Abstract
The trabecular meshwork (TM) and Schlemm’s canal generate the majority of outflow resistance; however, the distal regions of the conventional outflow pathway account for 25–50% of total resistance. Sections of distal vessels are surrounded by α-smooth muscle actin-containing cells, indicating that they may be vasoregulated. This study examined the effect of a potent vasodilator, nitric oxide (NO), and its physiological antagonist, endothelin-1 (ET-1), on the regulation of outflow resistance in the distal regions of the conventional outflow pathway. Using a physiological model of the conventional outflow pathway, human and porcine anterior segments were perfused in organ culture under constant flow conditions, while intrachamber pressure was continually monitored. For porcine anterior segments, a stable baseline outflow facility with TM intact was first achieved before anterior segments were removed and a trabeculotomy was performed. For human anterior segments, a trabeculotomy was immediately performed. In human anterior segments, 100 nM ET-1 significantly decreased distal outflow facility from 0.49 ± 0.26 to 0.31 ± 0.18 (mean ± SD) µl·min−1·mmHg, P < 0.01. Perfusion with 100 µM diethylenetriamine-NO in the presence of 1 nM ET-1 immediately reversed ET-1 effects, significantly increasing distal outflow facility to 0.54 ± 0.35 µl·min−1·mmHg, P = 0.01. Similar results were obtained in porcine anterior segment experiments. Therefore, data show a dynamic range of resistance generation by distal vessels in both the human and the porcine conventional outflow pathways. Interestingly, maximal contraction of vessels in the distal outflow tract of trabeculotomized eyes generated resistance very near physiological levels for both species having an intact TM.
Keywords: aqueous humor, distal outflow, glaucoma, outflow physiology
INTRODUCTION
Glaucoma is a neurodegenerative disease in which retinal ganglion cells are lost irreversibly (44), resulting in the second leading cause of blindness worldwide (45). Elevated intraocular pressure (IOP) is a major risk factor for retinal ganglion cell damage in glaucoma (32, 54), and IOP lowering currently is the only effective strategy for therapeutic intervention. IOP is generated as a function of the dynamic production of aqueous humor (AH) and its removal via two drainage pathways, the conventional and the unconventional. The conventional pathway is where resistance is generated and IOP is regulated, consisting of the trabecular meshwork (TM), Schlemm’s canal (SC), and distal vasculature, including collector channels, aqueous veins, and intrascleral venous plexus (15, 22, 50). Dysregulation of conventional outflow resistance results in ocular hypertension, often leading to the development of glaucoma. Interestingly, the majority of current daily pharmacological treatments do not target conventional outflow, but rather target the production of AH by the ciliary body, or drainage through the unconventional outflow pathway. Unfortunately, glaucoma treatments that target the conventional outflow pathway are often destructive, involve applying laser energy to the TM tissues or surgical removal/bypass of the TM and inner wall of SC (47).
While the TM and SC are responsible for generating the majority of outflow resistance in the eye, accounting for 50–75% of total (18, 48), studies have reproducibly shown that completely removing the TM and inner wall of SC still leaves 25–50% of resistance (48). Consistent with these data, newer surgical techniques that bypass the TM/SC (e.g., minimal invasive glaucoma surgeries) maximally lower IOP to the mid/high teens (i.e., 16–18 mmHg) (35, 37, 47); suggesting that there is still a significant level of resistance in the regions distal to TM/SC. Recent advances in optical coherence tomography have led to imaging and 3D reconstruction of the outflow pathway including SC and collector channels in humans (24), which has increased our knowledge about this portion of the outflow pathway. Further, it has been hypothesized that collector channels may play a role in AH outflow due to their anatomical variation and location (3). Portions of these distal vessels have been shown to contain α-smooth muscle actin (αSMA)-positive cells (9, 16, 43, 50), meaning they are likely vasoregulated similar to blood vessels. Further, acetylcholinergic (43) and nitrergic (50) nerves have been found associated with these distal vessels in mice and primates, indicating autonomic control.
In addition to autonomic control, vasoregulated vessels are subject to local mediators such as endothelin-1 (ET-1) and nitric oxide (NO). Released by the vascular endothelium, ET-1 is a peptide (41), whereas NO is a gas that can rapidly infuse across cell membranes. ET-1 is a potent vasoconstrictor (63) that acts through two receptors (ETA and ETB) to modulate vascular tone (53). NO is a vasodilator that acts through the soluble guanylate cyclase (sGC) pathway (5) to induce relaxation of vessels (19, 30, 39). Both ET-1 and NO have been shown to impact both conventional outflow resistance and IOP. Studies have shown a role for NO in decreasing IOP in mice, rabbits, dogs, monkeys, and humans (4, 7, 11, 21, 31, 40, 51). A study investigating latanoprostene bunod, a prostaglandin agonist and an NO donor, found that it was more effective in lowering IOP than a prostaglandin agonist alone (57). ET-1 has been found in many ocular tissues (14, 61) and appears to play a role in elevated IOP (17, 23, 42, 52). Theoretically, ET-1 can act in two regions of the conventional outflow tract to regulate IOP: ET-1 receptors in the TM cause contraction of the tissue (6, 10), which leads to an increase in IOP. ET-1 may also be acting on the distal vasculature to increase resistance post-TM and SC. However, with the TM intact, it is difficult to determine the contribution of TM versus distal vessels to total outflow resistance.
We therefore aimed to isolate the distal vasculature from the TM to elucidate whether ET-1 affects the distal vessels, the magnitude of its effects on these vessels, and therefore outflow resistance. NO has been shown to reverse the vasoconstrictor effects of ET-1 in other vasculature (58–60), and a similar effect may be occurring in the distal vessels of the eye. We tested the activity of these compounds in organ cultured human and porcine anterior segment preparations whereby the TM and SC inner wall have been removed.
MATERIALS AND METHODS
Human donor eye tissues.
Enucleated human eyes (10 pairs) were obtained from Miracles in Sight (Winston-Salem, NC) in accordance with the Declaration of Helsinki for research involving human tissue. Enucleated eyes were free of any known ocular disease and were stored in a moist chamber at 4°C until dissection and were used within 24 h of death. A summary of donor and tissue information is contained in Table 1.
Table 1.
