Abstract
After myocardial infarction, remodeling of the left ventricle involves a wound-healing orchestra involving a variety of cell types. In order for wound healing to be optimal, appropriate communication must occur; these cells all need to come in at the right time, be activated at the right time in the right amount, and know when to exit at the right time. When this occurs, a new homeostasis is obtained within the infarct, such that infarct scar size and quality are sufficient to maintain left ventricular size and shape. The ideal scenario does not always occur in reality. Often, miscommunication can occur between infarct and remote spaces, across the temporal wound-healing spectrum, and across organs. When miscommunication occurs, adverse remodeling can progress to heart failure. This review discusses current knowledge gaps and recent development of the roles of inflammation and the extracellular matrix in myocardial infarction remodeling. In particular, the macrophage is one cell type that provides direct and indirect regulation of both the inflammatory and scar-forming responses. We summarize current research efforts focused on identifying biomarker indicators that reflect the status of each component of the wound-healing process to better predict outcomes.
Keywords: extracellular matrix, fibroblast, inflammation, macrophage, proteomics, scar, systems biology
INTRODUCTION
Myocardial infarction (MI) occurs when ischemia is of sufficient duration to induce myocyte necrosis. If the time of ischemia is short and area of damage is small, the result is minimal damage to the myocardium. When the time of ischemia or the injury area is extensive, there is a variation in remodeling response across individuals in both humans and animal models that ranges from adequate wound healing to adverse remodeling that progresses to heart failure.
Once myocyte ischemia is past the point of no return and necrosis has initiated, the death of myocytes triggers an inflammatory response. The myocardium undergoes a wound-healing paradigm that involves first a proinflammatory and extracellular matrix (ECM)-degrading component to clear away the necrotic debris. The second step is anti-inflammatory and proreparative to turn off the inflammation and stimulate ECM deposition to form the new scar. Cells involved in this coordinated response include cardiac myocytes, neutrophils, macrophages, fibroblasts, endothelial cells, and nerve cells, with cross talk occurring across all cell types. Of these cell types, the macrophage and fibroblast have central roles in post-MI wound healing by providing mediators that span the whole period of the response.
When MI occurs, the best-case scenario is an optimized wound-healing response to generate an appropriate scar and minimize extension of damage to surrounding regions. Clinically, the current best treatment option is timely reperfusion, which will allow salvage of vulnerable myocytes in the border region of the infarct. In permanent occlusion coronary artery ligation animal models, which mimic patients who are not reperfused or who advance to heart failure, interventions that increase or decrease one component of the wound-healing process have consistently stimulated an adverse remodeling profile. This indicates that disruption of the spatial and temporal coordination of individual cell responses, even if beneficial to a certain cell type, can have a negative impact at the whole organ level.
There are numerous review articles summarizing the inflammatory and ECM components to post-MI remodeling (30, 99). Here, we highlight the current knowledge and focus on the key gaps that remain in our knowledge base.
INFLAMMATION
Miscommunication in inflammation includes signaling at the molecular and cellular levels, as shown in Table 1. The inflammatory response to MI has a molecular and cellular component, with a multitude of cytokines and chemokines involved (5, 20, 23, 28, 92, 124). Several groups have reported adverse MI remodeling in the setting of systemic inflammation (14, 23, 112). De Jesus et al. (11) have shown that preexisting atherosclerosis, either in apolipoprotein E (apoE)-null mice fed an atherogenic diet or simulated by elevated systemic LPS administration, exacerbates post-MI electrophysiological uncoupling and arrhythmias. Dutta et al. (19) reported the converse, that MI stimulates the progression of atherosclerosis. When chemokine (C-C motif) receptor (CCR)2 was silenced in apoE-null mice after MI, Ly-6Chigh monocyte recruitment was limited, and the resulting attenuation in infarct inflammation curbed post-MI remodeling (87). In a periodontal model of chronic systemic inflammation, a baseline elevation in the inflammatory status worsens MI remodeling characterized by earlier rupture as a result of elevated matrix metalloproteinase (MMP)-9 levels (12). Chronic inflammation stimulates macrophage secretion of chemokine (C-C motif) ligand (CCL)12, which impairs post-MI reparative fibroblast activation (14). The connection between inflammatory status and the MI response, therefore, is very strong.
Table 1.
Post-MI effect of perturbing inflammation components
| Perturbation | Effect | Mechanism |
|---|---|---|
| Reducing inflammation: | ↑LV physiology | IL-1α null: ↓innate immune response in cardiac fibroblasts |
| IL-1α null (79)TREM1 null (2)NF-κB null (60)TLR4 null (97)TRPV2 null (21)PS-presenting liposomes (39) | ↓LV remodeling | TREM1 null: ↓myeloid cell recruitment from the spleen and bone marrow; ↓CCR2+ proinflammatory but not proreparative CX3CR1+ monocyte infiltrationTLR4 null: ↓neutrophil infiltration/activationTRPV2 null: ↓macrophage migrationPS-liposomes: ↑macrophage secretion of IL-10 and TGF-β |
| Total neutrophil depletion (43, 44, 85, 86) | ↓LV physiology | ↓Necrotic myocyte removal |
| ↑Fibrosis | ↑Macrophage inflammatory response | |
| ↑Myofibroblast activation | ||
| Total macrophage depletion (120) | ↓LV physiology | Total: ↓necrotic/apoptotic cell removal |
| ↓Collagen deposition | ↓Fibroblast and endothelial cell activation | |
| M2-specific depletion (66) | ↓Neovascularization | M2: prolonged N1 neutrophil/M1 macrophage activation |
| Macrophage modulation: | ↑LV physiology | Wntless: ↑reparative M2 macrophages, neovascularization |
| Wntless null (98)Macrophage EP3R null (114)Macrophage EP3R transgenic (114)CD5L null (96) | ↓LV remodeling | EP3R null: monocyte TGF-β signaling/↓CX3CR1 and VEGF signaling, ↓Ly6Clow reparative monocyte activationEP3R transgenic: ↑angiogenesisCD5L null: ↓neutrophils, ↓collagen accumulation, ↓IL-1 receptor-associated kinase 4, NF-κB, myeloperoxidase, and inducible nitric oxide synthase |
Reference numbers are listed in parentheses. MI, myocardial infarction; TLR, Toll-like receptor; TREM1, triggering receptor expressed on myeloid cells 1; PS, phosphatidylserine; LV, left ventricular; CCR, chemokine (C-C motif) receptor; CX3CR, chemokine (C-X3-C motif) receptor; TGF-β, transforming growth factor-β; EP3R, E-prostanoid 3 receptor; ↑, increase; ↓, decrease.
For the cellular component, by numbers, neutrophils and macrophages are the predominant leukocytes that infiltrate into the infarcted left ventricle (LV) and are known participants in LV remodeling (109). While monocyte infiltration begins first, neutrophil infiltration quickly becomes the predominant leukocyte in the infarct zone by 24 h after MI (33, 44, 102). Neutrophils at day 1 post-MI release granule components (e.g., serine elastase and MMP-9) aimed at breaking down necrotic myocytes and clearing tissue debris (67, 86). Anti-inflammatory strategies that limit neutrophil infiltration reduce acute injury but exacerbate cardiac physiology and enhance the fibrotic response to promote progression to heart failure (43, 45). By day 7 post-MI, neutrophil numbers have begun to return toward baseline day 0 values. While neutrophils are primarily considered proinflammatory, we have reported that they undergo polarization, and anti-inflammatory (N2) neutrophils account for 20% of the neutrophils in the day 7 infarct (85). The N2 neutrophil is characterized by high expression of macrophage mannose receptor CD206 and IL-10 (85). Neutrophils, therefore, contribute directly to inflammation and directly and indirectly to cardiac repair after MI.
