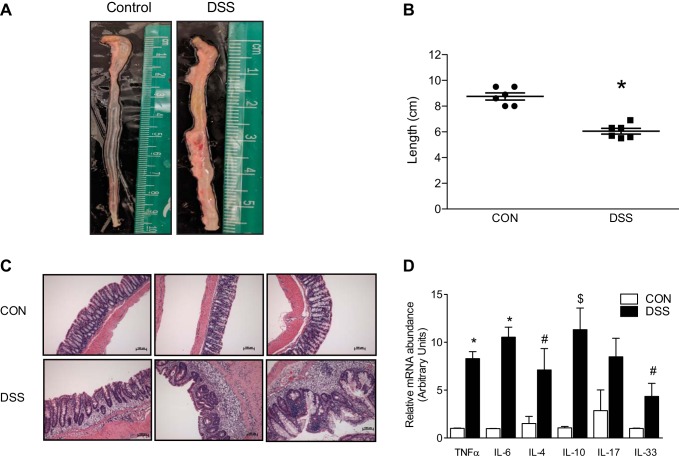
Model accuracy was evaluated using several parameters previously utilized to demonstrate consistent induction of DSS-colitis (19). Colon length in the DSS-colitis group exhibited a consistent shortening compared with control mice (Fig. 1A). Group data of measured colonic lengths from control mice were significantly longer than their DSS counterparts (8.75 ± 0.276 cm vs. 6.05 ± 0.221 cm, P < 0.001; Fig. 1B). Histological studies were also conducted to examine proper colitis induction. The whole thickness tissue isolated from control mice exhibited normal histological architecture (i.e., stacked test tubes) with intact surface epithelium and minimal inflammatory cells located in the lamina propria (Fig. 1C, top). Colonic mucosa from DSS-colitis animals exhibited substantial widening of the lamina propria as a result of an influx of inflammatory cells (Fig. 1C, bottom). Crypts in the colitic colon were also distorted, with an obvious enlargement of the underlying muscularis (Fig. 1C, bottom), which could have been the result of increased proliferation. However, this could also have been the result of colonic contraction and without a marker for proliferation cannot be excluded. Transcript abundance of acute-phase reactants was quantitated to further demonstrate active inflammation within the distal colon. Quantitative PCR (qPCR) demonstrated a significant increase in cytokine transcript abundance of TNFα (1.008 ± 0.033 vs. 8.293 ± 0.732, P < 0.0001) and IL-6 (0.980 ± 0.017 vs. 10.530 ± 1.045, P < 0.0001) from DSS-colitis samples (Fig. 1D). Besides the prototypical inflammatory cytokines, Th2-mediated cytokines were selected for study for their relevance to the clinical pathology, UC. IL-17 mRNA abundance was not significantly increased (1.000 ± 0.754 vs. 2.964 ± 0.676), however, transcript abundance of IL-4 (1.506 ± 0.747 vs. 7.109 ± 2.229, P < 0.05), IL-10 (1.062 ± 0.144 vs. 11.310 ± 2.262, P < 0.01), and IL-33 (0.998 ± 0.028 vs. 4.340 ± 1.367, P < 0.05) were all significantly increased in the DSS-colitis mice (Fig. 1D).
Fig. 1.
Evaluation of dextran sulfate sodium (DSS)-colitis in Balb/C mice. A: representative images of excised colons from either control or DSS-colitis cohorts. B: group data of measured colons from either control or colitic mice. Represented data are means ± SE of different groups, collected from 6 different animals in each respective group (*P < 0.001). C: hematoxylin and eosin staining of formalin-fixed colonic tissue. Each image represents a different animal from their corresponding treatment. DSS-colitis (bottom) demonstrates crypt dysplasia, leukocyte infiltration, and thickening of underlying serosa (×20, AxioImager). D: RT-qPCR analysis of multiple cytokines involved in the development of colitis. Asterisks (*) indicate significance of each individual cytokine transcript as compared with control. *P < 0.0001, compared with control; $P < 0.01, compared with control; #P < 0.05, compared with control.
Effect of chronic DSS-induced colitis on Ca2+-activated Cl− secretion.
