Abstract
Nitric oxide prevents hypertension yet enhances proximal tubule Na+ reabsorption. Nitric oxide synthase is inhibited by asymmetric dimethylarginine (ADMA) that is metabolized by dimethylarginine dimethylaminohydrolase (DDAH) whose type 1 isoform is expressed abundantly in the proximal tubule (PT). We hypothesize that ADMA metabolized by DDAH-1 inhibits fluid reabsorbtion (Jv) by the proximal tubule. S2 segments of the PT were microperfused between blocks in vivo to assess Jv in anesthetized rats. Compared with vehicle, microperfusion of ADMA or Nω-nitro-l-arginine methyl ester (l-NAME) in the proximal tubule reduced Jv dose dependently. At 10−4 mol/l both reduced Jv by ~40% (vehicle: 3.2 ± 0.7 vs. ADMA: 2.1 ± 0.5, P < 0.01 vs. l-NAME: 1.9 ± 0.4 nl·min−1·mm−1, P < 0.01; n = 10). Selective inhibition of DDAH-1 in rats with intravenous L-257 (60 mg/kg) given 2 h before and L-257 (10−5 mol/l) perfused in the proximal tubule for 5 min reduced Jv by 32 ± 4% (vehicle: 3.2 ± 0.5 vs. L-257: 2.2 ± 0.5 nl·min−1·mm−1; P < 0.01) and increased plasma ADMA by ≈50% (vehicle: 0.46 ± 0.03 vs. L-257: 0.67 ± 0.03 µmol/l, P < 0.0001) without changing plasma symmetric dimethylarginine. Compared with nontargeted control small-interference RNA, knock down of DDAH-1 in mice by 60% with targeted small-interference RNAs (siRNA) reduced Jv by 29 ± 5% (nontargeted siRNA: 2.8 ± 0.20 vs. DDAH-1 knockdown: 1.9 ± 0.31 nl·min−1·mm−1, P < 0.05). In conclusion, fluid reabsorption in the proximal tubule is reduced by tubular ADMA or by blocking its metabolism by DDAH-1. L-257 is a novel regulator of proximal tubule fluid reabsorption.
Keywords: L-257, micropuncture, nitric oxide, l-nitromethyl arginine, proximal tubule
INTRODUCTION
Nitric oxide (NO) relaxes blood vessels, prevents salt sensitivity (23), reduces sympathetic nervous system tone (11), reduces or prevents hypertension, and protects blood vessels, the heart, the kidney, and other organs from hypertensive damage (26). Indeed, a reduced renal expression of nitric oxide synthase (NOS)-1 has been related to progression of kidney disease in a wide range of animal models (3). NO causes vasodilation of renal afferent arterioles (13), inhibits the vasoconstrictive tubuloglomerular feedback response (24), and inhibits Na+ reabsorption in the thick ascending limb of the loop of Henle and the collecting ducts (7). Although blockade of NOS has variable effects on Na+ excretion, genetic deletion of NOS1 and -3 sharply reduces the reabsorption of fluid in the mouse proximal tubule in most (21), but not all, studies (18). The differences may relate to the effects of the NOS-1α and NOS-1β splice variants (14).
Asymmetric dimethylarginine (ADMA) is a cellular and circulating inhibitor of NOS (2) that is metabolized by dimethylaginine dimethylaminohydrolase (DDAH) whose type 1 isoform is heavily expressed in the proximal tubule (19). Although the function of DDAH in the kidney has not been studied, there are compelling clinical data linking it to chronic kidney disease (CKD). Thus, circulating levels of ADMA have been considered to be a uremic toxin and predict the progression of CKD (12, 28). However, activating polymorphisms of DDAH-1 (5) that reduce circulating ADMA are associated with protection from progression of CKD (5) and salt sensitivity (6). Thus, DDAH-1/ADMA/NO in the kidney may have effects independent of circulating ADMA. We tested the hypothesis that DDAH-1 regulates proximal tubule fluid reabsorption by regulating ADMA. First, rat renal proximal tubules were perfused with artificial tubular fluid (ATF) with graded addition of Nω-nitro-l-arginine methyl ester (l-NAME) to inhibit nitric oxide synthase isoforms or ADMA. Second, the effects of DDAH-1 were tested in rats administered with L-257, which is a specific DDAH-1 inhibitor (22). The findings were extended to a study in the mouse by gene silencing of DDAH-1 (19).