Macrophages have polarization profiles similar to neutrophils, with day 1 post-MI macrophages exhibiting a proinflammatory M1 profile (6, 25, 42). Over the first 7 days of MI, there is a transition from primarily M1 macrophages to primarily anti-inflammatory and then reparative M2 cells (75, 84). While the M1 and M2 classification system is frequently used to define pro- and anti-inflammatory macrophages, there is a growing effort to replace the M1/M2 nomenclature, as this naming was set up to describe the in vitro situation and does not apply to the MI scenario where there is a heterogeneous set of polarization stimuli (93).
In addition to known roles as phagocytic cells that remove necrotic debris and apoptotic neutrophils, macrophages have been assigned roles in facilitating electrical conduction in the atria and regulating diastolic pathophysiology in the setting of hypertension (46, 47, 57). In skin wound healing, macrophage-depleted mice showed a severely impaired wound morphology and delayed healing due to the persistence of large numbers of neutrophils, proinflammatory mediators (macrophage inflammatory protein-3, macrophage chemoattractant protein-1, IL-1b, and cycyooxygenase-2), impaired neovascularization, and fibroblast activation (37). Post-MI, macrophage-depleted mice show impaired wound healing and exacerbated LV remodeling, leading to high mortality and excessive LV dilation (120). Deletion of macrophage polarization components show similar negative phenotypes. For example, CCR5 deletion does not limit macrophage infiltration into the infarct region; rather, it attenuates both pro- and anti-inflammatory proteins, resulting in poor healing and increased rupture rates (131). Periodontal-induced chronic inflammation triggers macrophage secretion of CCL12 to inhibit fibroblast-mediated cardiac wound healing (14). Obesity superimposed on aging magnifies inflammation and delays the resolving response after MI (78). While total depletion of macrophages is detrimental to post-MI wound healing, in vivo silencing of the interferon regulatory factor 5 transcription factor reprograms the macrophage away from an inflammatory polarization status to attenuate inflammation and remodeling after MI (9). Likewise, infusion of anti-inflammatory IL-10 early post-MI shifts macrophage polarization and improves LV remodeling (56).
These studies highlight the role of the macrophage in cross talk with neutrophils, fibroblasts, and endothelial cells (33, 84). Neutrophil depletion is also detrimental to MI wound healing, in part because secretion of neutrophil gelatinase-associated lipocalin (NGAL; lipocalin 2) by neutrophils stimulates macrophage polarization toward the reparative phenotype (43). A number of reviews have summarized current efforts to target post-MI inflammation (5, 23, 28, 45, 103, 124). A major take-home message here is that modulation of some aspects of inflammation feedforward to modify other aspects. Caution should be given to strategies that totally block the entry of any one cell type, as this approach is likely overly simplistic. To date, inhibition of IL-1β is a target that shows promise in the post-MI setting in both animal models and humans (29, 94, 100). The consistency across species indicates that the inflammatory component is a common denominator and translational research focused on inflammation is likely to inform clinical practice.
SCAR FORMATION
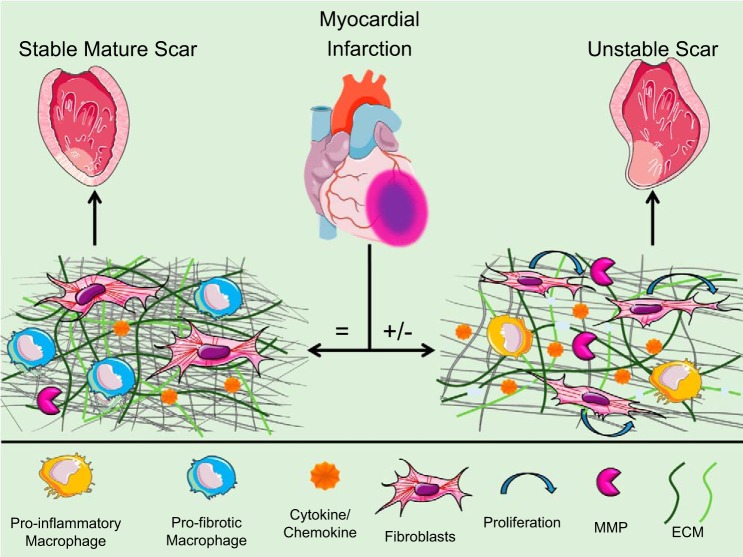
Miscommunication in ECM signaling also occurs at the molecular and cellular levels, as shown in Table 2 and Fig. 1. Major ECM components include structural proteins (collagens and fibronectin), accessory proteins [osteopontin, secreted protein acidic and rich in cysteine (SPARC), and thrombospondin-1 (TSP-1)], and MMPs. MMPs are a family of enzymes that degrade ECM constituents as well as many of the inflammatory mediators listed above (15). Tissue inhibitors of metalloproteinase (TIMPs) are the endogenous inhibitors of MMPs.
Table 2.
Post-MI effects of perturbing extracellular matrix components
| Perturbation | Effect | Mechanism |
|---|---|---|
| Collagen type VI null (80) | ↓Infarct size | Accelerated acute apoptosis, ↓chronic apoptosis |
| ↑LV physiology | ||
| MMP and TIMP null | Improved LV remodeling | MMP-2: ↓M2 macrophage and myofibroblast activation |
| MMP-2 (40) | MMP-7: ↓connexin-43 cleavage, ↑conduction velocity | |
| MMP-7 (8, 70) | MMP-9: ↓macrophage accumulation (late phase) | |
| MMP-9 (18, 69) | MMP28: ↓inflammation and ↓M2 macrophage and fibroblast activation | |
| MMP-28 (82) | TIMP null: ↑MMP activity | |
| TIMP-1 (49) | ||
| TIMP-2 (59) | ||
| TIMP-3 (38, 115) | ||
| TIMP-4 (64) | ||
| Macrophage-specific MMP-9 transgenic (90) | ↑LV physiology | ↓Expression of other MMPs, ↑expression of profibrotic genes |
| ↓LV inflammation | ||
| Cardiac-specific MMP-1 transgenic (61) | ↑LV remodeling | ↑Collagen degradation |
| Osteopontin null (117) | ↓LV physiology, collagen deposition | ↑MMP-2 and ↓MMP-9 activity |
| Secreted protein acidic and rich in cysteine null (88, 104) | ↑LV physiology early; ↑rupture later | ↑Connective tissue growth factor activity and collagen synthesis |
| Thrombospondin-1 null (24) | ↑LV dilation, inflammation | ↓Transforming growth factor-β and Smad-2 signaling |
Reference numbers are listed in parentheses. MI, myocardial infarction; LV, left ventricular; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; ↑, increase; ↓, decrease.
Fig. 1.
After myocardial infarction (MI), there is a sequential wound-healing response comprised of inflammation followed by scar formation. When components are aligned ( = ), a stable scar forms and a new homeostatic-like environment ensues. When miscommunication among the components occurs (+/−), the scar is unstable and adverse remodeling occurs. ECM, extracellular matrix; MMP, matrix metalloproteinase. [Modified from Ref. 14.]