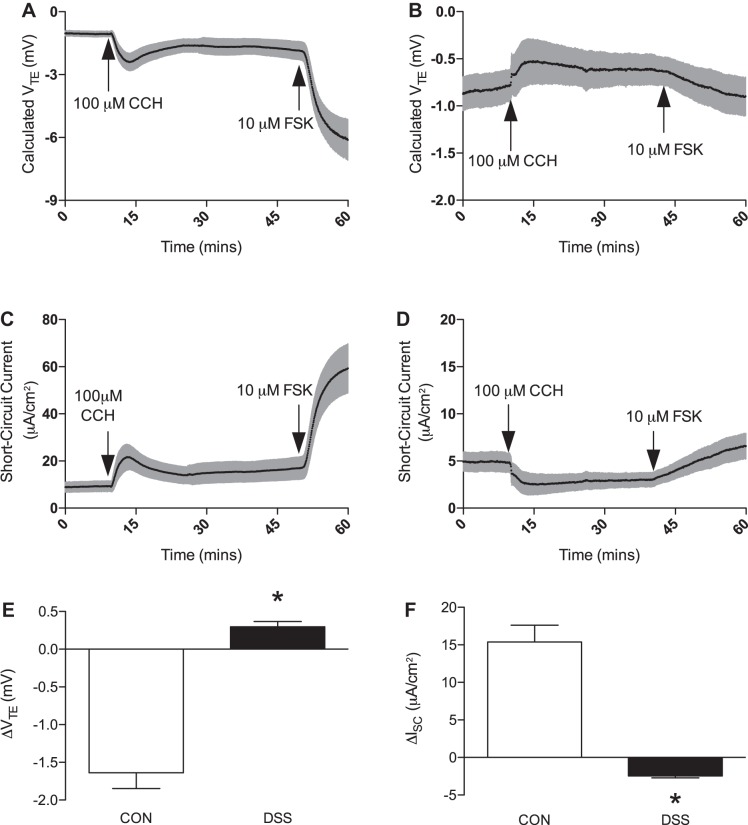
Control mucosa demonstrated a robust increase in Ca2+-activated Cl− secretion, which was measured as positive deflections in short-circuit current (ISC) (ΔISC, 15.40 ± 2.22 μA/cm2, n = 5; Fig. 2F). This was paralleled by a calculated hyperpolarization of transepithelial potential (ΔVTE, −1.64 ± 0.21 mV, n = 5; Fig. 2E) following 100 μM CCH application (Fig. 2, A and C). DSS-treated mucosa did elicit a response upon application of CCH; however, this response was significantly smaller in magnitude and opposite in apparent charge (Fig. 2, B and D). The decrease in measured ISC (ΔISC, −2.47 ± 0.25 μA/cm2, n = 5; Fig. 2F) most likely indicates activation of Ca2+-activated K+ channels, which are known to reside in the apical membrane of colonic epithelium (31, 32, 36, 42). This altered response to basolateral CCH could potentially be related to decreased synthesis of prostaglandin E2, which has previously been shown to uncouple CCH administration from apical Cl− secretion (7). Also, calculated VTE paralleled this response with a slight depolarization due to increased positive charge accumulating on the luminal side of the chamber (ΔVTE, 0.296 ± 0.07 mV, n = 5; Fig. 2E). Application of basolateral 10 μM FSK was administered to assess the tissues ability to respond to another physiological agonist (Fig. 2, C and D).
Fig. 2.
Effect of dextran sulfate sodium (DSS)-colitis on transepithelial potential and Cl− secretion. A: representative trace of calculated transepithelial potential (VTE) in control mice following application of 100 μM carbachol (CCH) and 10 μM forskolin (FSK). B: representative trace of calculated VTE in DSS-colitis mice following application of 100 μM CCH and 10 μM FSK. C: representative trace of elicited short-circuit current (ISC) in control mice following basolateral administration of 100 μM CCH and 10 μM FSK. D: representative trace of elicited ISC in DSS-colitis mice following basolateral administration of 100 μM CCH and 10 μM FSK. E: summarized data of calculated ΔVTE following CCH administration in either control (open bar) or DSS-colitis (closed bar) mice. DSS-colitis demonstrates a complete inhibition with an actual reversal in polarity as compared with control. F: group data of ΔISC from both control (open bar) and DSS-colitis (closed bar) groups following CCH-administration. DSS-colitis, in parallel to calculated ΔVTE, demonstrates an elicited current that is in opposition to that of the control cohort. Bar graphs of summarized data represent means ± SE of 5 different animals. *P < 0.001, compared with control.
Effect of acute DSS-induced colitis on Ca2+-activated Cl− secretion.
