Abstract
Up to date, the cervical cancer remains to be one of the leading gynecological malignancies worldwide. MicroRNAs (miRNAs) play critical roles in the process of tumor initiation and progression. However, miR-96 has rarely been investigated in human cervical carcinoma. We aimed to investigate the biological function and underlying molecular mechanism of miR-96 in human cervical carcinoma. MiR-96 levels were determined by qRT-PCR. Protein tyrosine phosphatase, non-receptor type 9 (PTPN9) mRNA and protein levels were investigated by qRT-PCR and western blotting. The cellular proliferation in cervical cells was monitored by CyQuant assay. Soft agar assay was employed to determine the tumorigenicity. 3′ UTR luciferase assay was used to validate the target gene of miR-96. SPSS was used to analyze statistical significance in different treatment. MiR-96 was dramatically upregulated in human cervical tumor tissues. Overexpression of miR-96 was found to significantly promote the cellular proliferation and tumorigenicity of cervical cells. Furthermore, we showed that PTPN9 was a direct target gene of miR-96 and had opposite effect to those of miR-96 on cervical cells. MiR-96 may promote the cellular proliferation and tumorigenicity of cervical cells by silencing PTPN9. Our study highlights an importantly regulatory role of miR-96 and suggests that an appropriate manipulation of miR-96 may be a new treatment of human cervical carcinoma in the future.
Keywords: MiR-96, Proliferation, PTPN9, Cervical carcinoma
1. Introduction
Human cervical cancer is the fourth leading cause of cancer death in women worldwide and is one of the main causes of cancer-related death in the developing countries (How et al., 2015, Jemal et al., 2008). In 2016, the new cases of cervical cancer diagnosis were around 12,990 and more than 4100 patients died from cervical cancer based on the cancer statistics study in the US (Siegel et al., 2016). In detection of advanced staged cervical cancer, the estimated 5- year survival rates decreased dramatically (Duenas-Gonzalez and Campbell, 2016). Despite extensive basic as well as clinical research efforts, very little is known regarding the molecular mechanisms of the cervical cancer. Therefore, it is important to understand the molecular mechanisms responsible for the cervical cancer for the diagnosis and treatment of cervical cancer.
MicroRNAs (miRNAs) have been known as critical regulators to modulate biological signaling cascades via the post-transcriptional gene regulation or protein degradation. They are single stranded noncoding small RNAs and conserved across species (Carroll et al., 2014, Ebert and Sharp, 2012, Krutzfeldt et al., 2006). Recent studies have demonstrated that miRNAs play important roles in various cancers and become as efficient prognostic biomarkers in developing new diagnostic methods and treatment strategies for patients (Hu et al., 2010, Wang et al., 2016a, Wang et al., 2016b). The role of miRNAs in human cervical carcinoma have also been discussed. For example, miR-138 has been reported as a potential biomarker and tumor suppressor in human cervical carcinoma by reversely correlated with TCF3 gene (Li et al., 2017). Also, miR-497 was shown to negatively regulate insulin-like growth factor and served as the tumor suppressor in cervical cancer (Luo et al., 2013). Although much is known about the miRNA profiles, the biological function of miRNAs in human cervical cancers has not yet been fully understood.
MiR-96 usually functions as an oncogene in the tumorigenesis. It has been investigated to be upregulated in multiple cancers, such as colorectal adenocarcinoma, bladder cancer, lung cancer, prostate cancer, and hepatocellular carcinoma (Guo et al., 2012, Haflidadottir et al., 2013, Rapti et al., 2016, Xu et al., 2013). FOXO1, FOXO3a, RECK, EphrinA5 and SAMD9 have been validated as the targets of miR-96 in different cancers (Carroll et al., 2014, Guo et al., 2014, Guttilla and White, 2009, He et al., 2014, Lin et al., 2010, Su et al., 2012, Wang et al., 2016a, Wang et al., 2016b, Xia et al., 2014). Thus, it is attractive to explore the underlying molecular mechanism and target gene of miR-96 in cervical cancer.
In this study, we observed that miR-96 expression was overexpressed in the human cervical cancer tissues which reversely correlated with the protein tyrosine phosphatase, non-receptor type 9 (PTPN9) expression. Next, we validated that PTPN9 was directly suppressed by miR-96. Subsequently, we found that miR-96 enhanced the cellular progression and tumorigenicity of cervical cancer cells. Moreover, upregulated PTPN9 had an opposite effect on the cervical cancer cells.
