Abstract
Intestinal absorption of the water-soluble vitamins biotin and pantothenic acid is carrier mediated and involves the sodium-dependent multivitamin transporter (SMVT; product of the SLC5A6 gene). We recently observed that intestinal-specific (conditional) knockout of the mouse Slc5a6 gene (SMVT-cKO) is associated with growth retardation, the development of spontaneous and severe inflammation, abnormal histology in the large intestine, altered gut permeability, and early death. Our aim in this study was to examine the possibility that biotin and pantothenic acid oversupplementation (BPS) of the SMVT-cKO mice could reverse the above-described abnormalities. BPS was provided in the drinking water to mice before conception, to dams during pregnancy and lactation, and to the SMVT-cKO mice throughout their life. Our findings showed that such a regimen prevents early death, as well as normalizes the growth rate, intestinal integrity, pathology, and inflammation in SMVT-cKO mice. These findings provide clear evidence for a role for biotin and/or pantothenic acid in the maintenance of normal intestinal integrity and health.
Keywords: biotin, mucosal inflammation, mucosal integrity, pantothenic acid, SMVT
INTRODUCTION
The sodium-dependent multivitamin transporter (SMVT) is a product of the SLC5A6 gene and is responsible for the transport of the water-soluble vitamins biotin and pantothenic acid, as well as the metabolite lipoate (27, 31). Biotin is an essential micronutrient that cannot be synthesized endogenously by mammalian cells and plays an important role in normal cellular metabolism, growth, and development. The vitamin is a coenzyme for multiple carboxylases involved in a variety of metabolic pathways, such as fatty acid synthesis, gluconeogenesis, and catabolism of branched chains amino acids (22, 23, 31). Biotin also plays a role in energy metabolism (i.e., ATP production), cellular oxidative stress regulation (20), and the expression of many genes (29). In addition, a role for biotin in normal immune function has been well documented (2, 3, 9, 15–17, 26). More recent findings have shown that biotin also plays a role in influencing the colonization/invasiveness of certain enteropathogenic bacteria (39), and in mediating the effect of probiotic bacteria on gut microbial community (36). Deficiency/suboptimal levels of biotin occur in a variety of conditions (5, 10, 13, 24, 37) and lead to serious clinical abnormalities which include neurological and dermatological disorders, as well as growth retardation. With regard to pantothenic acid, this vitamin also cannot be synthesized endogenously by mammalian cells. This micronutrient is required for the biosynthesis of coenzyme A and acyl carrier protein in mammalian cells, and therefore, it is important for carbohydrate, fat, and to a lesser extent protein metabolism (18, 32). Spontaneous pantothenic acid deficiency has not been reported in humans. With regard to lipoate, this substrate acts as an antioxidant, and mammalian cells can synthesize a considerable amount of this substrate endogenously (25).
We have been interested in understanding the mechanism and regulation of intestinal biotin absorption for over three decades (see reviews in Refs. 31–33). In recent investigations, we have generated a conditional (intestinal-specific) SMVT knockout (SMVT-cKO) mouse model (using the Cre-Lox approach) and used it to determine the relative contribution of the SMVT system toward intestinal carrier-mediated biotin uptake (12). Our findings showed that the SMVT is the only biotin transport system that operates in the intestine, and that its knockout leads to biotin deficiency. We also observed that all of the SMVT-cKO mice exhibited growth retardation and died within the first 10 wk of life. Furthermore, the knockout animals developed severe chronic active inflammation with abnormal histology in the large intestine, reminiscent of that seen in ulcerative colitis in humans, including focal cryptitis/crypt abscesses, focal low-grade adenomatous changes, and extensive submucosal edema (12). Further investigations of the observed intestinal inflammation in the SMVT-cKO mice showed 1) an increase in the level of expression of proinflammatory cytokines (i.e., TNF-α, and IFN-γ), 2) an increase in gut permeability, and 3) changes in the level of expression of important tight junction (TJ) proteins compared with their wild-type (WT) littermates (30).
Our aim in the current investigation was to determine whether the oversupplementation of biotin and pantothenic acid (BPS) to the SMVT-cKO mice could reverse the above-described abnormal phenotypes. For this, we provided high doses of biotin and pantothenic acid in drinking water to the dams of the SMVT-cKO mice during their pregnancy and lactation, as well as to the SMVT cKO mice throughout their life. Our results showed that such treatment leads to the complete reversal of abnormal phenotypes associated with the knockout mice, thus providing evidence for the important role that biotin and/or pantothenic acid play in the maintenance of normal intestinal integrity and physiology.