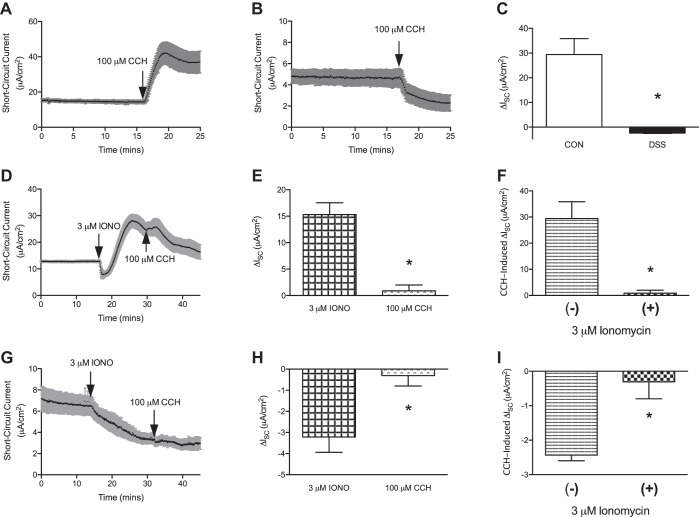
Following our initial observations in the Ussing chamber that recapitulated previously published work with the acute model of DSS, along with Western blot quantification of TMEM16A that demonstrated a decrease in expression (1.000 ± 0.13 vs. 0.065 ± 0.01, P < 0.05), we decided to utilize the acute model (16, 19). This was done to establish that changes in CCH-induced ISC were in fact due to aberrations in intracellular Ca2+ responses. However, we first needed to establish that our acute model of DSS-induced colitis was in agreement with previously published literature (16). As previously seen with our controls from the chronic model, control mucosa elicited a robust increase in ISC (ΔISC, 29.42 ± 6.42 μA/cm2, n = 6) when exposed to basolateral 100 μM CCH (Fig. 3, A and C). Also as expected, colonic mucosa isolated from animals that received DSS demonstrated a drop in baseline ISC (ΔISC, −2.43 ± 0.17 μA/cm2, n = 6) following basolateral 100 μM CCH (Fig. 3, B and C). To then establish that Ca2+ mediates the CCH-induced changes in ISC in control and DSS mucosa, 3 μM ionomycin (calcium ionophore, IONO) was added before basolateral CCH administration. IONO (3 μM) induced an increase in measured ISC in control mucosa (ΔISC, 15.30 ± 2.24 μA/cm2, n = 4; Fig. 3, D and E), which was followed by basolateral addition of 100 μM CCH. Administration of CCH was only able to minimally increase ISC (ΔISC, 0.90 ± 1.09 μA/cm2, n = 4; Fig. 3, D and E). And when compared with mucosa that was treated with CCH before IONO, the measured increase in ISC was significantly less (ΔISC, 29.42 ± 6.42 μA/cm2 vs. 0.90 ± 1.09 μA/cm2, P < 0.01; Fig. 3F). Colonic mucosa from DSS-colitis mice was then incubated with 3 μM IONO, which caused a drop in baseline ISC (ΔISC, −3.21 ± 0.73 μA/cm2, n = 4; Fig. 3, G and H). Following this, 100 μM CCH was administered to the basolateral surface of the tissue. CCH was able to elicit only a small drop in measured ISC (ΔISC, −0.31 ± 0.49 μA/cm2, n = 4; Fig. 3, G and H). Overall, this demonstrated a similar pattern to control mucosa of CCH-induced ISC being attenuated when following IONO administration (ΔISC, −3.21 ± 0.73 μA/cm2 vs. −0.31 ± 0.49 μA/cm2, P < 0.01; Fig. 3I). Taken together, these results indicate that CCH-induced changes in ISC are Ca2+ mediated in both control and DSS-exposed mucosa.
Fig. 3.
Effect of ionomycin on carbachol (CCH)-induced changes in short-circuit current (ISC) from control and acute dextran sulfate sodium (DSS)-colitis mice. A: representative trace of measured ISC following basolateral 100 μM CCH administration to control mucosa. B: representative trace of ISC from DSS-colitis mucosa following basolateral 100 μM CCH administration. C: group data of ΔISC following CCH-administration in control and DSS mice. D: representative trace of ISC from control mucosa that was stimulated with 3 μM ionomycin (IONO) and followed by 100 μM CCH. E: group data of ΔISC with 3 μM IONO followed by 100 μM CCH in control mucosa. F: group data of CCH-induced ΔISC with and without 3 μM IONO administration. G: representative trace of ISC from mucosa exposed to DSS administered 3 μM IONO and 100 μM CCH. H: group data of ΔISC from DSS-colitis in response to 3 μM IONO followed by 100 μM CCH administration. I: group data of DSS-colitis CCH-induced ΔISC with and without 3 μM IONO administration. All presented bar graphs are summarized data that represent means ± SE. *P < 0.01, compared with control.
Effect of chronic DSS-induced colitis on Cl− channel transcript abundance.
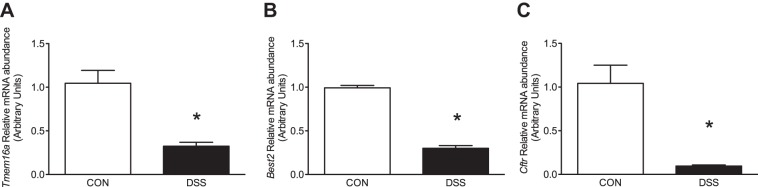
RT-qPCR analysis was utilized to assess transcript abundance of several Cl− channels known to be expressed in distal colonic epithelium (28, 37, 41). Two Ca2+-activated Cl− channels (Tmem16a, Best2) as well as the cystic fibrosis transmembrane regulator (Cftr) were selected for analysis. Tmem16a mRNA abundance was significantly decreased in epithelium isolated from DSS-colitis mice (1.045 ± 0.15 vs. 0.325 ± 0.04, P < 0.01; Fig. 4A), which may indicate a potential decrease in translated channel protein. However, Best2 (0.993 ± 0.03 vs. 0.301 ± 0.32, P < 0.001) and Cftr (1.042 ± 0.209 vs. 0.096 ± 0.013, P < 0.01) were also significantly decreased compared with control animals (Fig. 4, B and C).