While collagen- and fibronectin-null mice are both embryonically lethal, mice with cleavage-resistant collagen showed no effect on post-MI LV remodeling and mice that lack the fibronectin EDA domain showed improved survival and better remodeling compared with wild-type control mice (1, 76). Osteopontin-null mice show exaggerated LV dilation through reduced collagen accumulation in the scar region, which can be ameliorated with MMP inhibition (65, 117). Deletion of SPARC reduced early rupture and improved LV remodeling through day 3 post-MI but enhanced rupture and impaired LV remodeling through day 14 post-MI, indicating biphasic roles for SPARC in early macrophage polarization and later fibroblast entry (88, 104, 125). TSP-1 limits infarct expansion and plays an important role in the transition from MI to heart failure (24, 63).
A number of MMPs and all four TIMPs have been evaluated in the post-MI LV, including MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-12, MMP-13, MMP-14, and MMP-28 and all four TIMPs (15, 51, 54, 55, 72, 77, 126). Deletion of MMP-2, MMP-7, MMP-9, MMP-14, and MMP-28 overall improved LV remodeling through mechanisms ranging from effects on myocytes to inflammatory cells to endothelial cells (8, 17, 69, 70, 82, 132). MMP inhibition is complex for two reasons. First, the specific MMP that is inhibited and the time of its inhibition have a large effect on reparative outcomes. We will use MMP-9 as an example to illustrate this point (53). When MMP-9 is globally deleted from birth or is overexpressed in macrophages only, the net effect is improved LV remodeling (albeit through different mechanisms) (16, 18, 69, 90, 130). When MMP-9 is inhibited beginning at 3 h post-MI, inflammation resolution is delayed and the net effect is detrimental (52). The baseline and post-MI environments are also altered with age (7, 89, 116, 127, 128). To optimize scar formation, understanding how MMPs, MMP-9 in particular, coordinate inflammation with ECM deposition and cross linking is needed (122, 123).
Second, TIMP inhibition has shown beneficial effects on LV remodeling through effects on both MMPs and non-MMP roles (22). While TIMPs function through overlapping roles to endogenously inhibit MMPs and there is some compensation across their profiles, each TIMP has distinct mechanisms in LV remodeling. TIMP-1 deficiency accelerates post-MI LV remodeling, an effect that was pharmacologically modified with an MMP inhibitor (10, 49). Of note, TIMP-1 is an independent predictor of all-cause mortality, cardiac mortality, and MI (4). Deletion of TIMP-2 amplifies LV remodeling by enhancing activation of MMP-14 (59). TIMP-3-null mice show early MMP activation that leads to exacerbated systolic and diastolic dysfunction after MI through the activation of epidermal growth factor signaling, whereas TIMP-3 overexpression shows improvement by preventing ECM proteolysis (38, 58, 113). TIMP-4-null mice are susceptible to MI-induced rupture and mortality but not pressure overload pathology (64).
The ECM is an important modifier of fibroblast phenotype, with fibroblasts sensing changes to the ECM as an important cue (27). Depending on the ECM environment, fibroblasts can polarize through homeostatic, proinflammatory, anti-inflammatory, and reparative phenotypes to reset at a new homeostatic-like end point (81, 83, 106, 121). In the early post-MI proinflammatory environment, high concentrations of proinflammatory cytokines such as IL-1β and the generation of ECM fragments by MMPs induce an ECM-degrading fibroblast phenotype. Deposition of EDA-fibronectin, a common myofibroblast marker, activates myofibroblast differentiation in a feedforward manner (1, 119). In the later post-MI anti-inflammatory and reparative environments, growth factors such as transforming growth factor-β and the synthesis of new ECM induce an ECM-accumulating fibroblast phenotype (129).
A number of reviews have summarized current efforts to target post-MI ECM remodeling (26, 71, 81, 95, 110). Similar to inflammation, modulation of particular ECM proteins feeds forward to modify other aspects. To date, targeting specific MMPs has promise in the post-MI setting (15, 16, 51, 72, 111). Of interest, a collagen fragment generated post-MI by MMP-9 actually promotes wound healing by limiting dilation and promoting neovascularization when exogenously administrated after MI (73). The ECM, MMP, and TIMP environment is complex, and understanding how individual patterns fit into the entire LV effect, including cross talk among cell types, requires systems biology approaches to map the interplay (33, 34, 48, 72). More information on ECM remodeling is needed before approaches targeting ECM components can be translated.
FUTURE DIRECTIONS
There are four gaps to our understanding of how the inflammatory and ECM components interact in the MI wound-healing response. The four gaps are as follows: 1) harnessing the heterogeneity across cell subtypes to target most effective interventions, 2) linking molecular and cellular phenotypes to cell and tissue physiology to complete the knowledge maps, 3) figuring out how to best target endogenous and exogenous signaling pathways, and 4) translating findings to the clinic. In particular, sensors (marker subsets that define network status) are needed to better predict outcomes based on responses of the individual elements.
Heterogeneity Along the Response
While we have appreciated changes at the molecular and macrophage cell levels, molecular and cellular heterogeneity in polarization phenotypes along the MI time continuum is greater than previously realized. In addition to macrophage polarization (32, 35, 48, 75, 84), there is growing awareness for neutrophil and fibroblast polarization (41, 81, 83, 86, 91, 106, 107, 109, 118, 121). This is consistent with the polarization of the MI environment, which quickly transitions through phases, starting with the shift from homeostasis to inflammatory and then shifting to turn off inflammation and initiate repair. The final stage is a homeostatic-like end point, where a new homeostasis is maintained thereafter. To fill these gaps, there are three targets.
First, there is a need to map the molecular and cellular phenotypes, particularly during the early phase (i.e., first week in mice). Using omics approaches (transcriptomics, metabolomics, and proteomics) will allow the full molecular component to be defined, which will provide markers that best define tissue or cell phenotypes.
Second, there is a need for big data tools to harness the large data sets and distill the most informative details of the system (105). For example, identifying which genes, metabolites, proteins, and cell phenotypes provide the best diagnostic or prognostic information will focus attempts to provide mechanistic insights to those signaling pathways that most directly impact the system.
Third, there is a need to use computational models, which amplify the possible post-MI remodeling scenarios that can be studied and have the added benefit of limiting in vivo experiments. Computational modeling will also allow the introduction of multiple confounding factors, which more closely mimics the clinical scenario. While the possible permutations of intervention are extremely large, it is not possible to explore all using time-consuming in vivo approaches, which only evaluate a few factors at a time. Using knowledge maps to reduce the possible choices down to the most probable will focus and facilitate efforts.
Linking Molecular and Cellular Phenotypes to Cell and Tissue Physiology
Going beyond marker expression profiling is needed to build our understanding at the systems level and completely evaluate how the marker is influencing LV remodeling. To fill these gaps, there are two targets.
There is a need to understand the connections between molecular changes to cell physiology changes. Key cell physiology phenotypes that coordinate LV physiology phenotypes include activation of the inflammatory cascade for resident cardiac myocytes and fibroblasts, tissue clearance for neutrophils and macrophages, and ECM secretion for the fibroblast. Understanding cell activation and deactivation mechanisms is needed to better link molecular to cell to tissue pathophysiological responses to MI.