MATERIALS AND METHODS
Materials
All chemicals and reagents used in this study were purchased from commercial vendors and were of analytical/molecular biology grade. The specific primers used for PCR amplifications were from Sigma Genosys (Woodlands, TX). Anti-claudin-1 (Life Technologies; catalog no. 374900) and -2 (Life Technologies; catalog no. 325600), anti-zonula occludens (ZO)-1 (Santa Cruz Biotechnology; catalog no. sc-8146), and anti-myosin light chain kinase (MLCK) (Sigma-Aldrich; catalog no. M7905) antibodies were used in these investigations. Animal studies described in this study were approved by the Animal Care and Use Committee of the VA Medical Center (Long Beach, CA).
Methods
Animals: breeding of the conditional (intestinal-specific) SMVT-cKO mice and supplementation with biotin and pantothenic acid.
The SMVT-cKO mouse line was generated in our laboratory previously using the Cre/lox technology (12). The animals were genotyped as previously described (12). We used 20- to 24-wk-old SMVT-cKO mice and their sex-matched WT littermates as controls. For vitamin oversupplementation, we provided high levels of biotin and pantothenic acid (1 mM of each vitamin) in drinking water to mice before conception, to dams during pregnancy and lactation, and to their SMVT-cKO offspring and sex-matched WT littermates throughout their life span.
Intestinal permeability assay (FITC-dextran method).
Intestinal permeability was determined in vivo by measuring the appearance of FITC-dextran [molecular mass 4 kDa (FD4), Sigma-Aldrich] in the blood, as previously described (7, 30).
Quantitative real-time PCR.
Total RNA was extracted from mouse tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) following manufacturer protocol, and RT-PCR was performed as described before (12, 30) using the gene-specific primers for mouse TNF-α, IFN-γ, zonula occludens (ZO)-1, claudin -1 and -2, myosin light chain kinase (MLCK), mucin-1 (MUC-1), mucin-2 (MUC-2), mucin-3 (MUC-3), β-actin (used as an internal control for TJ protein and cytokines), and villin (used as an internal control for mucins) (Table 1). Relative gene expression was quantified by normalizing cycle threshold (Ct) values with the corresponding β-actin or villin.
Table 1.
List of primer sequences used for quantitative real-time PCR analysis
| Gene Name | Forward and Reverse Primer Sequences (5′–3′) |
|---|---|
| mClaudin-1 | TGTGGATGGCTGTCATTG; TGGCCAAATTCATACCTG |
| mClaudin-2 | TTAGCCCTGACCGAGAAAGA; AAAGGACCTCTCTGGTGCTG |
| mZO-1 | TTCAAAGTCTGCAGAGACAATAGC; TCACATTGCTTAGTCCAGTTCC |
| mMLCK | ACATGCTACTGAGTGGCCTCTCT; GGCAGACAGGACATTGTTTAAGG |
| mTNF-α | CATCTTCTCAAAATTCGAGTGACAA; TCGGAGTAGACAAGGTACAACCC |
| mIFN-γ | TCAAGTGGCATAGATGTGGAAGAA; TGGCTCTGCAGGATTTTCATG |
| mβ-actin | GGCTGTATTCCCCTCCATCG; CCAGTTGGTAACAATGCCATGT |
| mMUC-1 | GGTTGCTTTGGCTATCGTCTATTT; AAAGATGTCCAGCTGCCCATA |
| mMUC-2 | GTCCAGGGTCTGGATCACA; CAGATGGCAGTGAGCTGAGC |
| mMUC-3 | AATGTCAGTTGCAGCGAAGT; GGAGAACACGAGGAGGATCA |
| mVillin | CTCTCTCAACATCACCAC; TAGCCAGGACTACATAGCAG |
| mNOS2 | CGGAGCCTTTAGACCTCAACA; CCCTCGAAGGTGAGCTGAAC |
| mSOD-1 | GATGACTTGGGCAAAGGTGG; CTGCGCAATCCCATCACTC |
| mFMO-2 | CAGTTTCAGACCACTGTCA; TGTATTCGCGGCTATGGA |
| mLpo | GGGAGTGATACCTACACCA; CTAGGCTAGCATCCAGGA |
Western blot analysis.
For Western blot analysis, mouse tissue was homogenized in RIPA buffer (Sigma) with protease inhibitor cocktail (Roche). The soluble total protein homogenates were isolated by centrifugation at 8,000 g for 10 min, and an equal amount (45 μg) of the total proteins was loaded on a 4–12% mini gel (Invitrogen). The proteins were then transferred to a polyvinylidene difluoride membrane and probed simultaneously with mouse claudin-1 and -2, ZO-1, and MLCK antibodies (raised in mouse or rabbit) and monoclonal β-actin antibody (raised in mouse). The blots were then incubated with anti-rabbit/anti-mouse IR 800 dye and anti-mouse IR 680 dye (LI-COR) secondary antibodies (1:25,000) for 1 h at room temperature. Relative expression was quantified by comparing the fluorescence intensities in an Odyssey Infrared imaging system (LI-COR) using Odyssey application software (version 3.0) with respect to corresponding β-actin.
