Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is a life-threatening, highly prevalent monogenic disease caused by mutations in polycystin-1 (PC1) in 85% of patients. We have previously identified a COOH-terminal cleavage fragment of PC1, PC1-p30, which interacts with the transcription factor STAT6 to promote transcription. STAT6 is aberrantly active in PKD mouse models and human ADPKD, and genetic removal or pharmacological inhibition of STAT6 attenuates disease progression. High levels of IL-13, a STAT6-activating cytokine, are found in the cyst fluid of PKD mouse models and increased IL-13 receptors in ADPKD patient tissue, suggesting that a positive feedback loop exists between IL-13 and STAT6 is activated in cystic epithelial cells and contributes to disease progression. In this study, we aimed to identify genes aberrantly regulated by STAT6 to better understand how increased IL-13/STAT6 signaling may contribute to PKD progression. We demonstrate that the expression of periostin, galectin-3, and IL-24 is upregulated in various forms of PKD and that their aberrant regulation is mediated by IL-13 and STAT6 activity. Periostin and galectin-3 have previously been implicated in PKD progression. We support these findings by showing that periostin expression is increased after IL-13 treatment in kidney epithelial cells, that galectin-3 expression is increased after injecting IL-13 in vivo and that IL-24 expression is upregulated by both IL-13 treatment and PC1-p30 overexpression in mouse and human kidney cells. Overall, these findings provide insight into the possible mechanisms by which increased IL-13/STAT6 signaling contributes to PKD progression and suggest potential therapeutic targets.
Keywords: polycystic kidney disease, polycystin, STAT6
INTRODUCTION
Polycystic kidney disease (PKD) is characterized by renal fibrosis and the progressive expansion of numerous, fluid-filled cysts, which eventually displace and destroy normal renal parenchyma. Autosomal dominant polycystic kidney disease (ADPKD) is the most prevalent type of PKD, affecting between 1:500–1:1,000 individuals in the United States. A majority of ADPKD patients have mutations in the PKD1 gene, which encodes the large, multipass transmembrane protein, polycystin-1 (PC1) (9, 23, 65). Since its discovery, the normal and pathologic function of PC1 has been studied extensively, resulting in the identification of a multitude of protein interactions and downstream signaling pathways. However, the signaling pathways that are the most significant in the pathogenesis of ADPKD, and to the normal purpose of PC1, have remained unclear (65).
Signaling through STAT6 is one of the dysregulated pathways associated with PC1 in ADPKD (39, 47). Previously, we identified that the COOH-terminal cytoplasmic domain of PC1 interacts with the transcription factor STAT6 and the coactivator p100 (39). Under normal conditions, full-length PC1 has been found to localize to the primary cilium in renal epithelial cells. However, when conditions change, such as with fluid flow cessation, previous studies have shown that the cytoplasmic tail of PC1 is cleaved, resulting in the release of the soluble fragment PC1-p30 (10). This fragment then translocates to the nucleus, where it can coactivate transcription with STAT3 and STAT6 (39, 62, 74), as well as TCF and CHOP (42).
Canonical STAT6 signaling is initiated by the binding of IL-4 or IL-13 to their corresponding receptor, which leads to activation of STAT6 through tyrosine phosphorylation by a receptor-bound JAK (22). STAT6 is known to drive transcription of its own gene, the IL-4α and IL-13α1 receptor chains, and the IL-4/IL-13 cytokines (31, 35), which can lead to a positive feedback loop and persistent STAT6 pathway activation. We have previously reported that STAT6 is aberrantly activated in the cyst-lining epithelial cells of PKD mouse models and human ADPKD patients (39, 47). Our previous work has also shown that there is increased expression of the STAT6, IL-4α and IL-13α1 receptor chains in PKD, as well as elevated levels of IL-13 in the cyst lumens. These findings indicate that these genes are, indeed, transcriptional targets of STAT6 in renal cysts and that the IL-13 signaling pathway appears to be constitutively activated through a positive feedback loop between the IL-4/IL-13 receptor and STAT6 (47). Genetic ablation or pharmacological inhibition of STAT6 decreases disease severity, indicating that aberrant STAT6 activation is at least a partial driving force of renal cyst growth (47).
Given these previous data, we decided to investigate additional transcriptional targets of the IL-13/STAT6 signaling pathway to better understand how their aberrant regulation may promote PKD disease progression. We have recently identified the polymeric immunoglobulin receptor (pIgR) as a protein whose expression is regulated by the IL-13/STAT6 pathway in PKD (48). In this study, we identified three IL-13/STAT6-regulated genes with known or potential links to renal cyst growth: periostin, galectin-3, and IL-24. Periostin is a matricellular protein that can bind extracellular matrix and integrins to regulate cell signaling (6, 21, 37). Periostin has previously been shown to promote cell proliferation and fibrosis in PKD through integrin activation and to be upregulated in human ADPKD (68, 69). Additionally, periostin has been shown to be regulated by IL-4 and IL-13 in lung fibroblasts (64), suggesting that increased IL-13 activity may be the source of aberrant periostin expression in PKD.
Galectin-3 is a β-galactoside-binding lectin that is expressed in developing kidneys (7, 78) and has been shown to be upregulated (44) and promote fibrosis (24) after kidney injury. The protein has also been found to localize to cystic epithelial cells in both the cpk mouse model (11) and human autosomal recessive polycystic kidney disease (ARPKD) (78). Additionally, in a large clinical study, galectin-3 blood concentrations were found to be increased in diabetes and cardiovascular patients with progressive renal impairment (15). In macrophages, galectin-3 is required for their alternative (M2) activation by IL-4. IL-4 leads to increased expression of galectin-3 in macrophages independent of STAT6 but dependent of PI3K activation (40, 45). We hypothesized that elevated IL-13 in renal cyst fluid may promote IL-4/IL-13 receptor activation and subsequently, increased galectin-3 expression in PKD.
Lastly, IL-24 has been shown to play a role in wound healing, tissue integrity, and the response of epithelial cells to infections (50). The interleukin is also a regulator of apoptosis and cell death in cancer cells (14) and is known to signal through STAT3 (1), which is also overactivated in PKD (74). Together, these findings suggested that IL-24 might be important in PKD; however, to our knowledge, IL-24 has not been previously linked to the pathogenesis of PKD.
In this study, we found that periostin, galectin-3, and IL-24 are upregulated in multiple mouse models of PKD and that IL-13/STAT6 signaling plays a substantial role in their aberrant expressions. These findings suggest that the IL-13/STAT6 pathway orchestrates a complex set of cellular responses in renal epithelial cells that may play a role in injury repair under normal conditions, when its activity is properly regulated. However, when IL-13/STAT6 signaling is constitutively activated, such as in PKD, this pathway appears to promote renal cyst growth and disease progression. Identifying the pathways and genes connected to aberrant IL-13/STAT6 signaling in PKD may lead to novel therapeutic avenues.
MATERIALS AND METHODS
Cell lines and reagents.
Mouse inner medullary collecting duct (IMCD) cells were cultured in DMEM/F12 50:50 with 10% FBS (Omega Scientific, Tarzana, CA) and penicillin-streptomycin (Mediatech, Manassas, VA). Human embryonic kidney (HEK) 293 T and human kidney (HK2) cells were cultured in DMEM, supplemented with 10% FBS, l-glutamine, and penicillin-streptomycin. For authentication, cell lines were frequently replaced from low-passage frozen stocks, and their morphology was monitored. Testing to rule out the presence of mycoplasma was done by DAPI staining and fluorescence microscopy. All cells were originally obtained from American Type Culture Collection (Manassas, VA). Mouse and human IL-4 were obtained from R&D Systems (Minneapolis, MN). Mouse IL-13 was obtained from Cell Signaling Technology (Danvers, MA). Human IL-13 was obtained from eBioscience (San Diego, CA). Pyridone 6 (Sigma-Aldrich) was diluted in DMSO and applied to cells to a final concentration of 500 nM. A plasmid for the expression of PC1-p30 has been described previously (62) and has been made available through Addgene (plasmid no. 41520). It contains the sequence from 4107 to 4303 of human PKD1 followed by COOH-terminal Myc/His tags.
Animals.
All animal studies were performed in accordance with the University of California, Santa Barbara, Institutional Animal Care and Use Committee. Mice were maintained in standard vivarium conditions. Wt/bpk and STAT6−/− (Jackson Laboratories, Bar Harbor, ME) were crossed, as previously described (44). The human ADPKD orthologous Pkd1cond/cond:Nestin-Cre mouse model has been described previously (30, 55, 59). The Pkd1cond/−:Nestin-Cre mouse model is distinct in that it has a conditional and a knockout Pkd1 allele. Wild-type C57/BL6 mice were purchased from Jackson Laboratories and allowed to acclimate for 1 wk before intraperitoneal injection of IL-13.