There is a need to understand the connections that link individual cell physiology to LV tissue physiology. Key tissue physiology phenotypes include dilation (elevated end-systolic and end-diastolic dimensions), wall thinning, and ejection fraction. While we know individual cell effects in culture, how these combine in the complex in vivo environment is still being investigated.
Targeting Endogenous and Exogenous Signaling Pathways
There is a need to know how to intervene in a way that will change the molecular, cellular, and tissue phenotypes in predictable ways and is amenable to therapeutic intervention. This will tell us if the key drivers of LV remodeling have been identified and if there is translational potential. To fill these gaps, there are two targets. For molecular phenotypes, we need to identify the triggers that stimulate particular phenotypic outputs. Presently, IL-1β is a key candidate for inflammation, whereas IL-10 is a key candidate for anti-inflammation (5, 36, 50, 56, 62, 101).
There is a need to identify the subnetworks downstream of the candidates that may be more amenable to inhibition. If the main driver has off-target actions, inhibiting it may not be the best approach. For example, MMP inhibition, even if specific for a particular MMP, may not be as specific as targeting one of its downstream substrates.
Translating From Animal Models to Humans
While most interventions target endogenous mediators, consideration should also be given to using exogenous systems as intervention targets. For example, IL-4 is not elevated post-MI, yet the LV has the capability to respond to IL-4, which is a known in vitro anti-inflammation stimulus (108). With any intervention strategy, consideration should be given to unintentional consequences, and a good experimental design will consider possible secondary effects. A number of guidelines have recently been established to increase rigor and reproducibility in ischemia and infarction animal models, with antibody use, and in cardiac physiology measurements, all of which have an application here (3, 68, 74).
CONCLUSIONS
In summary, a large amount of data has been assimilated over the past two decades to increase our knowledge of inflammation and ECM roles in post-MI remodeling. In particular, the macrophage and fibroblast are the key cell types that regulate the inflammatory and scar formation processes. With focused systems biology approaches, future efforts can effectively translate strategies to improve LV remodeling.
GRANTS
We acknowledge funding from National Institutes of Health Grants GM-104357, GM-114833, GM-115428, HL-051971, HL-075360, HL-105324, and HL-129823 and Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Grant 5I01BX000505.
DISCLAIMERS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Veterans Administration.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.M., O.J.R., and M.L.L. edited and revised manuscript; A.J.M., O.J.R., and M.L.L. approved final version of manuscript; M.L.L. prepared figures; M.L.L. drafted manuscript.
REFERENCES
- 1.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res 108: 582–592, 2011. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 2.Boufenzer A, Lemarié J, Simon T, Derive M, Bouazza Y, Tran N, Maskali F, Groubatch F, Bonnin P, Bastien C, Bruneval P, Marie PY, Cohen R, Danchin N, Silvestre JS, Ait-Oufella H, Gibot S. TREM-1 mediates inflammatory injury and cardiac remodeling following myocardial infarction. Circ Res 116: 1772–1782, 2015. doi: 10.1161/CIRCRESAHA.116.305628. [DOI] [PubMed] [Google Scholar]
- 3.Brooks HL, Lindsey ML. Guidelines for authors and reviewers on antibody use in physiology studies. Am J Physiol Heart Circ Physiol 314: H724–H732, 2018. doi: 10.1152/ajpheart.00512.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 151: 1101.e1–1101.e8, 2006. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation 24: 24, 2017. doi: 10.1111/micc.12305. [DOI] [PubMed] [Google Scholar]
- 6.Chen B, Frangogiannis NG. Macrophages in the remodeling failing heart. Circ Res 119: 776–778, 2016. doi: 10.1161/CIRCRESAHA.116.309624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiao YA, Ramirez TA, Zamilpa R, Okoronkwo SM, Dai Q, Zhang J, Jin YF, Lindsey ML. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovasc Res 96: 444–455, 2012. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiao YA, Zamilpa R, Lopez EF, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. In vivo matrix metalloproteinase-7 substrates identified in the left ventricle post-myocardial infarction using proteomics. J Proteome Res 9: 2649–2657, 2010. doi: 10.1021/pr100147r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol 63: 1556–1566, 2014. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creemers EEJM, Davis JN, Parkhurst AM, Leenders P, Dowdy KB, Hapke E, Hauet AM, Escobar PG, Cleutjens JP, Smits JF, Daemen MJ, Zile MR, Spinale FG. Deficiency of TIMP-1 exacerbates LV remodeling after myocardial infarction in mice. Am J Physiol Heart Circ Physiol 284: H364–H371, 2003. doi: 10.1152/ajpheart.00511.2002. [DOI] [PubMed] [Google Scholar]
- 11.De Jesus NM, Wang L, Herren AW, Wang J, Shenasa F, Bers DM, Lindsey ML, Ripplinger CM. Atherosclerosis exacerbates arrhythmia following myocardial infarction: Role of myocardial inflammation. Heart Rhythm 12: 169–178, 2015. doi: 10.1016/j.hrthm.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeLeon-Pennell KY, de Castro Brás LE, Iyer RP, Bratton DR, Jin YF, Ripplinger CM, Lindsey ML. P. gingivalis lipopolysaccharide intensifies inflammation post-myocardial infarction through matrix metalloproteinase-9. J Mol Cell Cardiol 76: 218–226, 2014. doi: 10.1016/j.yjmcc.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeLeon-Pennell KY, Iyer RP, Ero OK, Cates CA, Flynn ER, Cannon PL, Jung M, Shannon D, Garrett MR, Buchanan W, Hall ME, Ma Y, Lindsey ML. Periodontal-induced chronic inflammation triggers macrophage secretion of Ccl12 to inhibit fibroblast-mediated cardiac wound healing. JCI Insight 2: 94207, 2017. doi: 10.1172/jci.insight.94207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLeon-Pennell KY, Meschiari CA, Jung M, Lindsey ML. Matrix metalloproteinases in myocardial infarction and heart failure. Prog Mol Biol Transl Sci 147: 75–100, 2017. doi: 10.1016/bs.pmbts.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLeon-Pennell KY, Tian Y, Zhang B, Cates CA, Iyer RP, Cannon P, Shah P, Aiyetan P, Halade GV, Ma Y, Flynn E, Zhang Z, Jin YF, Zhang H, Lindsey ML. CD36 Is a matrix metalloproteinase-9 substrate that stimulates neutrophil apoptosis and removal during cardiac remodeling. Circ Cardiovasc Genet 9: 14–25, 2016. doi: 10.1161/CIRCGENETICS.115.001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deschamps AM, Yarbrough WM, Squires CE, Allen RA, McClister DM, Dowdy KB, McLean JE, Mingoia JT, Sample JA, Mukherjee R, Spinale FG. Trafficking of the membrane type-1 matrix metalloproteinase in ischemia and reperfusion: relation to interstitial membrane type-1 matrix metalloproteinase activity. Circulation 111: 1166–1174, 2005. doi: 10.1161/01.CIR.0000157149.71297.3A. [DOI] [PubMed] [Google Scholar]