Estimation of biotin status.
Biotin status in BPS SMVT-cKO and WT littermates was estimated as described previously (6, 12, 19, 30) by measuring the total level of biotinylated proteins in the liver of these animals using Western blot analysis. In these investigations, the nitrocellulose membrane was incubated first with mouse anti-β-actin antibodies. This was followed by labeling the anti-β-actin primary antibodies with anti-mouse IR 680 dye (LI-COR) and the biotinylated proteins with avidin-IR 800 dye (LI-COR). Total biotinylated proteins were analyzed using an Odyssey Infrared imaging system (LI-COR).
Histopathologic analysis.
The cecum of SMVT-cKO mice and WT littermates was collected immediately after euthanasia and fixed in 10% formalin overnight and processed for staining as described before (12, 30).
Statistical analysis.
Data are presented as means ± SE of at least three separate experiments. Significance (calculated using the Student’s t-test) was set at P < 0.05.
RESULTS
Effect of Biotin and Pantothenic Acid Oversupplementation to SLC5A6 cKO (SMVT-cKO) Mice on Survival, Growth Rate, and General Phenotype
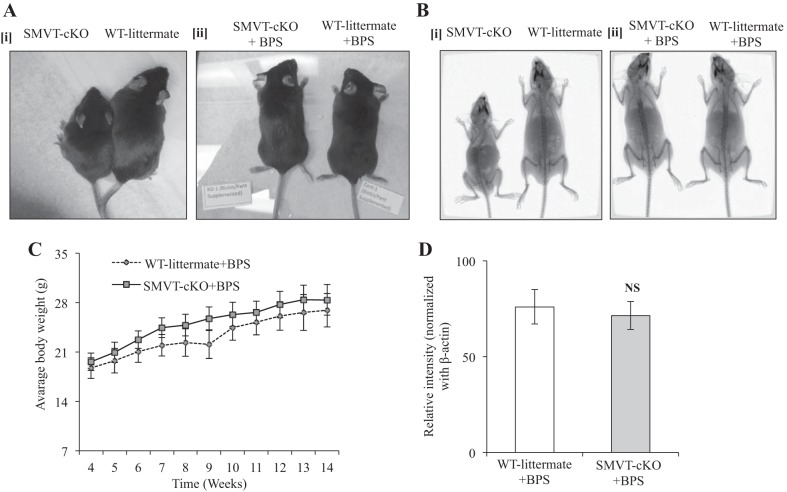
As seen before (12), the SMVT-cKO showed growth retardation (Fig. 1Ai), decreased bone density and length (Fig. 1Bi), and lethargic behavior and displayed a hunchback posture. In the present study, we examined the effect on the above-described abnormalities of BPS to dams during pregnancy and lactation, and to their SMVT-cKO mice and sex-matched WT littermates throughout their life span. The results showed that such supplementation prevents early death in the SMVT-cKO animals (mice were followed for up to 6 mo of age) and showed a similar phenotype to their WT littermates. The latter includes normal growth rate, normal skeletal development, no lethargic behavior, and normal posture (Fig. 1, A–C). Also, the previously observed biotin deficiency in the SMVT-cKO animals was corrected by BPS (Fig. 1D).
Fig. 1.
Effects of BPS to SMVT-cKO mice on growth rate, bone density, and biotin level. Ai: representative image of a SMVT-cKO mouse (left) and its sex-matched WT littermate (right) showing clear phenotypic differences in size and length that we have seen previously (12). Aii: representative image of BPS SMVT-cKO mouse (left) and its sex-matched BPS WT littermate (right) showing no difference in size and length. Bi: representative X-ray image of a SMVT-cKO mouse (left) and its sex-matched WT littermate (right) showing distinct difference in bone length. Bii: representative X-ray image of BPS SMVT-cKO mice (left) and its BPS WT littermate (right) showing no difference in bone size and length. C: growth chart showing no difference in weight gain of BPS SMVT-cKO mice compared with their BPS WT littermate. D: level of total biotinylated proteins in the liver (a measure of biotin status) of BPS SMVT-cKO mice and their BPS WT littermates. Data are means ± SE of at least three separate sets of mice. Abbreviations: BPS, biotin and pantothenic acid supplementation; cKO, conditional knockout of the mouse Slc5a6 gene; NS, not significant; SMVT, sodium-dependent multivitamin transporter; WT, wild type.
