Abstract
Interstitial cystitis/bladder pain syndrome is a chronic bladder condition associated with pain and voiding dysfunction that is often regarded as a neurogenic cystitis. Patient symptoms are correlated with the presence of urothelial lesions. We previously characterized a murine neurogenic cystitis model that recapitulates mast cell accumulation and urothelial lesions, and these events were dependent on TNF. To further explore the role of TNF in bladder inflammation and function, we generated a transgenic mouse model with chronic TNF overexpression in urothelium under the control of the uroplakin II (UPII) promoter. Transgenic mouse lines were maintained by backcross onto wild-type C57BL/6J mice and evaluated for pelvic tactile allodynia as a measure of visceral pain, urinary function, and urothelial lesions. TNF mRNA and protein were expressed at greater levels in bladders of UPII-TNF mice than in those of wild-type mice. UPII-TNF mice showed significantly increased urinary frequency and decreased void volume. UPII-TNF mice had increased urothelial apoptosis and loss of urothelial integrity consistent with urothelial lesions. Overexpression of TNF was also associated with pelvic tactile allodynia. Consistent with these findings, UPII-TNF mice exhibited increased bladder afferent activity in response to stretch ex vivo. In summary, UPII-TNF mice display significant pelvic pain, voiding dysfunction, urothelial lesions, and sensory input. Thus UPII-TNF mice are a model for characterizing mechanisms of interstitial cystitis symptoms and evaluating therapies.
Keywords: interstitial cystitis, pelvic pain, TNF, transgenic mouse model
INTRODUCTION
Interstitial cystitis (IC)/bladder pain syndrome is a chronic debilitating bladder disease of unknown etiology with no effective therapies. It affects as many as eight million patients in the United States, with women comprising ~90% of patients (2). IC is characterized by severe pelvic pain and voiding dysfunction, such as urinary frequency, urgency, and nocturia (20, 30). IC is often regarded as a neurogenic cystitis because of both dysfunction of micturition and the partial efficacy of sacral nerve stimulation or neuropharmacologic therapies in some patients, which suggests a neural component (10). The precise mechanism of IC is unclear, but because of the profound impact of pelvic pain on patients and the many unanswered clinical and pathogenesis questions in chronic pelvic pain, the National Institute of Diabetes and Digestive and Kidney Diseases established the Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network, its flagship effort to understand mechanisms underlying in urologic chronic pelvic pain syndrome. The MAPP Network is a multicenter network dedicated to clinical, epidemiologic, and mechanistic studies of chronic pelvic pain, including IC (9, 16). Recent studies using rodent models of neurogenic cystitis have identified pathways and mechanisms of bladder pathogenesis that may contribute to IC (14). Inoculation of rats with the attenuated Bartha’s strain of pseudorabies virus (PRV) through the tail base was shown to develop a cystitis accompanied by mast cell activation (11, 12). Activated mast cells can release proinflammatory cytokines, such as TNF and histamines. PRV-induced cystitis was not recapitulated by virus injection into the bladder, but was attenuated by either resection of bladder innervation or ablation of Barrington’s nucleus, a brain center of bladder control, thus demonstrating that PRV induces a centrally mediated, neurogenic cystitis. In mice, PRV inoculation at the tail base muscle was found to drive bladder pathology by mast cell production of tumor necrosis factor-α (TNF; 6–8).
TNF is a pleiotropic cytokine that is associated with diverse autoimmune disorders, such as Crohn’s disease and psoriasis (19, 32). TNF is a major component of the soluble factors released by mast cells, which have been implicated in various urinary bladder diseases, including bacterial infection, cancer, and IC (5, 21, 24). Using the rodent neurogenic cystitis model, we initially demonstrated that TNF promotes differential trafficking of bladder mast cells to lamina propria (6). That mast cell activation and release of TNF were found to drive urothelial apoptosis and lesion formation mediated by TNF receptor 1 and regulated on activation, normal T cell expressed, and secreted (RANTES; 7). Several investigators have begun to explore the role of TNF in IC pathogenesis. The possibility that TNF upregulation could result in apoptosis of urothelial cells in IC patients is consistent with the observation that TNF is overexpressed in bladder urothelium of patients with ulcerative IC (24).
To explore the effects of TNF independent of other mast cell factors, we generated transgenic mice (Tg) in which TNF is overexpressed under the control of the uroplakin II (UPII) promoter, a promoter previously shown to drive overexpression of a transgene specifically in the urothelium (26, 31, 35). Our data revealed that overexpression of TNF by urothelial cells in vivo caused significant pelvic pain behavior, voiding dysfunction, and urothelial lesions, which supports the potential role of TNF in the pathogenesis of IC.
MATERIALS AND METHODS
Production of UPII-TNF transgenic mice.
The UPII-TNF transgene construct was composed of mouse TNF cDNA sequence flanked by the murine UPII promoter and a simian virus 40 (SV40) 3′-polyadenylation sequence (Fig. 1A). Briefly, the UPII promoter was shuttled from plasmid pBluescript II as previous described (18), and the TNF cDNA was obtained in plasmid myosin heavy chain-TNF (pMHC-TNF; 4). The MHC promoter in pMHC-TNF was removed by Acc65I and EcoRV and replaced by 3.6-kb UPII promoter, which was excised as Acc65I-HindIII fragment from pBluescript II, with Klenow fragment (Promega, Madison, WI) and Clonesmart blunt cloning kits (Lucigen, Middleton, WI). The orientation of UPII promoter insertion was confirmed by DNA sequence analysis. The purified UPII-TNF fragment was excised from the plasmid with Acc65I and HindIII and then microinjected into the pronuclei of fertilized C57BL/6 oocytes by the Transgenic and Targeted Mutagenesis Laboratory at Northwestern University. Founder mice were backcrossed to C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME). All mice have the inbred genetic background of C57BL/6J maintained through a homozygous intercross strategy. All mice were used in accordance with the protocol approved by Northwestern University Institutional Animal Care and Use Committee. To screen for the transgene by PCR, genomic DNA was isolated from weanling tail clips, and transgene-specific primers (UPII, 5′-TCCCACTCCGAGACAAAATC-3′; TNF, 5′-AGGGTCTGGGCCATAGAACT-3′) were used with the following PCR conditions: 95°C (15 s), 55°C (15 s), and 72°C (60 s) for 40 cycles.