Animals were euthanized with ketamine/xylazine overdose solution followed by cervical dislocation. Tissues were harvested and fixed in 4% paraformaldehyde for 4 h twice, followed by dehydration in increasing concentrations of alcohol for 2 h twice each (70%, 95%, and 100%), 2 h in toluene, and then embedded for 4 h twice in paraffin.
Antibodies.
Well-validated antibodies from commercial vendors used in this study include anti-mouse periostin/OSF-2 (clone 345613) (R&D Systems, Minneapolis, MN), anti-mouse galectin-3/MAC-2 (clone M3/38) (American Type Culture Collection, Manassas, VA), anti-mouse IL-24 (clone 303308) (R&D Systems), anti-human IL-24 (AF1965) (R&D Systems), anti-phospho-STAT6 (Tyr-641) (sc-11762) (Santa Cruz Biotechnology, Dallas Texas), anti-STAT6 (sc-621) (Santa Cruz Biotechnology), β-actin (Sigma-Aldrich, St. Louis, MO), and HRP-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA). All antibodies were additionally validated by confirming that they recognize their antigens in bands of the expected molecular weights by immunoblotting.
Western blot analysis.
Protein lysates were created with lysis buffer containing, 2% SDS, 20% glycerol, 50 mM DTT, 1:300 phosphatase inhibitor cocktail 2 and 3 (Sigma Aldrich), 1:1,000 protease inhibitor cocktail (Sigma Aldrich), in 50 mM Tris pH 6.8. Lysates were separated at 100 V for 60 min and transferred on ice at 100 V for 100 min onto nitrocellulose membranes. Membranes were blocked in either 5% BSA or 5% milk in TBS with 0.05% Tween-20 (TBST) for 45 min at room temperature with agitation, then washed in TBST, and then incubated with the primary antibody in 1% BSA or 1% milk in TBST overnight at 4°C with rotation. The following day, membranes were washed in TBST and incubated in HRP-conjugated secondary antibody in 1% BSA or 1% milk in TBST for 45 min and then washed in TBST. Membranes were imaged using the Azure c300 imager or radiographic film.
Semiquantitative and quantitative RT-PCR.
IMCD cells were incubated with equivalent volume of vehicle control (DPBS), 100 ng/ml mouse IL-4 or 100 ng/ml mouse IL-13. HK2 cells were incubated with equivalent volume of vehicle control (DPBS), 10 ng/ml human IL-4 or 10 ng/ml human IL-13. Following cytokine treatment, total RNA was isolated using RNeasy Plus mini kit (Qiagen, Valencia, CA). Alternatively, total RNA was isolated from HEK293 T cells transfected with green fluorescent protein or PC1-p30 (TurboFect; Thermo Fisher Scientific, Pittsburgh, PA). Two micrograms of total RNA was used to make cDNA using M-MLV reverse transcriptase (Promega, Madison, WI), followed by quantitative PCR (qPCR) using GoTaq qPCR Master Mix (Promega) and the Stratagene Mx3000P real-time PCR System (Agilent Technologies, Santa Clara, CA). The following primers were used for quantitative PCR: human IL24, fwd 5′-TCATCGTGTCACAACTGCAA-3′ and rev 5′-GGCGCTGCTTAAAGAATGAC-3′; mouse periostin, fwd 5′-AGGGATTCGAACCCGGAGTCAC-3′ and rev 5′-TTTGAAGGTGCTGCCACGAACA-3′ (56); canine galectin-3, fwd 5′-TCCACTTTAACCCACGCTTC-3′ and rev 5′-TTCCCAGTTTGCTGATTTCC-3′ (51); human IL-24, fwd 5′-TCATCGTGTCACAACTGCAA-3′ and rev 5′-GGCGCTGCTTAAAGAATGAC-3′; mouse IL-24, fwd 5′-CAAGTGACAGGGGTGGTTCT-3′ and rev 5′-GCTTTCACCAAAGCGACTTC-3′; and cross species β-actin, fwd 5′-GAAGTGTGACGTTGACATCC-3′ and rev 5′-ACAGAGTACTTGCGCTCAGG-3′ (4). Semiquantitative RT-PCR performed using Taq 2× Master Mix (New England BioLabs, Ipswich, MA).
Immunofluorescence.
Five-micrometer sections from formaldehyde-fixed, paraffin-embedded kidneys were deparaffinized in xylene, rehydrated through a graded series of ethanol, and then subjected to antigen retrieval by pressure cooking in 10 mM sodium citrate, at pH 6. Sections incubated with blocking buffer (1% BSA in TBST) for 30 min at 37°C, followed by incubation in 0.1% Sudan Black B in 70% ethanol for 20 min at 25°C to quench autofluorescence. Sections were then washed in TBST and incubated overnight at 4°C with primary antibody in blocking buffer. They were then incubated with FITC-conjugated secondary antibody (Jackson Immunoresearch Laboratories) and rhodamine-labeled Dolichos biflorus agglutinin (DBA; Vector Laboratories, Burlingame, CA) diluted in blocking buffer for 1 h at 37°C, washed in TBST, followed by staining with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min at 25°C. Coverslips were mounted using ProLong Gold Antifade Reagent (Life Technologies, Grand Island, NY).
Microarray analysis.
Total RNA was collected from HEK293 T cells transfected with either a control plasmid or a plasmid encoding PC1-p30 using RNeasy Plus mini kit (Qiagen, Valencia, CA). RNA samples from six independent replicates of each condition were analyzed by Illumina Beadchip microarray at the Sanford Burnham Medical Research Institute core facility (La Jolla, CA). Briefly, the RNA was amplified and labeled using the Illumina TotalPrep RNA amplification kit (Life Technologies/Ambion) and were then analyzed on a Human HT-12v4 48K BeadChip. The Illumina BeadChips were analyzed using the manufacturer’s BeadArray Reader, and primary data were collected using the supplied Scanner software. Data analysis was done in three stages. First, expression intensities were calculated for each gene probed on the array for all hybridizations using Illumina’s BeadStudio 2 software. Second, intensity values were quality controlled and normalized: quality control was carried out by using the Illumina BeadStudio detection P value set to <0.05 as a cutoff. This removed genes, which were effectively absent from the array (that is, were not detected). All of the arrays were then normalized using the normalize.quantiles routine from the Affy package in Bioconductor. This procedure accounted for any variation in hybridization intensity between the individual arrays. Finally, these normalized data were imported into GeneSpring and analyzed for differentially expressed genes. The groups of biological replicates were described to the software, and significantly different expressed genes were determined on the basis of the results of the Welch t-test (parametric test, variances not assumed equal; P value cutoff 0.05), and fold difference changes in expression level. Average quantities of IL-24 gene transcript and standard errors were calculated from the biological replicates.
RESULTS
Periostin overexpression in PKD is dependent on STAT6 activity.
Periostin expression has been shown to be regulated by IL-4 and IL-13 in lung fibroblasts (64). Periostin is known to be upregulated in ADPKD and has been shown to increase cell proliferation in the disease via integrin interactions (68). Given these findings, we hypothesized that STAT6 activation in renal epithelial cells may lead to increased periostin expression. To test this idea, periostin mRNA expression was analyzed by quantitative PCR in mouse IMCD cells after treatment with IL-4 or IL-13. As shown in Fig. 1A, both IL-4 and IL-13 stimulation leads to increased periostin expression. Preventing STAT6 activation with the JAK inhibitor pyridone-6 blunts the IL-4/IL-13-induced periostin expression. These results suggest that STAT6 activity is both necessary and sufficient for increased periostin expression in this system.
Fig. 1.