2. Material and methods
2.1. Cell culture and tissue samples
HeLa cells were cultured in RPMI 1640 (Gibco) supplemented with 10% fetal bovine serum (FBS), penicillin and streptomycin (100 U/mL, and 100 μg/mL). The cells were incubated in the humidified chamber with 5% CO2 at 37 °C.
All tissue samples were collected in accordance the ethical guidelines of the Affiliated Liaocheng People’s Hospital of Shandong University
2.2. Overexpression of miR-96
Overexpression of miR-96 was achieved by transfecting cells with hsa-miR-96 mimic which was a synthetic double-stranded RNA oligonucleotide mimicking miR-96 precursor. The miR-96 was commercially available from life technologies (Carlsbad, CA). The transfection was performed with RNAiMAX reagent (life technologies). The miRNA vector control (miR-NC) was also commercially available from life technologies.
2.3. Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated from human tissues or cells using mirVanaTM miRNA isolation kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. The complement DNA (cDNA) was generated by RNA-to-cDNA kit (Applied Biosystems, Foster City, CA). The target gene of miR-96 was predicted using the miRbase Target (Griffiths- Jones et al., 2006). Assays to quantify miRNA target gene were performed by TaqMan gene specific probes (PTNP9, Applied Biosystems) following the manufacturer’s instructions. β-Actin was used an internal control. Assays to qualify miRNA expression was used by Taqman advanced miRNA cDNA synthesis kit (Applied Biosystems). The miR-96 levels were calculated relative to the U6 snRNA internal reference. The U6 cDNA was synthesized using a TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems).
2.4. Quantification of cell proliferation
The cell proliferation was qualified with CyQuant Assay Kit (Thermo Fisher Scientific) based on DNA fluorescence. Cell were seeded in the 96-well plate (5000 cells/well). The plates were frozen at the indicated time of incubation. The fresh prepared CyQuant solution was added to the well as described in assay instructions.
2.5. Soft agar colony formation assay
The soft agar colony formation assay is considered as the most stringent assay to monitor anchorage-independent growth in vitro. As previously described (Franken et al., 2006), the HeLa cells were seeded in the 6-well plates (1000 cells/well) following transfection. Fresh culture medium was replaced every 3 days. After the cells have formed sufficiently large clones, the cells were stained with crystal violet, and the numbers of colonies containing more than 50 cells were counted.
2.6. Luciferase assay
We constructed the luciferase reporter carrying the PTPN9 3′ UTR with the predicted potential binding site of miR-96 as previously described (Hong et al., 2016). For the luciferase assay, cells were seeded in a 96-well plate at a density of 2 × 104 cells per well. The cells were cotransfected with 0.3 μg firefly luciferase reporter plasmid, 0.15 μg β-galactosidase expression vector (Ambion, Austin, TX) and same amounts of miRNA vector control (miR-NC) or miR-96 by lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The cells were analyzed with luciferase assay kit (Promega) after 48 h. β-galactosidase expression vector was used as a transfection control.
2.7. Western blotting
The proteins were harvested after the cells and tissues were lysed with RIPA lysis buffer. All proteins were resolved on the commercially available 4–15% precast gels (Bio-Rad, Richmond, CA). After transferred onto the nitrocellulose membrane (Bio-Rad), the membrane was incubated with primary antibodies overnight at 4 °C. The membrane was washed and incubated with a horseradish peroxidase-conjugated secondary antibody. Protein levels were assessed by the enhanced chemiluminescence and exposure to the chemiluminescent film. β-actin was used as a loading control.
2.8. PTPN9 overexpression
As previously described (Hong et al., 2016), we purchased the mammalian expression plasmid (pReceiver-M02-PTPN9) designed to encode the full-length open reading frame (ORF) of human PTPN9 without the 3′ UTR from GeneCopoeia (Germantown, MD). An empty plasmid (pReceiver-M02) was used as a plasmid control (plasmid-NC). The overexpression plasmid of PTPN9 was transfected into cervical cells using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions.