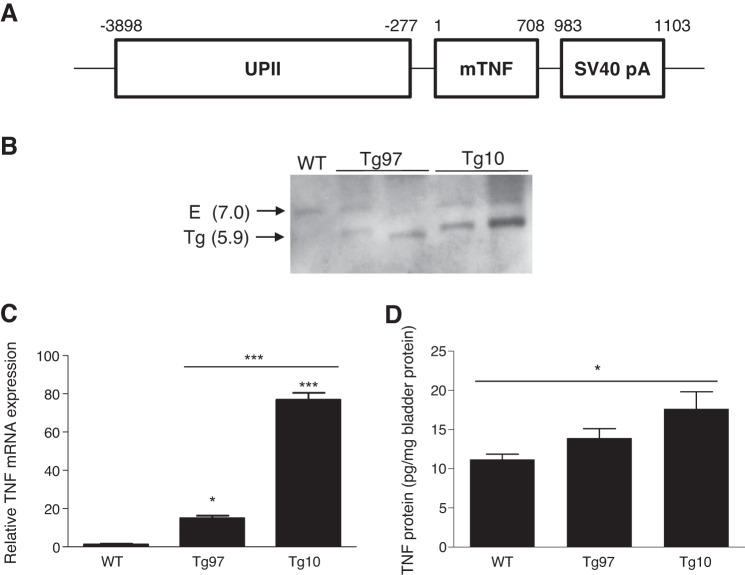
Fig. 1.
Structure and expression of uroplakin II (UPII)-TNF transgene. A: murine UPII promoter, murine TNF coding sequence (mTNF), and simian virus 40 polyadenylation sequence (SV40 pA) were cloned as indicated to generate the transgene construct. Numbers indicate length and position of each sequence. B: Southern blot of genomic DNA from wild-type (WT) and transgenic lines Tg97 and Tg10. A ~7-kb band from the endogenous (E) TNF gene and a ~5.9-kb band indicative of a transgene (Tg) were identified by HindIII digestion. C: TNF mRNA expression level in the urothelium of WT and transgenic mice, determined by quantitative PCR (qRT-PCR) relative to ribosomal protein L19. Urothelial TNF RNA was significantly greater in Tg10 bladder compared with that in WT and Tg97 (n = 3). D: TNF in the bladders of WT and transgenic mice. Only Tg10 bladders showed higher TNF protein levels than WT (n = 4–8). *P < 0.05, ***P < 0.0001.
Southern blot analysis.
Genomic DNA was extracted with Qiagen genomic DNA buffer set (Qiagen, Valencia, CA) according to the manufacturer’s handbook. Probes were labeled and blots were performed with DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics, Indianapolis, IN) according to the manufacturer’s manual. Briefly, genomic DNA (5–10 µg) was digested with HindIII and hybridized with a 204-bp TNF-specific probe generated from purified transgene DNA fragment. Transgene copy number was determined by densitometric scanning (ImageJ; 27).
Real-time RT-PCR.
Total RNA was extracted with RNeasy Plus Mini Kit (Qiagen), and RNA was reverse transcribed using iScript cDNASynthesis Kit (Bio-Rad, Hercules, CA), according to manufacturer’s directions. Quantitative real-time PCR analysis was performed using Bullseye EvaGreen qPCR Mastermix (MIDSCI, Valley Park, MO) in a Bio-Rad CFXConnect Real-Time System. The levels of mRNA were normalized to ribosomal protein L19 mRNA levels. We used the following primer for murine TNF: sense, GAACTGGCAGAAGAGGCACT; antisense, AGGGTCTGGGCCATAGAACT. Data were analyzed with Bio-Rad CFX Manager 3.1 software.
Detection of bladder TNF by ELISA.
Whole bladders of wild-type and UPII-TNF mice were homogenized in T-PER (Thermo Fisher Scientific, Waltham, MA) with protease inhibitors. Protein concentrations were measured by Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Mouse TNF was quantified by the Mouse TNF-α DuoSet ELISA Kit (R&D Systems, Minneapolis, MN) relative to a TNF-α standard.
Histology and immunohistochemistry.
All bladders were removed and processed by fixation and sectioning axially in the Northwestern University Mouse Histology & Phenotyping Laboratory, to account for nonuniform distribution of features between the bladder dome and trigone (e.g., sensory fibers). Bladders were embedded in paraffin, and hematoxylin-eosin-stained tissues were assessed by light microscopy on a Nikon E800 microscope equipped with a Spot Color RT camera (Diagnostic Instruments, Sterling Heights, MI). For anti-CGRP staining, bladder tissue sections were deparaffinized, rehydrated, and subjected to heat-induced epitope retrieval. Subsequently, the sections were incubated overnight with rabbit polyclonal anti-CGRP antibody (1:8,000; Sigma-Aldrich), respectively. After being washed three times, the slides were incubated with donkey anti-rabbit 488 secondary antibody (1:500, A-21206; Molecular Probes).
Images were acquired in Volocity software (PerkinElmer, Waltham, MA) using a Leica DMIRE2 microscope equipped with a Hamamatsu ORCAII camera. Within a given experiment, all images were acquired under identical exposure conditions from representative fields of view. To ensure appropriate representation of micrographs, composite figures were assembled from identically captured images before any manipulation. Composite figures were then adjusted as necessary in Photoshop using levels, brightness, and contrast in each RGB channel. Any changes were applied uniformly across all panels within a composite figure, and no images were modified in any way that alters qualitative interpretations of micrographs.
CGRP immunoreactivity was quantified in five randomly chosen fields captured from five nonserial sections for each bladder from five different mice. Images were captured in Volocity with exposure of 1 s, gain of 67, and offset of 4 under autocontrast. Staining intensity was determined in fibers of bladder sections using Volocity for CGRP-positive objects >1 µm in length to determine total pixel intensities. ImageJ (National Institutes of Health) was used to trace CGRP-positive axon length.
Apoptosis detection by terminal deoxynucleotidyl transferase dUTP nick end labeling.
Urothelial apoptosis was assessed in tissue sections with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) as previously described (8). Briefly, sections were deparaffinized, rehydrated, and incubated with 20 μg/ml nuclease-free proteinase K at 37°C. Sections were then rinsed with PBS and incubated at room temperature with blocking solution (3% BSA, 20% FBS). After incubation with TUNEL enzyme (Roche Diagnostics, Indianapolis, IN), slides were counterstained with DAPI and images were acquired using a Leica DMIRE2 microscope equipped with a Hamamatsu ORCAII camera. Suspected lesions were defined as three or more adjacent TUNEL-positive cells. Confirmation of lesions containing TUNEL-positive labeling was aided by removing the coverslips and staining for uroplakin III. Regions exhibiting 3+ TUNEL-positive cells and associated with a disruption in the normally intact urothelial layer were scored as lesions. Lesions were scored by staining of two nonserial sections from each bladder.