MATERIALS AND METHODS
Animals
The experiments were conducted under protocols approved by the Georgetown University Animal Care and Use Committee and performed according to the National Institutes of Health guidelines for the conduct of experiments in animals. Male Sprague-Dawley rats and C57Bl/6 mice were housed in cages kept in temperature-controlled units (25°C) with a 12:12-h light-dark cycle and maintained on a standard chow with free access to food and water.
Surgical Preparation
Rats were prepared for renal micropuncture under anesthesia with thiobarbital (Inactin, 80 mg/kg ip; Research Biochemicals) and infused with isotonic saline containing1% bovine serum albumin (Sigma Chemical, St. Louis, MO) at 1.5 ml/h to maintain euvolemia as described (24).
Mice were anesthetized with isoflurane (1.0% in room air, delivered by a pump; Univenter) and prepared for micropuncture as described (1, 4). Cannulas were placed in a jugular vein for infusion of isotonic saline containing 1.5% bovine serum albumin at 0.35 ml/h to maintain euvolemia (4). In both rats and mice, a femoral artery was cannulated for recording of mean arterial pressure (Powerlab; AD Instruments), and the left ureter was cannulated to collect urine from the left experimental kidney that was exposed by a flank incision and stabilized in a Lucite cup (Vestavia Scientific, Birmingham, AL) for micropuncture (1, 4, 24). The study commenced 60 min after surgery.
Microperfusion of Proximal Tubules of Rats and Mice
As described previously for rats (24) and mice (1, 4), a surface proximal tubule loop (S2 segment) was identified by injection from a “finding” pipette (8 µm outer diameter) containing artificial tubule fluid stained with the Fast Green FCF dye (0.1%; Sigma). An immobile grease block (Apiezon T, Manchester, UK) was injected in the tubule at the puncture site to stop tubular fluid flow. A perfusion pipette (8–10 µm outer diameter) was inserted immediately downstream from the block. The perfusion pipette was filled with ATF containing (mmol/l): 125 NaCl, 20 NaHCO3, 5 KCl, 1 MgSO4, 2 CaCl2, 1 NaH2PO4, 5 glucose, 4 urea, and [14C]inulin. It was connected to a calibrated nanoliter perfusion pump (Vestavia Scientific) to perfuse the segment of the proximal tubule for 2–4 min before timed fluid collections. The collections were made at a downstream site with a micropipette (8–10 µm outer diameter) after placement of a column of oil to block downstream flow. The samples were collected for 4 min and transferred to a constant-bore capillary tube whose length was measured with a micrometer to calculate the tubular fluid volume. Thereafter, the samples were injected into scintillation fluid, and the 14C activity was counted. Collected samples with <95% and >105% of microperfused [14C]inulin were discarded. The amount of microperfused inulin was estimated by the average of 14C activity in three samples perfused into a vial over 4 min. To determine the lengths of the perfused segments, tubules were filled with high-viscosity microfil (Flow Tech), the kidney was partially digested in 20% NaOH, and the length of the cast was measured under a dissecting microscope. Fluid reabsorbtion (Jv) was calculated by the difference in the rate of fluid perfusion and the rate of fluid collection factored by the length of the perfused nephron segment (1, 4, 24).
Construction and Administration of Small-Interference RNAs
These studies were performed in mice. RNAi duplexes of 21 nucleotides targeting the coding region of DDAH-1 (siDDAH-1) (Qiagen) were validated in vitro as described previously (19). The target site in the mouse DDAH-1 cDNA (GenBank accession no. NM_026993) of the construct selected was 673–693 (TGGCCGATTCTTTGCATTTAA). The nonsilencing control small-interference RNAs (siRNA, catalog no. 1027280; Qiagen) had no homology to any sequence in the mammalian genome. For injection of siRNA, mice were briefly anesthetized with 1–2% isofluorane, and a cannula was inserted in the femoral vein through which 25 µg of siRNA dissolved in 1 ml of TransIT-QR Hydrodynamic Delivery Solution (Mirus ZL) was injected rapidly over 5 s. The effects of this hydrodynamic DDAH-1 silencing were assessed by RNA analysis in the harvested kidney cortex after 48 h.
Protocols
Protocol 1. Microperfusion of ADMA or l-NAME in the proximal tubule of rats.