2.9. Statistics
All studies were performed on different samples from 3 independent cell preparations. The data was presented as mean ± SE. The expression differences were analyzed with two-tailed unpaired student’s t-test. A value of P < .05 was considered as the statistical significance by the calculation of SPSS (SPSS Inc., Chicago, IL).
3. Results
3.1. MiR-96 is up-regulated in human cervical carcinoma tissues
We first determined the expression patterns of miR-96 in human cervical carcinoma tissues. MiR-96 expression was observed to be dramatically up-regulated in the cancer tissues compared with adjacent noncancerous tissues (Fig. 1). We used 12 pairs of cervical cancer tissues and the adjacent noncancerous tissues.
Fig. 1.
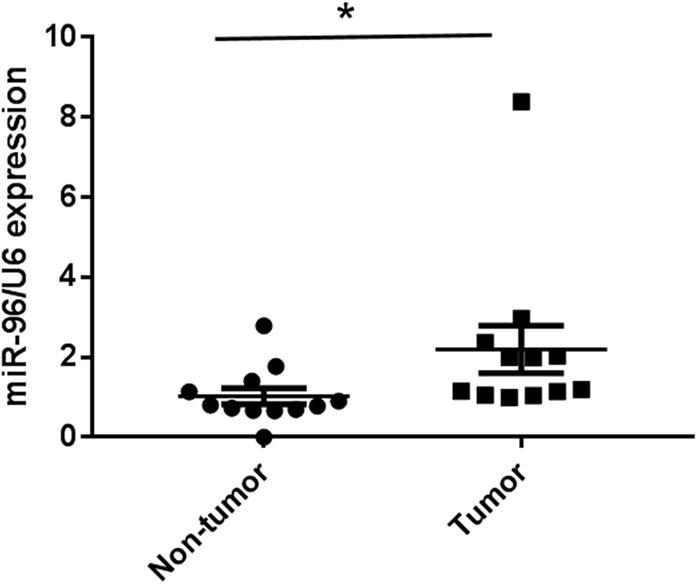
MiR-96 expression in cervical cancer tissues. Taqman qRT-PCR detection of miR-96 expression in 12 cases of cancer tissue and paired non-tumor tissues. U6 was employed as an internal loading control. *P < 0.05 versus non-tumor tissues.
3.2. MiR-96 up-regulation promotes cellular proliferation and tumorigenicity
To further explore the biological role of miR-96 in the development and progression of cervical carcinoma, we next overexpressed hsa-miR-96 in the HeLa cells to detect its effect on cellular proliferation. We found miR-96 overexpression significantly enhanced the growth rate of the cervical cells as compared with the miR-NC transfected cells (Fig.2A). Moreover, as seen in Fig.2B, overexpression of miR-96 also dramatically increased the anchorage independent growth in the cervical cells, as observed by the increase in colony numbers compared with untransfected cells. These results suggest that miR-96 up-regulation enhances cervical cell proliferation. The up-regulation of miR-96 could also enhance the tumorigenicity of cervical cancer cells in vitro.
Fig. 2.
miR-96 promote the cellular proliferation and tumorigenicity. (A) CyQuant assay was used to monitor the cellular proliferation. The HeLa cells were transfected with miR-NC or mir-96. CyQuant assay was performed as indicated time. *p < .05 versus Mock, **p < .05 versus miR-NC; (B) Soft agar assay was employed to determine the tumorigenicity in untransfected cells (Mock) and miR-96 transfected cells. Scale bar = 50 μm.