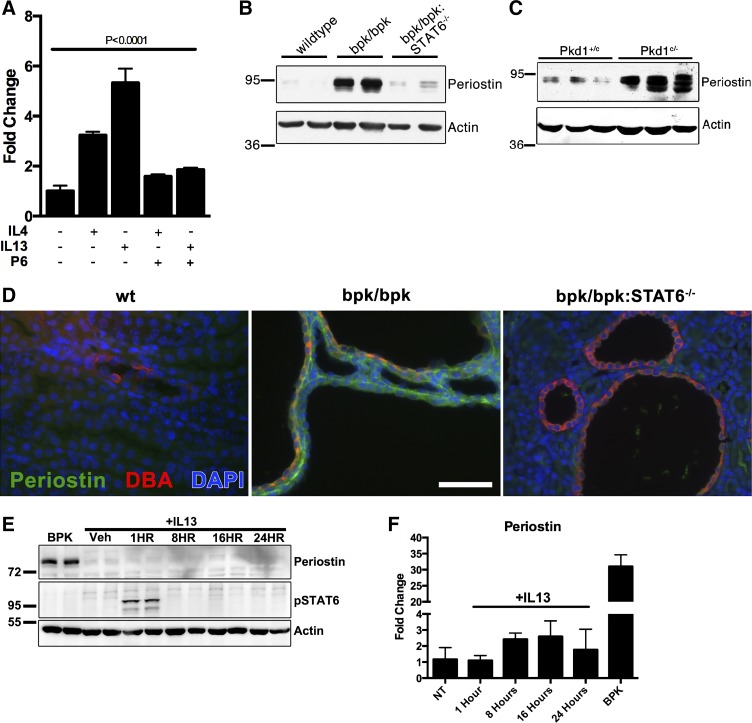
Elevated periostin expression in polycystic kidney disease (PKD) is dependent on IL-13 and STAT6 activity. A: quantitative PCR analysis of mouse periostin from the inner medullary collecting duct (IMCD) cells treated 24 h with vehicle control (PBS), 100 ng/ml mouse IL-4 or 100 ng/ml mouse IL-13, or with prior treatment of 500 nM pyridone-6 (P6). IL-13 treatment increases periostin expression (*P < 0.0001, one-way ANOVA with the Tukey post hoc test). B: immunoblot analysis of periostin in postnatal day 17 wild-type, bpk/bpk and bpk/bpk:STAT6−/− mouse kidney tissue lysates (representative of n = 3). C: immunoblot analysis of periostin in postnatal day 21 control (Pkd1+/c) and cystic (Pkd1c/−) mouse kidney tissue lysates (representative of n = 3). D: immunofluorescence analysis of mouse periostin (green) and Dolichos biflorus agglutinin (DBA, red) in postnatal day 17 wild-type, bpk/bpk and bpk/bpk:STAT6−/− mouse kidney sections. Nuclear staining with DAPI (blue) (scale bar = 100 μm). E: immunoblot of wild-type C57/BL6 mice injected with mouse IL-13 and probed for periostin and phospho-Tyr641 STAT6 (representative of three experimental treatments). F: quantitative PCR for periostin expression in mice from E.
To investigate the involvement of STAT6 in the regulation of periostin in vivo, we analyzed kidney lysates from the bpk/bpk and ADPKD orthologous Pkd1cond/−:Nestin-Cre (Pkd1c/−) mouse models. Periostin was markedly upregulated in the cystic kidneys of both the bpk/bpk (Fig. 1B) and the Pkd1c/− (Fig. 1C) mouse models, compared with wild-type controls, consistent with previous reports in the pcy/pcy mouse model (69). Importantly, genetic ablation of STAT6 in crossed bpk/bpk:STAT6−/− animals dramatically decreases the overexpression of periostin compared with kidneys of bpk/bpk animals that express STAT6 (Fig. 1B), demonstrating the necessity of STAT6 activity for periostin upregulation. Next, we investigated periostin expression by immunofluorescence microscopy and found that it localizes predominantly to the extracellular matrix surrounding renal cysts in bpk/bpk mice, an effect that is largely eliminated in the bpk/bpk:STAT6−/− animals (Fig. 1D). Given our previous findings that cyst growth is diminished in bpk/bpk:STAT6−/− animals, compared with unaltered bpk/bpk animals (47), these novel results suggest that increased periostin expression may be a component of the mechanism underlying STAT6-mediated renal cyst growth. Lastly, we tested the ability of STAT6 to activate periostin expression in wild-type mice injected with IL-13. Injection of IL-13 led to an increase in phosphorylated STAT6 but no increase in detectable periostin protein (Fig. 1E). Periostin mRNA expression was analyzed in these mice by quantitative PCR (Fig. 1F), showing a modest increase in periostin mRNA in response to IL-13 treatment. The expression is, however, still relatively low compared with the BPK mouse kidneys (Fig. 1F). Taken together, these data indicate that STAT6 activation in vivo is necessary and sufficient for increased periostin expression, but there may exist additional levels of posttranscriptional regulation.
STAT6 activation contributes to elevated galectin-3 expression in PKD.
Galectin-3 has previously been shown to be upregulated in cyst-lining cells in human ARPKD and the nonorthologous cpk mouse model (11, 78). We began by investigating whether galectin-3 expression is also increased in human ADPKD, in an orthologous PKD1 mouse model, and the nonorthologous bpk model. We found that galectin-3 expression was elevated in the renal tissue of human ADPKD patients (Fig. 2A), as well as, the bpk/bpk (Fig. 2B) and orthologous Pkd1c/− (Fig. 2C) mouse models. This result suggests that galectin-3 overexpression is a feature of many, if not all, forms of renal cystic diseases independent of the genetic cause.
Fig. 2.
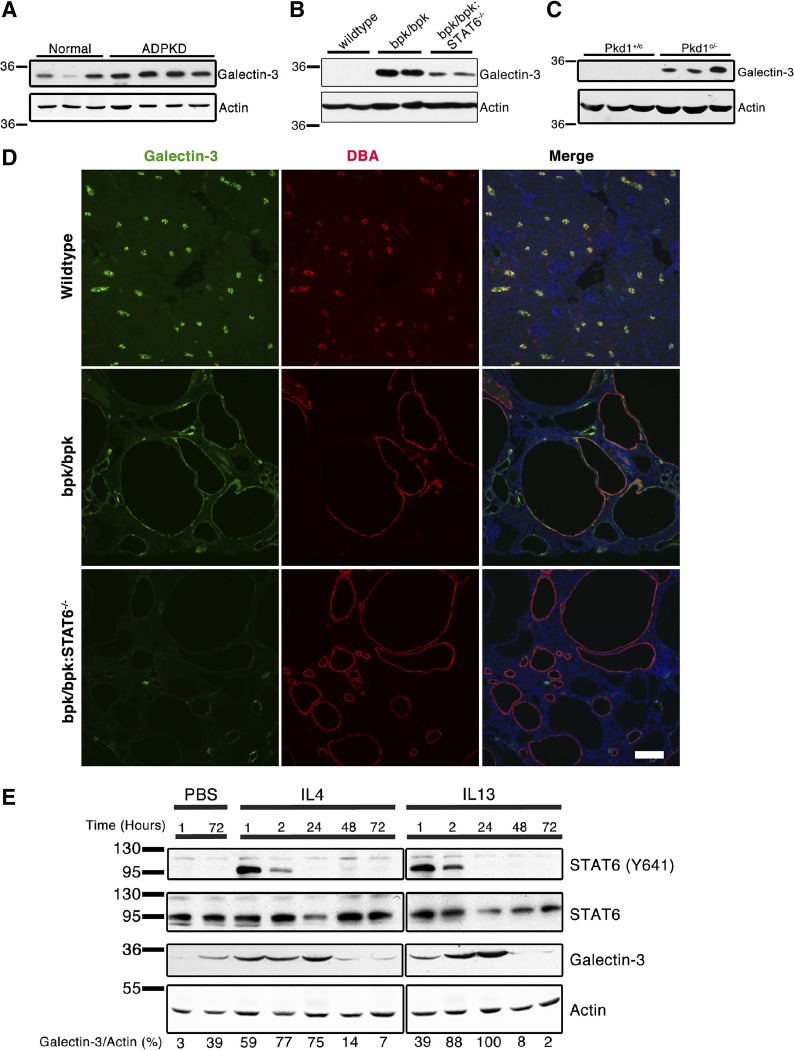
IL-13 and STAT6 activity contribute to increased galectin-3 expression in polycystic kidney disease (PKD). Immunoblot analysis of galectin-3 expression in normal and autosomal dominant (AD) PKD human patient kidney tissue lysates, from different patients per lane (A), postnatal day 17 wild-type, bpk/bpk and bpk/bpk:STAT6−/− mouse kidney tissue lysates (B), postnatal day 21 control (Pkd1+/c) and cystic (Pkd1c/−) mouse kidney tissue lysates (samples from different animals per lane) (C). D: immunofluorescence analysis of galectin-3 (green) and Dolichos biflorus agglutinin (DBA, red) in postnatal day 17 wild-type, bpk/bpk, and bpk/bpk:STAT6−/− mouse kidney sections. Nuclear staining with DAPI (blue) (Scale bar = 100 μm). E: immunoblot analysis of galectin-3 expression in wild-type C57BL/6 mouse kidney lysates after intraperitoneal injection of 1 μg of recombinant mouse IL-4 or IL-13. Galectin-3 signals were quantified (performed using Image Studio software) and were normalized to actin with the resulting values indicated underneath each lane. The blots shown were quantified from the same electrophoresis gel, but separated to improve presentation (representative of three experimental treatments).