- 18.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55–62, 2000. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, Majmudar MD, Lasitschka F, Etzrodt M, Waterman P, Waring MT, Chicoine AT, van der Laan AM, Niessen HW, Piek JJ, Rubin BB, Butany J, Stone JR, Katus HA, Murphy SA, Morrow DA, Sabatine MS, Vinegoni C, Moskowitz MA, Pittet MJ, Libby P, Lin CP, Swirski FK, Weissleder R, Nahrendorf M. Myocardial infarction accelerates atherosclerosis. Nature 487: 325–329, 2012. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science 356: 1026–1030, 2017. doi: 10.1126/science.aam7928. [DOI] [PubMed] [Google Scholar]
- 21.Entin-Meer M, Cohen L, Hertzberg-Bigelman E, Levy R, Ben-Shoshan J, Keren G. TRPV2 knockout mice demonstrate an improved cardiac performance following myocardial infarction due to attenuated activity of peri-infarct macrophages. PLoS One 12: e0177132, 2017. doi: 10.1371/journal.pone.0177132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan D, Creemers EE, Kassiri Z. Matrix as an interstitial transport system. Circ Res 114: 889–902, 2014. doi: 10.1161/CIRCRESAHA.114.302335. [DOI] [PubMed] [Google Scholar]
- 23.Francis Stuart SD, De Jesus NM, Lindsey ML, Ripplinger CM. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J Mol Cell Cardiol 91: 114–122, 2016. doi: 10.1016/j.yjmcc.2015.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation 111: 2935–2942, 2005. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 25.Frangogiannis NG. Emerging roles for macrophages in cardiac injury: cytoprotection, repair, and regeneration. J Clin Invest 125: 2927–2930, 2015. doi: 10.1172/JCI83191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frangogiannis NG. The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127: 1600–1612, 2017. doi: 10.1172/JCI87491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangogiannis NG. Fibroblast-extracellular matrix interactions in tissue fibrosis. Curr Pathobiol Rep 4: 11–18, 2016. doi: 10.1007/s40139-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol 30: 240–245, 2015. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frangogiannis NG. Interleukin-1 in cardiac injury, repair, and remodeling: pathophysiologic and translational concepts. Discoveries (Craiova) 3: e41, 2015. doi: 10.15190/d.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr Physiol 5: 1841–1875, 2015. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 32.Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res 102: 240–248, 2014. doi: 10.1093/cvr/cvu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frodermann V, Nahrendorf M. Neutrophil-macrophage cross-talk in acute myocardial infarction. Eur Heart J 38: 198–200, 2017. doi: 10.1093/eurheartj/ehw085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghasemi O, Ma Y, Lindsey ML, Jin YF. Using systems biology approaches to understand cardiac inflammation and extracellular matrix remodeling in the setting of myocardial infarction. Wiley Interdiscip Rev Syst Biol Med 6: 77–91, 2014. doi: 10.1002/wsbm.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glezeva N, Horgan S, Baugh JA. Monocyte and macrophage subsets along the continuum to heart failure: misguided heroes or targetable villains? J Mol Cell Cardiol 89: 136–145, 2015. doi: 10.1016/j.yjmcc.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 36.González-Domínguez É, Domínguez-Soto Á, Nieto C, Flores-Sevilla JL, Pacheco-Blanco M, Campos-Peña V, Meraz-Ríos MA, Vega MA, Corbí AL, Sánchez-Torres C. Atypical activin A and IL-10 production impairs human CD16+ monocyte differentiation into anti-inflammatory macrophages. J Immunol 196: 1327–1337, 2016. doi: 10.4049/jimmunol.1501177. [DOI] [PubMed] [Google Scholar]
- 37.Goren I, Allmann N, Yogev N, Schürmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 175: 132–147, 2009. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammoud L, Lu X, Lei M, Feng Q. Deficiency in TIMP-3 increases cardiac rupture and mortality post-myocardial infarction via EGFR signaling: beneficial effects of cetuximab. Basic Res Cardiol 106: 459–471, 2011. doi: 10.1007/s00395-010-0147-7. [DOI] [PubMed] [Google Scholar]
- 39.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 108: 1827–1832, 2011. doi: 10.1073/pnas.1015623108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashidani S, Tsutsui H, Ikeuchi M, Shiomi T, Matsusaka H, Kubota T, Imanaka-Yoshida K, Itoh T, Takeshita A. Targeted deletion of MMP-2 attenuates early LV rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol 285: H1229–H1235, 2003. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 41.Hermans KC, Daskalopoulos EP, Blankesteijn WM. The Janus face of myofibroblasts in the remodeling heart. J Mol Cell Cardiol 91: 35–41, 2016. doi: 10.1016/j.yjmcc.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Honold L, Nahrendorf M. Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res 122: 113–127, 2018. doi: 10.1161/CIRCRESAHA.117.311071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages toward a reparative phenotype. Eur Heart J 38: 187–197, 2017. [DOI] [PubMed] [Google Scholar]
- 44.Hoyer FF, Nahrendorf M. Neutrophil contributions to ischaemic heart disease. Eur Heart J 38: 465–472, 2017. doi: 10.1093/eurheartj/ehx017. [DOI] [PubMed] [Google Scholar]
- 45.Huang S, Frangogiannis NG. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol 175: 1377–1400, 2018. doi: 10.1111/bph.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M. Macrophages facilitate electrical conduction in the heart. Cell 169: 510–522.e20, 2017. doi: 10.1016/j.cell.2017.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hulsmans M, Sager HB, Roh JD, Valero-Muñoz M, Houstis NE, Iwamoto Y, Sun Y, Wilson RM, Wojtkiewicz G, Tricot B, Osborne MT, Hung J, Vinegoni C, Naxerova K, Sosnovik DE, Zile MR, Bradshaw AD, Liao R, Tawakol A, Weissleder R, Rosenzweig A, Swirski FK, Sam F, Nahrendorf M. Cardiac macrophages promote diastolic dysfunction. J Exp Med 215: 423–440, 2018. doi: 10.1084/jem.20171274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol 93: 149–155, 2016. doi: 10.1016/j.yjmcc.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikonomidis JS, Hendrick JW, Parkhurst AM, Herron AR, Escobar PG, Dowdy KB, Stroud RE, Hapke E, Zile MR, Spinale FG. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: effects of exogenous MMP inhibition. Am J Physiol Heart Circ Physiol 288: H149–H158, 2005. doi: 10.1152/ajpheart.00370.2004. [DOI] [PubMed] [Google Scholar]