Summary of human donor information
| Donor no. | Age, yr | Sex | TOD-TOE, h | TOD-TOP, h |
|---|---|---|---|---|
| 1590 | 78 | F | 5:28 | 14:48 |
| 76 | 74 | F | 4:20 | 13:32 |
| 605 | 58 | M | 3:45 | 20:55 |
| 818 | 68 | F | 5:38 | 16:07 |
| 1355 | 70 | F | 6:18 | 30:67 |
| 1473 | 68 | F | 3:45 | 11:59 |
| 1758 | 67 | F | 6:22 | 23:17 |
| 1906 | 86 | M | 4:48 | 14:03 |
| 2501 | 70 | M | 7:15 | 30:46 |
| 2728 | 73 | F | 6:37 | 16:17 |
| Mean ± SD | 71.2 ± 7.4 | 5:13 ± 1:20 | 19 ± 6:57 |
TOD, time of death; TOE, time of extraction; TOP, time of perfusion.
Enucleated porcine eyes (8 pairs) were obtained from Neese Country Sausage (Burlington, NC) within 6 h of death. Enucleated eyes were stored in a moist chamber at 4°C until dissection and were used within 10 h of death.
Anterior segment dissections.
Owing to the advanced age of the human donors and the limited tissue freshness, we decided a priori not to measure outflow facility before trabeculotomy. We were concerned that the additional manipulation, particularly reseating/resealing the sclera into the perfusion chamber, would severely limit the success rate on the human anterior segments, a valuable resource. On the other hand, we thought that anterior chambers from young, fresh, and easily obtained porcine donors justified the additional manipulations. Thus, we measured outflow facility before trabeculotomy in the porcine anterior segments.
Human eyes.
Dissections were performed as previously described (25, 26). Briefly, the eyes were hemisected at the equator, and vitreous and lens were removed. The iris was trimmed from the root, and the vascular and pigmented tissues of the ciliary body were removed. The TM and inner wall of SC was then carefully dissected away from the entire circumference (360 degrees) of the eye, leaving only the outer wall and distal portions of the outflow pathway intact. The area around the conjunctiva, ~1 mm posterior to the limbus, was lightly scored with a scalpel blade to minimize the potential influence of crushed episcleral veins (i.e., back pressure) on our measurements.
Porcine eyes.
Porcine eyes were hemisected at the equator. The vitreous and lens proper were removed, leaving the lens capsule in place to minimize pigment dispersal into the outflow pathway. The vascular tissues of the ciliary body and iris were carefully dissected out, with frequent rinsing to remove pigment. The lens capsule was then removed. The ciliary muscle and conventional outflow pathway tissues were left intact and the anterior segment was clamped into a perfusion chamber. After initial perfusion to establish a stable outflow facility, the anterior segments were removed from the perfusion chamber and the TM and inner wall of the angular aqueous plexus (AAP) were then dissected away from the whole circumference (360 degrees) of the eyes, leaving the distal regions of the conventional outflow pathway intact. The area around the limbus was lightly scored prior to initial perfusion, ~1 mm posterior to the limbus.
Anterior segment organ culture perfusions.
The anterior segment perfusion model (26) was used to study the effects of ET-1 and NO on the distal regions of the eye. A syringe pump (PHD 2000 Programmable Syringe Pump, Harvard Apparatus, Holliston, MA) was used to generate a constant inflow rate of 2.5 µl/min for human eyes and 4.5 µl/min for porcine eyes. A pressure transducer (Pressure Sensor 142PC01G, Honeywell, Golden Valley, MN) was used to measure intrachamber pressure every 2 min. Data were stored (Data Acquisition Logging Unit, Valitec, Dayton, OH) and used to calculate outflow facility. A reservoir was connected between the perfusion chambers and the pressure transducer to facilitate exchanges of media containing drug(s) into the chambers (~1 ml/min).
ET-1 and NO treatments.
Stable baseline facilities were obtained for 21 ± 4 h in human eyes to allow the system to settle before drug treatment. After stable baseline outflow facilities were obtained, anterior chambers were exchanged with media containing 100 nM ET-1 to precontract distal vessels. We reasoned that the distal vessels would have lost their basal tone and would have been maximally dilated due to severing any innervation of these vessels following death and enucleation. Therefore, we chose to precontract the distal vessels by pretreatment with ET-1. For this reason, we did not independently examine the effect of diethylenetriamine-nitric oxide (DETA-NO). After 2 h of perfusion, a second exchange was performed on both anterior segments with the experimental segment receiving media containing 100 µM DETA-NO and 1 nM ET-1 (10, 58, 59), while the contralateral (control) anterior segments received either 100 nM or 1 nM ET-1 as a control to maintain contraction. In the first set of experiments, we used 100 nM ET-1 for both the first and the second exchanges; however, upward drifting outflow facility over time in the presence of 100 nM ET-1 suggested vasospasm, so we decreased the ET-1 to 1 nM in a second set of experiments.
Histology.
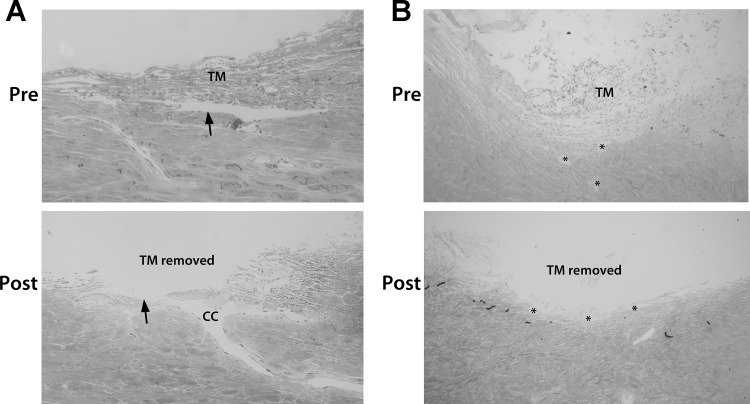
Approximately 12 h after the second exchange was conducted, the eyes were perfusion fixed. Thus, anterior chambers were exchanged with 3% paraformaldehyde in PBS and then were perfused at a constant flow rate of 2.5 µl/min for 1 h. Anterior segments were removed from chambers and submersion fixed overnight in 1% paraformaldehyde in PBS at 4°C. Wedges from each quadrant of the eye were processed using standard histology, with 1-µm radial sections being cut and stained with Toluidine blue. Images were captured and used to evaluate the dissection technique, ensuring that adequate TM was removed from the eyes (Fig. 1). To compare the trabeculotomized anterior segments with those containing an intact TM, we perfusion fixed eyes from additional experiments in which the TM was not removed and processed them for histological examination.