Effect of BPS to SMVT-cKO Mice on Intestinal Mucosal Morphology and Integrity
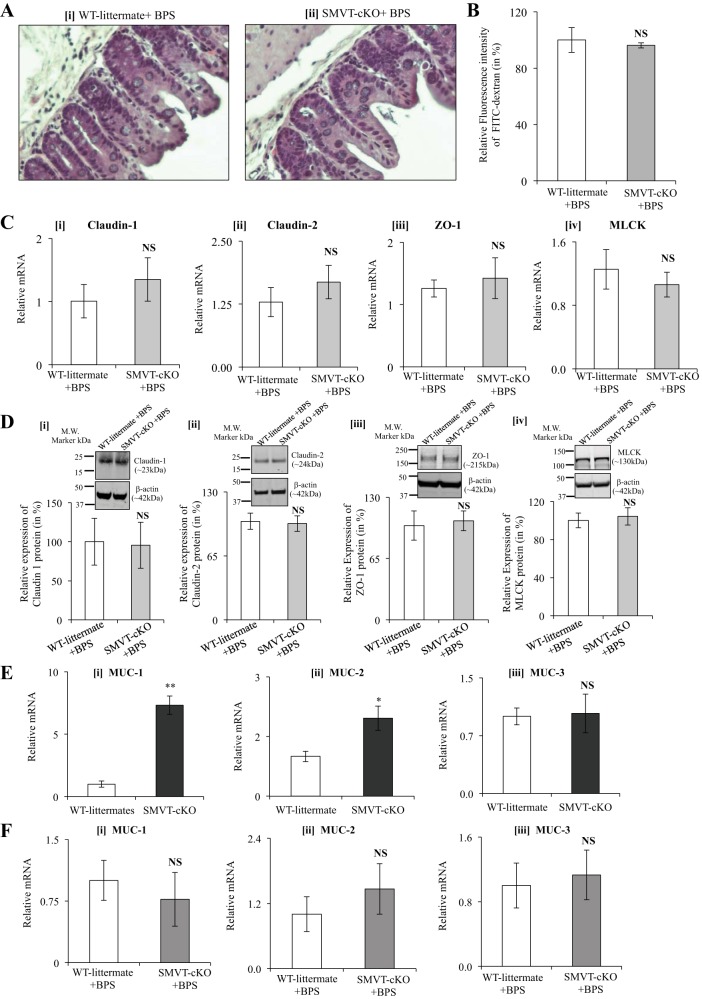
As mentioned earlier, SMVT-cKO mice showed histological abnormalities (neutrophil infiltration, focal cryptitis, and crypt abscesses in the cecum) and an increase in gut permeability associated with marked changes in the level of expression of important TJ proteins (a significant increase in expression of the “leaky” TJ proteins claudin-1 and -2, and a decrease in the level of expression of “tight” TJ protein ZO-1, as well as a significant induction in the level of expression of TJ regulator MLCK) (12, 30). In this study, we aimed to determine whether BPS could prevent such changes in intestinal permeability and expression of TJ proteins (and MLCK). The results showed that the cecal histology (Fig. 2A) and intestinal permeability (Fig. 2B) of SMVT-cKO mice were normal and similar to those of WT littermates. Similarly, BPS normalized the level of mRNA and protein expression of the TJ proteins claudin-1, claudin-2, and ZO-1, as well as MLCK in the cecum (Fig. 2, C and D) (and colon; data not shown) of SMVT-cKO mice similar to those seen in WT littermates.
Fig. 2.
A: histology of the cecum of BPS SMVT-cKO mice and their age- and sex-matched BPS WT littermates. A: representative section of the cecum of BPS WT littermate (i) and BPS SMVT-cKO mouse (ii) (hematoxylin and eosin, × 40). Animals of both groups showed normal cecal morphology. B: effect of BPS to SMVT-cKO mice on intestinal permeability. Intestinal permeability was determined using 4-kDa FITC-dextran method. Data are means ± SE of three pairs of BPS SMVT-cKO and WT littermates. C: effect of BPS to SMVT-cKO mice on the level of mRNA expression of TJ proteins and MLCK in the cecum. mRNA levels were determined by real-time RT-PCR, and data were normalized relative to β-actin. Data are means ± SE of at least three pairs of BPS SMVT-cKO mice and WT littermates. D: effect of BPS to SMVT-cKO mice on the level of protein expression of TJ proteins and MLCK in the cecum. Protein levels were determined by Western blot analysis, and data were normalized relative to β-actin expression as described in Methods. The graphs show relative protein expression of claudin-1 (i), claudin-2 (ii), ZO-1 (iii), and MLCK (iv) in cecum samples of SMVT-cKO mice and their WT littermates. Data are means ± SE of at least three separate sets of mice. E: expression of mRNA of mucin genes in the cecum of SMVT-cKO mice and their WT littermates. mRNA levels were determined by real-time RT-PCR, and data were normalized relative to villin. Data are means ± SE of at least three separate sets of mice (*P < 0.05; **P < 0.01; NS, not significant). F: effect of BPS to SMVT-cKO mice and their WT littermates on mRNA expression of mucins in the cecum. Level of mRNA expression of MUC-1 (i), MUC-2 (ii), and MUC-3 (iii) in cecum of BPS SMVT-cKO mice and their sex-matched BPS WT littermates. Data are means ± SE of at least three sets of mice. Abbreviations: BPS, biotin and pantothenic acid supplementation; cKO, conditional knockout of the mouse Slc5a6 gene; MLCK, myosin light chain kinase; MUC, mucin; SMVT, sodium-dependent multivitamin transporter; TJ, tight junction; WT, wild type; ZO-1, zonula occludens 1.