- 50.Ip WK, Hoshi N, Shouval DS, Snapper S, Medzhitov R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356: 513–519, 2017. doi: 10.1126/science.aal3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyer RP, de Castro Brás LE, Jin YF, Lindsey ML. Translating Koch’s postulates to identify matrix metalloproteinase roles in postmyocardial infarction remodeling: cardiac metalloproteinase actions (CarMA) postulates. Circ Res 114: 860–871, 2014. doi: 10.1161/CIRCRESAHA.114.301673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyer RP, de Castro Brás LE, Patterson NL, Bhowmick M, Flynn ER, Asher M, Cannon PL, Deleon-Pennell KY, Fields GB, Lindsey ML. Early matrix metalloproteinase-9 inhibition post-myocardial infarction worsens cardiac dysfunction by delaying inflammation resolution. J Mol Cell Cardiol 100: 109–117, 2016. doi: 10.1016/j.yjmcc.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iyer RP, Jung M, Lindsey ML. MMP-9 signaling in the left ventricle following myocardial infarction. Am J Physiol Heart Circ Physiol 311: H190–H198, 2016. doi: 10.1152/ajpheart.00243.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer RP, Jung M, Lindsey ML. Using the laws of thermodynamics to understand how matrix metalloproteinases coordinate the myocardial response to injury. Metalloproteinases Med 2: 75–82, 2015. doi: 10.2147/MNM.S74093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyer RP, Patterson NL, Fields GB, Lindsey ML. The history of matrix metalloproteinases: milestones, myths, and misperceptions. Am J Physiol Heart Circ Physiol 303: H919–H930, 2012. doi: 10.1152/ajpheart.00577.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol 112: 33, 2017. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kain V, Halade GV. Big eater macrophages dominate inflammation resolution following myocardial infarction. J Mol Cell Cardiol 87: 225–227, 2015. doi: 10.1016/j.yjmcc.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Kandalam V, Basu R, Abraham T, Wang X, Awad A, Wang W, Lopaschuk GD, Maeda N, Oudit GY, Kassiri Z. Early activation of matrix metalloproteinases underlies the exacerbated systolic and diastolic dysfunction in mice lacking TIMP3 following myocardial infarction. Am J Physiol Heart Circ Physiol 299: H1012–H1023, 2010. doi: 10.1152/ajpheart.00246.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kandalam V, Basu R, Abraham T, Wang X, Soloway PD, Jaworski DM, Oudit GY, Kassiri Z. TIMP2 deficiency accelerates adverse post-myocardial infarction remodeling because of enhanced MT1-MMP activity despite lack of MMP2 activation. Circ Res 106: 796–808, 2010. doi: 10.1161/CIRCRESAHA.109.209189. [DOI] [PubMed] [Google Scholar]
- 60.Kawano S, Kubota T, Monden Y, Tsutsumi T, Inoue T, Kawamura N, Tsutsui H, Sunagawa K. Blockade of NF-κB improves cardiac function and survival after myocardial infarction. Am J Physiol Heart Circ Physiol 291: H1337–H1344, 2006. doi: 10.1152/ajpheart.01175.2005. [DOI] [PubMed] [Google Scholar]
- 61.Kim HE, Dalal SS, Young E, Legato MJ, Weisfeldt ML, D’Armiento J. Disruption of the myocardial extracellular matrix leads to cardiac dysfunction. J Clin Invest 106: 857–866, 2000. doi: 10.1172/JCI8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King KR, Aguirre AD, Ye YX, Sun Y, Roh JD, Ng RP Jr, Kohler RH, Arlauckas SP, Iwamoto Y, Savol A, Sadreyev RI, Kelly M, Fitzgibbons TP, Fitzgerald KA, Mitchison T, Libby P, Nahrendorf M, Weissleder R. IRF3 and type I interferons fuel a fatal response to myocardial infarction. Nat Med 23: 1481–1487, 2017. doi: 10.1038/nm.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirk J, Cingolani OH. Thrombospondins in the transition from myocardial infarction to heart failure. J Mol Cell Cardiol 90: 102–110, 2016. doi: 10.1016/j.yjmcc.2015.12.009 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kytö V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285: 24487–24493, 2010. doi: 10.1074/jbc.M110.136820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krishnamurthy P, Peterson JT, Subramanian V, Singh M, Singh K. Inhibition of matrix metalloproteinases improves left ventricular function in mice lacking osteopontin after myocardial infarction. Mol Cell Biochem 322: 53–62, 2009. doi: 10.1007/s11010-008-9939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leblond AL, Klinkert K, Martin K, Turner EC, Kumar AH, Browne T, Caplice NM. Systemic and cardiac depletion of M2 macrophage through CSF-1R signaling inhibition alters cardiac function post myocardial infarction. PLoS One 10: e0137515, 2015. doi: 10.1371/journal.pone.0137515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation 103: 2181–2187, 2001. doi: 10.1161/01.CIR.103.17.2181. [DOI] [PubMed] [Google Scholar]
- 68.Lindsey ML, Bolli R, Canty JM Jr, Du XJ, Frangogiannis NG, Frantz S, Gourdie RG, Holmes JW, Jones SP, Kloner RA, Lefer DJ, Liao R, Murphy E, Ping P, Przyklenk K, Recchia FA, Schwartz Longacre L, Ripplinger CM, Van Eyk JE, Heusch G. Guidelines for experimental models of myocardial ischemia and infarction. Am J Physiol Heart Circ Physiol 314: H812–H838, 2018. doi: 10.1152/ajpheart.00335.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lindsey ML, Escobar GP, Dobrucki LW, Goshorn DK, Bouges S, Mingoia JT, McClister DM Jr, Su H, Gannon J, MacGillivray C, Lee RT, Sinusas AJ, Spinale FG. Matrix metalloproteinase-9 gene deletion facilitates angiogenesis after myocardial infarction. Am J Physiol Heart Circ Physiol 290: H232–H239, 2006. doi: 10.1152/ajpheart.00457.2005. [DOI] [PubMed] [Google Scholar]
- 70.Lindsey ML, Escobar GP, Mukherjee R, Goshorn DK, Sheats NJ, Bruce JA, Mains IM, Hendrick JK, Hewett KW, Gourdie RG, Matrisian LM, Spinale FG. Matrix metalloproteinase-7 affects connexin-43 levels, electrical conduction, and survival after myocardial infarction. Circulation 113: 2919–2928, 2006. doi: 10.1161/CIRCULATIONAHA.106.612960. [DOI] [PubMed] [Google Scholar]
- 71.Lindsey ML, Hall ME, Harmancey R, Ma Y. Adapting extracellular matrix proteomics for clinical studies on cardiac remodeling post-myocardial infarction. Clin Proteomics 13: 19, 2016. doi: 10.1186/s12014-016-9120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lindsey ML, Iyer RP, Jung M, DeLeon-Pennell KY, Ma Y. Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling. J Mol Cell Cardiol 91: 134–140, 2016. doi: 10.1016/j.yjmcc.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE. A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J Am Coll Cardiol 66: 1364–1374, 2015. doi: 10.1016/j.jacc.2015.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lindsey ML, Kassiri Z, Virag JAI, de Castro Brás LE, Scherrer-Crosbie M. Guidelines for measuring cardiac physiology in mice. Am J Physiol Heart Circ Physiol 314: H733–H752, 2018. doi: 10.1152/ajpheart.00339.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lindsey ML, Saucerman JJ, DeLeon-Pennell KY. Knowledge gaps to understanding cardiac macrophage polarization following myocardial infarction. Biochim Biophys Acta 2288–2292: 2016, 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindsey ML, Yoshioka J, MacGillivray C, Muangman S, Gannon J, Verghese A, Aikawa M, Libby P, Krane SM, Lee RT. Effect of a cleavage-resistant collagen mutation on left ventricular remodeling. Circ Res 93: 238–245, 2003. doi: 10.1161/01.RES.0000085580.45279.60. [DOI] [PubMed] [Google Scholar]
