Abstract
Ataxia telangiectasia mutated kinase (ATM) is activated in response to DNA damage. We have previously shown that ATM plays a critical role in myocyte apoptosis and cardiac remodeling after myocardial infarction (MI). Here, we tested the hypothesis that ATM deficiency results in autophagic impairment in the heart early during MI. MI was induced in wild-type (WT) and ATM heterozygous knockout (hKO) mice by ligation of the left anterior descending artery. Structural and biochemical parameters of the heart were measured 4 h after left anterior descending artery ligation. M-mode echocardiography revealed that MI worsens heart function, as evidenced by reduced percent ejection fraction and fractional shortening in both groups. However, MI-induced increase in left ventricular end-diastolic and end-systolic diameters and volumes were significantly lower in hKO hearts. ATM deficiency resulted in autophagic impairment during MI, as evidenced by decreased microtubule-associated protein light chain 3-II increased p62, decreased cathepsin D protein levels, and increased aggresome accumulation. ERK1/2 activation was only observed in WT-MI hearts. Activation of Akt and AMP-activated protein kinase (AMPK) was lower, whereas activation of glycogen synthase kinase (GSK)-3β and mammalian target of rapamycin (mTOR) was higher in hKO-MI hearts. Inhibition of ATM using KU-55933 resulted in autophagic impairment in cardiac fibroblasts, as evidenced by decreased light chain 3-II protein levels and formation of acidic vesicular organelles. This impairment was associated with decreased activation of Akt and AMPK but enhanced activation of GSK-3β and mTOR in KU-55933-treated fibroblasts. Thus, ATM deficiency results in autophagic impairment in the heart during MI and cardiac fibroblasts. This autophagic impairment may occur via the activation of GSK-3β and mTOR and inactivation of Akt and AMPK.
NEW & NOTEWORTHY Ataxia telangiectasia mutated kinase (ATM) plays a critical role in myocyte apoptosis and cardiac remodeling after myocardial infarction (MI). Here, we provide evidence that ATM deficiency results in autophagic impairment during MI. Further investigation of the role of ATM in autophagy post-MI may provide novel therapeutic targets for patients with ataxia telangiectasia suffering from heart disease.
Keywords: ataxia telangiectasia mutated kinase, autophagy, heart, myocardial infarction
INTRODUCTION
Heart failure is the leading cause of death worldwide. Cardiovascular disease accounted for ∼17.3 million deaths of 54 million total deaths worldwide in 2013. Despite having medications to manage the complications of heart failure, there is currently no treatment to alleviate the condition (18). Coronary artery disease (CAD) is the most common form of heart disease, with myocardial infarction (MI) as a serious outcome. Treatment of CAD and its progression to heart failure remains a challenging area of research (17).
Ataxia telangiectasia mutated kinase (ATM) is a ∼370-kDa serine/threonine kinase that resides primarily in the nucleus (19, 20, 40, 44, 45, 54). The main function of ATM is to control cell cycle progression after DNA damage, particularly double-strand breaks (1, 36). In response to DNA damage, ATM is activated and recruited to DNA double-strand breaks. Although ATM has mostly been reported as a nuclear protein involved in signaling pathways that control DNA damage recognition, it is also located in the cytoplasm, where it plays a role in several metabolic pathways (50, 54). Mutations in ATM cause a multisystemic disease known as ataxia telangiectasia (AT) (20, 40, 54). AT heterozygotes, individuals with an ATM mutation in one allele, make up a substantial portion of the population (∼1.4−2%) (25, 30). The incidence of AT is significantly higher in consanguineous populations (6). Although AT heterozygotes are spared from most of the symptoms of AT, they are more susceptible to ischemic heart disease (25, 30).
Macroautophagy (hereafter called autophagy) is a conserved physiological process in the body that ultimately results in the packaging of cytoplasmic components into autophagosomes that fuse to lysosomes for degradation (5, 23, 29, 37). Autophagy consist of three main phases: 1) induction and phagophore formation, 2) phagophore elongation and autophagosome formation, and 3) lysosomal fusion, degradation, and recycling (38). Autophagy is critical to cellular homeostasis under normal and stressful conditions and plays a crucial role in the development of diseases such as cancer and heart failure (5, 23, 37). In fact, autophagy has been suggested to play a significant role in cardiac remodeling during MI. Studies have shown that autophagy promotes the survival of cardiac myocytes, reduces infarct size, and attenuates adverse cardiac remodeling post-MI (24, 52). Moreover, defects in autophagy have been linked to cardiac dysfunction and heart failure (22). Thus, autophagy is considered cardioprotective and a potential therapeutic target for the treatment of heart disease.
Autophagic changes occur in mouse heart as early as 30 min after ligation of the left anterior descending coronary artery (LAD). The microtubule-associated protein light chain (LC)3-II-to-LC3-I ratio, an established indicator of autophagic turnover, peaks 4 h after LAD ligation (24), suggesting that autophagic activity increases in the ischemic heart during the early stages of MI. Previously, we have shown that ATM plays a critical role in myocyte apoptosis and cardiac remodeling after β-adrenergic receptor stimulation and MI (10, 11, 14–16). Here, we tested the hypothesis that deficiency of ATM impairs autophagic response in the heart early during MI (4 h after its onset). The data presented here suggest that ATM deficiency impairs autophagy in the heart during MI via the activation of glycogen synthase kinase (GSK)-3β and mammalian target of rapamycin (mTOR) and inactivation of Akt and AMP-activated protein kinase (AMPK).
MATERIALS AND METHODS
Vertebrate animals.
This investigation conformed with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996). All experiments were performed in accordance with protocols approved by the East Tennessee State University Committee on Animal Care. ATM-deficient mice (129 × black Swiss hybrid background), generated as previously described (4), were purchased from The Jackson Laboratory. Genotyping was performed by PCR using primers suggested by The Jackson Laboratory. Age-matched (∼4 mo old) male and female mice were used for the study. Since homozygous knockout mice die at ∼2 mo of age due to thymic lymphomas (4), the present study used ATM heterozygous knockout (hKO; deficient) mice. All mice undergoing surgery [sham operation (sham) and MI] received buprenorphine injections before surgery.
Myocardial infarction.
MI was performed as previously described (10). Briefly, mice were anesthetized with a mixture of isoflurane (2%) and oxygen (0.5 l/min) inhalation and ventilated using a rodent ventilator (Harvard Apparatus). Body temperature was maintained at ∼37°C using a heating pad. The heart was exposed by a left thoracotomy. The LAD was ligated using 7-0 polypropylene suture. Sham mice underwent the same procedure without ligation of the LAD. At the end of the study period (4 h after LAD ligation), isolated hearts were used for either histology or molecular analyses.
Echocardiography.
M-mode echocardiography images were obtained using transthoracic short-axis view imaging at the midpapillary level after 4 h of MI using a Vevo 1100 machine and with a 550D probe. M-mode tracings were used to calculate percent fractional shortening (%FS) and percent ejection fraction (%EF) and measure heart rate, left ventricular (LV) wall thicknesses, LV end-systolic diameter (LVESD), LV end-diastolic diameter (LVEDD), LV end-systolic volume (LVESV), and LV end-diastolic volume (LVEDV). An individual blinded to the experimental groups recorded the images, whereas a second individual assessed the images and calculated the structural and functional parameters of the heart.
Aggresome detection by histological staining.