ADMA or l-NAME were dissolved in ATF at 10−7 M to 10−4 mol/l and perfused in a rat proximal tubule between blocks. Alternate tubules were perfused with ATF + vehicle or ADMA or l-NAME.
Protocol 2. Blockade of DDAH-1 with L-257 in rats.
For each series, Jv was measured in a perfused proximal tubule of a rat after administration of vehicle or L-257. The optimal method for delivery of L-257 was assessed from the following three protocols: 1) proximal tubule perfusion of L-257 (10−5 mol/l) or vehicle; 2) intravenous injection of L-257 (60 mg/kg) or vehicle 2 h previous followed by tubular perfusion with vehicle, and 3) intravenous injection of L-257 or vehicle 2 h previous followed by tubular perfusion with L-257 or vehicle.
In separate groups, blood was collected for 2 h following bolus intravenous injection of vehicle or L-257 for measurements of plasma ADMA and symmetric dimethylarginine (SDMA) with a fully validated gas chromatography-mass spectrometry method and quantitated relative to deuterated standards (17).
Protocol 3. Gene silencing of DDAH-1 in mice.
The Jv of the perfused proximal tubule of C57/BL6 mice was assessed after intravenous injection 48 h previously of siRNA directed to DDAH-1 or nontargeted control siRNA, as described in detail previously (19). The kidney cortex was harvested to measure mRNA expression of DDAH-1.
RNA Extraction, cDNA Synthesis, and Real-Time PCR
RNA was extracted from harvested tissues using an RNeasy Mini Kit (Qiagen). The cDNA was synthesized using an iScript cDNA Synthesis kit (Bio-Rad). The gene expression for DDAH-1 was assessed with real-time PCR (StepOnePlus Real-time PCR System; ABI) using a FAM (6-carboxy-fluorescine dye)-labeled DDAH-1 Taqman probe assay (Mm01319453_ml; ABI) multiplexed with a VIC (fluorescine dye)-labeled 18S control probe. Relative amounts of mRNA, normalized by 18S rRNA, were calculated from threshold cycle numbers (CT, i.e., 2−ΔΔCT).
Drugs
ADMA and l-NAME were purchased from Sigma Chemical. L-257 is a fully validated DDAH-1 inhibitor that was synthesized in the laboratory of James Leiper of the MRC Clinical Sciences Center, London (22).
Statistical Analysis
Data are presented as means ± SE. The significance of differences within and between groups was evaluated using ANOVA followed by a Fisher’s post hoc test where appropriate. Results were considered significant at P < 0.05.
RESULTS
Proximal Tubule Fluid Reabsorption in Rats During Luminal Microperfusion of l-NAME or ADMA
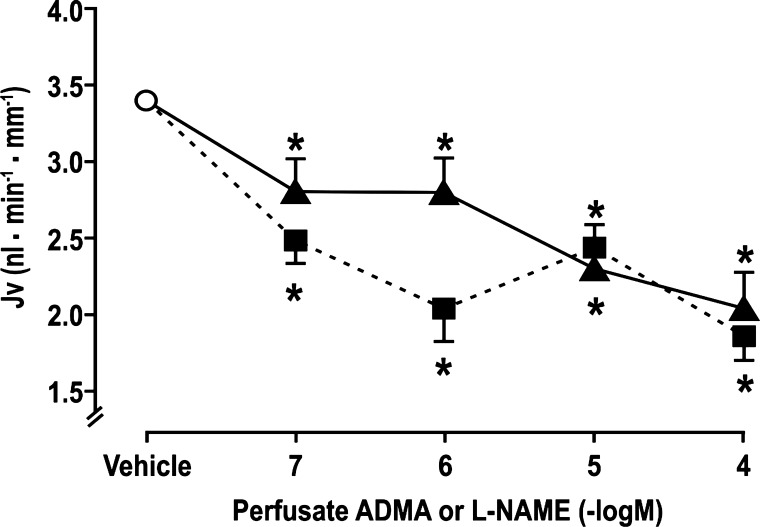
Microperfusion of l-NAME or ADMA reduced Jv similarly and dose dependently (Fig. 1). At the maximum dose tested of 10−4 mol/l, ADMA reduced Jv by 41 ± 5% (vehicle: 3.3 ± 0.5 vs. ADMA: 1.9 ± 0.4 nl·min−1·mm−1, P < 0.01; n = 10 tubules) and l-NAME by 38 ± 6% (vehicle: 3.3 ± 0.5 vs. l-NAME: 2.1 ± 0.5 nl·min−1·mm−1, P < 0.01; n = 10 tubules).