The ablation of the STAT6 gene in bpk/bpk mice leads to a marked reduction of galectin-3, compared with unaltered bpk/bpk mice (Fig. 2B). However, this alteration does not completely eliminate the original galectin-3 upregulation in the bpk/bpk mice (Fig. 2B), indicating that STAT6 activity is important, but not completely required, for galectin-3 expression. We next analyzed kidney sections by immunofluorescence microscopy. In wild-type kidneys, galectin-3 expression is primarily restricted to DBA-positive epithelial cells in the collecting duct (Fig. 2D), consistent with previous reports (11, 78). In cystic kidneys of bpk/bpk mice, galectin-3 is strongly expressed in DBA-positive cysts but also in cysts that do not express the collecting duct marker DBA and are likely derived from other tubule segments. Galectin-3 localizes to the cytoplasm of cyst-lining cells (Fig. 2D), consistent with its reported localization in human ARPKD and cpk mice (11, 78). Expression of galectin-3 in all cysts is strongly reduced, but not completely eliminated, in kidneys of bpk/bpk:STAT6−/− mice (Fig. 2D).
We further confirmed the relationship between IL-4/IL-13-induced STAT6 signaling and increased galectin-3 expression by injecting 8–10-wk-old wild-type (C57BL/6) mice with a single dose of 1 μg recombinant mouse IL-4 or IL-13 and tracking the changes in galectin-3 expression at various time points. As shown in Fig. 2E, both IL-4 and IL-13 activate STAT6 rapidly in vivo and lead to increased galectin-3 expression, peaking at ~24 h postinjection. Altogether, these results indicate that galectin-3 expression is significantly driven by the IL-4/IL-13/STAT6 pathway in the kidney, leading to overexpression in human ADPKD and mouse models.
IL-24 is overexpressed in PKD and regulated by both IL-13 signaling and the cleaved PC1 cytoplasmic tail (PC1-p30).
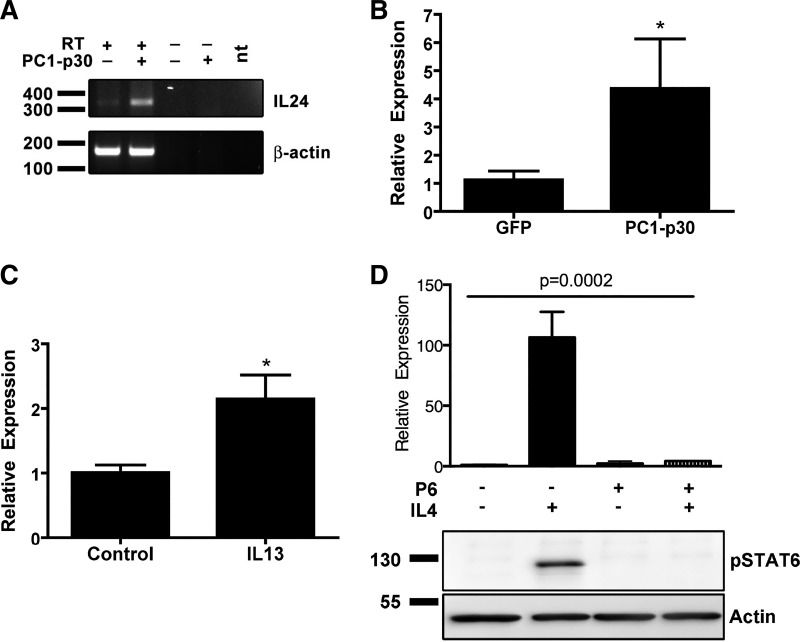
We have previously shown that PC1-p30 is, paradoxically, overexpressed in human ADPKD kidneys (39, 62) and that PC1-p30 can enhance STAT3 and STAT6 signaling (74). To identify genes regulated by PC1-p30, we compared the gene expression profiles by microarray analysis of HEK293 T cells expressing PC1-p30 vs. controls (six replicates each). No changes in expression of periostin or galectin 3 were noted in this analysis. However, one of the most highly upregulated genes was IL-24, which showed an increased mean fold change of 3.1 (SE ±0.48) over control. This regulation was confirmed by determining IL-24 mRNA levels in HEK293 T cells by semiquantitative RT-PCR (Fig. 3A) and quantitative PCR (Fig. 3B).
Fig. 3.
IL-24 expression is upregulated after PC1-p30 overexpression or IL-13 treatment. A: semiquantitative RT-PCR of IL-24 from PC1-p30 overexpression in HEK293 T cells. RT, reverse transcriptase; nt, no template. B: quantitative PCR analysis of IL-24 expression after overexpression of PC1-p30 in human embryonic kidney (HEK) 293 T cells (*P < 0.05, one-tailed t-test). C: quantitative PCR analysis of mouse IL-24 mRNA in mouse inner medullary collecting duct (IMCD) cells treated with vehicle control (PBS) or 100 ng/ml mouse IL-13 (*P < 0.05, one-tailed t-test). D, top: quantitative PCR of human kidney 2 (HK2) cells treated with vehicle (PBS), 100 ng human IL-4 for 24 h with or without prior treatment with 500 nM pyridone-6 (P = 0.0002, one-way ANOVA). D, bottom: immunoblot of HK2 cells treated with vehicle (PBS), 100 ng IL-4 for 1 h with or without 30 min prior treatment with 500 nM pyridone-6 (representative of n = 3).
To our knowledge, IL-24 has not been previously investigated in PKD. However, a link between IL-24 and PKD may be intriguing, as IL-24 expression has been shown to be regulated by the binding of STAT6 to the IL-24 gene promoter in immune cells (55). Furthermore, IL-24 binding to its receptor leads to STAT3 activation (1), a transcription factor shown by our laboratory and others to be aberrantly activated in PKD mouse models and human ADPKD, and likely be involved in promoting renal cyst growth (36, 62, 63).
To test whether IL-24 is regulated by IL-4/IL-13 signaling in renal epithelial cells, we treated two different kidney cell lines, mouse IMCD cells and human HK2 cells, with IL-4 or IL-13. Analysis of mRNA expression by qPCR showed that IL-24 expression is significantly upregulated in response to IL-4/IL-13 signaling in both cell lines (Fig. 3, C and D).
To test the necessity of STAT6 to activate IL-24 transcription, we inhibited JAK-dependent STAT6 activation in HK2 cells with pyridone-6 before treatment with IL-4. Pyridone-6-treated cells were unable to activate STAT6 following IL-4 treatment and showed a blunted expression of IL-24 (Fig. 3D). These data indicate that STAT6 activation is required for IL-24 expression.
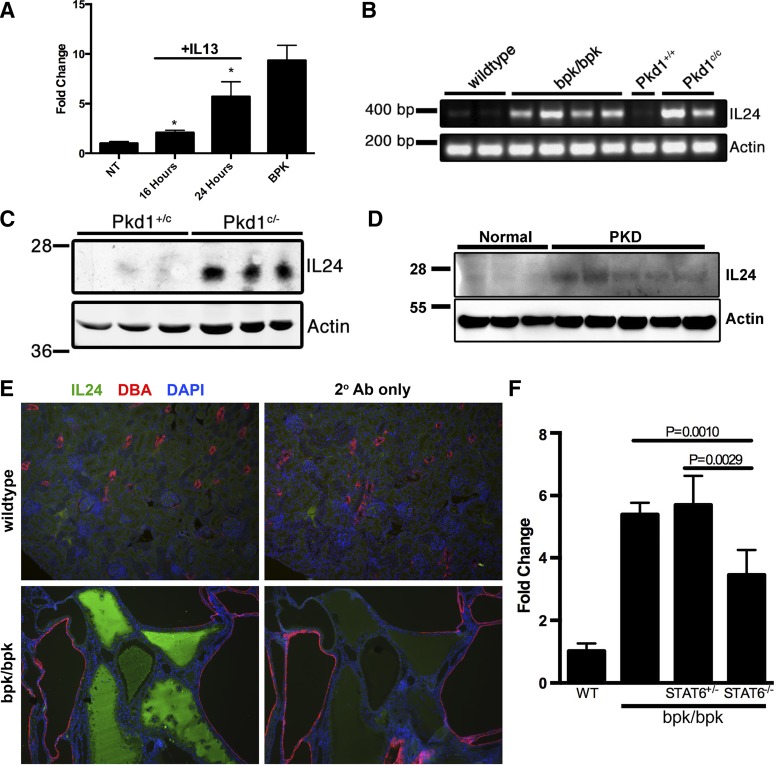
Next, we investigated IL-24 expression in various PKD mouse models. To test the ability of IL-13 to activate IL-24 in vivo, wild-type mice were treated with IL-13 followed by analysis of IL-24 expression by quantitative PCR (Fig. 4A). Treatment with IL-13 led to a time-dependent increase in IL-24 expression (Fig. 4A). Analysis of IL-24 mRNA expression by semiquantitative RT-PCR from total kidneys revealed that IL-24 mRNA is strongly overexpressed in both the bpk/bpk and the ADPKD orthologous Pkd1cond/cond:Nestin-Cre mouse models, compared with age-matched control kidneys (Fig. 4B). We also found that IL-24 protein expression is significantly increased in kidney lysates from the orthologous Pkd1c/− mouse model, compared with wild-type controls (Fig. 4C), verifying the changes seen in the mRNA expression. Increased IL-24 expression was also observed in human ADPKD kidney lysates compared with normal controls (Fig. 4D).