Paraffin-embedded heart sections were deparaffinized and rehydrated before staining. Tissue sections were immersed in xylene to remove paraffin. Subsequently, sections were rehydrated with decreasing ethanol concentrations (100%, 90%, and 80%). Sections were stained using Proteostat dye (2,000× dilution in PBS from the Proteostat Aggresomes Detection Kit, Enzo) for 3 min. Tissue sections were then washed in deionized water and destained using 1% acetic acid for 20 min. After being washed with deionized water and PBS, tissue sections were mounted using antifade mounting media and visualized using an EVOS FL Autofluorescence microscope (ThermoFisher Scientific). Images were acquired using a ×20 objective. The number of aggresomes were quantified in the entire LV area.
Western blot analysis.
LVs or cell lysates were prepared in RIPA buffer [10 mM Tris·HCl (pH 7.2), 158 mM NaCl, 1 mM EGTA, 0.1% SDS, 1% sodium deoxycholate, 1% Triton X-100, 1 mM sodium orthovanadate, and 0.2 mM phenylmethylsulfonyl fluoride] supplemented with protease inhibitors. Equal amounts of proteins (25 μg) were resolved using SDS-PAGE. Proteins were then transferred to a PVDF membrane. The membrane was blocked for 1 h using 5% nonfat dry milk and incubated overnight with primary antibodies against LC3B (1:1,000), p62 (1:1,000), phosphoraylated (p-)Akt (Ser473, 1:1,000), p-GSK-3β (Ser9, 1:1,000), p-mTOR (Ser2448, 1:1,000), p-ERK1/2 (1:1,000, Cell Signaling Technology), cathepsin D (1:1,000), or p-AMPK (Thr172, 1:1,000, Santa Cruz Biotechnology). Immune complexes were detected using the appropriate secondary antibodies and chemiluminescent reagents. Protein signals were visualized using ImageQuant LAS 500 imager and quantified using ImageQuant TL software (GE). Equal protein loading for phosphoproteins was verified by reprobing/probing the membranes using anti-Akt, anti-GSK-3β, anti-AMPK, anti-mTOR, anti-ERK1/2, and anti-GAPDH antibodies. Quantitative analyses showed no differences in total protein levels for these proteins among the groups. Therefore, all Western blot data are normalized using GAPDH as a loading control.
Fibroblast isolation and treatment.
Adult rat cardiac fibroblasts were isolated as previously described (53). Cells were grown to confluence in DMEM supplemented with 10% heat-inactivated FBS and serum starved for 5 h before use. Cells were then treated with the ATM inhibitor KU-55933 (KU; 100 μM, Tocris) for 4 h. Cells were maintained in serum-free DMEM for the duration of the treatment.
Evaluation of acidic vesicular organelles by acridine orange.
At the end of the study period, fibroblasts treated with KU for 4 h were incubated with 1 μg/ml acridine orange (Sigma) in serum-free DMEM for 15 min. Cells were then washed three times using PBS and observed using the EVOS FL Autofluorescence microscope (ThermoFisher Scientific). All images were acquired at ×20 magnification. The occurrence of acidic vesicular organelles (AVOs; orange fluorescence) was measured in 15 randomly chosen fields/dish for each experiment using NIS-Elements image analysis software (Nikon).
Statistical analysis.
Data are expressed as means ± SE. Data were analyzed using Student’s t-test or two-way ANOVA followed by the Student-Newman-Keuls test. P values of <0.05 were considered to be significant.
RESULTS
Echocardiographic measurements.
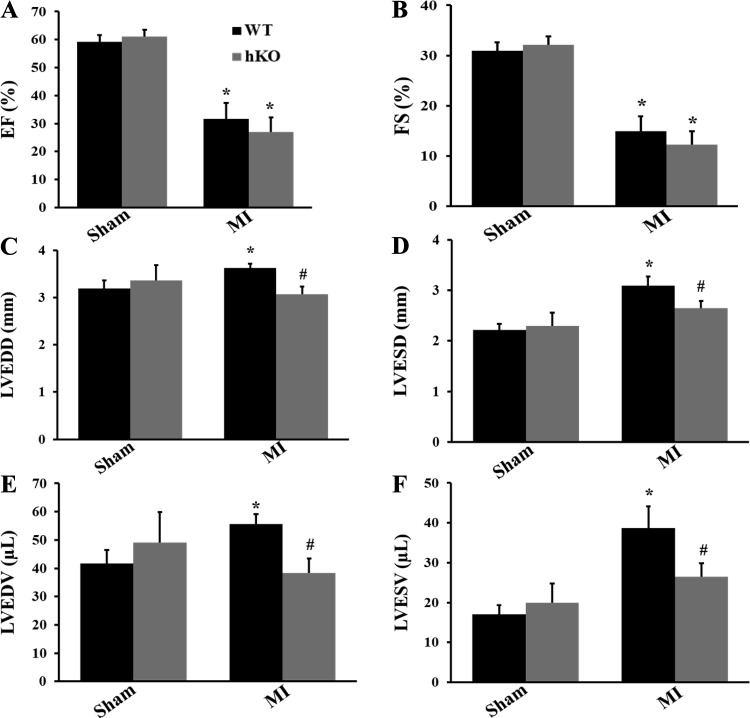
M-mode echocardiography showed no difference in echocardiographic parameters between the two sham groups. MI significantly decreased %EF (WT-sham group: 59.26 ± 2.39, hKO-sham group: 61.09 ± 2.43, WT-MI group: 31.59 ± 5.75, and hKO-MI group: 26.95 ± 5.16, P < 0.05 vs. the respective sham group, n = 5) and %FS (WT-sham group: 30.96 ± 1.66, hKO-sham group: 32.17 ± 1.69, WT-MI group: 14.95 ± 2.97, and hKO-MI group: 12.29 ± 2.69, P < 0.05 vs. the respective sham group; n = 5) in both groups compared with their respective sham groups. However, there was no significant difference in %EF and %FS between the two genotypes post-MI (Fig. 1, A and B). MI increased LVEDD (WT-sham group: 3.19 ± 0.17, hKO-sham group: 3.36 ± 0.33, and WT-MI group: 3.62 ± 0.10, P < 0.05 vs. the WT-sham group; hKO-MI group: 3.07 ± 0.16, P < 0.05 vs. the WT-MI group; n = 5), LVESD (WT-sham group: 2.21 ± 0.13, hKO-sham group: 2.30 ± 0.26, and WT-MI group: 3.09 ± 0.18, P < 0.05 vs. the WT-sham group; hKO-MI group: 2.65 ± 0.14, P < 0.05 vs. the WT-MI group; n = 5), LVEDV (WT-sham group: 41.70 ± 4.76, hKO-sham group: 49.10 ± 10.67, and WT-MI group: 55.67 ± 3.47, P < 0.05 vs. the WT-sham group; hKO-MI group: 38.41 ± 5.02, P < 0.05 vs. the WT-MI group; n = 5), and LVESV (WT-sham group: 17.06 ± 2.30, hKO-sham group: 19.90 ± 4.85, and WT-MI group: 36.68 ± 5.41, P < 0.05 vs. the WT-sham group; hKO-MI group: 26.44 ± 3.42, P < 0.05 vs. the WT-MI group; n = 5) in WT groups compared with the WT-sham group. However, there was no significant increase in LVEDD, LVESD, LVEDV, and LVESV in the hKO-MI group versus the hKO-sham group. In fact, these parameters were significantly lower in the hKO-MI group versus the WT-MI group (Fig. 1, C–F). Heart rates and posterior and septal wall thicknesses were not found to be significantly different between the two MI groups (data not shown).