Fig. 4.
Effect of dextran sulfate sodium (DSS)-colitis on transcript abundance of Cl− channels in murine colon. Transcript abundance was determined for transmembrane protein 16A (Tmem16a; A), bestrophin-2 (Best2; B), and Cftr (C) in control (open bars) or DSS-colitis mice (closed bars). Bar graphs of summarized data represent means ± SE of 5 different animals normalized to an endogenous control, Actb. *P < 0.001, compared with their respective control.
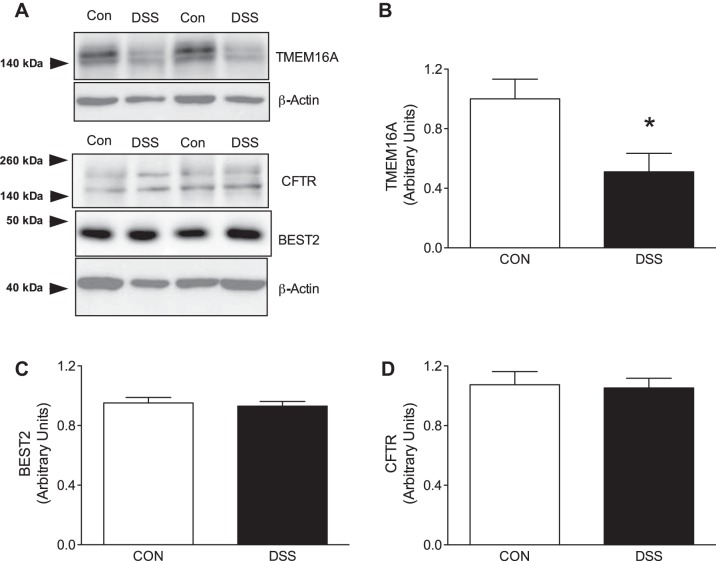
Effect of chronic DSS-induced colitis on Cl− channel protein expression in mucosal lysates.
Western blotting of epithelial lysates focused on different Cl− channels implicated to elicit a response to increasing intracellular levels of Ca2+ in colonic epithelium. Western blot technique was carried out on lysates to assess potential differences in protein expression between control and DSS mice. As previously seen with the RT-qPCR data, TMEM16A was significantly downregulated in DSS-colitis (1.001 ± 0.13 vs. 0.510 ± 0.12, P < 0.05; Fig. 5, A and B). However, Western blot quantification of BEST2, another CaCC, from the lysates demonstrated no difference between control and DSS groups (0.952 ± 0.04 vs. 0.930 ± 0.031, P > 0.05; Fig. 5, A and C). CFTR was also quantified via Western blot technique. This was to address previous observations that have described a mechanism by which CFTR is Ca2+ sensitive (5, 25a). Protein expression for CFTR was also unaltered (1.074 ± 0.09 vs. 1.053 ± 0.07, P > 0.05; Fig. 5, A and D) despite the mRNA transcript abundance being significantly decreased, similar to Best2 (Fig. 4, B and C).
Fig. 5.
Effect of dextran sulfate sodium (DSS)-colitis on Cl− channel protein expression in murine colon. A: representative images of protein expression of the different characterized Cl− channels from epithelial lysates. BEST2, bestrophin-2. B: transmembrane protein 16A (TMEM16A) protein expression normalized to β-actin for quantification of control and DSS-colitis groups. TMEM16A expression (closed bar) is significantly decreased in DSS-colitis mice as compared with control. C and D: summarized data of Western blot quantification of BEST2 and CFTR normalized to β-actin in control and DSS-colitis mice. Neither protein was significantly altered from the control cohort. Bar graphs of summarized data represent means ± SE of 5 different animals from each respective group. *P < 0.05, compared with their respective control.
Transcript and protein abundance of Gαq muscarinic signaling pathway.