Awake cystometry.
Cystometry measurements were made from conscious, unrestrained mice. Briefly, ethylene oxide-sterilized bladder catheters were constructed from 10–15-cm lengths of PE-10 tubing before surgery. Before sterilization by ethylene oxide, one end of the tubing was heat flared to serve as an anchor for implantation into the bladder. For surgery, mice were anesthetized with 2.5–4% isoflurane. One incision was made in the lower abdomen exposing the urinary bladder. A second incision was made in the midline of the upper back to serve as an exit point for the catheter. The distal end of the bladder catheter was then tunneled subcutaneously, from the abdominal incision and through the exit point on the back of the mouse, with the aid of a gavage needle. The catheter was then filled with saline, before being inserted into the dome of the bladder, and secured with an 8-0 nylon purse string suture. The incisions were closed with absorbable sutures for the muscle layers and prolene for the skin. The distal end of the catheter was trimmed to 5–10 cm, heat sealed, and placed in the subcutaneous space of the upper back incision before closing with prolene sutures. Postsurgery, mice were housed separately and buprenorphine was administered over a 3-day period to provide analgesia. After a 7- to 9-day recovery period, mice were briefly anesthetized (3–4% isoflurane), and the length of the distal end of the catheter was exposed. Cystometry measurements were made using a Small Animal Cystometry Laboratory Station (Catamount Research, St. Albans, VT), where the individual mouse was placed in a Plexiglas recording cage with a wire mesh bottom, and the catheter was connected to a syringe pump via a pressure transducer. Room temperature saline was infused at a rate of 12.5 μl/min to elicit repetitive urinary bladder contractions. A 90-min period was provided to allow the mouse to recover from the brief anesthesia and become accustomed to the novel environment. Measurements were made of the intercontraction interval and maximal voiding volume as the incidence of nonvoiding urinary bladder contractions per voiding cycle, over a fixed, 60-min period. For these studies, nonvoiding urinary bladder contractions were defined as rhythmic intravesical pressure rises (>5 mmHg). Mice were excluded from studies either when hematuria was observed on initial voids, when there was initial blockage of catheters, or because of mice biting through catheters. Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements. Mice were euthanized at the conclusion of study by isoflurane (5%) and thoracotomy.
Pelvic pain behavior.
Allodynia was quantified in response to von Frey filaments applied to the pelvic region, as previously described (33). Briefly, mice were adapted to the Plexiglas chamber (5–10 min), and each von Frey filament was applied 10 times to the pelvic region, stimulating different areas to avoid desensitization or “wind-up” effects.
In vitro sensory nerve recordings.
Mice were anesthetized with 5% isoflurane for 5 min, until unresponsive to touch, followed by cervical dislocation and exsanguination. For these experiments, control physiological saline solution contained (in mM) 37 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, 30 HEPES, 24 NaH2CO3, 1 NaH2PO4, and 11 dextrose (pH 7.2–7.4) and was gassed with O2. Urinary bladders were dissected from the mice with the associated nerves. Preparations were securely pinned at the neck of the bladder to the bottom of a Sylgard‐lined recording chamber [maintained at 37°C by a custom‐built heating stage and superfused with prewarmed oxygenated saline solution (1 ml/min)]. A midline incision was made exposing the interior of the bladder. A silk suture was secured to the bladder dome allowing the bladder to be stretched along its longitudinal axis. The free end of the suture was attached to the wheel of an Arduino microprocessor-controlled electric motor to provide a series of timed and precalibrated pull strengths. Force (10–500 mN) was applied to stretch the muscle (10-s duration, 1–5-min intervals). Each pull was replicated twice. Extracellular recordings of nerve activity from the nerves exiting the bladder on either the left- or right-hand side were made using a suction electrode (tip inner diameter 7–9 μm) connected to an A-M Systems model 1800 extracellular amplifier. Signals were fed into a personal computer using a Digidata 1200 A/D converter (Molecular Devices, Sunnyvale, CA). Responses were recorded and analyzed using winEDR Electrophysiology Disk Recorder software (John Dempster, Strathclyde University Software). Recordings were analyzed postexperiment, with automatic detection of events using a signal threshold of −4 times standard deviation calculated from event-free baseline noise levels and 0.5-ms duration with a 2-ms “dead time window” to avoid double detection of single events. During this process, detected events were visually screened for aberrant electrical noise. The times and amplitudes corresponding to the detected events were plotted. Histograms were made for the timing of events using Microsoft Excel, and the average frequencies of events were calculated for the first 4 s of the stretch.
Statistical analysis.
Results were expressed as means ± SE. When the data were compared between two groups, they were analyzed with the Student’s t-test; data compared from >2 groups were analyzed by either Kruskal-Wallis test or two-way ANOVA followed by a posttest comparison using Bonferroni’s multiple-comparison test. All analyses were done using Prism software (GraphPad Software, La Jolla, CA). A value of P < 0.05 was considered statistically significant.
RESULTS
Development of UPII-TNF mice overexpressing TNF in the urothelium.
To determine the role of TNF in urinary bladder dysfunction and pelvic pain, we generated transgenic mice overexpressing TNF constitutively in urothelium (Fig. 1A). In these animals, TNF overexpression is controlled by the UPII promoter. This promoter has been shown to direct selective expression of β-galactosidase, monocyte chemoattractant protein-1, and nerve growth factor in bladder and is upregulated immediately after birth (18, 26, 31). Purified UPII-TNF DNA fragments were microinjected into the male pronucleus of single-cell fertilized mouse embryos (zygotes). Microinjected embryos were then surgically transferred into the reproductive tract of a pseudopregnant female who carried the pregnancy to term. Two transgenic founder mice were produced and were backcrossed with C57BL/6 to establish parental UPII-TNF mice, which we designated Tg97 and Tg10. UPII-TNF transgene integration was shown by Southern blot analysis of HindIII-digested genomic DNA (Fig. 1B). With the use of a TNF-specific probe, the endogenous TNF gene appeared as an ~7-kb HindIII fragment in both wild-type and transgenic lineages. Other ~5.9-kb bands were only detected in both transgenic lines, indicating a unique, integrated transgene. Comparing the signal intensity of this 5.9-kb band with ~7-kb HindIII fragments as single-copy endogenous TNF gene, Tg97 has ~1.5 copies per haploid genome compared with Tg10, which has ~15.6 copies per haploid genome. Consistent with Southern blot analysis demonstrating integrated transgenes, quantitative real-time PCR revealed overexpression of TNF mRNA transcript in the bladders of transgenic mice, with significantly higher TNF mRNA in Tg10 bladders than in Tg97 bladders (Fig. 1C). To confirm elevated expression at the level of bladder protein, TNF was quantified by ELISA. Tg97 bladders expressed only slightly higher TNF protein levels than wild-type tissue, whereas Tg10 bladders exhibited significant overexpression of TNF (Fig. 1D).