Fig. 1.
Echocardiographic analysis of heart function. Myocardial infarction (MI) was performed in wild-type (WT) and heterozygous knockout (hKO) mice. Indexes of cardiac function, percent ejection fraction (%EF; A), percent fractional shortening (%FS; B), left ventricular (LV) end-diastolic diameter (LVEDD; C), LV end-systolic diameter (LVESD; D), LV end-diastolic volume (LVEDV; E), and LV end-systolic volume (LVESV; F) were calculated using echocardiographic images 4 h after left anterior descending coronary artery ligation. n = 5. *P < 0.05 vs. the respective sham group; #P < 0.05 vs. the WT-MI group.
Expression of autophagy-related proteins in cardiac tissue.
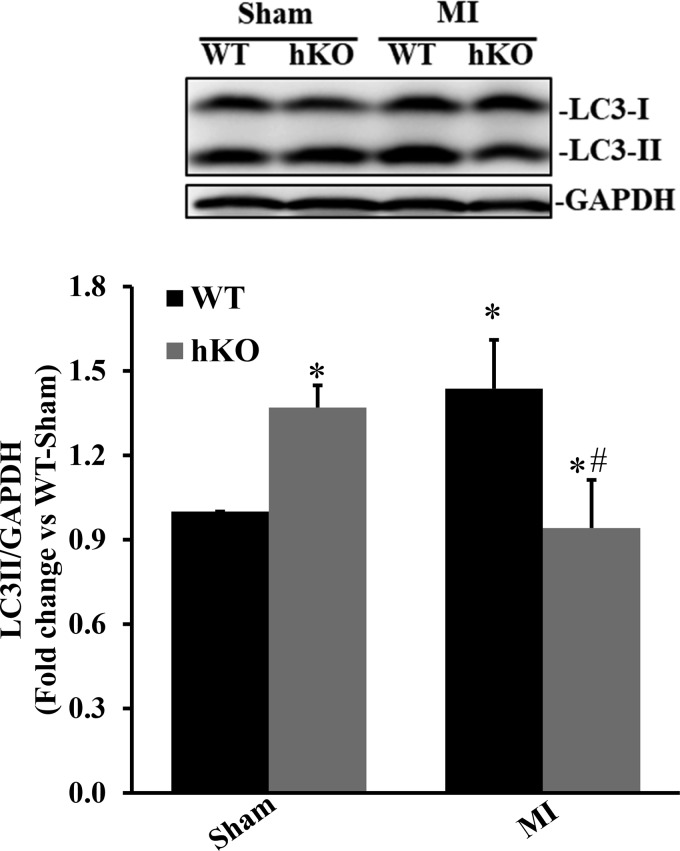
LC3 is considered one of the most important autophagy markers since it is essential to autophagosome formation (39). In mammalian cells, LC3 is processed into LC3-I, a soluble form of LC3. LC3-I is then modified to LC3-II, a membrane-bound form of LC3, by the addition of phosphatidylethanolamine. To date, LC3-II is the only well-characterized protein that is localized specifically to autophagosomes throughout the autophagy process from autophagosome formation to lysosomal degradation. Thus, measuring the levels of LC3-II protein is suggested to be a good marker for early autophagosome formation (39). Western blot analysis of LV lysates using an anti-LC3 antibody revealed a significant increase in LC3-II protein levels in the hKO-sham group versus the WT-sham group (Fig. 2). LC3-II protein levels were significantly increased in the WT-MI group compared with the WT-sham group. In contrast, LC3-II protein levels were significantly lower in the hKO-MI group compared with the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 4–7; Fig. 2).
Fig. 2.
Ataxia telangiectasia mutated kinase deficiency reduces microtubule-associated protein light chain (LC)3-II protein levels during 4 h of myocardial infarction (MI). Total left ventricular lysates, prepared from sham and MI hearts, were analyzed by Western blot analysis using LC3B antibodies. Top: Western blot exhibiting immunostaining for LC3-I, LC3-II, and GAPDH. Bottom: quantitative analysis of LC3-II normalized to GAPDH. WT, wild-type; hKO, heterozygous knockout. n = 4−7. *P < 0.05 vs. the respective sham group; #P < 0.05 vs. the WT-MI group.
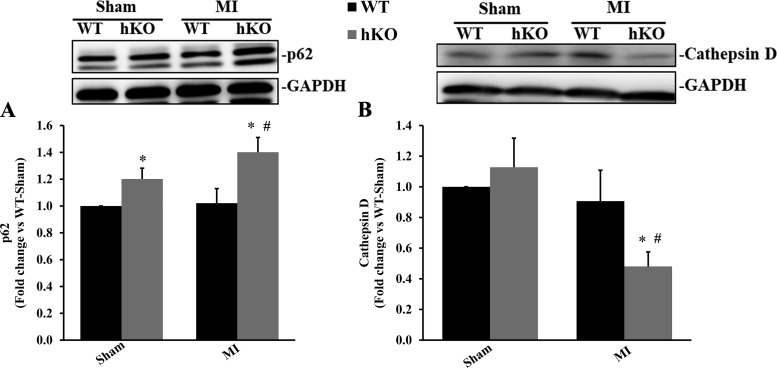
p62 is an autophagy receptor, meaning that it recognizes and transports misfolded proteins to the autophagosome for degradation by lysosomes (33). Cathepsin D, a lysosomal protease, is involved in lysosomal degradation of misfolded proteins (49). Both p62 and cathepsin D are critical to autophagic clearance or the degradation of autophagolysosomes. p62 protein levels were significantly higher in the hKO-sham group compared with its WT counterpart (Fig. 3A). p62 protein levels remained unchanged in the WT-MI group. However, p62 protein levels were significantly greater in the hKO-MI group versus the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 5–7; Fig. 3A). Cathepsin D protein levels were not significantly different between the two sham groups. Cathepsin D protein levels remained unchanged in the WT-MI group. However, MI led to a significant decrease in cathepsin D levels in the hKO group versus the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 4–5; Fig. 3B).
Fig. 3.
Ataxia telangiectasia mutated kinase deficiency increases p62 protein levels and decreases cathepsin D protein levels during 4 h of myocardial infarction (MI). Total left ventricular lysates, prepared from sham and MI hearts, were analyzed by Western blot analysis using anti-p62 (A) and anti-cathepsin D (B) antibodies. Both p62 bands were analyzed. A and B, top: Western blots exhibiting immunostaining for p62, cathepsin D, and GAPDH. A and B, bottom: quantitative analyses of p62 and cathepsin D protein levels normalized to GAPDH. WT, wild-type; hKO, heterozygous knockout. n = 4−7. *P < 0.05 vs. the respective sham group; #P < 0.05 vs. the WT-MI group.
Activation of signaling molecules related to autophagy.