Fig. 1.
Histology showing conventional outflow pathway pre- and post-trabeculotomy in human and porcine eyes. Radial sections (1 µm) from perfusion-fixed human (A) and porcine (B) eyes were taken with the trabecular meshwork (TM) intact and after trabeculotomy. Following trabeculotomy in human eyes, all of the TM and inner wall of Schlemm’s canal were removed, leaving only the outer wall (arrows) and the distal regions present. The images shown were taken from anterior segments from different donors. For porcine eyes, the angular aqueous plexus (AAP) can be seen in both sections (asterisks); however, parts of the AAP are open to the anterior chamber in the post-trabeculotomy section, whereas others are not. CC, collector channel.
Statistical analysis.
Data are presented as mean ± SD. The paired Student’s t-test was used to compare absolute values of outflow facility between conditions.
RESULTS
Human anterior segment perfusions.
In 7 pairs of human anterior segments, a stable baseline outflow facility (C) was first achieved following trabeculotomy. Unlike human anterior segments with the TM and SC inner wall intact, preparations without these tissues obtained a stable baseline C very quickly (4 ± 1.5 h). Control anterior segments showed a mean baseline C of 0.43 ± 0.1 µl·min−1·mmHg, while experimental anterior segments had a mean baseline C of 0.54 ± 0.1 µl·min−1·mmHg. There was no statistical difference in baseline C between the two groups (P = 0.87). Average time from beginning of perfusion to the first exchange was 21 ± 4 h.
Following the exchange with media containing 100 nM ET-1, facility decreased by 0.14 ± 0.1 µl·min−1·mmHg to 0.29 ± 0.2 µl·min−1·mmHg, P < 0.01 in control anterior segments and by 0.21 ± 0.2 µl·min−1·mmHg to 0.33 ± 0.2 µl·min−1·mmHg, P < 0.01 in experimental anterior segments relative to the facility before ET-1. There was no significant difference in the ET-1 response between control and experimental groups (P = 0.5). After 2 ± 0.3 h, experimental anterior segments were exchanged with media containing 100 µM DETA-NO and 1 nM ET-1, which rapidly increased C to a mean of 0.54 ± 0.4 µl·min−1·mmHg (P < 0.05), an increase of 0.21 ± 0.2 µl·min−1·mmHg. The contralateral control anterior segments were instead exchanged with media containing 100 nM ET-1, which maintained a C of 0.32 ± 0.1 µl·min−1·mmHg (P < 0.05). Interestingly, we observed that in the control anterior segments, C steadily increased over time (6 ± 3.5 h) in the continued presence of 100 nM ET-1. Data are summarized graphically in Figure 2 and Table 2.
Fig. 2.

Outflow facility data from perfused human anterior segments. A: perfusion trace summary showing combined outflow facility data from 7 pairs of human anterior segments, normalized to baseline measurements. Endothelin-1 (ET-1; 100 nM) was exchanged into both anterior segments of the pair at ~2 h. Diethylenetriamine-nitric oxide (DETA-NO; 100 µM) was then exchanged into the experimental segment at ~4 h in the presence of ET-1 (1 nM), whereas ET-1 (100 nM) was exchanged into the contralateral segment. Data are presented as mean ± SD (solid lines ± shaded regions). B: box and whisker plot summary of outflow facility data collected at each stage of the human anterior segment perfusion. Data show an average (represented by the + in the box of each data set) facility value of 0.23 ± 0.06 µl·min−1·mmHg in anterior segments with the trabecular meshwork (TM) intact reported in previous studies [Ψthe outflow facility data were obtained from 73 anterior segments that had been perfused and previously published by our laboratory (8, 13, 46, 55, 56)]. Trabeculotomized eyes (TRAB) showed a facility value of 0.49 ± 0.26 µl·min−1·mmHg (control and experimental data). ET-1 treatment decreased facility to 0.31 ± 0.18 µl·min−1·mmHg (control and experimental data), and DETA-NO reversed this decrease in facility, achieving a value of 0.54 ± 0.35 µl·min−1·mmHg (experimental data). The box represents the range of the values from the 25th to the 75th percentile, whereas the error bars show the minimum and maximum values of each data set. The horizontal line in the box represents the median value.
Table 2.
Summary of facility values in human eyes treated with 100 nM ET-1
| Facility (C) | With TM | Trabeculotomy (Baseline) | ET-1 (100 nM) | ET-1 (100 nM) (Second Intervention) | DETA-NO (100 µM) + ET-1 (1 nM) (Second Intervention) |
|---|---|---|---|---|---|
| Control (n = 7) | 0.23 ± 0.06* | 0.43 ± 0.1 | 0.29 ± 0.2 (P < 0.01)† | 0.32 ± 0.1 (P = 0.13)† | NA |
| Experimental (n = 7) | NA | 0.54 ± 0.1 | 0.33 ± 0.2 (P < 0.01)† | NA | 0.54 ± 0.4 (P < 0.05)† |
| P value (control vs. experimental) | NA | P = 0.87 | P = 0.5 | NA | P < 0.05 |
| P value | NA | NA | NA | P < 0.05 (Trab vs. ET-1) | NA |
ET-1, endothelin-1; NA, not applicable; TM, trabecular meshwork; Trab, trabeculotomized eyes.
This outflow facility data was obtained from 73 anterior segments that had been perfused and previously published by our laboratory (8, 13, 46, 55, 56).
P values indicated are a comparison of the indicated facility value with respect to the first facility value to the left of the indicated value on the table.
Owing to possible vasospasm caused by prolonged high concentrations of 100 nM ET-1, we performed a second set of experiments using 3 pairs of human eyes that differed in design only with respect to the second exchange in which control anterior segments were exchanged with media containing the lower dose of 1 nM ET-1. In this second set of experiments, 100 nM ET-1 decreased facility by 0.18 ± 0.13 µl·min−1·mmHg from 0.56 ± 0.1 µl·min−1·mmHg to 0.38 ± 0.2 µl·min−1·mmHg (P = 0.07) in control anterior segments and by 0.24 ± 0.1 µl·min−1·mmHg from 0.66 ± 0.1 µl·min−1·mmHg to 0.42 ± 0.2 µl·min−1·mmHg (P < 0.05) in experimental anterior segments compared with pre-ET-1 facility. There was no significant difference in the ET-1 response between control and experimental anterior segments (P = 0.2). After 2 ± 0.3 h, experimental anterior segments were exchanged with media containing 100 µM DETA-NO and 1 nM ET-1, which rapidly increased C in experimental anterior segments to a mean of 0.54 ± 0.2 µl·min−1·mmHg (P < 0.05), an increase of 0.11 ± 0.04 µl·min−1·mmHg. The contralateral control anterior segments were exchanged with media containing 1 nM ET-1, which achieved a C of 0.33 ± 0.2 µl·min−1·mmHg (P = 0.07). In this set of experiments, the ET-1-mediated decrease in facility was maintained at 0.33 ± 0.2 µl·min−1·mmHg for a longer period of time (57 ± 39 h) in the control eyes. Data are summarized in Fig. 3 and Table 3.