Uropathological changes in UPII-TNF mice.
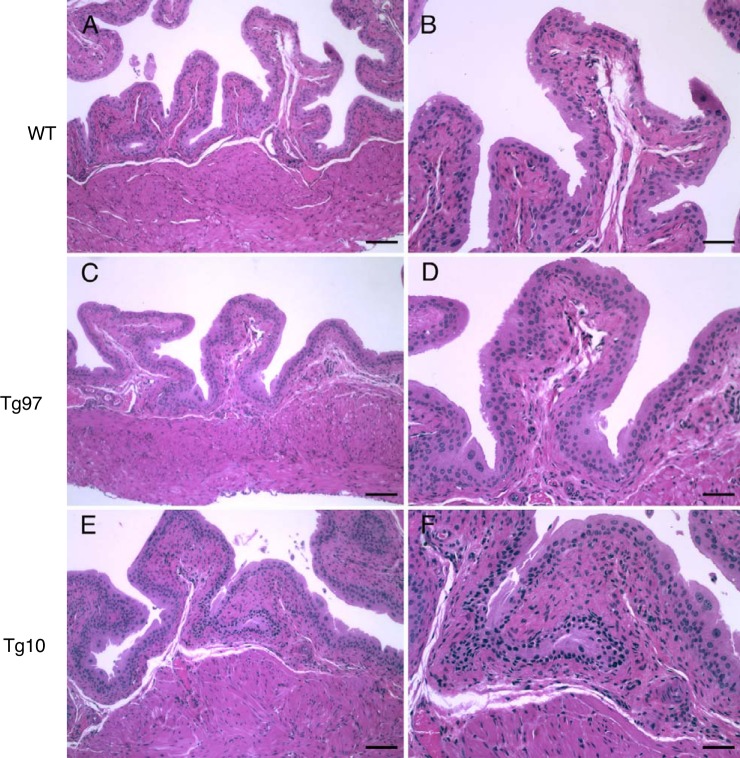
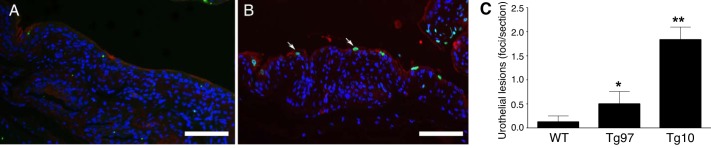
UPII-TNF mice displayed normal development and activity without clinical symptoms. Wild-type and UPII-TNF mice have similar body and urinary bladder weight and have no difference in bladder weight-to-body weight ratio (data not shown). Hematoxylin-eosin-stained bladder sections of transgenic mice showed normal transitional epithelium, lamina propria, and detrusor, suggesting that UPII-TNF mice had normal urinary bladder development while TNF was overexpressed in urothelium (Fig. 2, A–F). Since our previous studies indicated that TNF released from mast cells can lead to urothelial lesions (8), we examined the bladder for evidence of urothelial apoptosis using TUNEL staining. Urothelial apoptosis was minimal in bladders of wild-type mice (Fig. 3A). However, in UPII-TNF mice, apoptotic cells were evident and found in foci with disrupted integrity of uroplakin staining characteristic of urothelial lesions, reminiscent of punctate lesions in IC bladders (Fig. 3B). Indeed, urothelial lesions were significantly increased in bladders of both Tg97 and Tg10 transgenic lines (Fig. 3C).
Fig. 2.
Bladder histology of wild-type (WT) and uroplakin II-TNF transgenic mice. Hematoxylin-eosin-stained cross sections of bladders from WT (A and B) and transgenic lines Tg97 (C and D) and Tg10 (E and F) appeared similar. Scale bars: 100 µm (A, C, and E) and 50 µm (B, D, and F).
Fig. 3.
Overexpression of TNF results in urothelial apoptosis and lesions. Representative bladder sections from wild-type mice (WT; A) and transgenic line Tg10 (B) were stained for uroplakin III (red) and for apoptosis using transferase-mediated dUTP nick end labeling (TUNEL, green). The arrows indicate TUNEL-positive superficial cells. C: lesions were quantified from TUNEL staining of two nonserial sections from each bladder (n = 5). A positive lesion was defined as three or more adjacent TUNEL-positive cells. Scale bars: 100 µm. *P < 0.05, **P < 0.001.
Clinical studies suggest that mast cells may be involved in the pathophysiology of IC, and mast cells mediate bladder inflammatory processes (24). To investigate whether uropathological changes of UPII-TNF mice were associated with increased local inflammation, bladder sections were stained with toluidine blue to reveal granulated mast cells. Similar numbers of mast cells were observed in the bladders of both wild-type and UPII-TNF mice (data not shown), suggesting that urothelial TNF does not mediate mast cell recruitment to the mouse bladder. No obvious interstitial edema, vasodilation, or leukocytic infiltrate were observed in the UPII-TNF urinary bladder (Fig. 2), suggesting that TNF overexpression in the urothelium does not generate pronounced local or systemic inflammatory processes.
Altered bladder sensory innervation in UPII-TNF mice.