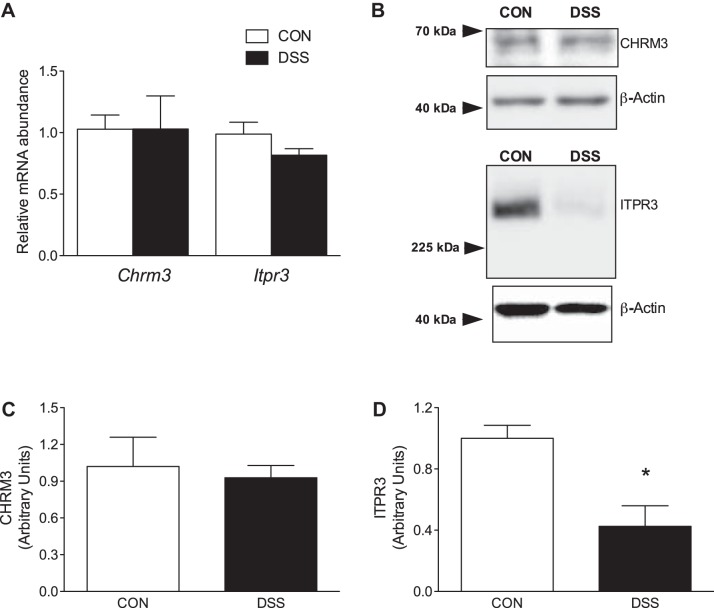
Previous work has shown that the decreased CCH-stimulated ISC in DSS-colitis was attributable to aberrations in signaling of pro-secretory stimuli (1, 18). To assess this from the standpoint of activation by CCH, mRNA abundance, as well as protein expression, of the cholinergic receptor M3 receptor (CHRM3), a prototypical Gαq G protein-coupled receptor, was quantified in the distal colon. mRNA transcript abundance for Chrm3 was unchanged between control and DSS-colitis groups (1.028 ± 0.12 vs. 1.029 ± 0.27, P > 0.05; Fig. 6A). Western blot quantification of CHRM3 was also unchanged between the different experimental groups (1.021 ± 0.24 vs. 0.928 ± 0.09, P > 0.05; Fig. 6, B and C). While signal transduction is initiated through CHRM3, canonical Gαq signaling also includes the production of the second messenger IP3, which leads to the release of Ca2+ via binding to the inositol trisphosphate receptor (ITPR3). Although Itpr3 transcript abundance was not significantly decreased (0.988 ± 0.09 vs. 0.816 ± 0.05, P > 0.05), protein expression of ITPR3 was significantly downregulated (1.000 ± 0.09 vs. 0.426 ± 0.13, P < 0.01) in DSS-colitis mice compared with their parallel controls (Fig. 6, A, B, and D).
Fig. 6.
Characterization of Gαq muscarinic signaling pathway. A: transcript abundance of cholinergic receptor muscarinic 3 receptor (Chrm3) and inositol trisphosphate receptor (Itpr3) from control and dextran sulfate sodium (DSS)-colitis groups. mRNA abundance of either transcript was not significantly different from the control cohort (P > 0.05). B: representative images of Western blots for CHRM3 and ITPR3 with corresponding loading controls for normalization. C: summarized data of CHRM3 protein expression normalized to β-actin. CHRM3 expression in DSS-colitis was unaltered as compared with control counterparts. D: group data of ITPR3 protein expression normalized to β-actin from control and DSS-colitis. ITPR3 expression was significantly downregulated in DSS-colitis. Bar graphs of summarized data represent means ± SE of 5 different animals from each respective group. *P < 0.01, compared with control.
Immunohistochemistry of TMEM16A in distal colon of control and DSS mice.
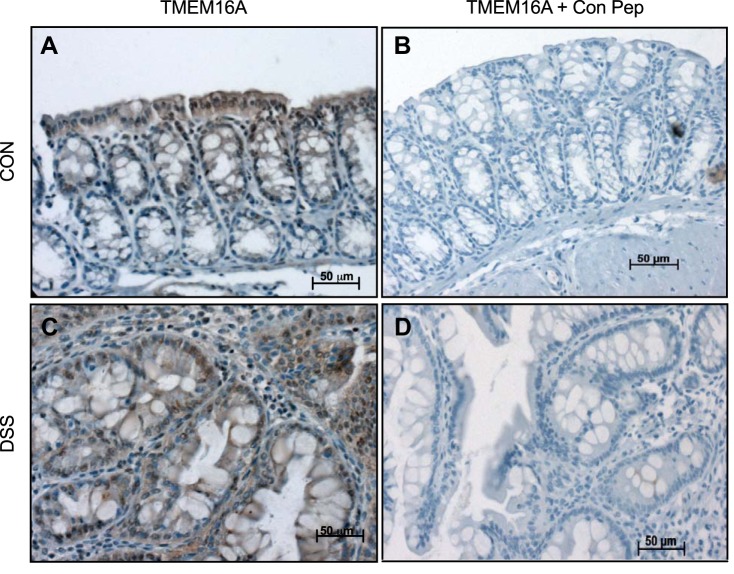
Immunohistochemistry (IHC) of TMEM16A was performed to follow-up the finding of decreased protein expression with immunoblotting (Fig. 5, A and B) and to determine the exact localization of that protein. IHC performed with anti-TMEM16A antibody on control tissue demonstrated staining of the surface epithelium with additional staining of the upper crypts (Fig. 7A). However, DSS-exposed tissue that was incubated with anti-TMEM16A antibody demonstrated substantially less membrane staining of the epithelium, with more generalized staining of the cytoplasm (Fig. 7C). This generalized staining, especially in DSS-colitis, is most likely subapical vesicles that have not been properly trafficked to the plasma membrane; however, the staining could also be potential splice variants of TMEM16A located in cellular organelles. Control peptide experiments incubated with the anti-TMEM16A antibody were run in parallel for both groups. The addition of peptide to either control or DSS-colitis tissue was devoid of staining, indicating specificity of the antibody, instead of possible nonspecific binding to similar epitopes (Fig. 7, B and D).
Fig. 7.