Fig. 4.
IL-24 upregulation in polycystic kidney disease (PKD) mouse models. A: quantitative PCR of IL-24 from IL-13-treated C57/BL6 mice. B: semiquantitative RT-PCR of extracted total RNA from wild-type and cystic kidney tissue of the bpk/bpk and orthologous Pkd1c/c mouse models. C: immunoblot analysis of IL-24 expression in wild-type (Pkd1+/c) and cystic (Pkd1c/−) mouse kidney tissue lysates (representative of n = 2). D: immunoblot of IL-24 from normal and PKD human kidney lysates (representative of n = 3). E: immunofluorescence staining for IL-24 in wild-type and bpk/bpk mouse kidney sections (scale bar = 50 μm). F: quantitative PCR from bpk/bpk mice with and without STAT6 expression (P values calculated using two-tailed t-test).
Additionally, we analyzed renal tissue sections from wild-type and bpk/bpk mice by immunofluorescence microscopy to visualize IL-24 protein in vivo. In wild-type kidneys, we observed very little IL-24 staining (Fig. 4E). In contrast, we detected strong IL-24 immunosignals in the protein casts within renal cyst lumens in bpk/bpk mice (Fig. 4E). Costaining with the distal tubule marker DBA revealed that IL-24 staining is absent in DBA-positive cysts (Fig. 4E). To examine the effect of STAT6 on the expression of IL-24, bpk/bpkSTAT6−/− kidneys were analyzed by quantitative PCR (Fig. 4F). Deletion of both STAT6 alleles led to a decrease in renal IL-24 expression compared with BPK mice expressing STAT6 (Fig. 4F). These results suggest that IL-24 is expressed by epithelial cells lining specific cysts and that it is secreted apically into cyst lumens, where it may accumulate and stimulate cells in an autocrine and paracrine fashion.
DISCUSSION
The purpose of this investigation was to identify molecular mechanisms that may explain how aberrantly activated IL-13 and STAT6 signaling in PKD contributes to renal cyst growth. We found that periostin, a protein previously linked to disease progression in PKD, is regulated by IL-13 and STAT6 signaling in renal epithelial cells and in PKD mouse models. We show that galectin-3 expression is elevated in multiple mouse models of PKD and human ADPKD patients and that the activity of the IL-13/STAT6 pathway play significant roles in these changes. We found that the expression of the cytokine IL-24, which had not been previously investigated in PKD, can be regulated by PC1-p30 and IL-13 in renal epithelial cells, and that IL-24 is upregulated in mouse models of PKD.
Periostin.
We report that cultured renal epithelial cells increase periostin expression in response to IL-4/IL-13, and is inhibited by pyridone-6 (Fig. 1A), which is consistent with previous data in other cell types (64, 80). Periostin has previously been shown to be highly overexpressed in renal tissue of human ADPKD and ARPKD patients, and in the cpk, jck, and pcy mouse models of PKD (68, 69). Our finding that periostin is also strongly overexpressed in the bpk/bpk mouse model, which has a defect in the Bicc1 gene (12), and the ADPKD orthologous Pkd1c/− mouse model, which involves conditional knockout and null-Pkd1 alleles, further solidifies these previous findings and suggests that periostin may be universally overexpressed in most, if not all, forms of renal cystic diseases, irrespective of the affected gene. We found that STAT6 gene ablation in bpk/bpk mice leads to a substantial reduction of periostin expression, to a level that is comparable to wild-type kidneys (Fig. 1B). This suggests that STAT6 activity is absolutely required for elevated periostin expression in renal cysts.
On the other hand, STAT6 activity may not be sufficient for periostin protein expression in normal kidneys. When normal mice were challenged with a single dose of IL-13, we observed a modest increase in periostin mRNA (Fig. 1F) but no detectable increase in periostin protein expression (Fig. 1E). The explanation may simply be that more persistent STAT6 activation is required to lead to the accumulation of detectable extracellular periostin protein levels. It is also possible that periostin expression is regulated by additional posttranscriptional mechanisms that may be unaffected by STAT6.
Crossing the pcy mouse model of PKD with periostin-null mice leads to reduced renal cyst growth, proliferation, fibrosis, and mTOR activity, as well as partial preservation of renal function and extended survival (69). These findings are interesting in light of our previous findings that STAT6 knockout improves PKD disease progression in the bpk/bpk mouse (47). Given that periostin expression is largely abolished in bpk/bpk mice lacking STAT6 (Fig. 1B), it is plausible that some of the beneficial effects of STAT6 inhibition or genetic ablation on disease severity may be due to the loss of periostin expression.
The normal purpose of periostin upregulation in renal epithelial cells after STAT6 activation remains unclear. Periostin has been reported to have multiple functions, including the promotion of epithelial to mesenchymal transition, fibrosis, and cell proliferation (56, 68, 69), all of which, if aberrantly upregulated, may play a role in PKD. Periostin is also known to play a role in the development of the periodontal ligament (25), cardiac valves (33), the kidney, and the ureter (60). The protein has been linked to pathological roles in cancer (34, 43, 53) and to subepithelial fibrosis in bronchial asthma (64).
Periostin was found to undergo vitamin K-dependent posttranslational modification of glutamic acid residues to γ-carboxyglutamic acid (Gla) (13). The ability of Gla to bind Ca2+, as well as the high periostin expression in the periosteum, suggests a role for periostin in tissue calcification, although it is still unclear whether periostin promotes or prevents calcification (18, 58). Interestingly, the vitamin-K-dependent enzyme responsible for the Gla posttranslational modification, γ-glutamyl carboxylase, is expressed in kidney tubule epithelial cells, and its enzyme activity is upregulated after kidney injury, suggesting a role for a Gla-modified protein in kidney injury (2, 19). In this regard, periostin expression is upregulated after glomerular injury, as well as in chronic kidney diseases, where it is secreted into urine and can serve as a biomarker (56, 57). On the basis of the idea that cellular changes in PKD represent the aberrant activation of changes that are normally activated in response to renal injury (72, 73), we suggest that periostin plays an important, yet currently undefined, role in renal injury repair.
Galectin-3.
Galectin-3 is a β-galactoside-binding lectin that can be found in numerous subcellular locations, including the nucleus, mitochondria, vesicles, and cytoplasm, and is known to associate extracellularly with the cell surface and the extracellular matrix (16). It has numerous ascribed roles in the regulation of proliferation, apoptosis, cell adhesion, migration, signaling, pre-mRNA splicing, transcription, and protein sorting (16). Galectin-3 has also been shown to regulate M2 macrophage differentiation (40). In the kidney, galectin-3 is expressed during renal development (7, 78). In adult kidneys, galectin-3 is expressed at low levels in some distal or collecting tubule cells (44, 78). Galectin-3-null mice are viable and have only mild kidney defects, including a reduction of glomeruli by ~11% (5).