Fig. 3.

Outflow facility data from human anterior segments exposed to 1 nM endothelin-1 (ET-1). A: perfusion trace summary showing combined outflow facility data from 3 pairs of human anterior segments, normalized to baseline measurements. ET-1 (100 nM) was exchanged into both anterior segments of the pair at ~2 h. Diethylenetriamine-nitric oxide (DETA-NO; 100 µM) was then exchanged into the experimental segment at ~4 h in the presence of ET-1 (1 nM), whereas ET-1 (1 nM) was exchanged into the contralateral segment. Data are presented as mean ± SD (solid lines ± shaded regions). B: box and whisker plot summarizing the facility values obtained at each stage of the human anterior segment perfusions. Data show an average (represented by the + in the box of each data set) facility value of 0.23 ± 0.06 µl·min−1·mmHg in anterior segments with the trabecular meshwork (TM) intact reported in previous studies [Ψthe outflow facility data were obtained from 73 anterior segments that had been perfused and previously published by our laboratory (8, 13, 46, 55, 56)]. Trabeculotomized eyes (TRAB) showed a facility value of 0.61 ± 0.12 µl·min−1·mmHg (control and experimental data). ET-1 treatment decreased facility to 0.40 ± 0.15 µl·min−1·mmHg (control and experimental data), and DETA-NO reversed this decrease in facility, achieving a value of 0.54 ± 0.15 µl·min−1·mmHg (experimental data). The box represents the range of the values from the 25th to the 75th percentile, whereas the error bars show the minimum and maximum values of each data set. The horizontal line in the box represents the median value.
Table 3.
Summary of facility data in human eyes treated with 1 nM ET-1
| Facility (C) | With TM | Trabeculotomy (Baseline) | ET-1 (100 nM) | ET-1 (1 nM) (Second Intervention) | DETA-NO (100 µM) + ET-1 (1 nM) (Second Intervention) |
|---|---|---|---|---|---|
| Control (n = 3) | 0.23 ± 0.06* | 0.56 ± 0.1 | 0.38 ± 0.2 (P = 0.07)† | 0.33 ± 0.2 (P = 0.1)† | NA |
| Experimental (n = 3) | NA | 0.66 ± 0.1 | 0.42 ± 0.2 (P < 0.05)† | NA | 0.54 ± 0.2 (P < 0.05)† |
| P value (control vs. experimental) | NA | P = 0.21 | P = 0.21 | NA | P < 0.05 |
| P value | NA | NA | NA | P = 0.07 (Trab vs ET-1) | NA |
ET-1, endothelin-1; NA, not applicable; TM, trabecular meshwork; Trab, trabeculotomized eyes.
This outflow facility data was obtained from 73 anterior segments that had been perfused and previously published by our laboratory (8, 13, 46, 55, 56).
P values indicated are a comparison of the indicated facility value with respect to the first facility value to the left of the indicated value on the table.
When considering data from all human anterior segment perfusions, ET-1 increased outflow resistance by 71% and DETA-NO inhibited ET-1 vasoconstriction activity by 66%.
For the purpose of comparisons between the C of eyes pre- and posttrabeculotomy, we used published data from our laboratory whereby C from 73 perfused human anterior segments with TM intact was obtained (8, 13, 46, 55, 56). The average age of the donor eyes in our study was 71.2 ± 7.4 yr, and the average age of the donors from the previously published data was 73.7 ± 5 yr; there was no significant difference between the donor ages in these studies (P = 0.5). On the basis of the facility values with the TM intact from previous studies, we found that resistance in the distal vessels was 49 ± 22% of total resistance, which was consistent with previously reported data (18, 48).
Porcine anterior segment perfusions.
We next tested whether a similar contribution of distal vessel regulation of conventional C could be observed in anterior segments from porcine eyes. The identical protocol was followed as described above with one additional step. We first obtained C data from anterior segments with the TM and inner wall of the angular aqueous plexus (porcine equivalent of the SC) intact. In 8 pairs of porcine anterior segments, a stable C with the TM intact was achieved post-washout for 28 ± 4 h before trabeculotomy. We observed that, on average, anterior segments achieved a stable C with the TM intact of 0.24 ± 0.1 µl·min−1·mmHg in control anterior segments and 0.29 ± 0.1 µl·min−1·mmHg in experimental anterior segments following washout period of 9 ± 2 h. During washout, C increased from 0.17 ± 0.04 µl·min−1·mmHg to 0.24 ± 0.1 µl·min−1·mmHg in control anterior segments and from 0.23 ± 0.1 µl·min−1·mmHg to 0.29 ± 0.1 µl·min−1·mmHg in experimental anterior segments. These outflow facilities with the TM intact were not significantly different from each other (P = 0.17). Posttrabeculotomy, a stable baseline was achieved within 2.3 ± 0.3 h and maintained for 11 ± 2 h before drug treatment. Following trabeculotomy, mean C increased to 0.69 ± 0.1 µl·min−1·mmHg in control anterior segments and to 0.75 ± 0.1 µl·min−1·mmHg in experimental anterior segments. There was no significant difference between C of the control and experimental eyes after trabeculotomy (P = 0.34).
After treatment with 100 nM ET-1, C decreased to 0.50 ± 0.1 µl·min−1·mmHg in control eyes and 0.47 ± 0.1 µl·min−1·mmHg in experimental eyes. There was no significant difference in the ET-1 response between control and experimental anterior segments (P = 0.5). DETA-NO effectively reversed ET-1 effects, with an average facility of 0.73 ± 0.1 µl·min−1·mmHg following DETA-NO treatment. Similar to the human eyes, the higher dose of ET-1 resulted in a gradual facility increase over time (4 ± 1 h). Facility data are graphically compared in Fig. 4 and Table 4.
Fig. 4.