In related studies, we examined the level of expression of mucin (mostly MUC-1, MUC-2, and MUC-3) in SMVT-cKO and whether BPS causes any observed changes. We did this because accumulating evidence suggests that the intestinal inflammation that occurs in inflammatory bowel disease (IBD) and in experimental colitis is associated with aberrant expression of mucins, leading to an imbalanced mucus barrier (1, 4, 11, 28, 34). The results showed a significant increase in the level of mRNA expression of MUC-1 (P < 0.01) and MUC-2 (P < 0.05) (but not MUC-3) in SMVT-cKO mice (Fig. 2E), and this was normalized following BPS (Fig. 2F).
Effect of BPS to SMVT-cKO Mice on Intestinal Mucosal Inflammation
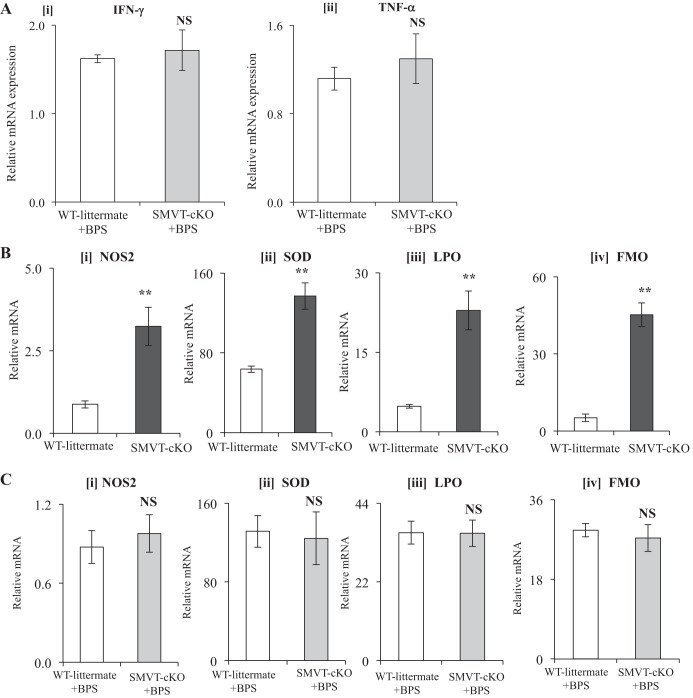
As mentioned earlier, we observed a significant induction in the level of expression of proinflammatory cytokines (IFN-γ and TNF-α) in the cecum of SMVT-cKO mice compared with their sex-matched WT littermates (30). Thus, in this study we examined whether BPS to SMVT-cKO mice could normalize the level of expression of IFN-γ and TNF-α in the cecal mucosa. We focused on these proinflammatory cytokines because of the important role they play in intestinal inflammation in IBD patients (21, 38). Our results showed that BPS indeed normalizes the level of these proinflammatory cytokines in the cecal mucosa of the SMVT-cKO mice to levels comparable to those in BPS-treated WT littermates (Fig. 3A).
Fig. 3.
A: effect of BPS of SMVT-cKO mice on the level of mRNA expression of proinflammatory cytokines in the cecum. mRNA levels were determined by real-time RT-PCR, and data were normalized relative to β-actin. Data are means ± SE of at least three separate sets of mice (NS, not significant). B: expression of mRNA of oxidative stress-responsive genes in the cecum of SMVT-cKO mice and their WT littermates. mRNA levels were determined by real-time RT-PCR and data were normalized relative to β-actin. Data are means ± SE of at least three separate sets of mice (**P < 0.01; NS, not significant). C: effect of BPS to SMVT-cKO mice and their WT littermates on mRNA expression of oxidative stress-responsive genes in the cecum. mRNA levels were determined by real-time RT-PCR, and data were normalized relative to β-actin. Data are means ± SE of at least three separate sets of mice. Abbreviations: BPS, biotin and pantothenic acid supplementation; cKO, conditional knockout of the mouse Slc5a6 gene; FMO, flavin-containing monooxygenase; LPO, lipid peroxidation; NOS, nitric oxide synthase; SMVT, sodium-dependent multivitamin transporter; SOD, superoxide dismutase; WT, wild type.