Fig. 1.
Nω-nitro-l-arginine methyl ester (l-NAME) and asymmetric dimethylarginine (ADMA) inhibit fluid reabsorption (Jv) in the rat perfused proximal tubule in vivo during microperfusion and recollection of artificial tubular fluid + vehicle (open circle), + l-NAME (solid triangles and continuous lines), or + ADMA (solid squares and broken lines). *P < 0.05 compared with artificial tubular fluid + vehicle.
Proximal Tubule Fluid Reabsorption in Rats After Inhibition of DDAH-1 with L-257
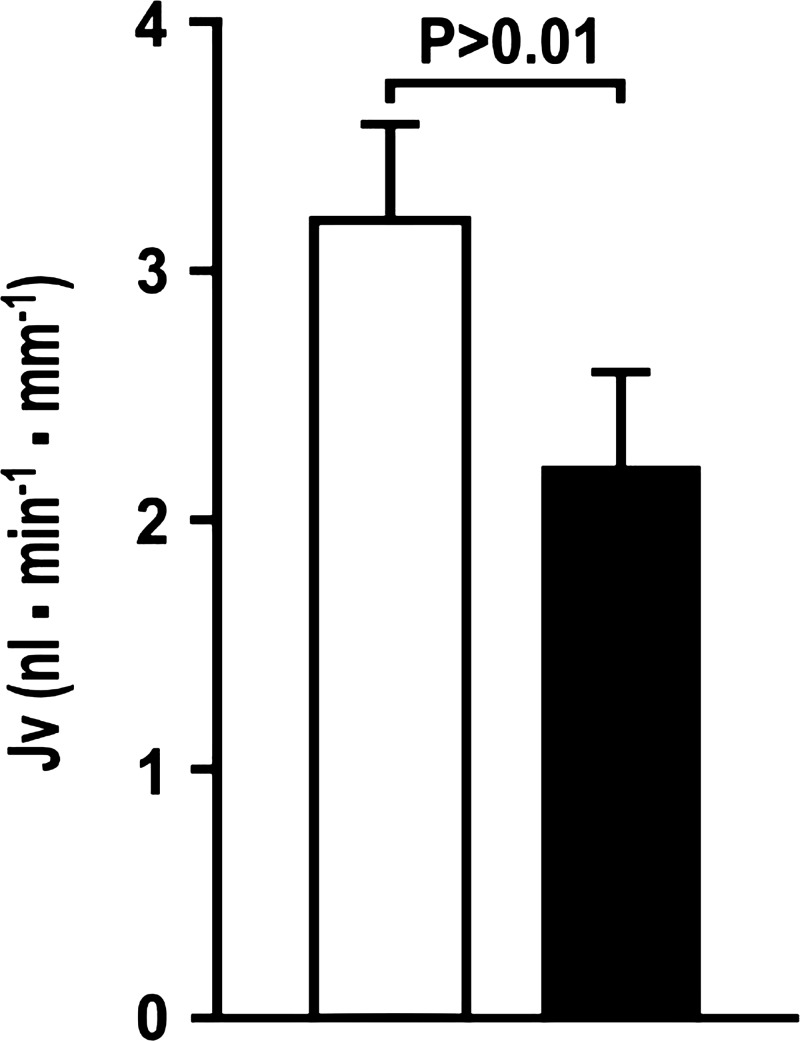
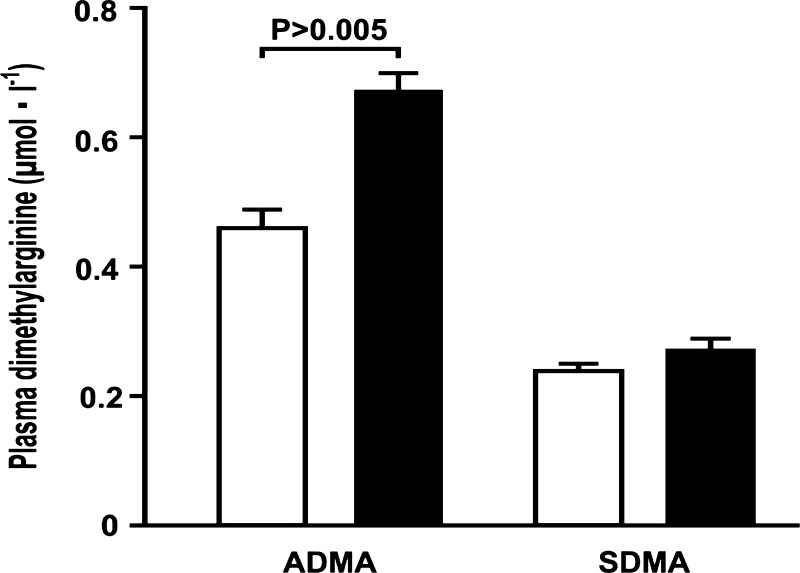
L-257 delivered by intravenous injection 2 h before experimentation and by direct perfusion of the proximal tubule reduced the Jv by 32 ± 4% (vehicle: 3.2 ± 0.4 vs. L-257: 2.2 ± 0.5 nl·min−1·mm−1; P < 0.01; n = 8 tubules). (Fig. 2). Direct tubular perfusion of L-257 or sole intravenous administration of L-257 did not change Jv consistently (data not shown). Intravenous administration of L-257 to rats 2 h previously increased plasma ADMA by 50% (vehicle: 0.46 ± 0.03 vs. L-257: 0.67 ± 0.03 µmol/l; P < 0.0001) without changing SDMA (Fig. 3).
Fig. 2.