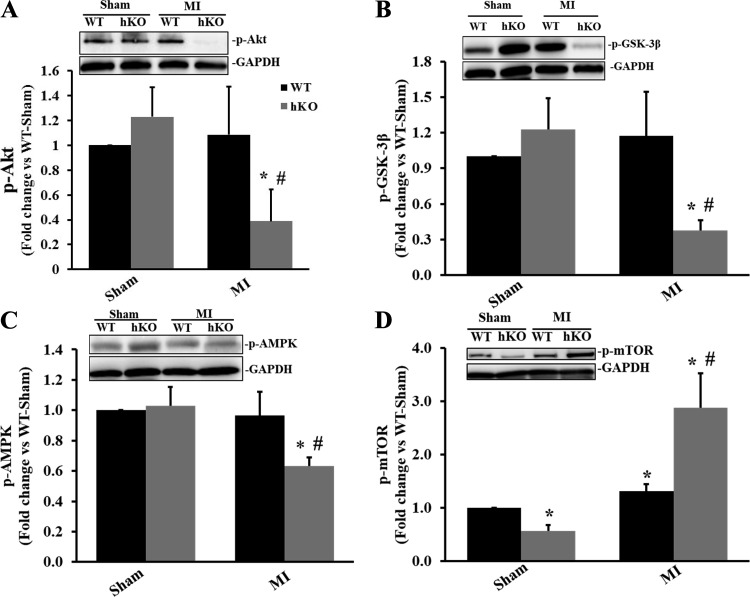
Typically, Akt signaling acts as a positive regulator of autophagy (47). However, there are several downstream targets of Akt, such as GSK-3β, AMPK, and mTOR, that can act to either upregulate or downregulate the autophagy pathway. Inactivation (phosphorylation) of GSK-3β induces autophagy (7, 35, 51), whereas activation (phosphorylation) of AMPK stimulates autophagy (27) and activation (phosphorylation) of mTOR inhibits autophagy (27, 28). Phosphorylation of Akt remained unchanged in the sham groups and in the WT group post-MI. However, phosphorylation of Akt was significantly lower in the hKO-MI group compared with the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 5–7; Fig. 4A). Akt acts as an upstream regulator of GSK-3β, phosphorylating it at Ser9 and inactivating it (13). Phosphorylation of GSK-3β was significantly lower in the hKO-MI group compared with the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 5–7; Fig. 4B). AMPK phosphorylation was significantly lower in the hKO-MI group compared with the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 5–7; Fig. 4C). mTOR phosphorylation was significantly lower in the hKO-sham group versus the WT-sham group. MI significantly increased mTOR phosphorylation in both genotypes versus their respective sham groups. However, phosphorylation of mTOR was significantly greater in the hKO-MI group compared with the WT-MI group (P < 0.05 vs. the respective sham group and P < 0.05 vs. the WT-MI group, n = 4–5; Fig. 4D).
Fig. 4.
Ataxia telangiectasia mutated kinase deficiency affects the activation of Akt, glycogen synthase kinase (GSK)-3β, AMP-activated protein kinase (AMPK), and mammalian target of rapamycin (mTOR) during 4 h of myocardial infarction (MI). Total left ventricular lysates, prepared from sham and MI hearts, were analyzed by Western blot analysis using anti-phosphorylated (p)-Akt (A), anti-p-GSK-3β (B), anti-p-AMPK (C), and anti-p-mTOR (D) antibodies. A–D, top: Western blots exhibiting immunostaining for p-Akt, p-GSK-3β, p-AMPK, p-mTOR, and GAPDH. A–D, bottom: quantitative analyses of p-Akt, p-GSK-3β, p-AMPK, and p-mTOR normalized to GAPDH. WT, wild-type; hKO, heterozygous knockout. n = 4−7. *P < 0.05 vs. the respective sham group; #P < 0.05 vs. the WT-MI group.
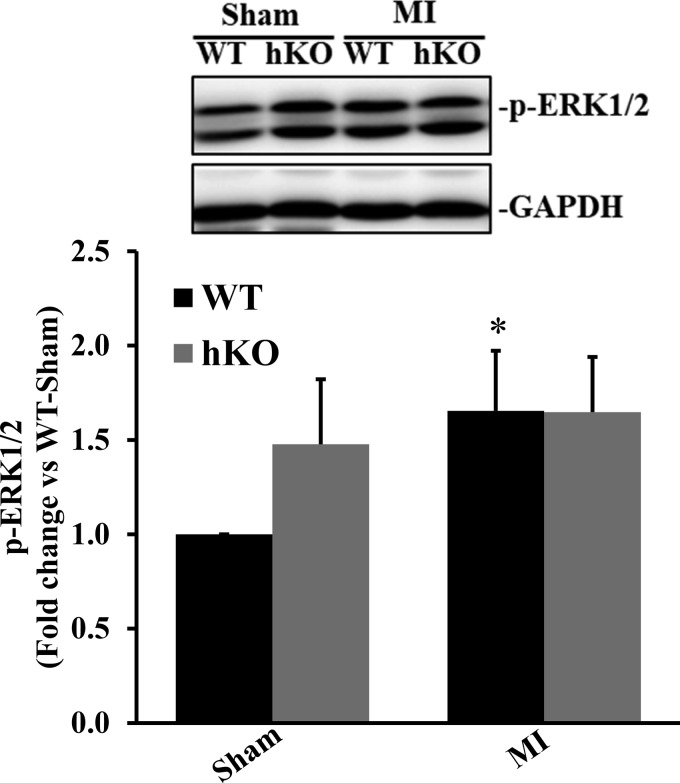
ERK1/2 activation acts as a positive regulator of autophagy (47). ERK1/2 phosphorylation was not significantly different between the two sham groups. ERK1/2 phosphorylation was significantly higher in the WT-MI group compared with the WT-sham group (P < 0.05, n = 7; Fig. 5). It remained unchanged between the hKO-sham and hKO-MI groups.
Fig. 5.
ERK1/2 activation during 4 h of myocardial infarction (MI). Total left ventricular lysates, prepared from sham and MI hearts, were analyzed by Western blot analysis using phospho-specific ERK1/2 antibodies. Top: Western blot exhibiting immunostaining for phosphorylated (p-)ERK1/2 and GAPDH. Bottom: quantitative analysis of p-ERK1/2 normalized to GAPDH. WT, wild-type; hKO, heterozygous knockout. n = 7. *P < 0.05 vs. the respective sham group.
Aggresome formation in cardiac tissue.
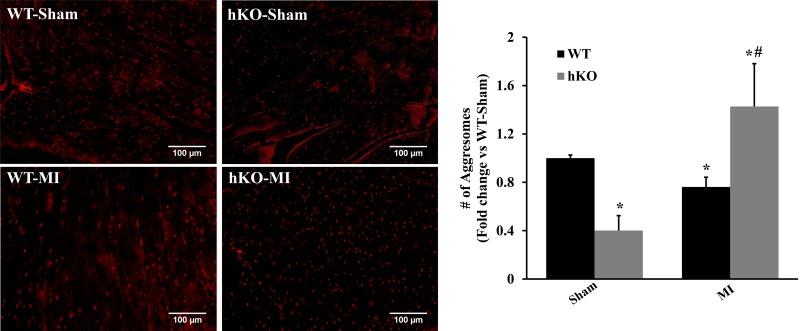
Aggresomes are aggregates of misfolded proteins within cells, which can ultimately be degraded by the autophagy pathway (46). The number of aggresomes was significantly lower in the hKO-sham group compared with the WT-sham group. MI led to decreased number of aggresomes in the WT-MI group versus the WT-sham group. However, the number of aggresomes was significantly higher in the hKO-MI group compared with the hKO-sham and WT-MI groups (P < 0.05 vs. the respective sham groups and P < 0.05 vs. the WT-MI group, n = 3; Fig. 6).