In related studies, we compared the expression of nitric oxide synthase (NOS) and reactive oxygen species (ROS) in the cecum of the SMVT-cKO mice to their WT littermates, and whether BPS could reverse any observed effect. We focused on these parameters because active intestinal inflammation is known to be accompanied by an increase in the level of expression of the inducible nitric oxide synthase (NOS2) and oxidative stress -responsive genes [namely, superoxide dismutase-1 (SOD1); flavin-containing monooxygenase-2 (FMO-2); lipid peroxidation (LPO)] (8, 14). The results showed a significant (P < 0.01 for all) induction in the level of expression NOS2 and as well as FMO2, SOD1, and LPO in the cecum of the SMVT-cKO mice (Fig. 3B) with complete normalization in BPS-treated SMVT-cKO mice compared with WT littermates (Fig. 3C).
DISCUSSION
Our aim in this study was to determine whether BPS to SMVT-cKO mice could reverse the abnormalities seen in these animals, such as impairment of growth rate, spontaneous development of large intestinal inflammation and abnormal pathology, and early death (12, 30). We initiated BPS before conception and continued this regimen throughout pregnancy and lactation of the dams and throughout the life of the offspring to ensure that sufficient biotin and pantothenic acid were provided to the SMVT-cKO animals at all times. Our results showed that such supplementation normalizes all of the above-described abnormalities, i.e., early death of the animals, impairment in their growth rate and skeletal development as well as the development of inflammation and abnormal pathology in their intestine. The abnormalities in gut permeability, level of expression of important TJ proteins and MLCK, the mucosal inflammation, and the changes in the level of expression of proinflammatory cytokines and stress response genes in the SMVT-cKO animals were all normalized by BPS. These findings clearly demonstrate the important role that biotin and/or pantothenic acid plays in maintaining normal intestinal integrity and health. A role for biotin in the maintenance of normal intestinal homeostasis has recently been well documented in related studies from our laboratory, where significant changes in large intestinal integrity, combined with the development of spontaneous inflammation, were observed in mice made biotin deficient via dietary means (30). Also, a role for biotin in normal immune function has been well described by us and others previously (2, 3, 9, 15–17). As to pantothenic acid, there is little known about its role in the maintenance of intestinal integrity/homeostasis. Further studies are required to address the latter issue. [We were not able to provide supplementation of biotin and pantothenic acid separately because of the inherited difficulties in generating sufficient numbers of the SMVT-cKO animals. Such difficulties could be circumvented with the use of a tamoxifen-inducible SMVT-cKO approach, an approach that should be tried in future investigations.
Of relevance to the findings in the current investigation is the recent identification by our group (35) of a loss of function mutations in the SLC5A6 gene in a 15-mo-old child who demonstrated growth impairment, variable immunodeficiency, and bone abnormalities, among other symptoms, a situation that was significantly improved following oversupplementation of the child with high doses of biotin and pantothenic acid.
In summary, results of this investigation provide further support to previous findings on the role of biotin and pantothenic acid in the maintenance of normal intestinal integrity and health.
GRANTS
Support for this study was provided by grants from the Department of Veterans Affairs and the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases Grants DK58057 and DK56061 and National Institute on Alcohol Abuse and Alcoholism Grant AA018071).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S. and H.M.S. conceived and designed research; S.S., R.K., A.G., M.S., and N.W.G.L. performed experiments; S.S., R.K., A.G., N.W.G.L., and H.M.S. analyzed data; S.S., A.G., N.W.G.L., and H.M.S. interpreted results of experiments; S.S., R.K., A.G., M.S., N.W.G.L., and H.M.S. prepared figures; S.S., N.W.G.L., and H.M.S. drafted manuscript; S.S., M.S., N.W.G.L., and H.M.S. edited and revised manuscript; S.S., R.K., A.G., M.S., N.W.G.L., and H.M.S. approved final version of manuscript.