Immunohistochemistry of transmembrane protein 16A (TMEM16A) in colonic tissue sections. A: immunohistochemistry of TMEM16A in control tissue demonstrates pronounced expression and localization of the protein to surface epithelium and upper crypts (top, left). B. DSS-colitis tissue stained for TMEM16A exhibits less staining in epithelial cells and a more diffuse signal in the lamina propria (bottom, left). C and D: both cohorts of tissue were run in parallel with a control peptide specific for the anti-TMEM16A antibody. Both images demonstrate a lack of staining when coincubated with control peptide. Images were captured at ×20 magnification on the AxioImager (Carl Zeiss, Germany).
DISCUSSION
Utilizing the previously described model of colitis in mice (20, 39), five alternating weeks of 4% DSS administration was able to induce epithelial dysplasia, crypt distortion, and increased inflammatory infiltrate in the lamina propria, similar to the histopathological features observed in UC (2). Previously, acute induction of DSS-colitis has resulted in decreased CCH-stimulated Ca2+-activated Cl− secretion in murine distal colon (3, 16). However, these losses in Ca2+-activated Cl− secretion have been attributed to dysfunctional muscarinic receptor signaling (1) or to membrane depolarization related to decreased functioning of basolateral K+ channels (16). These previous works were also conducted before the discovery of the novel CaCC, TMEM16A (6, 34, 40). TMEM16A exhibits a similar electrophysiological profile to that found in cultured and native human bronchial epithelium (6). Also, global and tissue-specific knockout studies of TMEM16A in mice have demonstrated an absence of Ca2+-activated Cl− secretion (4, 28).
In this study, our findings demonstrate a significant attenuation in Ca2+-activated Cl− secretion in the colitic colon that is in part due to the downregulation of TMEM16A. This conclusion is evidenced by the use of a Ca2+ ionophore (ionomycin) that demonstrates a complete lack of Ca2+-activated Cl− secretion (Fig. 3) as well as significantly decreased mRNA abundance and protein expression of TMEM16A in the DSS-induced colitic colon (Figs. 4 and 5). In addition, other potential canonical and noncanonical CaCCs were also examined (5, 41). Transcript abundance was decreased for both Best2 and Cftr (Fig. 4); however, the protein expression of these Cl− channels in whole cell lysates was unchanged in the colitic colon compared with control littermates (Fig. 5). Also, transcript abundance and protein expression of CHRM3 were unchanged in DSS-induced colitis (Fig. 6), which is in agreement with a more recent publication (16). Taken together, our results indicate that decreased expression of TMEM16A following DSS administration substantially blunts Ca2+-activated Cl− secretion in murine distal colon.
While TMEM16A expression was substantially downregulated, the protein expression of ITPR3 was also significantly decreased in the DSS-colitis cohort (Fig. 6). And while this could contribute to the attenuation in CCH-stimulated Cl− secretion in DSS-colitis, a previous observation with a tissue-specific knockout of TMEM16A in colonic epithelium demonstrated decreased CCH-stimulated Ca2+ release as measured by fura-2 (33). Schreiber et al. (33) did put forth one possibility that TMEM16A may provide a physical tether for ITPR3 to the plasma membrane and that loss of TMEM16A could disrupt this architecture. Also, a prior study study demonstrated a physical interaction of TMEM16A and ITPR3 in nociceptive neurons (17). Taken together with our findings, the diminished expression of TMEM16A in colonic epithelium could cause a destabilization of ITPR3 in endoplasmic reticular membranes, leading to premature degradation of the receptor. This notion is supported by the fact that mRNA abundance of Itpr3 (0.988 ± 0.09 vs. 0.816 ± 0.05, P > 0.05) is not significantly decreased in DSS-colitis (Fig. 6). This would potentially indicate a nongenomic mechanism of decreased protein expression, possibly a protein-protein interaction between ITPR3 and TMEM16A.
Although it is most likely that the downregulation of TMEM16A is a result of transcriptional regulation, it is uncertain as to what mechanism mediates this regulation of the channel. Several groups have previously shown that IL-4 and IL-13, Th2 immune mediators, were able to significantly increase Ca2+-activated Cl− secretion in bronchial epithelium (6, 43). Another group incubated IL-4 and IL-13 on immortalized colonic epithelium (T84), and whereas IL-13 did not seem to have an effect, IL-4 was able to substantially inhibit the CCH-stimulated ISC in a dose-dependent manner (44). Another study incubated IL-10 on T84 monolayers and was able to demonstrate a similar dose-dependent inhibition of CCH-stimulated ISC (24). Together, this provides support that Th2 signaling in colonic epithelium may oppose the effects that are observed in respiratory epithelium. Also, it is worth noting that our results demonstrate a significant increase in transcript abundance of several prototypical Th2 cytokines (Fig. 1D), which could lead to the decreased expression of TMEM16A.