In this study, we demonstrate very strong upregulation of galectin-3 in the kidneys of human ADPKD tissue (Fig. 2A), the bpk/bpk (Fig. 2B), and ADPKD orthologous Pkd1c/− (Fig. 2C) mouse models. This finding is consistent with reported galectin-3 overexpression in human ARPKD, multicystic dysplastic kidneys, the Han:SPRD Cy rat, and the cpk mouse model (8, 11, 78), suggesting that this protein is highly expressed in most, if not all, renal cystic diseases, irrespective of the affected gene. Furthermore, we show that treatment with IL-4 and IL-13 increases galectin-3 expression in vivo (Fig. 2D), implying a direct link between STAT6 activity and galectin-3 expression. However, in the bpk/bpk:STAT6−/− mice, we observed a significant, but not complete, reduction of galectin-3 protein expression, compared with the unaltered bpk/bpk animals (Fig. 2B). This indicates that while STAT6 is likely involved in the expression of galectin-3 in renal cysts, it is not absolutely essential. Since the IL-4/IL-13 receptor also signals to the PI3K pathway, in addition to STAT6, we suggest that the upregulation of galectin-3 in PKD may also be dependent on PI3K activation. This would be consistent with previous findings in immune cells, where IL-4 regulates galectin-3 expression through PI3K activation, independent of STAT6 (40). The reduced galectin-3 expression in the bpk/bpk:STAT6−/− mice would then likely be the result of blunting the positive feedback loop involving IL-13, the IL-4/IL-13 receptor, and STAT6, which leads to decreased IL-13 in these mice (47), and would, therefore, also blunt PI3K activation. There are a variety of other mechanisms by which galectin-3 can be regulated and, thus, may explain the remaining galectin-3 expression in the bpk/bpk:STAT6−/− kidneys. Mechanisms reported to upregulate galectin-3 include hypoxia, via HIF-1α (81), the transcription factors RUNX1 and RUNX2 (82), and β1 integrin signaling (41, 71).
Another possibility is that the changes in galectin-3 expression between wild-type and bpk/bpk mice are not specific to PKD, but rather a result of the substantial increases in inflammation and fibrosis that are associated with PKD. Galectin-3 is known to be involved in these processes through several mechanisms. It promotes the mitogenesis of fibroblasts and secretion of fibrotic proteins (16), and regulates macrophage phenotypes by inducing M2 macrophage differentiation through a positive feedback loop (40). Galectin-3ʼs involvement in macrophage regulation is particularly relevant, given the fact that M2 macrophages have been shown to be deleterious in PKD (28, 61). Galectin-3 upregulation is also associated with multiple inflammatory pathologies, including numerous types of cancer, asthma, heart disease, obesity, arthritis, and parasitic infections (17, 38, 52, 67, 75, 76). Galectin-3 is expressed in the glomeruli of a systemic lupus erythmatosis mouse model (27) and is strongly upregulated in tubule cells after ischemia/reperfusion or folic acid injury in rats (44), and unilateral ureteral obstruction (UUO) in mice (24). Galectin-3-null kidneys show reduced fibrosis after UUO, although it is believed that galectin-3 expressed by macrophages is responsible for the promotion of fibrosis, as reintroduction of wild-type macrophages increases fibrosis (24).
Despite the presumed positive role of galectin-3 in fibrosis, this protein may paradoxically play an inhibitory role in renal cyst growth. Galectin-3 has been shown to inhibit cyst growth in Madin-Darby canine kidney cells cultured in three-dimensional matrices (3), and genetic loss of galectin-3 has been shown to promote disease progression in the cpk mouse model of PKD (11). Taken together, our results suggest that elevated galectin-3 expression in PKD is driven by a combination of IL-13-mediated STAT6 and PI3K activity, but the exact role of galectin-3 in disease progression remains to be identified.
Interleukin-24.
We found that overexpression of PC1-p30 or treatment with IL-13 increases the expression of IL-24 in renal epithelial cells (Fig. 3, A–D) and in vivo (Fig. 4A). Furthermore, we observed significant upregulation of IL-24 mRNA and protein levels in human PKD, and mouse models of PKD (Fig. 4, B–D). Interestingly, a microarray analysis of the Han:SPRD Cy rat also revealed upregulation of the IL-24 gene (8), indicating that IL-24 may be commonly overexpressed in renal cystic disease. We observed a marked increase in IL-24 in bpk/bpk mice compared with wild-type controls. IL-1β, which has been found in renal cyst fluid (20), has also been reported to increase expression of IL-24, through p38 MAPK activation (49), and may contribute to IL-24 expression in PKD.
It is currently difficult to assign a role for IL-24 in PKD because the physiological function of IL-24 has remained largely unknown. Increased expression of IL-24 has been found in several diseases, including psoriasis, rheumatoid arthritis, and inflammatory bowel disease (1, 32, 54). When artificially expressed using viral vectors, IL-24 has been shown to be a strong inducer of cell death of tumor cells, which has led to its evaluation as a clinical cancer therapeutic (14). Other studies indicate that IL-24 is not a cell-death inducer by itself but rather acts as a sensitizer to TLR-mediated cell death under conditions of viral infection (77). Interestingly, activation of CD14/TLR4 signaling has been proposed to play a role in the pathogenesis of PKD (83). It is possible that IL-24 may modulate TLR signaling in PKD and may contribute to the high rate of apoptosis of cyst-lining cells.
IL-24 signals via two different heterodimeric receptor complexes, IL-20R1/IL-20R2 and IL-22R1/IL-20R2, both of which lead to activation of STAT3 (1, 70). Therefore, it is possible that IL-24 contributes to the strong activation of STAT3 observed in renal cysts (36, 62, 63). Furthermore, the presence of IL-24 in cyst lumens (Fig. 4E) may suggest the possibility of persistent IL-24-receptor/STAT3 activation. However, it is currently unknown whether cyst-lining epithelial cells express IL-24 receptors. The possible role of IL-24 in STAT3 activation in PKD requires further investigation.
In conclusion, our findings suggest that aberrantly increased STAT6 activity, via IL-13 signaling, in PKD leads to strong overexpression of four proteins: periostin, galectin-3, IL-24 (this study), and the pIgR (48). These proteins are overexpressed in polycystic kidneys caused by a wide variety of genetic defects, suggesting that IL-13 signaling, and subsequent STAT6 activation, may orchestrate a coordinated expression of a set of genes as a general feature accompanying renal cyst growth. While the exact roles of periostin, galectin-3, and IL-24 are still largely unclear, a common denominator among their proposed functions is a role in the regulation of innate immune responses. The IL-13/STAT6 signaling pathway is a promising target for PKD therapy, especially since inhibitory compounds are already being developed for the treatment of asthma, in which IL-13 signaling is also aberrantly activated (26, 29, 46).
GRANTS
This work was supported by grants from the National Institutes of Health (R01-DK-078043, R01-DK-109563) to T. Weimbs and gifts from the Lillian Goldman Charitable Trust and the Amy P. Goldman Foundation to the University of California, Santa Barbara, to support the work of T. Weimbs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.E.O., J.D.W., J.A.T. and T.W. provided conception and design of research experiments. E.E.O., J.D.W. and J.A.T. performed experiments. N.D. conducted the microarray experiments and provided analyzed data. E.E.O., J.D.W., J.A.T. and T.W. interpreted results of experiments and prepared figures. E.E.O., J.D.W., J.A.T. and T.W. drafted, revised and edited manuscript. T.W. directed the research as the principal investigator and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Katie Lee and Sirpi Nackeeran for help with experiments.