REFERENCES
- 1.Ahn DH, Crawley SC, Hokari R, Kato S, Yang SC, Li JD, Kim YS. TNF-α activates MUC2 transcription via NF-κB but inhibits via JNK activation. Cell Physiol Biochem 15: 29–40, 2005. doi: 10.1159/000083636. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal S, Agrawal A, Said HM. Biotin deficiency enhances the inflammatory response of human dendritic cells. Am J Physiol Cell Physiol 311: C386–C391, 2016. doi: 10.1152/ajpcell.00141.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Báez-Saldaña A, Díaz G, Espinoza B, Ortega E. Biotin deficiency induces changes in subpopulations of spleen lymphocytes in mice. Am J Clin Nutr 67: 431–437, 1998. doi: 10.1093/ajcn/67.3.431. [DOI] [PubMed] [Google Scholar]
- 4.Beatty PL, Plevy SE, Sepulveda AR, Finn OJ. Cutting edge: transgenic expression of human MUC1 in IL-10−/− mice accelerates inflammatory bowel disease and progression to colon cancer. J Immunol 179: 735–739, 2007. doi: 10.4049/jimmunol.179.2.735. [DOI] [PubMed] [Google Scholar]
- 5.Bonjour JP. Biotin. Handbook of Vitamins: Nutritional Biochemical and Clinical Aspects, edited by Machlin LJ. New York: Marcel Dekker, 1984, p. 403–435. [Google Scholar]
- 6.Bogusiewicz A, Stratton SL, Ellison DA, Mock DM. Biotin accounts for less than half of all biotin and biotin metabolites in the cerebrospinal fluid of children. Am J Clin Nutr 88: 1291–1296, 2008. doi: 10.3945/ajcn.2008.26525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandl K, Rutschmann S, Li X, Du X, Xiao N, Schnabl B, Brenner DA, Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proc Natl Acad Sci USA 106: 3300–3305, 2009. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkstra G, Moshage H, van Dullemen HM, de Jager-Krikken A, Tiebosch AT, Kleibeuker JH, Jansen PL, van Goor H. Expression of nitric oxide synthases and formation of nitrotyrosine and reactive oxygen species in inflammatory bowel disease. J Pathol 186: 416–421, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 9.Elahi A, Sabui S, Narasappa NN, Agrawal S, Lambrecht NW, Agrawal A, Said HM. Biotin deficiency induces Th1- and Th17-mediated proinflammatory responses in human CD4+ T lymphocytes via activation of the mTOR signaling pathway. J Immunol 200: 2563–2570, 2018. doi: 10.4049/jimmunol.1701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Banares F, Abad-Lacruz A, Xiol X, Gine JJ, Dolz C, Cabre E, Esteve M, Gonzalez-Huix F, Gassull MA. Vitamin status in patients with inflammatory bowel disease. Am J Gastroenterol 84: 744–748, 1989. [PubMed] [Google Scholar]
- 11.Furr AE, Ranganathan S, Finn OJ. Aberrant expression of MUC1 mucin in pediatric inflammatory bowel disease. Pediatr Dev Pathol 13: 24–31, 2010. doi: 10.2350/08-06-0479.1. [DOI] [PubMed] [Google Scholar]
- 12.Ghosal A, Lambrecht N, Subramanya SB, Kapadia R, Said HM. Conditional knockout of the Slc5a6 gene in mouse intestine impairs biotin absorption. Am J Physiol Gastrointest Liver Physiol 304: G64–G71, 2013. doi: 10.1152/ajpgi.00379.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause KH, Bonjour JP, Berlit P, Kochen W. Biotin status of epileptics. Ann NY Acad Sci 447: 297–313, 1985. doi: 10.1111/j.1749-6632.1985.tb18447.x. [DOI] [PubMed] [Google Scholar]
- 14.Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol 201: 28–36, 2003. doi: 10.1002/path.1409. [DOI] [PubMed] [Google Scholar]
- 15.Kuroishi T. Regulation of immunological and inflammatory functions by biotin. Can J Physiol Pharmacol 93: 1091–1096, 2015. doi: 10.1139/cjpp-2014-0460. [DOI] [PubMed] [Google Scholar]
- 16.Kuroishi T, Endo Y, Muramoto K, Sugawara S. Biotin deficiency up-regulates TNF-α production in murine macrophages. J Leukoc Biol 83: 912–920, 2008. doi: 10.1189/jlb.0607428. [DOI] [PubMed] [Google Scholar]
- 17.Kung JT, Mackenzie CG, Talmage DW. The requirement for biotin and fatty acids in the cytotoxic T-cell response. Cell Immunol 48: 100–110, 1979. doi: 10.1016/0008-8749(79)90103-5. [DOI] [PubMed] [Google Scholar]
- 18.Lederer WH, Kumar M, Axelrod AE. Effects of pantothenic acid deficiency on cellular antibody synthesis in rats. J Nutr 105: 17–25, 1975. doi: 10.1093/jn/105.1.17. [DOI] [Google Scholar]
- 19.Lewis B, Rathman S, McMahon R. Dietary biotin intake modulates the pool of free and protein-bound biotin in rat liver. J Nutr 131: 2310–2315, 2001. doi: 10.1093/jn/131.9.2310. [DOI] [PubMed] [Google Scholar]