Combined and normalized facility data from porcine anterior segment perfusions. A: combined perfusion data showing outflow facility in 8 porcine anterior segment pairs normalized to baseline. Endothelin-1 (ET-1; 100 nM) was exchanged into both anterior segment chambers of the pair at ~2 h. Diethylenetriamine-nitric oxide (DETA-NO; 100 µM) was then exchanged into the experimental chamber at ~4 h in the presence of ET-1 (1 nM), whereas ET-1 (100 nM) was exchanged into the contralateral segment. Data are presented as mean ± SD (solid lines ± shaded regions). B: box and whisker plot showing the facility values collected at each stage of the porcine anterior segment perfusions. Data show a facility value of 0.28 ± 0.13 µl·min−1·mmHg in anterior segments with the trabecular meshwork (TM) intact (control and experimental data). Trabeculotomized eyes (TRAB) showed a facility value of 0.72 ± 0.32 µl·min−1·mmHg (control and experimental data). ET-1 treatment decreased facility to 0.48 ± 0.21 µl·min−1·mmHg (control and experimental data), and DETA-NO reversed this decrease in facility achieving a value of 0.73 ± 0.21 µl·min−1·mmHg (experimental data). The mean is indicated by the cross in the box. The box represents the range of the values from the 25th to the 75th percentile, whereas the error bars show the minimum and maximum values of each data set. The horizontal line in the box represents the median value.
Table 4.
Summary of facility data in porcine eyes
| Facility (C) | With TM | Trabeculotomy (Baseline) | ET-1 (100 nM) | ET-1 (100 nM) (Second Intervention) | DETA-NO (100 µM) + ET-1 (1 nM) (Second Intervention) |
|---|---|---|---|---|---|
| Control (n = 8) | 0.24 ± 0.1 | 0.69 ± 0.1 (P < 0.01)† | 0.50 ± 0.1 (P < 0.01)† | 0.61 ± 0.3 (P = 0.16)† | NA |
| Experimental (n = 8) | 0.29 ± 0.1 | 0.75 ± 0.1 (P < 0.01)† | 0.47 ± 0.1 (P < 0.01)† | NA | 0.73 ± 0.1 (P < 0.01)† |
| P value (control vs. experimental) | P = 0.31 | P = 0.34 | P = 0.46 | NA | P = 0.15 |
| P value | NA | NA | NA | P < 0.01 (Trab vs ET-1) | NA |
ET-1, endothelin-1; NA, not applicable; TM, trabecular meshwork.
P values indicated are a comparison of the indicated facility value with respect to the first facility value to the left of the indicated value on the table.
DISCUSSION
The primary goal of this study was to determine the contribution of the vasculature distal to the TM and inner wall of SC to conventional outflow resistance. We used a pharmacological approach in a physiological model system, employing the vasoconstrictor, ET-1 and the vasodilator, NO in trabeculotomized human and porcine anterior segments in organ culture. Nitric oxide decreased outflow resistance by 61 ± 17% in human and 63 ± 17% in porcine anterior segments. In contrast, ET-1 increased outflow resistance by 74 ± 59% in human and 54 ± 40% in porcine anterior segments. In fact, maximal contraction of distal vasculature restored 59% of measured porcine outflow resistance and an estimated 89% of human outflow resistance.
A recently published study was the first to show pharmacological regulation of the distal vessels using a potassium channel opener, Cromakalim prodrug 1 (CKLP1), which decreased pressure by ~50% in trabeculotomized human anterior segments (49). Similar to NO, CKLP1 is a vasodilator and equally efficacious at decreasing pressure (increasing C). In the present study, we extended this observation by showing the vasomotive range of the distal vessels by first precontracting with ET-1, which increased pressure (decreased C). Once precontracted fully, we relaxed vessels with a concentration of DETA-NO (100 µM) that maximally increases C (16). In our experiments, we found that NO restored C to baseline levels (before drug treatments). We reasoned that precontraction of distal vessels in postmortem eyes was necessary due to severing of nerves during enucleation, which would reduce/eliminate basal tone. Interestingly, Roy Chowdhury et al. observed vasodilator effects of CKLP1 (increased C) without precontracting the distal vessels, indicating that there was some vascular tone in the vessels before treatment in their preparations. This may be due to the younger age of the eyes in this study compared with ours (~54 yr compared with ~71 yr), and commencement of perfusion earlier (~14 h compared with ~21 h). The younger age and the freshness of the tissue may have allowed the distal vessels to retain basal tone. Additional studies are needed to test this hypothesis.
Our data using the NO donor, DETA-NO, are consistent with previously published data indicating that NO increased C and decreased IOP in mice, rabbits, dogs, monkeys, and humans (4, 7, 11, 21, 31, 40, 51). SC cells express endothelial nitric oxide synthase (eNOS), and produce NO in response to shear stress (1) caused by elevations of IOP and subsequent narrowing of SC. The NO produced in SC may travel to and dilate the distal regions of the conventional outflow pathway to normalize IOP. However, the eNOS pathway may be compromised in some forms of glaucoma. Genome-wide association studies have found a number of eNOS polymorphisms associated with an increased risk for primary open-angle glaucoma across different ethnicities (2, 12, 27–29, 33, 34, 62). Therefore, delivering NO to the distal regions of the conventional outflow pathway represents a curative therapeutic avenue.
An interesting observation is that both human and porcine eyes responded similarly to ET-1 and NO, despite the known physiological and anatomical differences between the species. The human eyes responded in a more consistent manner than the porcine eyes. We think this may be due to differences in the anatomy of Schlemm’s canal compared with the angular aqueous plexus (AAP). SC has a continuous luminal opening around the circumference of the eye while the AAP is a series of discontinuous loops around the circumference of the eye. Depending on the location of these loops in reference to the TM, there may be more or less resistance provided, and they may also have a different magnitude of response to the ET-1 and/or NO. Therefore, some of the variability observed in the porcine data may be attributable to the dissections. If the tissue removed opens the AAP to the anterior chamber, C would be more effectively increased or decreased by our pharmacological interventions. However, if the AAP remains covered with layers of tissue or if pieces of TM are lodged into the AAP opening, the perfused drugs may not be as effective at regulating distal vessels. This may result in a blunted or delayed response to the drugs, leading to the variability we observed in our data.