REFERENCES
- 1.Andoh A, Shioya M, Nishida A, Bamba S, Tsujikawa T, Kim-Mitsuyama S, Fujiyama Y. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J Immunol 183: 687–695, 2009. doi: 10.4049/jimmunol.0804169. [DOI] [PubMed] [Google Scholar]
- 2.Angayarkanni N, Selvam R. Enhanced renal vitamin-K-dependent γ-glutamyl carboxylase activity in experimental rat urolithiasis. Eur Urol 33: 116–120, 1998. doi: 10.1159/000019523. [DOI] [PubMed] [Google Scholar]
- 3.Bao Q, Hughes RC. Galectin-3 expression and effects on cyst enlargement and tubulogenesis in kidney epithelial MDCK cells cultured in three-dimensional matrices in vitro. J Cell Sci 108: 2791–2800, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Barlucchi L, Leri A, Dostal DE, Fiordaliso F, Tada H, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. Canine ventricular myocytes possess a renin-angiotensin system that is upregulated with heart failure. Circ Res 88: 298–304, 2001. doi: 10.1161/01.RES.88.3.298. [DOI] [PubMed] [Google Scholar]
- 5.Bichara M, Attmane-Elakeb A, Brown D, Essig M, Karim Z, Muffat-Joly M, Micheli L, Eude-Le Parco I, Cluzeaud F, Peuchmaur M, Bonvalet JP, Poirier F, Farman N. Exploring the role of galectin 3 in kidney function: a genetic approach. Glycobiology 16: 36–45, 2006. doi: 10.1093/glycob/cwj035. [DOI] [PubMed] [Google Scholar]
- 6.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14: 608–616, 2002. doi: 10.1016/S0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 7.Bullock SL, Johnson TM, Bao Q, Hughes RC, Winyard PJ, Woolf AS. Galectin-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J Am Soc Nephrol 12: 515–523, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Burtey SJ, Riera M, Fontés M. Overexpression of complement-component genes in Han:SPRD rats a model of polycystic kidney disease. Kidney Int 73: 1324–1325, 2008. doi: 10.1038/ki.2008.50. [DOI] [PubMed] [Google Scholar]
- 9.Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. J Cell Biol 191: 701–710, 2010. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hieseberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114: 1433–1443, 2004. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu MG, Johnson TM, Woolf AS, Dahm-Vicker EM, Long DA, Guay-Woodford L, Hillman KA, Bawumia S, Venner K, Hughes RC, Poirier F, Winyard PJ. Galectin-3 associates with the primary cilium and modulates cyst growth in congenital polycystic kidney disease. Am J Pathol 169: 1925–1938, 2006. doi: 10.2353/ajpath.2006.060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cogswell C, Price SJ, Hou X, Guay-Woodford LM, Flaherty L, Bryda EC. Positional cloning of jcpk/bpk locus of the mouse. Mamm Genome 14: 242–249, 2003. doi: 10.1007/s00335-002-2241-0. [DOI] [PubMed] [Google Scholar]
- 13.Coutu DL, Wu JH, Monette A, Rivard GE, Blostein MD, Galipeau J. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem 283: 17991–18001, 2008. doi: 10.1074/jbc.M708029200. [DOI] [PubMed] [Google Scholar]
- 14.Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel DT, Fisher PB. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-targeted toxicity. Cytokine Growth Factor Rev 21: 381–391, 2010. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drechsler C, Delgado G, Wanner C, Blouin K, Pilz S, Tomaschitz A, Kleber ME, Dressel A, Willmes C, Krane V, Krämer BK, März W, Ritz E, van Gilst WH, van der Harst P, de Boer RA. Galectin-3, renal function, and clinical outcomes: results from the LURIC and 4D studies. J Am Soc Nephrol 26: 2213–2221, 2015. doi: 10.1681/ASN.2014010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta 1760: 616–635, 2006. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Filer A, Bik M, Parsonage GN, Fitton J, Trebilcock E, Howlett K, Cook M, Raza K, Simmons DL, Thomas AM, Salmon M, Scheel-Toellner D, Lord JM, Rabinovich GA, Buckley CD. Galectin 3 induces a distinctive pattern of cytokine and chemokine production in rheumatoid synovial fibroblasts via selective signaling pathways. Arthritis Rheum 60: 1604–1614, 2009. doi: 10.1002/art.24574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fortunati D, Reppe S, Fjeldheim AK, Nielsen M, Gautvik VT, Gautvik KM. Periostin is a collagen associated bone matrix protein regulated by parathyroid hormone. Matrix Biol 29: 594–601, 2010. doi: 10.1016/j.matbio.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Friedman PA, Mitch WE, Silva P. Localization of renal vitamin K-dependent gamma-glutamyl carboxylase to tubule cells. J Biol Chem 257: 11037–11040, 1982. [PubMed] [Google Scholar]
- 20.Gardner KD Jr, Burnside JS, Elzinga LW, Locksley RM. Cytokines in fluids from polycystic kidneys. Kidney Int 39: 718–724, 1991. doi: 10.1038/ki.1991.87. [DOI] [PubMed] [Google Scholar]
- 21.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for α(V)β3 and α(V)β5 integrins and promotes cell motility. Cancer Res 62: 5358–5364, 2002. [PubMed] [Google Scholar]
- 22.Goenka S, Kaplan MH. Transcriptional regulation by STAT6. Immunol Res 50: 87–96, 2011. doi: 10.1007/s12026-011-8205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PC, Torres VE. Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol 172: 288–298, 2008. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res 14: 1239–1249, 1999. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 26.Ingram JL, Kraft M. IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J Allergy Clin Immunol 130: 829–842, 2012. doi: 10.1016/j.jaci.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 27.Kang EH, Moon KC, Lee EY, Lee YJ, Lee EB, Ahn C, Song YW. Renal expression of galectin-3 in systemic lupus erythematosus patients with nephritis. Lupus 18: 22–28, 2009. doi: 10.1177/0961203308094361. [DOI] [PubMed] [Google Scholar]
- 28.Karihaloo A, Koraishy F, Huen SC, Lee Y, Merrick D, Caplan MJ, Somlo S, Cantley LG. Macrophages promote cyst growth in polycystic kidney disease. J Am Soc Nephrol 22: 1809–1814, 2011. doi: 10.1681/ASN.2011010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kasaian MT, Miller DK. IL-13 as a therapeutic target for respiratory disease. Biochem Pharmacol 76: 147–155, 2008. doi: 10.1016/j.bcp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Kipp KR, Rezaei M, Lin L, Dewey EC, Weimbs T. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 310: F726–F731, 2016. doi: 10.1152/ajprenal.00551.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotanides H, Reich NC. Interleukin-4-induced STAT6 recognizes and activates a target site in the promoter of the interleukin-4 receptor gene. J Biol Chem 271: 25555–25561, 1996. doi: 10.1074/jbc.271.41.25555. [DOI] [PubMed] [Google Scholar]
- 32.Kragstrup TW, Otkjaer K, Holm C, Jørgensen A, Hokland M, Iversen L, Deleuran B. The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 41: 16–23, 2008. doi: 10.1016/j.cyto.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev 103: 183–188, 2001. doi: 10.1016/S0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 34.Kudo Y, Siriwardena BS, Hatano H, Ogawa I, Takata T. Periostin: novel diagnostic and therapeutic target for cancer. Histol Histopathol 22: 1167–1174, 2007. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Kaminski N, Dolganov G, Grunig G, Koth L, Solomon C, Erle DJ, Sheppard D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am J Respir Cell Mol Biol 25: 474–485, 2001. doi: 10.1165/ajrcmb.25.4.4522. [DOI] [PubMed] [Google Scholar]
- 36.Leonhard WN, van der Wal A, Novalic Z, Kunnen SJ, Gansevoort RT, Breuning MH, de Heer E, Peters DJ. Curcumin inhibits cystogenesis by simultaneous interference of multiple signaling pathways: in vivo evidence from a Pkd1-deletion model. Am J Physiol Renal Physiol 300: F1193–F1202, 2011. doi: 10.1152/ajprenal.00419.2010. [DOI] [PubMed] [Google Scholar]
- 37.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, Granger DN. Periostin mediates vascular smooth muscle cell migration through the integrins αvβ3 and αvβ5 and focal adhesion kinase (FAK) pathway. Atherosclerosis 208: 358–365, 2010. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.López E, del Pozo V, Miguel T, Sastre B, Seoane C, Civantos E, Llanes E, Baeza ML, Palomino P, Cárdaba B, Gallardo S, Manzarbeitia F, Zubeldia JM, Lahoz C. Inhibition of chronic airway inflammation and remodeling by galectin-3 gene therapy in a murine model. J Immunol 176: 1943–1950, 2006. doi: 10.4049/jimmunol.176.3.1943. [DOI] [PubMed] [Google Scholar]