Fig. 6.
Ataxia telangiectasia mutated kinase increases aggresome accumulation in the left ventricle (LV) during 4 h of myocardial infarction (MI). Left: proteostat dye-stained images obtained from the LV of wild-type (WT) and heterozygous knockout (hKO) hearts 4 h after MI. Left: red fluorescent staining indicates aggresomes. Right: quantitative analysis of aggresomes in the LV region. n = 3. *P < 0.05 vs. the respective sham group; #P < 0.05 vs. the WT-MI group.
The autophagic response in cardiac fibroblasts.
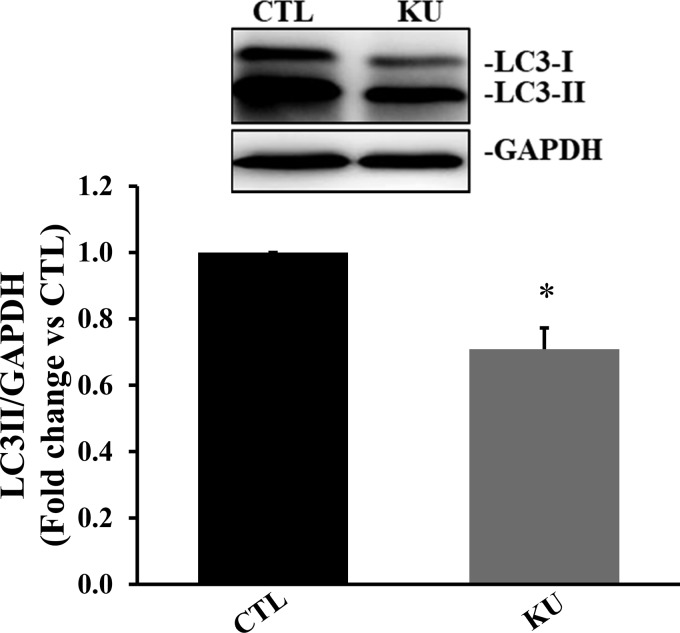
Cardiac fibroblast-specific deletion of ATM in mice has been shown to play a pivotal role in doxorubicin-induced cardiotoxicity (55). In fact, cardiac fibroblasts serve as a major cell type involved in the deposition of fibrosis in the heart post-MI (48). To investigate whether inhibition of ATM affects the autophagic response of cardiac fibroblasts, serum-starved cultures of cardiac fibroblasts were treated with KU (100 μM) for 4 h. Western blot analyses of cell lysates showed a significant decrease in LC3-II protein levels in KU-treated cells (P < 0.05 vs. the control group, n = 3; Fig. 7). ATM inhibition had no effect on p62 and cathepsin D protein levels (data not shown).
Fig. 7.
Inhibition of ataxia telangiectasia mutated kinase reduces microtubule-associated protein light chain (LC)3-II protein levels in cardiac fibroblasts. Serum-starved cardiac fibroblasts were treated with KU-55933 (KU; 100 µm, ataxia telangiectasia mutated kinase inhibitor) for 4 h. Total cell lysates were analyzed by Western blot analysis using LC3B antibodies. Top: Western blot exhibiting immunostaining for LC3-I, LC3-II, and GAPDH. Bottom: quantitative analysis of LC3-II normalized to GAPDH. n = 3. *P < 0.05 vs. the control (CTL) group.
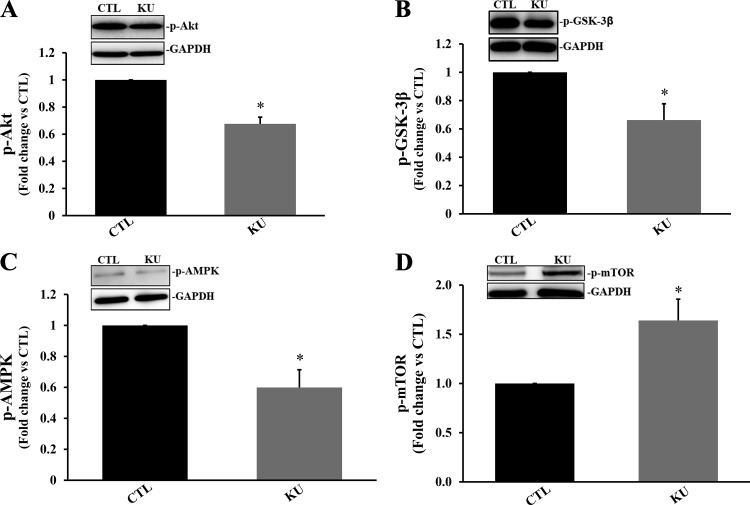
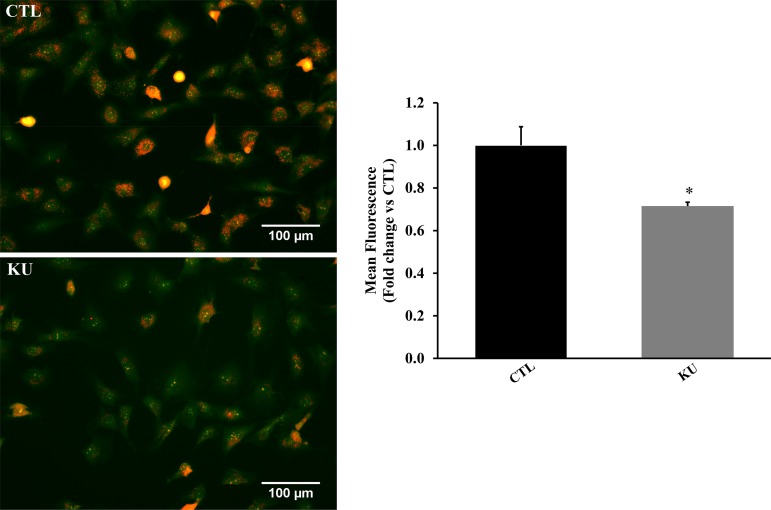
KU treatment significantly decreased phosphorylation of Akt, GSK-3β, and AMPK (P < 0.05 vs. the control group, n = 3–4; Fig. 8, A–C). However, it significantly increased mTOR phosphorylation (P < 0.05 vs. the control group, n = 3; Fig. 8D). The formation of AVOs is a key characteristic of cells that have passed through autophagy. AVOs, such as autolysosomes, increase upon autophagy induction (43). KU significantly decreased AVO formation in cardiac fibroblasts 4 h posttreatment (P < 0.05 vs. the control group, n = 3; Fig. 9).
Fig. 8.
Inhibition of ataxia telangiectasia mutated kinase affects the activation of Akt, glycogen synthase kinase (GSK)-3β, AMP-activated protein kinase (AMPK), and mammalian target of rapamycin (mTOR) in cardiac fibroblasts. Serum-starved cardiac fibroblasts were treated with KU-55933 (KU; 100 µm, ataxia telangiectasia mutated kinase inhibitor) for 4 h. Total cell lysates were analyzed by Western blot analysis using anti-phosphorylated (p-)Akt (A), anti-p-GSK-3β (B), anti-p-AMPK (C), and anti-p-mTOR (D) antibodies. A–D, top: Western blots exhibiting immunostaining for p-Akt, p-GSK-3β, p-AMPK, p-mTOR, and GAPDH. A–D, bottom: quantitative analyses of p-Akt, p-GSK-3β, p-AMPK, and p-mTOR normalized to GAPDH. n = 3–4. *P < 0.05 vs. the control (CTL) group.