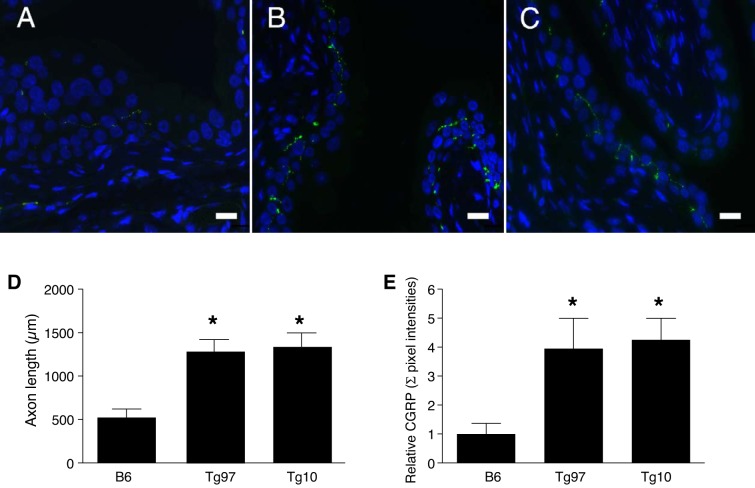
Calcitonin gene-related peptide (CGRP) is a peptide neurotransmitter, and unmyelinated C fiber sensory afferents expressing CGRP are abundant in the bladder and are increased in murine models of urinary dysfunction (26). To investigate whether overexpressed TNF in the urothelium alters sensory nerve innervation, we stained bladder tissue for CGRP immunoreactivity. CGRP-positive fibers were observed in the urothelium and lamina propria (Fig. 4, A–C). Image analysis revealed significantly increased CGRP-positive axonal length and expression level in UPII-TNF mice (Fig. 4, D and E). These findings suggest that TNF can enhance the unmyelinated CGRP-dependent C fiber sensory afferent function in the bladder.
Fig. 4.
CGRP-positive nerve innervation in bladders of uroplakin II-TNF mice. Representative bladder sections from wild-type mice (B6; A) and transgenic lines Tg97 (B) and Tg10 (C) were stained for CGRP. D: immunostaining axonal length was measured by ImageJ. E: immunostaining pixel intensity was measure by Volocity software (n = 5 slices/5 mice). Scale bars: 100 µm. Values are means ± SE. *P < 0.05.
Abnormal voiding activity in UPII-TNF mice.
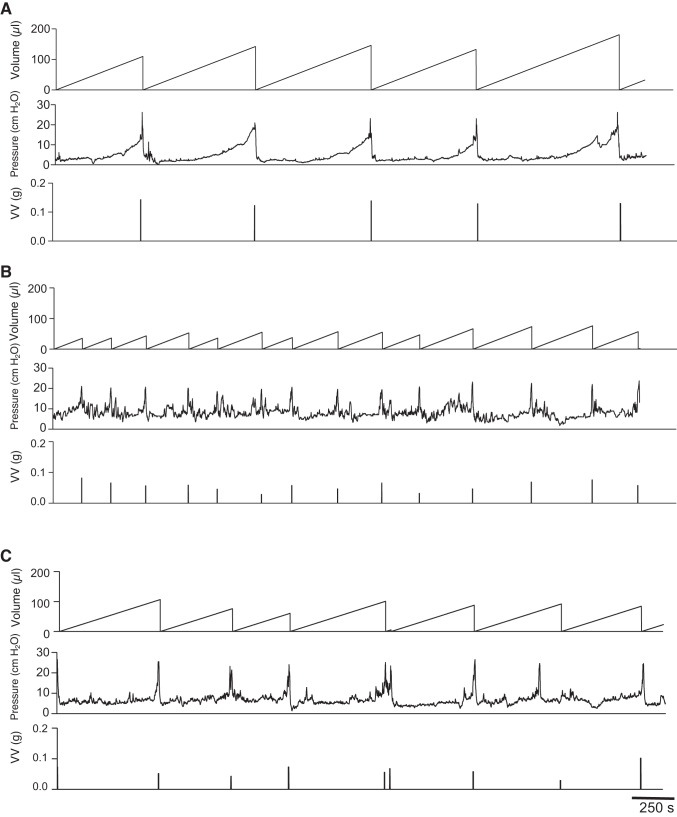
To investigate whether the overexpression of TNF in urothelium can result in bladder dysfunction, we evaluated the urinary voiding patterns in wild-type and UPII-TNF mice in vivo by performing awake cystometry (26). Figure 5 shows representative awake cystometrograms from conscious, freely moving wild-type and UPII-TNF mice; each panel contains a continuous record of volume of saline infused (reset to 0 after each micturition), bladder pressure, and volume of urine voided. In contrast to wild-type mice (Fig. 5A), both Tg97 and Tg10 mice displayed increased urinary frequency and significantly reduced infused volume, voided volume, and intercontraction intervals (Fig. 5, B and C, and Table 1). There were no differences in the nonvoiding bladder contractions between wild-type and UPII-TNF mice. A decrease in nonvoiding urinary bladder contraction was observed in Tg10 mice, but this effect did not reach statistical significance. In addition, there were no differences in threshold pressure or minimum, average, or maximum pressure detected between wild-type and UPII-TNF mice.
Fig. 5.
Abnormal bladder function in uroplakin II-TNF transgenic mice. Representative awake cystometry of wild-type mice (A) and transgenic lines Tg97 (B) and Tg10 (C). Volume infused, bladder pressure, and voided volume (VV, g) are displayed.
Table 1.
Urodynamic findings in wild-type and UPII-TNF mice by awake cystometry
| WT | Tg97 | Tg10 | |
|---|---|---|---|
| n | 7 | 6 | 6 |
| Infused volume, µl | 131 ± 23 | 59 ± 32† | 93 ± 38* |
| Voided volume, µl | 121 ± 15 | 57 ± 11* | 76.7 ± 15.1* |
| Threshold pressure, cmH2O | 18 ± 8 | 14 ± 7 | 13 ± 2 |
| Maximum pressure, cmH2O | 32 ± 14 | 27 ± 13 | 24 ± 5 |
| Average pressure, cmH2O | 9.8 ± 3.8 | 10.6 ± 6.4 | 8.5 ± 1.9 |
| Minimum pressure, cmH2O | 4.9 ± 2.7 | 6.4 ± 4.4 | 5.4 ± 1.4 |
| Nonvoiding contractions/cycle | 1.0 ± 1.4 | 0.9 ± 1.5 | 0.3 ± 3 |
| Intercontraction interval, s | 626 ± 41 | 283 ± 59* | 449 ± 75* |
Values are means ± SE; n = no. of mice. UPII, uroplakin II. Statistical comparison of wild-type (WT) and transgenic (Tg97 and Tg10) mice using an unpaired t-test:
P < 0.05;
P < 0.01.
Dose-dependent pelvic pain in UPII-TNF mice.