- 39.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell 10: 57–69, 2006. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 40.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, Haslett C, Forbes SJ, Sethi T. Regulation of alternative macrophage activation by galectin-3. J Immunol 180: 2650–2658, 2008. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 41.Margadant C, van den Bout I, van Boxtel AL, Thijssen VL, Sonnenberg A. Epigenetic regulation of galectin-3 expression by β1 integrins promotes cell adhesion and migration. J Biol Chem 287: 44684–44693, 2012. doi: 10.1074/jbc.M112.426445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merrick D, Chapin H, Baggs JE, Yu Z, Somlo S, Sun Z, Hogenesch JB, Caplan MJ. The γ-secretase cleavage product of polycystin-1 regulates TCF and CHOP-mediated transcriptional activation through a p300-dependent mechanism. Dev Cell 22: 197–210, 2012. doi: 10.1016/j.devcel.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch 459: 465–475, 2011. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishiyama J, Kobayashi S, Ishida A, Nakabayashi I, Tajima O, Miura S, Katayama M, Nogami H. Up-regulation of galectin-3 in acute renal failure of the rat. Am J Pathol 157: 815–823, 2000. doi: 10.1016/S0002-9440(10)64595-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Novak R, Dabelic S, Dumic J. Galectin-1 and galectin-3 expression profiles in classically and alternatively activated human macrophages. Biochim Biophys Acta 1820: 1383–1390, 2012. doi: 10.1016/j.bbagen.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 46.Oh CK, Geba GP, Molfino N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur Respir Rev 19: 46–54, 2010. doi: 10.1183/09059180.00007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 108: 18067–18072, 2011. doi: 10.1073/pnas.1111966108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsan EE, Matsushita T, Rezaei M, Weimbs T. Exploitation of the polymeric immunoglobulin receptor for antibody targeting to renal cyst lumens in polycystic kidney disease. J Biol Chem 290: 15,679–15,686, 2015. doi: 10.1074/jbc.M114.607929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Otkjaer K, Holtmann H, Kragstrup TW, Paludan SR, Johansen C, Gaestel M, Kragballe K, Iversen L. The p38 MAPK regulates IL-24 expression by stabilization of the 3′ UTR of IL-24 mRNA. PLoS One 5: e8671, 2010. doi: 10.1371/journal.pone.0008671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol 29: 71–109, 2011. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 51.Poland PA, Rondanino C, Kinlough CL, Heimburg-Molinaro J, Arthur CM, Stowell SR, Smith DF, Hughey RP. Identification and characterization of endogenous galectins expressed in Madin-Darby canine kidney cells. J Biol Chem 286: 6780–6790, 2011. doi: 10.1074/jbc.M110.179002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quattroni P, Li Y, Lucchesi D, Lucas S, Hood DW, Herrmann M, Gabius HJ, Tang CM, Exley RM. Galectin-3 binds Neisseria meningitidis and increases interaction with phagocytic cells. Cell Microbiol 14: 1657–1675, 2012. doi: 10.1111/j.1462-5822.2012.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci 66: 2219–2230, 2009. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, Kasman I, Winer J, Modrusan Z, Danilenko DM, Ouyang W. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol 178: 2229–2240, 2007. doi: 10.4049/jimmunol.178.4.2229. [DOI] [PubMed] [Google Scholar]
- 55.Sahoo A, Lee CG, Jash A, Son JS, Kim G, Kwon HK, So JS, Im SH. Stat6 and c-Jun mediate Th2 cell-specific IL-24 gene expression. J Immunol 186: 4098–4109, 2011. doi: 10.4049/jimmunol.1002620. [DOI] [PubMed] [Google Scholar]
- 56.Satirapoj B, Wang Y, Chamberlin MP, Dai T, LaPage J, Phillips L, Nast CC, Adler SG. Periostin: novel tissue and urinary biomarker of progressive renal injury induces a coordinated mesenchymal phenotype in tubular cells. Nephrol Dial Transplant 27: 2702–2711, 2012. doi: 10.1093/ndt/gfr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen K, Lindenmeyer MT, Gaspert A, Eichinger F, Neusser MA, Kretzler M, Segerer S, Cohen CD. Periostin is induced in glomerular injury and expressed de novo in interstitial renal fibrosis. Am J Pathol 179: 1756–1767, 2011. doi: 10.1016/j.ajpath.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shanmugam I, Sinha A, Norris RA, Markwald R, Vyavahare N. Periostin as an early marker for elastin mediated vascular smooth muscle cell calcification. Cardiovascular System 1: 2, 2013. doi: 10.7243/2052-4358-1-2. [DOI] [Google Scholar]
- 59.Shillingford JM, Piontek KB, Germino GG, Weimbs T. Rapamycin ameliorates PKD resulting from conditional inactivation of Pkd1. J Am Soc Nephrol 21: 489–497, 2010. doi: 10.1681/ASN.2009040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sorocos K, Kostoulias X, Cullen-McEwen L, Hart AH, Bertram JF, Caruana G. Expression patterns and roles of periostin during kidney and ureter development. J Urol 186: 1537–1544, 2011. doi: 10.1016/j.juro.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 61.Swenson-Fields KI, Vivian CJ, Salah SM, Peda JD, Davis BM, van Rooijen N, Wallace DP, Fields TA. Macrophages promote polycystic kidney disease progression. Kidney Int 83: 855–864, 2013. doi: 10.1038/ki.2012.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T. Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci USA 108: 7985–7990, 2011. doi: 10.1073/pnas.1103816108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J. Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20: 4143–4154, 2011. doi: 10.1093/hmg/ddr338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118: 98–104, 2006. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 65.Torres VE, Harris PC. Polycystic kidney disease in 2011: Connecting the dots toward a polycystic kidney disease therapy. Nat Rev Nephrol 8: 66–68, 2012. doi: 10.1038/nrneph.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Stijn CM, van den Broek M, van de Weerd R, Visser M, Taşdelen I, Tefsen B, van Die I. Regulation of expression and secretion of galectin-3 in human monocyte-derived dendritic cells. Mol Immunol 46: 3292–3299, 2009. doi: 10.1016/j.molimm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 68.Wallace DP, Quante MT, Reif GA, Nivens E, Ahmed F, Hempson SJ, Blanco G, Yamaguchi T. Periostin induces proliferation of human autosomal dominant polycystic kidney cells through alphaV-integrin receptor. Am J Physiol Renal Physiol 295: F1463–F1471, 2008. doi: 10.1152/ajprenal.90266.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace DP, White C, Savinkova L, Nivens E, Reif GA, Pinto CS, Raman A, Parnell SC, Conway SJ, Fields TA. Periostin promotes renal cyst growth and interstitial fibrosis in polycystic kidney disease. Kidney Int 85: 845–854, 2014. doi: 10.1038/ki.2013.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang M, Liang P. Interleukin-24 and its receptors. Immunology 114: 166–170, 2005. doi: 10.1111/j.1365-2567.2005.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weber M, Sporrer D, Weigert J, Wanninger J, Neumeier M, Wurm S, Stögbauer F, Kopp A, Bala M, Schäffler A, Buechler C. Adiponectin downregulates galectin-3 whose cellular form is elevated whereas its soluble form is reduced in type 2 diabetic monocytes. FEBS Lett 583: 3718–3724, 2009. [Erratum in FEBS Lett 587: 2483, 2013.] doi: 10.1016/j.febslet.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 72.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293: F1423–F1432, 2007. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 73.Weimbs T. Third-hit signaling in renal cyst formation. J Am Soc Nephrol 22: 793–795, 2011. doi: 10.1681/ASN.2011030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weimbs T, Olsan EE, Talbot JJ. Regulation of STATs by polycystin-1 and their role in polycystic kidney disease. JAKSTAT 2: e23650, 2013. doi: 10.4161/jkst.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, Schnitzbauer A, Schäffler A, Aslanidis C, Schölmerich J, Buechler C. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab 95: 1404–1411, 2010. doi: 10.1210/jc.2009-1619. [DOI] [PubMed] [Google Scholar]
- 76.Weir RA, Petrie CJ, Murphy CA, Clements S, Steedman T, Miller AM, McInnes IB, Squire IB, Ng LL, Dargie HJ, McMurray JJ. Galectin-3 and cardiac function in survivors of acute myocardial infarction. Circ Heart Fail 6: 492–498, 2013. doi: 10.1161/CIRCHEARTFAILURE.112.000146. [DOI] [PubMed] [Google Scholar]
- 77.Weiss R, Sachet M, Zinngrebe J, Aschacher T, Krainer M, Hegedus B, Walczak H, Bergmann M. IL-24 sensitizes tumor cells to TLR3-mediated apoptosis. Cell Death Differ 20: 823–833, 2013. doi: 10.1038/cdd.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winyard PJ, Bao Q, Hughes RC, Woolf AS. Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol 8: 1647–1657, 1997. [DOI] [PubMed] [Google Scholar]
- 80.Yuyama N, Davies DE, Akaiwa M, Matsui K, Hamasaki Y, Suminami Y, Yoshida NL, Maeda M, Pandit A, Lordan JL, Kamogawa Y, Arima K, Nagumo F, Sugimachi M, Berger A, Richards I, Roberds SL, Yamashita T, Kishi F, Kato H, Arai K, Ohshima K, Tadano J, Hamasaki N, Miyatake S, Sugita Y, Holgate ST, Izuhara K. Analysis of novel disease-related genes in bronchial asthma. Cytokine 19: 287–296, 2002. doi: 10.1006/cyto.2002.1972. [DOI] [PubMed] [Google Scholar]
- 81.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1α is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res 22: 1851–1861, 2007. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 82.Zhang HY, Jin L, Stilling GA, Ruebel KH, Coonse K, Tanizaki Y, Raz A, Lloyd RV. RUNX1 and RUNX2 upregulate Galectin-3 expression in human pituitary tumors. Endocrine 35: 101–111, 2009. doi: 10.1007/s12020-008-9129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou J, Ouyang X, Cui X, Schoeb TR, Smythies LE, Johnson MR, Guay-Woodford LM, Chapman AB, Mrug M. Renal CD14 expression correlates with the progression of cystic kidney disease. Kidney Int 78: 550–560, 2010. doi: 10.1038/ki.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]