Fig. 9.
Inhibition of ataxia telangiectasia mutated kinase decreases acidic vesicular organelle formation in cardiac fibroblasts. Cardiac fibroblasts were treated with KU-55933 (KU; 100 µm) for 4 h and then stained with acridine orange dye. Left: orange fluorescent staining indicates acidic vesicular organelles. Right: quantitative analysis of acidic vesicular organelles in cardiac fibroblasts. n = 3. *P < 0.05 vs. the control (CTL) group.
DISCUSSION
ATM typically becomes activated in the presence of double-stranded DNA breaks (1, 36). Thus, most of the studies regarding ATM and autophagy have been in relation to the DNA damage response. This is the first study investigating the role of ATM in cardiac autophagy, particularly during MI. A major finding of this study is that ATM deficiency results in autophagic impairment during 4 h of MI. This is evidenced by decreased LC3-II, increased p62, decreased cathepsin D protein levels, and enhanced aggresome accumulation. In accordance with in vivo data, ATM inhibition also resulted in autophagic impairment in adult cardiac fibroblasts, as shown by a reduction in LC3-II protein expression and a decrease in AVO formation. Furthermore, we provide evidence that ATM deficiency may exert its effect on autophagy during MI via the activation of GSK-3β and mTOR and inactivation of Akt and AMPK, as phosphorylation of Akt, GSK-3β, and AMPK decreased during MI, whereas mTOR phosphorylation increased during MI.
MI worsened heart function during 4 h of MI, as evidenced by a decrease in %EF and %FS in both groups compared with their respective sham group. This is consistent with the observations of Andrade et al. (3) in CD1 mice, where MI led to decreased %EF and %FS 4 h after LAD ligation. Previously, we have provided evidence that ATM deficiency improves LV function 1 day after LAD ligation, as observed by hKO-MI hearts exhibiting higher %EF and %FS coupled with lower LVESV and LVEDV compared with the WT-MI group (10). Here, %EF and %FS remained unchanged between the two MI groups 4 h after LAD ligation. The observed nonsignificant difference in %EF and %FS between WT and hKO hearts may relate to an early observation time point (4 vs. 24 h after LAD ligation).
Autophagy has been suggested to be cardioprotective, reducing infarct size and attenuating adverse cardiac remodeling post-MI (24, 52). Moreover, defects in autophagy have been linked to cardiac dysfunction and heart failure (22). Thus, autophagy is typically upregulated post-MI (2, 24, 52). Specifically, autophagy is induced in the acute phase of MI but impaired in the later phase of MI (24, 52). However, the mechanism(s) by which these autophagic changes occur post-MI is unknown. In our study, MI resulted in autophagic induction early during MI in WT hearts, as evidenced by high LC3-II protein levels coupled with reduced aggregate accumulation. Interestingly, Akt, GSK-3β, and AMPK phosphorylation was unaffected in WT hearts during MI. However, phosphorylation of mTOR (activation) and activation of ERK1/2 were significantly higher in WT hearts during MI. Several studies have suggested that ERK1/2 signaling positively regulates autophagy (21, 47, 57). However, the involvement of mTOR in ERK1/2-mediated autophagic changes is not completely clear. Zhang et al. (56) showed that mTOR inhibition results in ERK1/2 activation that induces autophagy in hepatocellular carcinoma. Choi et al. (9) demonstrated that ERK1/2 activation induced autophagy in luteal cells independent of mTOR activation. Additionally, ERK1/2 activation results in autophagic impairment in irinotecan-induced steatohepatitis (34). In the present study, we provide evidence that ERK1/2 activation may be involved in the induction of autophagy in WT hearts during MI. However, further investigations are needed to define the correlation between ERK1/2 and mTOR activation.
ATM has been suggested to play a role in autophagy, particularly after DNA damage in cancer models. ATM has been shown to be necessary for autophagy induction in response to ionizing radiation in Hela cells, a human cervical cancer cell line (32). ATM-mediated phosphorylation of phosphatase and tensin homolog promoted autophagy in cancer cells in response to DNA damage-causing agents (8). ATM/AMPK signaling promoted autophagy induction in mouse spermatocytes in response to cadmium-induced oxidative stress (31). In the present study, we provide evidence that ATM is critical in cardiac autophagy at basal levels and during MI. ATM deficiency induces autophagy at basal levels, as indicated by increased LC3-II protein levels coupled with an accumulation of p62. However, the number of aggresomes was lower in the hKO-sham group versus the WT-sham group. Thus, it is possible that under basal conditions, ATM deficiency results in enhanced autophagic flux; such enhancement could result in the accumulation of LC3-II and p62 and the rapid degradation of aggresomes. During MI, ATM deficiency resulted in reduced LC3-II, increased p62, decreased cathepsin D protein levels, and enhanced aggresome accumulation. Similarly, ATM inhibition resulted in a reduction in the LC3-II protein levels in cardiac fibroblasts. Although inhibition of ATM had no effect on p62 and cathepsin D protein expression in cardiac fibroblasts, the formation of acidic vesicular organelles was reduced. Together, these results suggest that ATM deficiency impairs autophagy in the heart at all three stages (autophagy induction, autophagosome formation, and autophagolysosome degradation) during MI and in cardiac fibroblasts. In ATM-deficient hearts during MI, the initiation of autophagy is lower, as evidenced by decreased LC3-II. The enhanced accumulation of p62 and aggresomes is likely due to decreased cathepsin D levels, indicating an impairment in aggresome degradation. In KU-treated cardiac fibroblasts, the initiation of autophagy is decreased, as evidenced by decreased LC3-II levels. The decrease in AVOs coupled with no changes in p62 and cathepsin D suggests a lack of autophagosome formation and subsequent degradation. Thus, ATM deficiency impairs autophagy in the heart during MI and in cardiac fibroblasts by inhibiting autophagy induction, autophagosome formation, and autophagolysosome degradation. These findings ultimately reveal that ATM is critical in cardiac autophagy regulation in the presence and absence of an insult.
The Akt/GSK-3β pathway is well known as a prosurvival pathway, as it typically prevents apoptotic signaling (12). Akt signaling has also been implicated in the regulation of autophagy. Akt activation induces autophagy in the brain after middle cerebral artery occlusion, resulting in neuroprotection (42). Pharmacological inhibition of GSK-3β induces prosurvival autophagic signals in human pancreatic cancer cells (35). Additionally, knockdown of GSK-3β increases basal autophagy and AMPK signaling in human aortic endothelial cells (51). AMPK functions as an energy sensor in cells that is activated by ATP depletion or glucose starvation (41). In the heart, AMPK activation is critical to the heart’s response to stresses such as ischemia (41). Pharmacological activation of AMPK has cardioprotective effects, protecting the heart against ischemia-reperfusion injury by maintaining ATP levels postischemia and reducing infarct size (26). AMPK activation promotes autophagy (27). Under conditions of low energy levels and hypoxia, AMPK inhibits mTOR (the master regulator of autophagy) activity via the phosphorylation of Raptor (7, 28). In the present study, we provide evidence that autophagic enhancement during ATM deficiency under basal conditions does not involve alterations in Akt, GSK-3β, or AMPK phosphorylation, as those parameters remained unchanged in ATM-deficient hearts. However, mTOR phosphorylation was lower in ATM-deficient hearts, indicating that the enhanced autophagic response in ATM-deficient hearts under basal conditions may be mTOR dependent. ATM deficiency may cause autophagic impairment during MI and in cardiac fibroblasts via the activation of GSK-3β and mTOR and inactivation of Akt and AMPK. We suggest that inactivation of Akt causes a reduction in GSK-3β phosphorylation, thus activating GSK-3β. Increased GSK-3β activity may result in a decline in AMPK phosphorylation and activation. This decline in AMPK activation results in mTOR activation that ultimately inhibits the autophagic response. However, further investigations are necessary to confirm the relationship between the activation of GSK-3β and mTOR and inactivation of Akt and AMPK in the regulating autophagy during MI in ATM-deficient hearts.