Visceral pain is typically associated with tactile allodynia and corresponding tactile hypersensitivity (15). To quantify bladder-associated pelvic pain, we assessed allodynia of the pelvic region in response to mechanical stimulation with von Frey filaments as previously reported (33). Pelvic stimulation evoked responses, including pelvic withdrawal, jumping, and pelvic licking/scratching. Compared with wild-type controls, Tg97 mice exhibited a modest increase in pelvic sensitivity, whereas Tg10 exhibited significantly enhanced sensitivity to pelvic stimulation, suggesting that chronic expression TNF in the bladder contributes to pelvic pain severity (Fig. 6A).
Fig. 6.
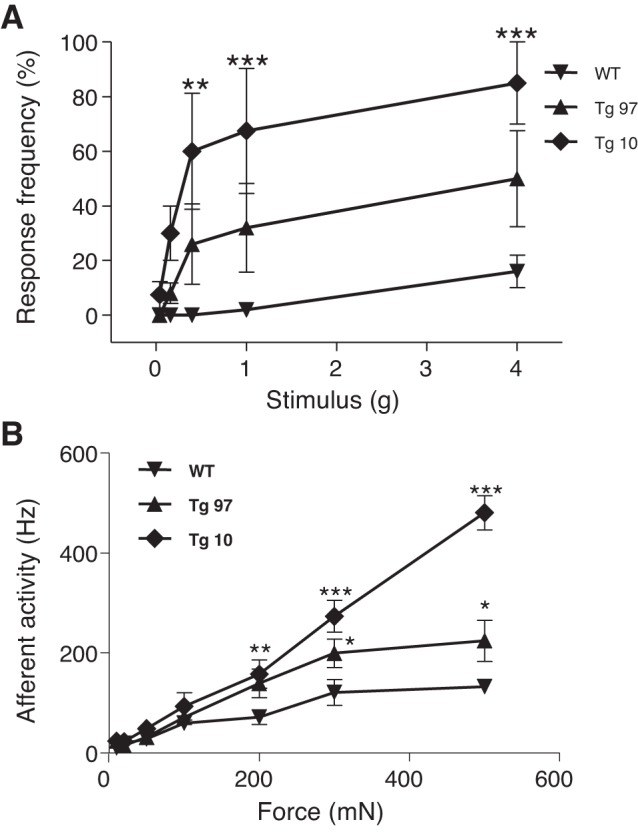
Properties of bladder sensation in wild-type (WT) and uroplakin II-TNF transgenic mice. A: referred allodynia was quantified as response to pelvic stimulation using von Frey filaments. B: firing rates of bladder afferents evoked by stretch in ex vivo bladders from WT mice and transgenic lines Tg97 and Tg10. Two-way ANOVA with Bonferroni’s posttest. Data represent means ± SE. *P < 0.05, **P < 0.001, ***P < 0.0001.
Afferent hyperactivity in UPII-TNF mice.
Since UPII-TNF mice have chronic allodynia, we quantified bladder afferent nerve activity. Measurements were made of the neuronal firing rates of afferent nerves in response to bladder stretch over a range of forces using an ex vivo bladder preparation. Neuronal firing rates increased in response to increasing the strength of the stretch stimulus (10–500 mN). We observed increased firing rates in response to stretch in the Tg97 and Tg10 mice compared with the wild-type mice (Fig. 6B), indicating increased sensitivity to stretch in the UPII-TNF mice and consequent increased sensory output.
DISCUSSION
Prior studies in vitro and in murine PRV neurogenic cystitis implicated mast cell TNF as a major mediator of bladder responses similar to those observed in bladders of IC patients (1, 6–8). Here, we report the development of transgenic mouse lines with bladder-specific overexpression of TNF using the urothelium-specific promoter for uroplakin II in combination with a TNF cDNA (13, 17). Southern blot analysis revealed differential integration of the transgene construct among founder lines where the UPII-TNF line Tg10 had more integrated copies of the TNF transgene than the Tg97 line (Fig. 1). Consistent with higher TNF transgene copy number, both Tg97 and Tg10 mice had increased bladder TNF expression at the mRNA level (Fig. 1), whereas only Tg10 mice had significantly elevated TNF protein by ELISA, relative to wild-type mice. Nonetheless, the UPII-TNF lines represent a panel for examining the potential role of TNF dosage in bladder pathogenesis and urologic symptoms.
PRV neurogenic cystitis is associated with leukocytic influx and TNF- and RANTES-dependent accumulation of mast cells in the lamina propria (6–8). TNF also plays a critical regulator role in innate immunity against bacterial urinary tract infection (25, 34). Bladder-resident lymphocyte antigen 6 complex-negative (Ly6C−) macrophages were shown to attract circulating Ly6C+ phagocytes that release C-X-C motif chemokine 2, which in turn activated matrix metalloproteinase 9 secretion by neutrophils to enable transepithelial migration. In contrast, here we did not observe a leukocytic infiltrate or increased numbers of mast cells in bladders of UPII-TNF mice (Fig. 2 and data not shown). This does not exclude the possibility that UPII-TNF bladders have modest leukocytic influx, as TNF-positive macrophages are known to reside in the lamina propria (29). Nonetheless, this suggests that the cellular source of TNF may influence lamina propria accumulation of mast cells and other leukocytes. Thus, autocrine urothelial TNF may not induce RANTES and/or other factors and thereby fail to recruit mast cells to the lamina propria, or other factors released by initial mast cell activation may be required to properly induce urothelial RANTES secretion to trigger mast cell trafficking.
The accumulation of mast cells proximal to urothelium and consequent high local concentrations of TNF were associated with the formation of urothelial lesions and loss of barrier function in PRV neurogenic cystitis (7, 8). Here, we observed significantly increased urothelial apoptosis in UPII-TNF mice (Fig. 3), consistent with the role of TNF as an inducer of urothelial apoptosis (8). Although PRV cystitis results in mast cell activation and TNF-dependent urothelial apoptosis, other mast cell factors conceivably contribute to compromised urothelial function. The data reported here suggest that TNF alone is sufficient to induce urothelial dysfunction.