Blockade of dimethylarginine dimethylaminohydrolase with L-257 in the rat reduces absolute proximal tubule fluid reabsorption (Jv) 2 h after iv injection of L-257 (60 mg/kg) and during tubule perfusion of L-257 (10−5 mol/l; solid boxes) compared with corresponding administration of vehicle (open boxes).
Fig. 3.
Injection of L-257 iv increases asymmetric dimethylarginine (ADMA) selectively. Plasma levels of ADMA or symmetric dimethylarginine (SDMA) 2 h after iv injection of vehicle (open boxes) or L-257 (solid boxes).
Proximal Tubule Fluid Reabsorption in Mice After Knockdown of DDAH-1 with siRNA
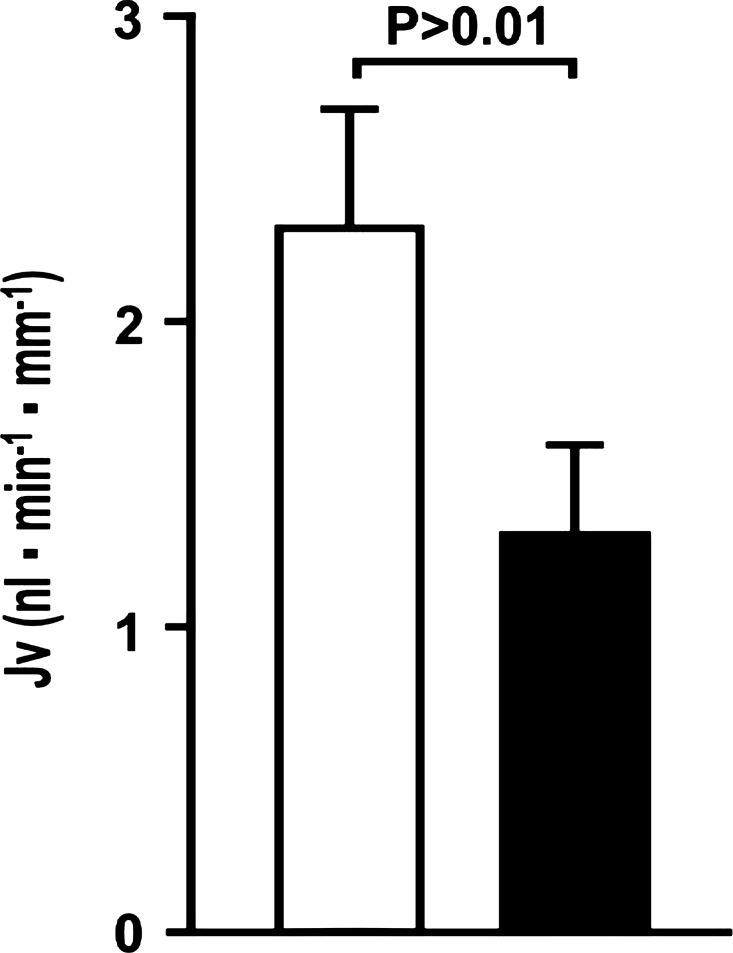
Injection of siRNA for DDAH-1 in rats produced quite variable knockdown of DDAH-1 mRNA in the kidney, whereas the knockdown in mice was more consistent. Therefore, mice were selected for this protocol. Rapid bolus intravenous injections of siRNA directed to DDAH-1, compared with nontargeted siRNA, given 48 h before experimentation to mice reduced the expression of mRNA to DDAH-1 in the renal cortex by 55 ± 5% and reduced Jv in the proximal tubule by 43 ± 5% (siControl: 2.3 ± 0.4 vs. siDDAH-1: 1.3 ± 0.3 nl·min−1·mm−1, P < 0.01; n = 6) (Fig. 4).