- 20.Madsen CT, Sylvestersen KB, Young C, Larsen SC, Poulsen JW, Andersen MA, Palmqvist EA, Hey-Mogensen M, Jensen PB, Treebak JT, Lisby M, Nielsen ML. Biotin starvation causes mitochondrial protein hyperacetylation and partial rescue by the SIRT3-like deacetylase Hst4p. Nat Commun 6: 7726, 2015. doi: 10.1038/ncomms8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-α and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol 81: 301–305, 1990. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon RJ. Biotin in metabolism and molecular biology. Annu Rev Nutr 22: 221–239, 2002. doi: 10.1146/annurev.nutr.22.121101.112819. [DOI] [PubMed] [Google Scholar]
- 23.Mock DM. Biotin. Handbook of Vitamins, edited by Zempleni J, McCormick DB, Suttie JW. New York: CRC, 2006, p. 361–377. [Google Scholar]
- 24.Mock DM, deLorimer AA, Liebman WM, Sweetman L, Baker H. Biotin deficiency: an unusual complication of parenteral alimentation. N Engl J Med 304: 820–823, 1981. doi: 10.1056/NEJM198104023041405. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa T, Yasuno R, Wada H. Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett 498: 16–21, 2001. doi: 10.1016/S0014-5793(01)02469-3. [DOI] [PubMed] [Google Scholar]
- 26.Okabe N, Urabe K, Fujita K, Yamamoto T, Yao T, Doi S. Biotin effects in Crohn’s disease. Dig Dis Sci 33: 1495–1496, 1988. doi: 10.1007/BF01537009. [DOI] [PubMed] [Google Scholar]
- 27.Prasad PD, Wang H, Kekuda R, Fujita T, Fei YJ, Devoe LD, Leibach FH, Ganapathy V. Cloning and functional expression of a cDNA encoding a mammalian sodium-dependent vitamin transporter mediating the uptake of pantothenate, biotin, and lipoate. J Biol Chem 273: 7501–7506, 1998. doi: 10.1074/jbc.273.13.7501. [DOI] [PubMed] [Google Scholar]
- 28.Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut 35: 353–359, 1994. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Melendez R, Zempleni J. Regulation of gene expression by biotin (review). J Nutr Biochem 14: 680–690, 2003. doi: 10.1016/j.jnutbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Sabui S, Bohl JA, Kapadia R, Cogburn K, Ghosal A, Lambrecht NW, Said HM. Role of the sodium-dependent multivitamin transporter (SMVT) in the maintenance of intestinal mucosal integrity. Am J Physiol Gastrointest Liver Physiol 311: G561–G570, 2016. doi: 10.1152/ajpgi.00240.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Said HM. Biotin: biochemical, physiological and clinical aspects. Subcell Biochem 56: 1–19, 2012. doi: 10.1007/978-94-007-2199-9_1. [DOI] [PubMed] [Google Scholar]
- 32.Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J 437: 357–372, 2011. doi: 10.1042/BJ20110326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Said HM, Trebble T. Intestinal digestion and absorption of micronutrients. Slesenger and Fordtran Gastrointestinal and Liver Disease, edited by Feldman M, Friedman LS, Brandt LJ. New York: Elsevier, 2015, p. 1765–1788. [Google Scholar]
- 34.Saitoh H, Takagaki K, Nakamura T, Munakata A, Yoshida Y, Endo M. Characterization of mucin in whole-gut lavage fluid obtained from patients with inflammatory bowel disease. Dig Dis Sci 41: 1768–1774, 1996. doi: 10.1007/BF02088743. [DOI] [PubMed] [Google Scholar]
- 35.Subramanian VS, Constantinescu AR, Benke PJ, Said HM. Mutations in SLC5A6 associated with brain, immune, bone, and intestinal dysfunction in a young child. Hum Genet 136: 253–261, 2017. doi: 10.1007/s00439-016-1751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugahara H, Odamaki T, Fukuda S, Kato T, Xiao JZ, Abe F, Kikuchi J, Ohno H. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Sci Rep 5: 13548, 2015. doi: 10.1038/srep13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweetman L, Nyhan WL. Inheritable biotin-treatable disorders and associated phenomena. Annu Rev Nutr 6: 317–343, 1986. doi: 10.1146/annurev.nu.06.070186.001533. [DOI] [PubMed] [Google Scholar]
- 38.Vitale S, Strisciuglio C, Pisapia L, Miele E, Barba P, Vitale A, Cenni S, Bassi V, Maglio M, Del Pozzo G, Troncone R, Staiano A, Gianfrani C. Cytokine production profile in intestinal mucosa of paediatric inflammatory bowel disease. PLoS One 12: e0182313, 2017. doi: 10.1371/journal.pone.0182313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B, Feng L, Wang F, Wang L. Enterohemorrhagic Escherichia coli senses low biotin status in the large intestine for colonization and infection. Nat Commun 6: 6592, 2015. doi: 10.1038/ncomms7592. [DOI] [PMC free article] [PubMed] [Google Scholar]