- 77.Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther 30: 31–41, 2012. doi: 10.1111/j.1755-5922.2010.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lopez EF, Kabarowski JH, Ingle KA, Kain V, Barnes S, Crossman DK, Lindsey ML, Halade GV. Obesity superimposed on aging magnifies inflammation and delays the resolving response after myocardial infarction. Am J Physiol Heart Circ Physiol 308: H269–H280, 2015. doi: 10.1152/ajpheart.00604.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, Müller O, Vergely C, Zeller M, Tardivel A, Schneider P, Pacher P, Liaudet L. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. J Immunol 194: 499–503, 2015. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luther DJ, Thodeti CK, Shamhart PE, Adapala RK, Hodnichak C, Weihrauch D, Bonaldo P, Chilian WM, Meszaros JG. Absence of type VI collagen paradoxically improves cardiac function, structure, and remodeling after myocardial infarction. Circ Res 110: 851–856, 2012. doi: 10.1161/CIRCRESAHA.111.252734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma Y, de Castro Brás LE, Toba H, Iyer RP, Hall ME, Winniford MD, Lange RA, Tyagi SC, Lindsey ML. Myofibroblasts and the extracellular matrix network in post-myocardial infarction cardiac remodeling. Pflugers Arch 466: 1113–1127, 2014. doi: 10.1007/s00424-014-1463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma Y, Halade GV, Zhang J, Ramirez TA, Levin D, Voorhees A, Jin YF, Han HC, Manicone AM, Lindsey ML. Matrix metalloproteinase-28 deletion exacerbates cardiac dysfunction and rupture after myocardial infarction in mice by inhibiting M2 macrophage activation. Circ Res 112: 675–688, 2013. doi: 10.1161/CIRCRESAHA.111.300502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML. Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38: 448–458, 2017. doi: 10.1016/j.tips.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res 191: 15–28, 2018. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma Y, Yabluchanskiy A, Iyer RP, Cannon PL, Flynn ER, Jung M, Henry J, Cates CA, Deleon-Pennell KY, Lindsey ML. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 110: 51–61, 2016. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma Y, Yabluchanskiy A, Lindsey ML. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis Tissue Repair 6: 11, 2013. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Majmudar MD, Keliher EJ, Heidt T, Leuschner F, Truelove J, Sena BF, Gorbatov R, Iwamoto Y, Dutta P, Wojtkiewicz G, Courties G, Sebas M, Borodovsky A, Fitzgerald K, Nolte MW, Dickneite G, Chen JW, Anderson DG, Swirski FK, Weissleder R, Nahrendorf M. Monocyte-directed RNAi targeting CCR2 improves infarct healing in atherosclerosis-prone mice. Circulation 127: 2038–2046, 2013. doi: 10.1161/CIRCULATIONAHA.112.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, Nguyen N, Jin YF, Bradshaw AD, Lindsey ML. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 301: H497–H505, 2011. doi: 10.1152/ajpheart.01070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meschiari CA, Ero OK, Pan H, Finkel T, Lindsey ML. The impact of aging on cardiac extracellular matrix. Geroscience 39: 7–18, 2017. doi: 10.1007/s11357-017-9959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meschiari CA, Jung M, Iyer RP, Yabluchanskiy A, Toba H, Garrett MR, Lindsey ML. Macrophage overexpression of matrix metalloproteinase-9 in aged mice improves diastolic physiology and cardiac wound healing after myocardial infarction. Am J Physiol Heart Circ Physiol 314: H224–H235, 2018. doi: 10.1152/ajpheart.00453.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagalingam RS, Safi HA, Czubryt MP. Gaining myocytes or losing fibroblasts: Challenges in cardiac fibroblast reprogramming for infarct repair. J Mol Cell Cardiol 93: 108–114, 2016. doi: 10.1016/j.yjmcc.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 92.Nahrendorf M, Frantz S, Swirski FK, Mulder WJ, Randolph G, Ertl G, Ntziachristos V, Piek JJ, Stroes ES, Schwaiger M, Mann DL, Fayad ZA. Imaging systemic inflammatory networks in ischemic heart disease. J Am Coll Cardiol 65: 1583–1591, 2015. doi: 10.1016/j.jacc.2015.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nahrendorf M, Swirski FK. Abandoning M1/M2 for a network model of macrophage function. Circ Res 119: 414–417, 2016. doi: 10.1161/CIRCRESAHA.116.309194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, Golenbock D, Gresnigt MS, Heneka MT, Hoffman HM, Hotchkiss R, Joosten LA, Kastner DL, Korte M, Latz E, Libby P, Mandrup-Poulsen T, Mantovani A, Mills KH, Nowak KL, O’Neill LA, Pickkers P, van der Poll T, Ridker PM, Schalkwijk J, Schwartz DA, Siegmund B, Steer CJ, Tilg H, van der Meer JW, van de Veerdonk FL, Dinarello CA. A guiding map for inflammation. Nat Immunol 18: 826–831, 2017. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nielsen SH, Mouton AJ, DeLeon-Pennell KY, Genovese F, Karsdal M, Lindsey ML. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol pii: S0945-053X(17)30268-8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nishikido T, Oyama J, Shiraki A, Komoda H, Node K. Deletion of apoptosis inhibitor of macrophage (AIM)/CD5L attenuates the inflammatory response and infarct size in acute myocardial infarction. J Am Heart Assoc 5: e002863, 2016. doi: 10.1161/JAHA.115.002863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oyama J, Blais C Jr, Liu X, Pu M, Kobzik L, Kelly RA, Bourcier T. Reduced myocardial ischemia-reperfusion injury in toll-like receptor 4-deficient mice. Circulation 109: 784–789, 2004. doi: 10.1161/01.CIR.0000112575.66565.84. [DOI] [PubMed] [Google Scholar]
- 98.Palevski D, Levin-Kotler LP, Kain D, Naftali-Shani N, Landa N, Ben-Mordechai T, Konfino T, Holbova R, Molotski N, Rosin-Arbesfeld R, Lang RA, Leor J. Loss of macrophage Wnt secretion improves remodeling and function after myocardial infarction in mice. J Am Heart Assoc 6: 6, 2017. doi: 10.1161/JAHA.116.004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Prabhu SD, Frangogiannis NG. The biological basis for cardiac repair after myocardial infarction: from inflammation to fibrosis. Circ Res 119: 91–112, 2016. doi: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ, Kastelein J, Koenig W, Genest J, Lorenzatti A, Varigos J, Siostrzonek P, Sinnaeve P, Fonseca F, Nicolau J, Gotcheva N, Yong H, Urina-Triana M, Milicic D, Cifkova R, Vettus R, Anker SD, Manolis AJ, Wyss F, Forster T, Sigurdsson A, Pais P, Fucili A, Ogawa H, Shimokawa H, Veze I, Petrauskiene B, Salvador L, Cornel JH, Klemsdal TO, Medina F, Budaj A, Vida-Simiti L, Kobalava Z, Otasevic P, Pella D, Lainscak M, Seung KB, Commerford P, Dellborg M, Donath M, Hwang JJ, Kultursay H, Flather M, Ballantyne C, Bilazarian S, Chang W, East C, Forgosh L, Harris B, Ligueros M; CANTOS Trial Group . Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet 391: 319–328, 2018. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 101.Sager HB, Heidt T, Hulsmans M, Dutta P, Courties G, Sebas M, Wojtkiewicz GR, Tricot B, Iwamoto Y, Sun Y, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Targeting interleukin-1β reduces leukocyte production after acute myocardial infarction. Circulation 132: 1880–1890, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sager HB, Hulsmans M, Lavine KJ, Moreira MB, Heidt T, Courties G, Sun Y, Iwamoto Y, Tricot B, Khan OF, Dahlman JE, Borodovsky A, Fitzgerald K, Anderson DG, Weissleder R, Libby P, Swirski FK, Nahrendorf M. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 119: 853–864, 2016. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res 167: 152–166, 2016. doi: 10.1016/j.trsl.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 206: 113–123, 2009. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Scruggs SB, Watson K, Su AI, Hermjakob H, Yates JR III, Lindsey ML, Ping P. Harnessing the heart of big data. Circ Res 116: 1115–1119, 2015. doi: 10.1161/CIRCRESAHA.115.306013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol 70: 74–82, 2014. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shinde AV, Frangogiannis NG. Mechanisms of fibroblast activation in the remodeling myocardium. Curr Pathobiol Rep 5: 145–152, 2017. doi: 10.1007/s40139-017-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shintani Y, Ito T, Fields L, Shiraishi M, Ichihara Y, Sato N, Podaru M, Kainuma S, Tanaka H, Suzuki K. IL-4 as a repurposed biological drug for myocardial infarction through augmentation of reparative cardiac macrophages: proof-of-concept data in mice. Sci Rep 7: 6877, 2017. doi: 10.1038/s41598-017-07328-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 17: 248–261, 2017. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 110.Spinale FG, Frangogiannis NG, Hinz B, Holmes JW, Kassiri Z, Lindsey ML. Crossing into the next frontier of cardiac extracellular matrix research. Circ Res 119: 1040–1045, 2016. doi: 10.1161/CIRCRESAHA.116.309916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spinale FG, Villarreal F. Targeting matrix metalloproteinases in heart disease: lessons from endogenous inhibitors. Biochem Pharmacol 90: 7–15, 2014. doi: 10.1016/j.bcp.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339: 161–166, 2013. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takawale A, Zhang P, Azad A, Wang W, Wang X, Murray AG, Kassiri Z. Myocardial overexpression of TIMP3 after myocardial infarction exerts beneficial effects by promoting angiogenesis and suppressing early proteolysis. Am J Physiol Heart Circ Physiol 313: H224–H236, 2017. doi: 10.1152/ajpheart.00108.2017. [DOI] [PubMed] [Google Scholar]
- 114.Tang J, Shen Y, Chen G, Wan Q, Wang K, Zhang J, Qin J, Liu G, Zuo S, Tao B, Yu Y, Wang J, Lazarus M, Yu Y. Activation of E-prostanoid 3 receptor in macrophages facilitates cardiac healing after myocardial infarction. Nat Commun 8: 14656, 2017. doi: 10.1038/ncomms14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian H, Cimini M, Fedak PW, Altamentova S, Fazel S, Huang ML, Weisel RD, Li RK. TIMP-3 deficiency accelerates cardiac remodeling after myocardial infarction. J Mol Cell Cardiol 43: 733–743, 2007. doi: 10.1016/j.yjmcc.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Toba H, Cannon PL, Yabluchanskiy A, Iyer RP, D’Armiento J, Lindsey ML. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am J Physiol Heart Circ Physiol 312: H375–H383, 2017. doi: 10.1152/ajpheart.00633.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res 88: 1080–1087, 2001. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 118.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair 6: 5, 2013. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ulrich MM, Janssen AM, Daemen MJ, Rappaport L, Samuel JL, Contard F, Smits JF, Cleutjens JP. Increased expression of fibronectin isoforms after myocardial infarction in rats. J Mol Cell Cardiol 29: 2533–2543, 1997. doi: 10.1006/jmcc.1997.0486. [DOI] [PubMed] [Google Scholar]
- 120.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170: 818–829, 2007. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.van Nieuwenhoven FA, Turner NA. The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul Pharmacol 58: 182–188, 2013. doi: 10.1016/j.vph.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Voorhees AP, DeLeon-Pennell KY, Ma Y, Halade GV, Yabluchanskiy A, Iyer RP, Flynn E, Cates CA, Lindsey ML, Han HC. Building a better infarct: modulation of collagen cross-linking to increase infarct stiffness and reduce left ventricular dilation post-myocardial infarction. J Mol Cell Cardiol 85: 229–239, 2015. doi: 10.1016/j.yjmcc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Voorhees AP, Han HC. A model to determine the effect of collagen fiber alignment on heart function post myocardial infarction. Theor Biol Med Model 11: 6, 2014. doi: 10.1186/1742-4682-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Westman PC, Lipinski MJ, Luger D, Waksman R, Bonow RO, Wu E, Epstein SE. Inflammation as a driver of adverse left ventricular remodeling after acute myocardial infarction. J Am Coll Cardiol 67: 2050–2060, 2016. doi: 10.1016/j.jacc.2016.01.073. [DOI] [PubMed] [Google Scholar]
- 125.Wu RX, Laser M, Han H, Varadarajulu J, Schuh K, Hallhuber M, Hu K, Ertl G, Hauck CR, Ritter O. Fibroblast migration after myocardial infarction is regulated by transient SPARC expression. J Mol Med (Berl) 84: 241–252, 2006. doi: 10.1007/s00109-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 126.Yabluchanskiy A, Li Y, Chilton RJ, Lindsey ML. Matrix metalloproteinases: drug targets for myocardial infarction. Curr Drug Targets 14: 276–286, 2013. [PMC free article] [PubMed] [Google Scholar]
- 127.Yabluchanskiy A, Ma Y, Chiao YA, Lopez EF, Voorhees AP, Toba H, Hall ME, Han HC, Lindsey ML, Jin YF. Cardiac aging is initiated by matrix metalloproteinase-9-mediated endothelial dysfunction. Am J Physiol Heart Circ Physiol 306: H1398–H1407, 2014. doi: 10.1152/ajpheart.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yabluchanskiy A, Ma Y, DeLeon-Pennell KY, Altara R, Halade GV, Voorhees AP, Nguyen NT, Jin YF, Winniford MD, Hall ME, Han HC, Lindsey ML. Myocardial infarction superimposed on aging: MMP-9 deletion promotes M2 macrophage polarization. J Gerontol A Biol Sci Med Sci 71: 475–483, 2016. doi: 10.1093/gerona/glv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zamilpa R, Chiao YA, Dai Q, Bansal A, Lindsey ML. Cardiac fibroblast functions following myocardial infarction: cause and effect roles of MMPs and TIMPs. In: The Cardiac Fibroblast, edited by Turner NA. Kerala, India: Research Signpost, 2011, p. 75–90. [Google Scholar]
- 130.Zamilpa R, Ibarra J, de Castro Brás LE, Ramirez TA, Nguyen N, Halade GV, Zhang J, Dai Q, Dayah T, Chiao YA, Lowell W, Ahuja SS, D’Armiento J, Jin YF, Lindsey ML. Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. J Mol Cell Cardiol 53: 599–608, 2012. doi: 10.1016/j.yjmcc.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zamilpa R, Kanakia R, Cigarroa J IV, Dai Q, Escobar GP, Martinez H, Jimenez F, Ahuja SS, Lindsey ML. CC chemokine receptor 5 deletion impairs macrophage activation and induces adverse remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 300: H1418–H1426, 2011. doi: 10.1152/ajpheart.01002.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zamilpa R, Lopez EF, Chiao YA, Dai Q, Escobar GP, Hakala K, Weintraub ST, Lindsey ML. Proteomic analysis identifies in vivo candidate matrix metalloproteinase-9 substrates in the left ventricle post-myocardial infarction. Proteomics 10: 2214–2223, 2010. doi: 10.1002/pmic.200900587. [DOI] [PMC free article] [PubMed] [Google Scholar]