Using an experimental paradigm previously described in human cardiac vasculature (58–60), we regulated distal vessels with the vasoconstrictor ET-1 and a NO donor, DETA-NO. Our experimental setup differed from these studies as we used ex vivo organ culture, whereas these studies used isolated vessels. Therefore, the time scale over which the effects of ET-1 and DETA-NO occurred differed from the previous studies in which effects were typically seen within minutes, compared with hours for our own. Despite these differences in experimental setup, we achieved similar results using this pharmacological paradigm.
In both human and porcine anterior segments, we noticed that prolonged exposure (>4 h) to 100 nM ET-1 resulted in decreased responsiveness (i.e., increased C) over time. ET-1 has a short half-life of <1 h; however, its biological effects are typically seen to last longer than that. However, a previous study showed that vasospasm is induced in vessels by higher doses of ET-1 (~100 nM), whereas lower doses (~1 nM) caused vasoregulation (64). Data suggest that at the higher dose of ET-1, the vessels underwent vasospasm and were unable to sustain that level of vascular tone for an extended time, which ultimately led to vessel relaxation. To test this idea, we conducted experiments using a lower dose of ET-1 (1 nM) in the control eyes, whereby we found that the lower dose of ET-1 sustained its effectiveness (decreased C) over a much longer period of time (6 ± 3.5 h @ 100 nM vs. 57 ± 39 h @ 1 nM).
We found that the NO donor, DETA-NO, maintained an increase in C in experimental anterior segments over a time period of ~13.5 ± 6.5 h. NO typically has a typical half-life of 2–6 s in vascularized tissue. However, its half-life is also dependent on concentration of NO, and the presence of hemoglobin and oxygen. In the absence of hemoglobin, which is the case in much of the conventional outflow tract, NO has been demonstrated to have a half-life of 445 s (20). On the basis of an estimated volume of Schlemm’s canal of 0.2 µl and a conventional outflow rate of 2.4 µl/min (38), AH in SC would reach the distal vasculature in ~5 s. As this time is much shorter than the half-life of NO in the absence of hemoglobin, this indicates that NO produced in SC by endothelial cells would likely reach the distal vessels, where it may affect a vasoactive response. The NO donor used in this set of experiments, DETA-NO, has been shown to release NO over a period of ~20 h (36). Our data showed an effective time of DETA-NO of ~13.5 h. This is consistent with the previous studies showing the release of NO by DETA-NO.
Conclusions.
Previous studies in human eyes have shown that resistance in the distal regions of the conventional outflow pathway account for 25–50% of total outflow resistance. The data in the present study indicate that regulation of distal vessels using the physiological vasoregulators ET-1 and NO dramatically affect outflow resistance, demonstrating a dynamic range of vasomotion. Our data suggest that the distal vessels likely play an important role in the regulation of outflow resistance, particularly after microinvasive glaucoma surgeries and possibly in the pathophysiology of some forms of glaucoma. These vessels may play a role in the active regulation of IOP and resistance through vasoconstriction and vasodilation; they show a range of outflow facility regulation comparable to that seen by total outflow facility. Taken together, distal outflow resistance represents a viable target for therapeutic interventions to treat people with glaucoma.
GRANTS
This research was supported by NIH National Eye Institute Grant EY-022359, EY-005722 and the Research to Prevent Blindness Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.M. and W.M.D. performed experiments; F.M. analyzed data; F.M., D.R.O., and W.D.S. interpreted results of experiments; F.M. prepared figures; F.M. drafted manuscript; F.M., W.M.D., D.R.O., and W.D.S. edited and revised manuscript; F.M., W.M.D., D.R.O., and W.D.S. approved final version of manuscript; D.R.O. and W.D.S. conceived and designed research.
ACKNOWLEDGMENTS
The authors thank Dr. Simon John, Dr. Ross Ethier, Dr. Krish Kizhatil, and Dr. Joseph Sherwood for helpful discussion regarding the data.
REFERENCES
- 1.Ashpole NE, Overby DR, Ethier CR, Stamer WD. Shear stress-triggered nitric oxide release from Schlemm’s canal cells. Invest Ophthalmol Vis Sci 55: 8067–8076, 2014. doi: 10.1167/iovs.14-14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ayub H, Khan MI, Micheal S, Akhtar F, Ajmal M, Shafique S, Ali SH, den Hollander AI, Ahmed A, Qamar R. Association of eNOS and HSP70 gene polymorphisms with glaucoma in Pakistani cohorts. Mol Vis 16: 18–25, 2010. [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley MD, Hann CR, Fautsch MP. Anatomical variation of human collector channel orifices. Invest Ophthalmol Vis Sci 57: 1153–1159, 2016. doi: 10.1167/iovs.15-17753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borghi V, Bastia E, Guzzetta M, Chiroli V, Toris CB, Batugo MR, Carreiro ST, Chong WK, Gale DC, Kucera DJ, Jia L, Prasanna G, Ongini E, Krauss AH, Impagnatiello F. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther 26: 125–132, 2010. doi: 10.1089/jop.2009.0120. [DOI] [PubMed] [Google Scholar]
- 5.Braughler JM, Mittal CK, Murad F. Purification of soluble guanylate cyclase from rat liver. Proc Natl Acad Sci USA 76: 219–222, 1979. doi: 10.1073/pnas.76.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cellini M, Versura P, Zamparini E, Bendo E, Campos EC. Effects of endothelin-1 and flunarizine on human trabecular meshwork cell contraction. Exp Biol Med (Maywood) 231: 1081–1084, 2006. [PubMed] [Google Scholar]
- 7.Chang JY, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR, Overby DR. Role of nitric oxide in murine conventional outflow physiology. Am J Physiol Cell Physiol 309: C205–C214, 2015. doi: 10.1152/ajpcell.00347.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel Stamer W, Chan DW, Ross Ethier C. Targeted gene transfer to Schlemm’s canal by retroperfusion. Exp Eye Res 84: 843–849, 2007. doi: 10.1016/j.exer.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Kater AW, Shahsafaei A, Epstein DL. Localization of smooth muscle and nonmuscle actin isoforms in the human aqueous outflow pathway. Invest Ophthalmol Vis Sci 33: 424–429, 1992. [PubMed] [Google Scholar]