Previous studies have demonstrated the importance for TMEM16A in mucociliary clearance in murine airways (28, 30). Furthermore, poor hydration of the mucus layer between secretory epithelium and resident bacteria has been shown to promote an environment of bacterial overgrowth, leading to an increased interaction of nonhost with host (25, 27). This potentially highlights the impact that TMEM16A may have on colonic inflammation in UC. Utilizing these previous observations, we speculate that decreased TMEM16A may exacerbate colonic inflammation via an increase in interaction between resident immune cells and the microbiota. From a clinical perspective, an increase in Ca2+-activated Cl− secretion could decrease this interaction and help to ameliorate further inflammation induced via this mechanism.
In conclusion, our findings demonstrate a significant decrease in Ca2+-activated Cl− secretion in a DSS-induced colitic colon. The CaCC TMEM16A is significantly decreased in the DSS-induced colitis cohort of mice, whereas other Cl− channels (BEST2, CFTR) were unchanged at the protein level. The significant decrease in ITPR3 may be the result of a loss of TMEM16A stabilization through protein-protein interactions. Also, we speculate that the loss of TMEM16A in colonic epithelium may contribute to the inflammatory response.
GRANTS
Support for this study was provided by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-104791 (V. M. Rajendran).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.S.R., A.J.N., E.A.M., A.B.S., A.D.H., and V.M.R. conceived and designed research; T.S.R. performed experiments; T.S.R. analyzed data; T.S.R. interpreted results of experiments; T.S.R. and V.M.R. prepared figures; T.S.R. and V.M.R. drafted manuscript; T.S.R., A.J.N., and V.M.R. edited and revised manuscript; T.S.R., A.J.N., E.A.M., A.B.S., A.D.H., and V.M.R. approved final version of manuscript.
REFERENCES
- 1.Sayer B, Lu J, Green C, Söderholm JD, Akhtar M, McKay DM. Dextran sodium sulphate-induced colitis perturbs muscarinic cholinergic control of colonic epithelial ion transport. Br J Pharmacol 135: 1794–1800, 2002. doi: 10.1038/sj.bjp.0704633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appleman HD. What are the critical histologic features in the diagnosis of ulcerative colitis? Inflamm Bowel Dis 14, Suppl 2: S164–S165, 2008. doi: 10.1002/ibd.20586. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Navarro R, Ballester I, Zarzuelo A, Sánchez de Medina F. Disturbances in epithelial ionic secretion in different experimental models of colitis. Life Sci 76: 1489–1501, 2005. doi: 10.1016/j.lfs.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Benedetto R, Ousingsawat J, Wanitchakool P, Zhang Y, Holtzman MJ, Amaral M, Rock JR, Schreiber R, Kunzelmann K. Epithelial chloride transport by CFTR requires TMEM16A. Sci Rep 7: 12397, 2017. doi: 10.1038/s41598-017-10910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billet A, Hanrahan JW. The secret life of CFTR as a calcium-activated chloride channel. J Physiol 591: 5273–5278, 2013. doi: 10.1113/jphysiol.2013.261909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 322: 590–594, 2008. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 7.Carew MA, Thorn P. Carbachol-stimulated chloride secretion in mouse colon: evidence of a role for autocrine prostaglandin E2 release. Exp Physiol 85: 67–72, 2000. doi: 10.1111/j.1469-445X.2000.01947.x. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O’Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63: 827–834, 1990. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 9.Cliff WH, Frizzell RA. Separate Cl− conductances activated by cAMP and Ca2+ in Cl−-secreting epithelial cells. Proc Natl Acad Sci USA 87: 4956–4960, 1990. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe SE, Luthra GK, Perdue MH. Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut 41: 785–792, 1997. doi: 10.1136/gut.41.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmsathaphorn K, Pandol SJ. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77: 348–354, 1986. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz-Granados N, Howe K, Lu J, McKay DM. Dextran sulfate sodium-induced colonic histopathology, but not altered epithelial ion transport, is reduced by inhibition of phosphodiesterase activity. Am J Pathol 156: 2169–2177, 2000. doi: 10.1016/S0002-9440(10)65087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickinson KE, Frizzell RA, Sekar MC. Activation of T84 cell chloride channels by carbachol involves a phosphoinositide-coupled muscarinic M3 receptor. Eur J Pharmacol 225: 291–298, 1992. doi: 10.1016/0922-4106(92)90102-2. [DOI] [PubMed] [Google Scholar]
- 15.Galietta LJV. The TMEM16 protein family: a new class of chloride channels? Biophys J 97: 3047–3053, 2009. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota CL, McKay DM. Loss of Ca-mediated ion transport during colitis correlates with reduced ion transport responses to a Ca-activated K channel opener. Br J Pharmacol 156: 1085–1097, 2009. doi: 10.1111/j.1476-5381.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin X, Shah S, Liu Y, Zhang H, Lees M, Fu Z, Lippiat JD, Beech DJ, Sivaprasadarao A, Baldwin SA, Zhang H, Gamper N. Activation of the Cl− channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci Signal 6: ra73, 2013. doi: 10.1126/scisignal.2004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kachur JF, Keshavarzian A, Sundaresan R, Doria M, Walsh R, de las Alas MM, Gaginella TS. Colitis reduces short-circuit current response to inflammatory mediators in rat colonic mucosa. Inflammation 19: 245–259, 1995. doi: 10.1007/BF01534465. [DOI] [PubMed] [Google Scholar]