In summary, WT hearts exhibited autophagy induction during MI, a phenomenon most likely because of ERK1/2 activation. Under basal conditions, ATM deficiency results in an enhanced autophagic response that appears to be mTOR dependent. In addition, ATM deficiency results in autophagic impairment in all phases (autophagy induction, autophagosome formation, and autophagolysosome degradation) during MI and in cardiac fibroblasts via the activation of GSK-3β and mTOR and inactivation of Akt and AMPK. It should be emphasized that our data on ATM deficiency and cardiac autophagy are obtained 4 h following LAD ligation. The experimental time point should be extended beyond 4 h to investigate the long-term effects of ATM deficiency on autophagic changes in the heart.
GRANTS
This work was supported by Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Merit Review Awards BX002332 and BX000640, National Institutes of Health (NIH) Grant R15-HL-129140, and funds from the Institutional Research and Improvement account (to K. Singh) and NIH Grant C06-RR-0306551.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.R.T., M.S., and K.S. conceived and designed research; P.R.T., S.L.S., S.D., and C.C.C. performed experiments; P.R.T., S.L.S., and C.C.C. analyzed data; P.R.T. and K.S. interpreted results of experiments; P.R.T. prepared figures; P.R.T. drafted manuscript; P.R.T., M.S., and K.S. edited and revised manuscript; P.R.T., S.L.S., S.D., C.C.C., M.S., and K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The technical help received from Barbara A. Connelly is appreciated.
REFERENCES
- 1.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196, 2001. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 2.Aisa Z, Liao GC, Shen XL, Chen J, Li L, Jiang SB. Effect of autophagy on myocardial infarction and its mechanism. Eur Rev Med Pharmacol Sci 21: 3705–3713, 2017. [PubMed] [Google Scholar]
- 3.Andrade JN, Tang J, Hensley MT, Vandergriff A, Cores J, Henry E, Allen TA, Caranasos TG, Wang Z, Zhang T, Zhang J, Cheng K. Rapid and efficient production of coronary artery ligation and myocardial infarction in mice using surgical clips. PLoS One 10: e0143221, 2015. doi: 10.1371/journal.pone.0143221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171, 1996. doi: 10.1016/S0092-8674(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 5.Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res 120: 1812–1824, 2017. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 6.Broides A, Nahum A, Mandola AB, Rozner L, Pinsk V, Ling G, Yerushalmi B, Levy J, Givon-Lavi N. Incidence of typically severe primary immunodeficiency diseases in consanguineous and non-consanguineous populations. J Clin Immunol 37: 295–300, 2017. doi: 10.1007/s10875-017-0378-6. [DOI] [PubMed] [Google Scholar]
- 7.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett 584: 1287–1295, 2010. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JH, Zhang P, Chen WD, Li DD, Wu XQ, Deng R, Jiao L, Li X, Ji J, Feng GK, Zeng YX, Jiang JW, Zhu XF. ATM-mediated PTEN phosphorylation promotes PTEN nuclear translocation and autophagy in response to DNA-damaging agents in cancer cells. Autophagy 11: 239–252, 2015. doi: 10.1080/15548627.2015.1009767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi J, Jo M, Lee E, Choi D. ERK1/2 is involved in luteal cell autophagy regulation during corpus luteum regression via an mTOR-independent pathway. Mol Hum Reprod 20: 972–980, 2014. doi: 10.1093/molehr/gau061. [DOI] [PubMed] [Google Scholar]
- 10.Daniel LL, Daniels CR, Harirforoosh S, Foster CR, Singh M, Singh K. Deficiency of ataxia telangiectasia mutated kinase delays inflammatory response in the heart following myocardial infarction. J Am Heart Assoc 3: e001286, 2014. doi: 10.1161/JAHA.114.001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniel LL, Scofield SLC, Thrasher P, Dalal S, Daniels CR, Foster CR, Singh M, Singh K. Ataxia telangiectasia-mutated kinase deficiency exacerbates left ventricular dysfunction and remodeling late after myocardial infarction. Am J Physiol Heart Circ Physiol 311: H445–H452, 2016. doi: 10.1152/ajpheart.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3β signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab 26: 1479–1489, 2006. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- 13.Fang X, Yu SX, Lu Y, Bast RC Jr, Woodgett JR, Mills GB. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci USA 97: 11960–11965, 2000. doi: 10.1073/pnas.220413597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster CR, Daniel LL, Daniels CR, Dalal S, Singh M, Singh K. Deficiency of ataxia telangiectasia mutated kinase modulates cardiac remodeling following myocardial infarction: involvement in fibrosis and apoptosis. PLoS One 8: e83513, 2013. doi: 10.1371/journal.pone.0083513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster CR, Singh M, Subramanian V, Singh K. Ataxia telangiectasia mutated kinase plays a protective role in β-adrenergic receptor-stimulated cardiac myocyte apoptosis and myocardial remodeling. Mol Cell Biochem 353: 13–22, 2011. doi: 10.1007/s11010-011-0769-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster CR, Zha Q, Daniel LL, Singh M, Singh K. Lack of ataxia telangiectasia mutated kinase induces structural and functional changes in the heart: role in β-adrenergic receptor-stimulated apoptosis. Exp Physiol 97: 506–515, 2012. doi: 10.1111/j.1469-445X.2011.01034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res 58: 88–111, 2008. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JHY, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics-2017 update: a report from the american heart association. Circulation 135: e146–e603, 2017. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viniegra JG, Martínez N, Modirassari P, Hernández Losa J, Parada Cobo C, Sánchez-Arévalo Lobo VJ, Aceves Luquero CI, Álvarez-Vallina L, Ramón y Cajal S, Rojas JM, Sánchez-Prieto R. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem 280: 4029–4036, 2005. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 20.Halaby MJ, Hibma JC, He J, Yang DQ. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell Signal 20: 1555–1563, 2008. doi: 10.1016/j.cellsig.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 21.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43: 67–93, 2009. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilkun O, Boudina S. Cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Curr Pharm Des 19: 4806–4817, 2013. doi: 10.2174/1381612811319270003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jimenez RE, Kubli DA, Gustafsson ÅB. Autophagy and mitophagy in the myocardium: therapeutic potential and concerns. Br J Pharmacol 171: 1907–1916, 2014. doi: 10.1111/bph.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanamori H, Takemura G, Goto K, Maruyama R, Ono K, Nagao K, Tsujimoto A, Ogino A, Takeyama T, Kawaguchi T, Watanabe T, Kawasaki M, Fujiwara T, Fujiwara H, Seishima M, Minatoguchi S. Autophagy limits acute myocardial infarction induced by permanent coronary artery occlusion. Am J Physiol Heart Circ Physiol 300: H2261–H2271, 2011. doi: 10.1152/ajpheart.01056.2010. [DOI] [PubMed] [Google Scholar]