- 10.Dismuke WM, Liang J, Overby DR, Stamer WD. Concentration-related effects of nitric oxide and endothelin-1 on human trabecular meshwork cell contractility. Exp Eye Res 120: 28–35, 2014. doi: 10.1016/j.exer.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dismuke WM, Mbadugha CC, Ellis DZ. NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel. Am J Physiol Cell Physiol 294: C1378–C1386, 2008. doi: 10.1152/ajpcell.00363.2007. [DOI] [PubMed] [Google Scholar]
- 12.Emam WA, Zidan HE, Abdulhalim BE, Dabour SA, Ghali MA, Kamal AT. Endothelial nitric oxide synthase polymorphisms and susceptibility to high-tension primary open-angle glaucoma in an Egyptian cohort. Mol Vis 20: 804–811, 2014. [PMC free article] [PubMed] [Google Scholar]
- 13.Ethier CR, Wada S, Chan D, Stamer WD. Experimental and numerical studies of adenovirus delivery to outflow tissues of perfused human anterior segments. Invest Ophthalmol Vis Sci 45: 1863–1870, 2004. doi: 10.1167/iovs.03-1133. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Durango R, Rollín R, Mediero A, Roldán-Pallares M, García Feijo J, García Sánchez J, Fernández-Cruz A, Rípodas A. Localization of endothelin-1 mRNA expression and immunoreactivity in the anterior segment of human eye: expression of ETA and ETB receptors. Mol Vis 9: 103–109, 2003. [PubMed] [Google Scholar]
- 15.Francis AW, Kagemann L, Wollstein G, Ishikawa H, Folz S, Overby DR, Sigal IA, Wang B, Schuman JS. Morphometric analysis of aqueous humor outflow structures with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 53: 5198–5207, 2012. doi: 10.1167/iovs.11-9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez JM Jr, Ko MK, Hong YK, Weigert R, Tan JCH. Deep tissue analysis of distal aqueous drainage structures and contractile features. Sci Rep 7: 17071, 2017. doi: 10.1038/s41598-017-16897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granstam E, Wang L, Bill A. Effects of endothelins (ET-1, ET-2 and ET-3) in the rabbit eye; role of prostaglandins. Eur J Pharmacol 194: 217–223, 1991. doi: 10.1016/0014-2999(91)90108-3. [DOI] [PubMed] [Google Scholar]
- 18.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol 69: 783–801, 1963. doi: 10.1001/archopht.1963.00960040789022. [DOI] [PubMed] [Google Scholar]
- 19.Gruetter CA, Gruetter DY, Lyon JE, Kadowitz PJ, Ignarro LJ. Relationship between cyclic guanosine 3′:5′-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther 219: 181–186, 1981. [PubMed] [Google Scholar]
- 20.Hakim TS, Sugimori K, Camporesi EM, Anderson G. Half-life of nitric oxide in aqueous solutions with and without haemoglobin. Physiol Meas 17: 267–277, 1996. doi: 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- 21.Heyne GW, Kiland JA, Kaufman PL, Gabelt BT. Effect of nitric oxide on anterior segment physiology in monkeys. Invest Ophthalmol Vis Sci 54: 5103–5110, 2013. doi: 10.1167/iovs.12-11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye: An Atlas and Textbook. Philadelphia, PA: Saunders, 1971. [Google Scholar]
- 23.Holló G, Lakatos P, Vargha P. Immediate increase in aqueous humour endothelin 1 concentration and intra-ocular pressure after argon laser trabeculoplasty in the rabbit. Ophthalmologica 214: 292–295, 2000. doi: 10.1159/000027507. [DOI] [PubMed] [Google Scholar]
- 24.Huang AS, Belghith A, Dastiridou A, Chopra V, Zangwill LM, Weinreb RN. Automated circumferential construction of first-order aqueous humor outflow pathways using spectral-domain optical coherence tomography. J Biomed Opt 22: 66010, 2017. doi: 10.1117/1.JBO.22.6.066010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson DH, Tschumper RC. The effect of organ culture on human trabecular meshwork. Exp Eye Res 49: 113–127, 1989. doi: 10.1016/0014-4835(89)90080-8. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DH, Tschumper RC. Human trabecular meshwork organ culture. A new method. Invest Ophthalmol Vis Sci 28: 945–953, 1987. [PubMed] [Google Scholar]
- 27.Kang JH, Wiggs JL, Haines J, Abdrabou W, Pasquale LR. Reproductive factors and NOS3 variant interactions in primary open-angle glaucoma. Mol Vis 17: 2544–2551, 2011. [PMC free article] [PubMed] [Google Scholar]
- 28.Kang JH, Wiggs JL, Rosner BA, Haines J, Abdrabou W, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with hypertension, alcohol intake, and cigarette smoking. Arch Ophthalmol 129: 773–780, 2011. doi: 10.1001/archophthalmol.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JH, Wiggs JL, Rosner BA, Hankinson SE, Abdrabou W, Fan BJ, Haines J, Pasquale LR. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci 51: 971–979, 2010. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukovetz WR, Holzmann S, Wurm A, Pöch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol 310: 129–138, 1979. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- 31.Larsson LI, Maus TL, Brubaker RF, Nathanson JA. Topically applied hydralazine: effects on systemic cardiovascular parameters, blood-aqueous barrier, and aqueous humor dynamics in normotensive humans. J Ocul Pharmacol Ther 11: 145–156, 1995. doi: 10.1089/jop.1995.11.145. [DOI] [PubMed] [Google Scholar]
- 32.Leske MC, Connell AM, Wu SY, Hyman LG, Schachat AP. Risk factors for open-angle glaucoma. The Barbados Eye Study. Arch Ophthalmol 113: 918–924, 1995. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 33.Liao Q, Wang DH, Sun HJ. Association of genetic polymorphisms of eNOS with glaucoma. Mol Vis 17: 153–158, 2011. [PMC free article] [PubMed] [Google Scholar]