Chronic dosing or transgenic overexpression of nerve growth factor in the bladder leads to increased density of CGRP-positive sensory fibers (26, 36). Similarly, UPII-TNF mice exhibit increased density of CGRP-positive fibers in the bladder (Fig. 4), suggesting that inflammatory and neurotrophic factors can elicit similar remodeling of peripheral sensory components. Although quantitative image analysis indicates that both Tg97 and Tg10 bladders exhibit comparable increases in CGRP fiber density relative to controls, the two UPII-TNF transgenic lines have different physiologic manifestations. Awake cystometry revealed significantly reduced infused volume and voided volume per voiding cycle in Tg97 and Tg10 mice, and UPII-TNF mice also exhibit reduced intercontraction intervals (Fig. 5 and Table 1). However, UPII-TNF lines distinguish themselves at the level of pelvic pain (Fig. 6). Tg10 mice exhibit significant pelvic allodynia, whereas Tg97 mice do not. These findings correspond to markedly increased afferent activity in response to stretch of Tg10 bladders and only a modest increase in the responses of Tg97 bladders to stretch. Together, these findings raise the possibility that TNF-mediated sensory remodeling in the bladder differentially affects voiding and pain in a dose-dependent manner. That such sensory remodeling happens over longer times may explain why chronic TNF expression, as in UPII-TNF mice, results in allodynia, whereas TNF is dispensable for pain in acute neurogenic cystitis (22).
UPII-TNF mice demonstrate that TNF alone can trigger key events in bladder pathogenesis and urologic dysfunction, suggesting that TNF is a therapeutic target in urologic symptoms clinically. For example, in a clinically relevant murine model of type 2 diabetes that develops diabetic bladder dysfunction resulting from secondary upregulation of TNF signaling, anti-TNF therapy reversed bladder dysfunction by reducing nonvoiding contractions and restoring normal void volumes (29). Consistent with anecdotal reports by patients, a recent clinical trial of adalimumab in IC observed a significant improvement in symptoms by several assessment measures (3). That adalimumab therapy for IC failed to achieve significance compared with placebo likely speaks more to the large placebo effect in pelvic pain, approaching or exceeding 40%, or the possibility that IC represents a complex syndrome with potentially multiple etiologies and underlying mechanisms (3). Alternatively, anti-TNF therapy may be most efficacious for urologic conditions in combination with other mechanistically targeted therapies. For example, mast cells are associated with patient symptoms in IC (28), and antihistamines specific for histamine receptor H2R effectively blocked pelvic pain in neurogenic cystitis (22, 23). Such a strategy may prove useful in clinical disease where TNF is a critical mediator within a complex inflammatory milieu that mediates urologic symptoms.
GRANTS
Studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Awards R01-DK-066112 (D. J. Klumpp), U01-DK-82342 (MAPP Research Network; D. J. Klumpp and A. J. Schaeffer), and 1R01-DK-095775 (T. J. Searl) and generous salary support of T. J. Searl by Dr. Alfred George Jr., Chairperson of Pharmacology.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
W.Y., T.J.S., A.J.S., and D.J.K. conceived and designed research; W.Y., T.J.S., and R.Y. performed experiments; W.Y., T.J.S., and D.J.K. analyzed data; W.Y., T.J.S., A.J.S., and D.J.K. interpreted results of experiments; W.Y., T.J.S., and R.Y. prepared figures; W.Y., R.Y., and D.J.K. drafted manuscript; W.Y., T.J.S., A.J.S., and D.J.K. edited and revised manuscript; D.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Tung-Tien Sun of New York University Medical School for kindly providing the UPII-lacZ plasmid and Dr. Brett Giroir of the University of Texas Southwestern Medical Center for kindly providing the MHC-TNF plasmid. We also thank Dr. Gerald Herrera of Catamount Research and Development for expert training in awake cystometry.
REFERENCES
- 1.Batler RA, Sengupta S, Forrestal SG, Schaeffer AJ, Klumpp DJ. Mast cell activation triggers a urothelial inflammatory response mediated by tumor necrosis factor-α. J Urol 168: 819–825, 2002. doi: 10.1016/S0022-5347(05)64750-7. [DOI] [PubMed] [Google Scholar]
- 2.Berry SH, Elliott MN, Suttorp M, Bogart LM, Stoto MA, Eggers P, Nyberg L, Clemens JQ. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol 186: 540–544, 2011. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosch PC. A randomized, double-blind, placebo controlled trial of adalimumab for interstitial cystitis/bladder pain syndrome. J Urol 191: 77–82, 2014. doi: 10.1016/j.juro.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 4.Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, Thompson M, Giroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation 97: 1375–1381, 1998. doi: 10.1161/01.CIR.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 5.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity 38: 349–359, 2013. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MC, Blunt LW, Pins MR, Klumpp DJ. Tumor necrosis factor promotes differential trafficking of bladder mast cells in neurogenic cystitis. J Urol 175: 754–759, 2006. doi: 10.1016/S0022-5347(05)00171-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen MC, Keshavan P, Gregory GD, Klumpp DJ. RANTES mediates TNF-dependent lamina propria mast cell accumulation and barrier dysfunction in neurogenic cystitis. Am J Physiol Renal Physiol 292: F1372–F1379, 2007. doi: 10.1152/ajprenal.00472.2006. [DOI] [PubMed] [Google Scholar]
- 8.Chen MC, Mudge CS, Klumpp DJ. Urothelial lesion formation is mediated by TNFR1 during neurogenic cystitis. Am J Physiol Renal Physiol 291: F741–F749, 2006. doi: 10.1152/ajprenal.00081.2006. [DOI] [PubMed] [Google Scholar]
- 9.Clemens JQ, Mullins C, Kusek JW, Kirkali Z, Mayer EA, Rodríguez LV, Klumpp DJ, Schaeffer AJ, Kreder KJ, Buchwald D, Andriole GL, Lucia MS, Landis JR, Clauw DJ; MAPP Research Network Study Group . The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol 14: 57, 2014. doi: 10.1186/1471-2490-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanno P. Bladder pain syndrome (interstitial cystitis) and related disorders. In: Campbell-Walsh Urology, edited by Wein A, Kavoussi L, Novick A, Partin A, Peters C. Philadelphia, PA: Elsevier, 2012, p. 357–401.e18. doi: 10.1016/B978-1-4160-6911-9.00012-8. [DOI] [Google Scholar]