Fig. 4.
Knockdown of dimethylarginine dimethylaminohydrolase with small-interfering RNA (siRNA) reduces absolute proximal tubule fluid reabsorption (Jv) in the mouse proximal tubules 48 h after iv injections of siRNA to DDAH-1 (solid boxes) compared with nontargeted siRNA (open boxes).
DISCUSSION
We confirm that L-257 is an effective inhibitor of DDAH-1 and increases plasma levels of ADMA by 50% (22). The main new findings are that ADMA is as effective as l-NAME in reducing Jv of the rat perfused proximal tubule. Maximal concentrations of each drug reduced Jv by ≈40%, which was similar to the reduction of Jv of 32% following pharmacological inhibition of DDAH-1 with L-257 in rats or reduction of Jv of 43% following gene silencing of DDAH-1 in mice.
ADMA is produced by hydrolysis of methyl arginine moieties in proteins after methylation by protein arginine methyl transferases (15). Its plasma levels are primarily regulated by metabolism by DDAH (2, 10). ADMA also can be metabolized by alanine-gluyoxilate amino transferase II (2). However, the finding that the proximal tubule fluid reabsorption was inhibited similarly by direct microperfusion of ADMA or by pharmacological inhibition of ADMA metabolism by DDAH-1 or silencing of the DDAH-1 gene demonstrates the importance of DDAH-1 for regulation of ADMA in the proximal tubule.
DDAH-1 is heavily expressed in the liver and the proximal tubule (9, 16). Measurements of ADMA extraction across organs in vivo has shown that the kidney and the liver are the prime sites for clearance of plasma ADMA (2, 10, 15). The large increase in plasma ADMA after knockdown of DDAH-1 in this study is consistent with these findings. SDMA is not metabolized by DDAH (2). Therefore, the finding that plasma levels of ADMA were increased, but SDMA were unchanged, after L-257 provides further evidence of the specific effect of L-257 to inhibit DDAH (22). The failure of L-257 to reduce Jv significantly when perfused directly in the proximal tubule may relate to the limited time of tubular exposure of ~5 min. This may have been insufficient for ADMA to accumulate effectively after inhibition of its metabolism by blocking DDAH-1. In contrast, DDAH-2 is expressed in the vascular endothelium, distal nephron, and macula densa of the kidney. Unlike DDAH-1, knockdown of DDAH-2 in the rat impairs endothelial function and reduces the expression of endothelial NOS but does not change plasma levels of ADMA (19). However, the DDAH-1 knockout mouse has endothelial dysfunction (27), suggesting differences between rats and mice in the specific roles of the DDAH isoforms. In this study, DDAH-1 was inhibited by two distinct means (pharmacological and gene knockdown) in rats and mice. The similar effects of these to reduce proximal tubule fluid reabsorption by 30–45% suggest an important role for DDAH-1 in the proximal tubules of both species. The expression of DDAH-1, and consequently the plasma and tissue levels of ADMA, are regulated by reactive oxygen species both in vitro (8) and in vivo (20, 25) and thereby have been linked to pathophysiology of hypertension and CKD (25).
We acknowledge some limitations of our study. DDAH-1 was blocked pharmacologically in rats and by gene knockout in mice. However, the similar effects on proximal fluid reabsorption suggest no major species difference. Inhibition or silencing of DDAH-1 will have systemic effects that could influence proximal tubule reabsorption. However, DDAH-1 silencing over 2 h does not change blood pressure significantly (19). Moreover, ADMA perfused in the nephron had a similar effect to reduce proximal tubule reabsorption as did systemic inhibition of its metabolism by DDAH-1 blockade or gene knockdown.