- 25.Khanna KK, Lavin MF, Jackson SP, Mulhern TD. ATM, a central controller of cellular responses to DNA damage. Cell Death Differ 8: 1052–1065, 2001. doi: 10.1038/sj.cdd.4400874. [DOI] [PubMed] [Google Scholar]
- 26.Kim AS, Miller EJ, Wright TM, Li J, Qi D, Atsina K, Zaha V, Sakamoto K, Young LH. A small molecule AMPK activator protects the heart against ischemia-reperfusion injury. J Mol Cell Cardiol 51: 24–32, 2011. doi: 10.1016/j.yjmcc.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest 125: 25–32, 2015. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy 3: 181–206, 2007. doi: 10.4161/auto.3678. [DOI] [PubMed] [Google Scholar]
- 30.Lavin MF, Khanna KK, Beamish H, Spring K, Watters D, Shiloh Y. Relationship of the ataxia-telangiectasia protein ATM to phosphoinositide 3-kinase. Trends Biochem Sci 20: 382–383, 1995. doi: 10.1016/S0968-0004(00)89083-0. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Luo X, Zhu Y, Zhao L, Li L, Peng Q, Ma M, Gao Y. ATM signals to AMPK to promote autophagy and positively regulate DNA damage in response to cadmium-induced ROS in mouse spermatocytes. Environ Pollut 231: 1560–1568, 2017. doi: 10.1016/j.envpol.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 32.Liang N, Jia L, Liu Y, Liang B, Kong D, Yan M, Ma S, Liu X. ATM pathway is essential for ionizing radiation-induced autophagy. Cell Signal 25: 2530–2539, 2013. doi: 10.1016/j.cellsig.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu HL, Yang C, Liu HF. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett 21: 29, 2016. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahli A, Saugspier M, Koch A, Sommer J, Dietrich P, Lee S, Thasler R, Schulze-Luehrmann J, Luehrmann A, Thasler WE, Müller M, Bosserhoff A, Hellerbrand C. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut 0: 1–11, 2017. [DOI] [PubMed] [Google Scholar]
- 35.Marchand B, Arsenault D, Raymond-Fleury A, Boisvert FM, Boucher MJ. Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem 290: 5592–5605, 2015. doi: 10.1074/jbc.M114.616714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282: 1893–1897, 1998. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 37.Meijer AJ, Codogno P. Autophagy: regulation and role in disease. Crit Rev Clin Lab Sci 46: 210–240, 2009. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- 38.Mizushima N. Autophagy: process and function. Genes Dev 21: 2861–2873, 2007. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 39.Mizushima N, Yoshimori T. How to interpret LC3 immunoblotting. Autophagy 3: 542–545, 2007. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 40.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci USA 98: 1676–1681, 2001. doi: 10.1073/pnas.98.4.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi D, Young LH. AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab 26: 422–429, 2015. doi: 10.1016/j.tem.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qi ZF, Luo YM, Liu XR, Wang RL, Zhao HP, Yan F, Song ZJ, Luo M, Ji XM. AKT/GSK3β-dependent autophagy contributes to the neuroprotection of limb remote ischemic postconditioning in the transient cerebral ischemic rat model. CNS Neurosci Ther 18: 965–973, 2012. doi: 10.1111/cns.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasul A, Yu B, Khan M, Zhang K, Iqbal F, Ma T, Yang H. Magnolol, a natural compound, induces apoptosis of SGC-7901 human gastric adenocarcinoma cells via the mitochondrial and PI3K/Akt signaling pathways. Int J Oncol 40: 1153–1161, 2012. doi: 10.3892/ijo.2011.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotman G, Shiloh Y. ATM: from gene to function. Hum Mol Genet 7: 1555–1563, 1998. doi: 10.1093/hmg/7.10.1555. [DOI] [PubMed] [Google Scholar]
- 45.Schneider JG, Finck BN, Ren J, Standley KN, Takagi M, Maclean KH, Bernal-Mizrachi C, Muslin AJ, Kastan MB, Semenkovich CF. ATM-dependent suppression of stress signaling reduces vascular disease in metabolic syndrome. Cell Metab 4: 377–389, 2006. doi: 10.1016/j.cmet.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Shen D, Coleman J, Chan E, Nicholson TP, Dai L, Sheppard PW, Patton WF. Novel cell- and tissue-based assays for detecting misfolded and aggregated protein accumulation within aggresomes and inclusion bodies. Cell Biochem Biophys 60: 173–185, 2011. doi: 10.1007/s12013-010-9138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sridharan S, Jain K, Basu A. Regulation of autophagy by kinases. Cancers (Basel) 3: 2630–2654, 2011. doi: 10.3390/cancers3022630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talman V, Ruskoaho H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 365: 563–581, 2016. doi: 10.1007/s00441-016-2431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tatti M, Motta M, Di Bartolomeo S, Scarpa S, Cianfanelli V, Cecconi F, Salvioli R. Reduced cathepsins B and D cause impaired autophagic degradation that can be almost completely restored by overexpression of these two proteases in Sap C-deficient fibroblasts. Hum Mol Genet 21: 5159–5173, 2012. doi: 10.1093/hmg/dds367. [DOI] [PubMed] [Google Scholar]
- 50.Watters D, Kedar P, Spring K, Bjorkman J, Chen P, Gatei M, Birrell G, Garrone B, Srinivasa P, Crane DI, Lavin MF. Localization of a portion of extranuclear ATM to peroxisomes. J Biol Chem 274: 34277–34282, 1999. doi: 10.1074/jbc.274.48.34277. [DOI] [PubMed] [Google Scholar]
- 51.Weikel KA, Cacicedo JM, Ruderman NB, Ido Y. Knockdown of GSK3β increases basal autophagy and AMPK signalling in nutrient-laden human aortic endothelial cells. Biosci Rep 36: e00382, 2016. doi: 10.1042/BSR20160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, He L, Chen F, He X, Cai Y, Zhang G, Yi Q, He M, Luo J. Impaired autophagy contributes to adverse cardiac remodeling in acute myocardial infarction. PLoS One 9: e112891, 2014. doi: 10.1371/journal.pone.0112891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1β. J Biol Chem 279: 39513–39519, 2004. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 54.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol 2: 893–898, 2000. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 55.Zhan H, Aizawa K, Sun J, Tomida S, Otsu K, Conway SJ, Mckinnon PJ, Manabe I, Komuro I, Miyagawa K, Nagai R, Suzuki T. Ataxia telangiectasia mutated in cardiac fibroblasts regulates doxorubicin-induced cardiotoxicity. Cardiovasc Res 110: 85–95, 2016. doi: 10.1093/cvr/cvw032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Yang H, Yue S, He G, Qu S, Zhang Z, Ma B, Ding R, Peng W, Zhang H, Yang Z, Dou K, Tao K, Li X. The mTOR inhibition in concurrence with ERK1/2 activation is involved in excessive autophagy induced by glycyrrhizin in hepatocellular carcinoma. Cancer Med 6: 1941–1951, 2017. doi: 10.1002/cam4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu B, Zhou Y, Xu F, Shuai J, Li X, Fang W. Porcine circovirus type 2 induces autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in PK-15 cells. J Virol 86: 12003–12012, 2012. doi: 10.1128/JVI.01434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]