- 11.Jasmin L, Janni G, Manz HJ, Rabkin SD. Activation of CNS circuits producing a neurogenic cystitis: evidence for centrally induced peripheral inflammation. J Neurosci 18: 10016–10029, 1998. doi: 10.1523/JNEUROSCI.18-23-10016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasmin L, Janni G, Ohara PT, Rabkin SD. CNS induced neurogenic cystitis is associated with bladder mast cell degranulation in the rat. J Urol 164: 852–855, 2000. doi: 10.1016/S0022-5347(05)67326-0. [DOI] [PubMed] [Google Scholar]
- 13.Kerr DE, Liang F, Bondioli KR, Zhao H, Kreibich G, Wall RJ, Sun TT. The bladder as a bioreactor: urothelium production and secretion of growth hormone into urine [see comments]. Nat Biotechnol 16: 75–79, 1998. doi: 10.1038/nbt0198-75. [DOI] [PubMed] [Google Scholar]
- 14.Lai H, Gereau RW IV, Luo Y, O’Donnell M, Rudick CN, Pontari M, Mullins C, Klumpp DJ. Animal models of urologic chronic pelvic pain syndromes: findings from the Multidisciplinary Approach to the Study of Chronic Pelvic Pain Research Network. Urology 85: 1454–1465, 2015. doi: 10.1016/j.urology.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 92: 335–342, 2001. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 16.Landis JR, Williams DA, Lucia MS, Clauw DJ, Naliboff BD, Robinson NA, van Bokhoven A, Sutcliffe S, Schaeffer AJ, Rodriguez LV, Mayer EA, Lai HH, Krieger JN, Kreder KJ, Afari N, Andriole GL, Bradley CS, Griffith JW, Klumpp DJ, Hong BA, Lutgendorf SK, Buchwald D, Yang CC, Mackey S, Pontari MA, Hanno P, Kusek JW, Mullins C, Clemens JQ; MAPP Research Network Study Group . The MAPP Research Network: design, patient characterization and operations. BMC Urol 14: 58, 2014. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr, Sivasubramanian N, Mann DL, Reid MB. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102: 1690–1696, 2000. doi: 10.1161/01.CIR.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 18.Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proc Natl Acad Sci USA 92: 679–683, 1995. doi: 10.1073/pnas.92.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peyrin-Biroulet L, Fiorino G, Buisson A, Danese S. First-line therapy in adult Crohn’s disease: who should receive anti-TNF agents? Nat Rev Gastroenterol Hepatol 10: 345–351, 2013. doi: 10.1038/nrgastro.2013.31. [DOI] [PubMed] [Google Scholar]
- 20.Rabin C, O’Leary A, Neighbors C, Whitmore K. Pain and depression experienced by women with interstitial cystitis. Women Health 31: 67–81, 2000. doi: 10.1300/J013v31n04_05. [DOI] [PubMed] [Google Scholar]
- 21.Rao Q, Chen Y, Yeh CR, Ding J, Li L, Chang C, Yeh S. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget 7: 7842–7855, 2016. doi: 10.18632/oncotarget.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudick CN, Bryce PJ, Guichelaar LA, Berry RE, Klumpp DJ. Mast cell-derived histamine mediates cystitis pain. PLoS One 3: e2096, 2008. doi: 10.1371/journal.pone.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudick CN, Schaeffer AJ, Klumpp DJ. Pharmacologic attenuation of pelvic pain in a murine model of interstitial cystitis. BMC Urol 9: 16, 2009. doi: 10.1186/1471-2490-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology 69, Suppl: 34–40, 2007. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 25.Schiwon M, Weisheit C, Franken L, Gutweiler S, Dixit A, Meyer-Schwesinger C, Pohl JM, Maurice NJ, Thiebes S, Lorenz K, Quast T, Fuhrmann M, Baumgarten G, Lohse MJ, Opdenakker G, Bernhagen J, Bucala R, Panzer U, Kolanus W, Gröne HJ, Garbi N, Kastenmüller W, Knolle PA, Kurts C, Engel DR. Crosstalk between sentinel and helper macrophages permits neutrophil migration into infected uroepithelium. Cell 156: 456–468, 2014. doi: 10.1016/j.cell.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675, 2012. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomaszewski JE, Landis JR, Russack V, Williams TM, Wang LP, Hardy C, Brensinger C, Matthews YL, Abele ST, Kusek JW, Nyberg LM; Interstitial Cystitis Database Study Group . Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology 57, Suppl 1: 67–81, 2001. doi: 10.1016/S0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Cheng Z, Cristofaro V, Li J, Xiao X, Gomez P, Ge R, Gong E, Strle K, Sullivan MP, Adam RM, White MF, Olumi AF. Inhibition of TNF-α improves the bladder dysfunction that is associated with type 2 diabetes. Diabetes 61: 2134–2145, 2012. doi: 10.2337/db11-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins KE, Eberhart N, Hilton L, Suttorp MJ, Hepner KA, Clemens JQ, Berry SH. Depressive disorders and panic attacks in women with bladder pain syndrome/interstitial cystitis: a population-based sample. Gen Hosp Psychiatry 33: 143–149, 2011. doi: 10.1016/j.genhosppsych.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu S, Wang X, Wang Y, Lutgendorf S, Bradley C, Schrepf A, Kreder K, O’Donnell M, Luo Y. Transgenic mice expressing MCP-1 by the urothelium demonstrate bladder hypersensitivity, pelvic pain and voiding dysfunction: a multidisciplinary approach to the Study of Chronic Pelvic Pain Research Network Animal Model Study. PLoS One 11: e0163829, 2016. doi: 10.1371/journal.pone.0163829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamauchi PS, Bissonnette R, Teixeira HD, Valdecantos WC. Systematic review of efficacy of anti-tumor necrosis factor (TNF) therapy in patients with psoriasis previously treated with a different anti-TNF agent. J Am Acad Dermatol 75: 612–618.e616, 2016. doi: 10.1016/j.jaad.2016.02.1221. [DOI] [PubMed] [Google Scholar]
- 33.Yang W, Rudick CN, Hoxha E, Allsop SA, Dimitrakoff JD, Klumpp DJ. Ca2+/calmodulin-dependent protein kinase II is associated with pelvic pain of neurogenic cystitis. Am J Physiol Renal Physiol 303: F350–F356, 2012. doi: 10.1152/ajprenal.00077.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zec K, Volke J, Vijitha N, Thiebes S, Gunzer M, Kurts C, Engel DR. Neutrophil migration into the infected uroepithelium is regulated by the crosstalk between resident and helper macrophages. Pathogens 5: 15, 2016. doi: 10.3390/pathogens5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang ZT, Pak J, Shapiro E, Sun TT, Wu XR. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res 59: 3512–3517, 1999. [PubMed] [Google Scholar]
- 36.Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol 7: 9, 2007. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]