In conclusion, ADMA and its metabolism by DDAH-1 are important determinants of proximal tubule fluid reabsorption in rats and mice.
Perspective
Luminal ADMA inhibited rat proximal tubule fluid reabsorption at 10−7 mol/l, which is at the plasma level of ADMA of 3 × 10−7 mol/l recorded in this study. A 55% knockdown of DDAH-1 in the mouse reduced proximal tubule reabsorption significantly. This reduction in DDAH-1 expression is equivalent to that reported in earlier studies in rats infused with angiotensin II that was attributed to reactive oxygen species (8, 20, 25). Thus, these findings establish ADMA as a physiological inhibitor of proximal tubule reabsorption, and DDAH-1 as an important physiological regulator of proximal tubule function in vivo in rats and mice. Moreover, DDAH-1 and ADMA may contribute to the pathophysiology of hypertension and conditions associated with oxidative stress.
GRANTS
This study was supported by National Institutes of Health Grants DK-49870, DK-109272, and HL-68686 to C. S. Wilcox and W. J. Welch and by funds from the George E. Schreiner Chair of Nephrology, the Georgetown Hypertension Center, and the Smith-Kogod Family Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.B., M.A., Z.L., J.T., J.L., W.J.W., and C.S.W. conceived and designed research; T.B. and Z.L. performed experiments; T.B., M.A., Z.L., J.L., and W.J.W. analyzed data; T.B., M.A., J.T., and J.L. interpreted results of experiments; T.B., M.A., Z.L., J.T., J.L., W.J.W., and C.S.W. approved final version of manuscript; J.T. and C.S.W. prepared figures; W.J.W. and C.S.W. drafted manuscript; C.S.W. edited and revised manuscript.
REFERENCES
- 1.Araujo M, Welch WJ. Tubuloglomerular feedback is decreased in COX-1 knockout mice after chronic angiotensin II infusion. Am J Physiol Renal Physiol 298: F1059–F1063, 2010. doi: 10.1152/ajprenal.00547.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigoni F, Ahmetaj B, Leiper J. The biology and therapeutic potential of the DDAH/ADMA pathway. Curr Pharm Des 16: 4089–4102, 2010. doi: 10.2174/138161210794519246. [DOI] [PubMed] [Google Scholar]
- 3.Baylis C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr Opin Nephrol Hypertens 21: 1–6, 2012. doi: 10.1097/MNH.0b013e32834d54ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell TD, Luo Z, Welch WJ. Glomerular tubular balance is suppressed in adenosine type 1 receptor-deficient mice. Am J Physiol Renal Physiol 299: F1158–F1163, 2010. doi: 10.1152/ajprenal.00202.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplin B, Nitsch D, Gill H, Hoefield R, Blackwell S, MacKenzie D, Cooper JA, Middleton RJ, Talmud PJ, Veitch P, Norman J, Wheeler DC, Leiper JM. Circulating methylarginine levels and the decline in renal function in patients with chronic kidney disease are modulated by DDAH1 polymorphisms. Kidney Int 77: 459–467, 2010. doi: 10.1038/ki.2009.463. [DOI] [PubMed] [Google Scholar]
- 6.Defagó MD, Gu D, Hixson JE, Shimmin LC, Rice TK, Gu CC, Jaquish CE, Liu DP, He J, Kelly TN. Common genetic variants in the endothelial system predict blood pressure response to sodium intake: the GenSalt study. Am J Hypertens 26: 643–656, 2013. doi: 10.1093/ajh/hps099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garvin JL, Herrera M, Ortiz PA. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: clinical implications. Annu Rev Physiol 73: 359–376, 2011. doi: 10.1146/annurev-physiol-012110-142247. [DOI] [PubMed] [Google Scholar]
- 8.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension 56: 498–504, 2010. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onozato ML, Tojo A, Leiper J, Fujita T, Palm F, Wilcox CS. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes 57: 172–180, 2008. doi: 10.2337/db06-1772. [DOI] [PubMed] [Google Scholar]
- 10.Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293: H3227–H3245, 2007. doi: 10.1152/ajpheart.00998.2007. [DOI] [PubMed] [Google Scholar]
- 11.Patel K, Chen Y, Dennehy K, Blau J, Connors S, Mendonca M, Tarpey M, Krishna M, Mitchell JB, Welch WJ, Wilcox CS. Acute antihypertensive action of nitroxides in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol 290: R37–R43, 2006. doi: 10.1152/ajpregu.00469.2005. [DOI] [PubMed] [Google Scholar]