- 34.Magalhães da Silva T, Rocha AV, Lacchini R, Marques CR, Silva ES, Tanus-Santos JE, Rios-Santos F. Association of polymorphisms of endothelial nitric oxide synthase (eNOS) gene with the risk of primary open angle glaucoma in a Brazilian population. Gene 502: 142–146, 2012. doi: 10.1016/j.gene.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 35.Malvankar-Mehta MS, Iordanous Y, Chen YN, Wang WW, Patel SS, Costella J, Hutnik CM. iStent with phacoemulsification versus phacoemulsification alone for patients with glaucoma and cataract: a meta-analysis. PLoS One 10: e0131770, 2015. doi: 10.1371/journal.pone.0131770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mooradian DL, Hutsell TC, Keefer LK. Nitric oxide (NO) donor molecules: effect of NO release rate on vascular smooth muscle cell proliferation in vitro. J Cardiovasc Pharmacol 25: 674–678, 1995. doi: 10.1097/00005344-199504000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Mosaed S, Dustin L, Minckler DS. Comparative outcomes between newer and older surgeries for glaucoma. Trans Am Ophthalmol Soc 107: 127–133, 2009. [PMC free article] [PubMed] [Google Scholar]
- 38.Moses RA. Circumferential flow in Schlemm’s canal. Am J Ophthalmol 88: 585–591, 1979. doi: 10.1016/0002-9394(79)90519-1. [DOI] [PubMed] [Google Scholar]
- 39.Napoli SA, Gruetter CA, Ignarro LJ, Kadowitz PJ. Relaxation of bovine coronary arterial smooth muscle by cyclic GMP, cyclic AMP and analogs. J Pharmacol Exp Ther 212: 469–473, 1980. [PubMed] [Google Scholar]
- 40.Nathanson JA. Nitrovasodilators as a new class of ocular hypotensive agents. J Pharmacol Exp Ther 260: 956–965, 1992. [PubMed] [Google Scholar]
- 41.O’Brien RF, Robbins RJ, McMurtry IF. Endothelial cells in culture produce a vasoconstrictor substance. J Cell Physiol 132: 263–270, 1987. doi: 10.1002/jcp.1041320210. [DOI] [PubMed] [Google Scholar]
- 42.Okada K, Sugiyama K, Haque MS, Taniguchi T, Kitazawa Y. The effects of endothelin-1 on intraocular pressure and pupillary diameter in rabbits. Jpn J Ophthalmol 39: 233–241, 1995. [PubMed] [Google Scholar]
- 43.Overby DR, Bertrand J, Schicht M, Paulsen F, Stamer WD, Lütjen-Drecoll E. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci 55: 3727–3736, 2014. doi: 10.1167/iovs.13-13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res 18: 39–57, 1999. doi: 10.1016/S1350-9462(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 45.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90: 262–267, 2006. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos RF, Stamer WD. Effects of cyclic intraocular pressure on conventional outflow facility. Invest Ophthalmol Vis Sci 49: 275–281, 2008. doi: 10.1167/iovs.07-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol 10: 189–206, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res 8: 1233–1240, 1989. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- 49.Roy Chowdhury U, Rinkoski TA, Bahler CK, Millar JC, Bertrand JA, Holman BH, Sherwood JM, Overby DR, Stoltz KL, Dosa PI, Fautsch MP. Effect of Cromakalim Prodrug 1 (CKLP1) on Aqueous humor dynamics and feasibility of combination therapy with existing ocular hypotensive agents. Invest Ophthalmol Vis Sci 58: 5731–5742, 2017. doi: 10.1167/iovs.17-22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selbach JM, Rohen JW, Steuhl KP, Lütjen-Drecoll E. Angioarchitecture and innervation of the primate anterior episclera. Curr Eye Res 30: 337–344, 2005. doi: 10.1080/02713680590934076. [DOI] [PubMed] [Google Scholar]
- 51.Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci 52: 9438–9444, 2011. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sugiyama K, Haque MS, Okada K, Taniguchi T, Kitazawa Y. Intraocular pressure response to intravitreal injection of endothelin-1 and the mediatory role of ETA receptor, ETB receptor, and cyclooxygenase products in rabbits. Curr Eye Res 14: 479–486, 1995. doi: 10.3109/02713689509003759. [DOI] [PubMed] [Google Scholar]
- 53.Sumner MJ, Cannon TR, Mundin JW, White DG, Watts IS. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. Br J Pharmacol 107: 858–860, 1992. doi: 10.1111/j.1476-5381.1992.tb14537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Topouzis F, Wilson MR, Harris A, Founti P, Yu F, Anastasopoulos E, Pappas T, Koskosas A, Salonikiou A, Coleman AL. Risk factors for primary open-angle glaucoma and pseudoexfoliative glaucoma in the Thessaloniki eye study. Am J Ophthalmol 152: 219–228 e211, 2011. doi: 10.1016/j.ajo.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 55.Wan Z, Brigatti L, Ranger-Moore J, Ethier CR, Stamer WD. Rate of change in central corneal thickness: a viability indicator for conventional drainage tissues in organ culture. Exp Eye Res 82: 1086–1093, 2006. doi: 10.1016/j.exer.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 56.Wan Z, Woodward DF, Cornell CL, Fliri HG, Martos JL, Pettit SN, Wang JW, Kharlamb AB, Wheeler LA, Garst ME, Landsverk KJ, Struble CS, Stamer WD. Bimatoprost, prostamide activity, and conventional drainage. Invest Ophthalmol Vis Sci 48: 4107–4115, 2007. doi: 10.1167/iovs.07-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weinreb RN, Ong T, Scassellati Sforzolini B, Vittitow JL, Singh K, Kaufman PL; VOYAGER study group . A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. Br J Ophthalmol 99: 738–745, 2015. doi: 10.1136/bjophthalmol-2014-305908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley KE, Davenport AP. Nitric oxide-mediated modulation of the endothelin-1 signalling pathway in the human cardiovascular system. Br J Pharmacol 132: 213–220, 2001. doi: 10.1038/sj.bjp.0703834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiley KE, Davenport AP. Novel nitric oxide donors reverse endothelin-1-mediated constriction in human blood vessels. J Cardiovasc Pharmacol 36, Suppl 1: S151–S152, 2000. doi: 10.1097/00005344-200036051-00047. [DOI] [PubMed] [Google Scholar]
- 60.Wiley KE, Davenport AP. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery. Br J Pharmacol 133: 568–574, 2001. doi: 10.1038/sj.bjp.0704119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wollensak G, Schaefer HE, Ihling C. An immunohistochemical study of endothelin-1 in the human eye. Curr Eye Res 17: 541–545, 1998. doi: 10.1076/ceyr.17.5.541.5187. [DOI] [PubMed] [Google Scholar]
- 62.Xiang Y, Dong Y, Li X, Tang X. Association of common variants in eNOS gene with primary open angle glaucoma: a meta-analysis. J Ophthalmol 2016: 1348347, 2016. doi: 10.1155/2016/1348347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 64.Zhao J, van Helden DF. ET-1-associated vasomotion and vasospasm in lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol 140: 1399–1413, 2003. doi: 10.1038/sj.bjp.0705573. [DOI] [PMC free article] [PubMed] [Google Scholar]