- 19.Kanthesh BM, Sandle GI, Rajendran VM. Enhanced K+ secretion in dextran sulfate-induced colitis reflects upregulation of large conductance apical K+ channels (BK; Kcnma1). Am J Physiol Cell Physiol 305: C972–C980, 2013. doi: 10.1152/ajpcell.00165.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp 60: e3678, 2012. doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Luo D, Broad LM, Bird G, Putney JW Jr. Signaling pathways underlying muscarinic receptor-induced [Ca2+]i oscillations in HEK293 cells. J Biol Chem 276: 5613–5621, 2001. doi: 10.1074/jbc.M007524200. [DOI] [PubMed] [Google Scholar]
- 24.Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 113: 151–159, 1997. doi: 10.1016/S0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 25.Johansson ME, Sjövall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10: 352–361, 2013. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Namkung W, Finkbeiner WE, Verkman AS. CFTR-adenylyl cyclase I association responsible for UTP activation of CFTR in well-differentiated primary human bronchial cell cultures. Mol Biol Cell 21: 2639–2648, 2010. doi: 10.1091/mbc.E09-12-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanda Kumar NS, Singh SK, Rajendran VM. Mucosal potassium efflux mediated via Kcnn4 channels provides the driving force for electrogenic anion secretion in colon. Am J Physiol Gastrointest Liver Physiol 299: G707–G714, 2010. doi: 10.1152/ajpgi.00101.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun 72: 6040–6049, 2004. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J Biol Chem 284: 28698–28703, 2009. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol 321: 141–149, 2008. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Rock JR, O’Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, Grubb BR. Transmembrane protein 16A (TMEM16A) is a Ca2+-regulated Cl− secretory channel in mouse airways. J Biol Chem 284: 14875–14880, 2009. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Sandle GI, Hayslett JP, Binder HJ. Effect of glucocorticoids on rectal transport in normal subjects and patients with ulcerative colitis. Gut 27: 309–316, 1986. doi: 10.1136/gut.27.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandle GI, Rajendran VM. Cyclic AMP-induced K+ secretion occurs independently of Cl− secretion in rat distal colon. Am J Physiol Cell Physiol 303: C328–C333, 2012. doi: 10.1152/ajpcell.00099.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006. doi: 10.1681/ASN.2005101111. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber R, Faria D, Skryabin BV, Wanitchakool P, Rock JR, Kunzelmann K. Anoctamins support calcium-dependent chloride secretion by facilitating calcium signaling in adult mouse intestine. Pflügers Arch 467: 1203–1213, 2015. doi: 10.1007/s00424-014-1559-2. [DOI] [PubMed] [Google Scholar]
- 34.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell 134: 1019–1029, 2008. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scudieri P, Caci E, Bruno S, Ferrera L, Schiavon M, Sondo E, Tomati V, Gianotti A, Zegarra-Moran O, Pedemonte N, Rea F, Ravazzolo R, Galietta LJV. Association of TMEM16A chloride channel overexpression with airway goblet cell metaplasia. J Physiol 590: 6141–6155, 2012. doi: 10.1113/jphysiol.2012.240838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflügers Arch 459: 645–656, 2010. doi: 10.1007/s00424-009-0781-9. [DOI] [PubMed] [Google Scholar]
- 37.Thiagarajah JR, Broadbent T, Hsieh E, Verkman AS. Prevention of toxin-induced intestinal ion and fluid secretion by a small-molecule CFTR inhibitor. Gastroenterology 126: 511–519, 2004. doi: 10.1053/j.gastro.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc 2: 541–546, 2007. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 40.Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455: 1210–1215, 2008. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 41.Yu K, Lujan R, Marmorstein A, Gabriel S, Hartzell HC. Bestrophin-2 mediates bicarbonate transport by goblet cells in mouse colon. J Clin Invest 120: 1722–1735, 2010. doi: 10.1172/JCI41129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Halm ST, Halm DR. Role of the BK channel (KCa1.1) during activation of electrogenic K+ secretion in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 303: G1322–G1334, 2012. doi: 10.1152/ajpgi.00325.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Wang X, Wang H, Jiao J, Li Y, Fan E, Zhang L, Bachert C. TMEM16A-mediated mucin secretion in IL-13-induced nasal epithelial cells from chronic rhinosinusitis patients. Allergy Asthma Immunol Res 7: 367–375, 2015. doi: 10.4168/aair.2015.7.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zünd G, Madara JL, Dzus AL, Awtrey CS, Colgan SP. Interleukin-4 and interleukin-13 differentially regulate epithelial chloride secretion. J Biol Chem 271: 7460–7464, 1996. doi: 10.1074/jbc.271.13.7460. [DOI] [PubMed] [Google Scholar]