- 12.Schepers E, Speer T, Bode-Böger SM, Fliser D, Kielstein JT. Dimethylarginines ADMA and SDMA: the real water-soluble small toxins? Semin Nephrol 34: 97–105, 2014. doi: 10.1016/j.semnephrol.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: roles of and NO. Am J Physiol Renal Physiol 279: F302–F308, 2000. doi: 10.1152/ajprenal.2000.279.2.F302. [DOI] [PubMed] [Google Scholar]
- 14.Tain YL, Ghosh S, Krieg RJ, Baylis C. Reciprocal changes of renal neuronal nitric oxide synthase-α and -β associated with renal progression in a neonatal 5/6 nephrectomized rat model. Pediatr Neonatol 52: 66–72, 2011. doi: 10.1016/j.pedneo.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res 60: 448–460, 2009. doi: 10.1016/j.phrs.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tojo A, Welch WJ, Bremer V, Kimoto M, Kimura K, Omata M, Ogawa T, Vallance P, Wilcox CS. Colocalization of demethylating enzymes and NOS and functional effects of methylarginines in rat kidney. Kidney Int 52: 1593–1601, 1997. doi: 10.1038/ki.1997.490. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson JA, Caplin B, Boruc O, Bruce-Cobbold C, Cutillas P, Dormann D, Faull P, Grossman RC, Khadayate S, Mas VR, Nitsch DD, Wang Z, Norman JT, Wilcox CS, Wheeler DC, Leiper J. Reduced renal methylarginine metabolism protects against progressive kidney damage. J Am Soc Nephrol 26: 3045–3059, 2015. doi: 10.1681/ASN.2014030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vallon V, Traynor T, Barajas L, Huang YG, Briggs JP, Schnermann J. Feedback control of glomerular vascular tone in neuronal nitric oxide synthase knockout mice. J Am Soc Nephrol 12: 1599–1606, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res 101: 627–635, 2007. doi: 10.1161/CIRCRESAHA.107.158915. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Luo Z, Wang X, Jose PA, Falck JR, Welch WJ, Aslam S, Teerlink T, Wilcox CS. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: effects of tempol. Hypertension 56: 950–955, 2010. doi: 10.1161/HYPERTENSIONAHA.110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang T. Role of iNOS and eNOS in modulating proximal tubule transport and acid-base balance. Am J Physiol Renal Physiol 283: F658–F662, 2002. doi: 10.1152/ajprenal.00243.2001. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Lambden S, Taylor V, Sujkovic E, Nandi M, Tomlinson J, Dyson A, McDonald N, Caddick S, Singer M, Leiper J. Pharmacological inhibition of DDAH1 improves survival, haemodynamics and organ function in experimental septic shock. Biochem J 460: 309–316, 2014. doi: 10.1042/BJ20131666. [DOI] [PubMed] [Google Scholar]
- 23.Welch WJ, Mendonca M, Blau J, Karber A, Dennehy K, Patel K, Lao YS, José PA, Wilcox CS. Antihypertensive response to prolonged tempol in the spontaneously hypertensive rat. Kidney Int 68: 179–187, 2005. doi: 10.1111/j.1523-1755.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- 24.Welch WJ, Wilcox CS. Macula densa arginine delivery and uptake in the rat regulates glomerular capillary pressure. Effects of salt intake. J Clin Invest 100: 2235–2242, 1997. doi: 10.1172/JCI119761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox CS. Asymmetric dimethylarginine and reactive oxygen species: unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension 59: 375–381, 2012. doi: 10.1161/HYPERTENSIONAHA.111.187310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther 126: 119–145, 2010. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang P, Hu X, Xu X, Chen Y, Bache RJ. Dimethylarginine dimethylaminohydrolase 1 modulates endothelial cell growth through nitric oxide and Akt. Arterioscler Thromb Vasc Biol 31: 890–897, 2011. doi: 10.1161/ATVBAHA.110.215640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zoccali C. Traditional and emerging cardiovascular and renal risk factors: an epidemiologic perspective. Kidney Int 70: 26–33, 2006. doi: 10.1038/sj.ki.5000417. [DOI] [PubMed] [Google Scholar]