3.3. PTPN9 is repressed by miR-96
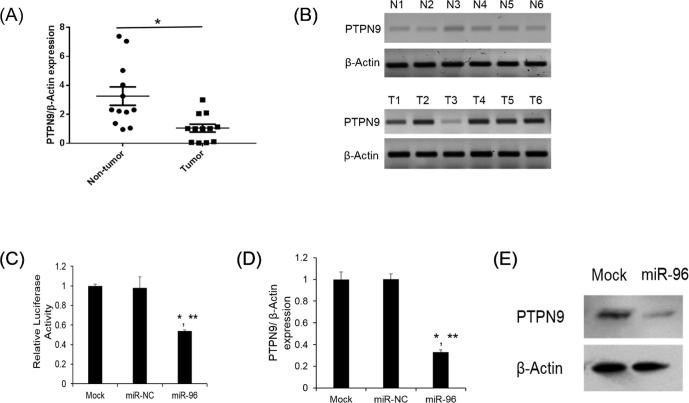
To decipher the molecular mechanism of how miR-96 contributes to cervical cancer progression, we used miRbase Target to predict target genes of miR-96. Among those candidates, PTPN9 was found to be frequently downregulated in other cancers (Hong et al., 2016, Su et al., 2012, Yuan et al., 2010). We first determined the PTPN9 mRNA level in tissue samples. PTPN9 mRNA and protein expression was found to downregulated in human cervical tumor tissues compared with the normal tissues (Fig.3A, B). We next verified whether PTPN9 is a potential target of miR-96 by luciferase assay. As seen in Fig.3C, miR-96 significantly decreased the luciferase activity of PTPN9 3′ UTR as compared to the untransfected cells (Mock). MiR-NC also had no effect on the luciferase activity of PTPN9 3′ UTR (Fig.3C). PTPN9 mRNA and protein expression was also found to be dramatically decreased after miR-96 overexpression (Fig.3D, E). Our results indicate that PTPN9 is a target of miR-96.
Fig. 3.
PTPN9 is the direct target of miR-96. (A) Taqman qRT-PCR detection of PTPN9 expression in 12 cases of cancer tissue and paired non-tumor tissues. β-Actin was used as an internal reference. *P < .05 versus non-tumor tissues; (B) western blot was used to detect the PTPN9 protein expression from 6 randomly chosen non-tumorous tissues or tumor tissues, respectively. β-Actin was used as a loading control. (C) 3′ UTR luciferase reporter assay. The HeLa cells were cotransfected with PTPN9 3′ UTR reporter with miR-NC or mir-96. Luciferase assays were performed after 48 h. The β-galactosidase activity was used for normalization. *p < .05 versus Mock, **p < .05 versus miR-NC; (D) relative PTPN9 mRNA expression level was analyzed by Taqman qRT-PCR in HeLa cells transfected with miR-NC or miR-96. *p < .05 versus Mock, ** p < .05 versus miR-NC; (E) Western blot analysis forPTPN9 in the HeLa cells after transfected with miR-96. Mock was used as a negative control.
3.4. PTPN9 has opposite effect of miR-96 in vitro
To investigate whether miR-96 may regulate cellular proliferation and tumorigenicity, we assessed the biological role of PTPN9 on cellular proliferation and tumorigenicity by overexpression of PTPN9 in cervical cells. As seen in Fig.4A, B, PTPN9 mRNA and protein expression was shown to be significantly increased compared to untransfected cells and cells transfected with plasmid-NC. After PTPN9 overexpression, the cellular proliferation and tumorigenicity ability significantly decreased in the cervical cells (Fig.4C, D). These results suggest that miR-96 regulate the proliferation and tumorigenicity through a PTPN9 dependent manner.
Fig. 4.
PTPN9 overexpression repressed the cellular proliferation and tumorigenicity. (A) Taqman qRT-PCR was used to analyze PTPN9 mRNA levels after PTPN9 overexpression. *p < .05 versus Mock, **p < .05 versus plasmid-NC; (B) Western blot was employed to detect PTPN9 protein expression after PTPN9 overexpression; (C) After 48 h of transfection, the untransfected (Mock) and transfected cells were cultured for the indicated time. The cellular proliferation was determined by CyQuant assay. *p < .05 versus Mock, **p < .05 versus plasmid-NC; (D) Soft agar assay was employed to detect the tumorigenicity in Mock and miR-96 transfected cells. Scale bar = 50 μm.
4. Discussion
Many researchers have reported that the expression and functions of miRNAs are cell and tissue specific. Since the dysfunctions of miRNAs is also commonly associated with the initial and developmental stages of human cancers, the correction of miRNA expression may emerge as a potential therapeutic strategy. In this study, miR-96 was found to be upregulated in human cervical cancer tissues and it could promote cervical cancer cell proliferation and tumorigenicity. In addition, we validated that PTPN9 is directly targeted by miR-96. PTPN9 overexpression was shown to have opposite effect of miR-96 on cervical cancer cells in vitro.
Taken together, these data indicate that mir-96 enhances cellular proliferation of human cervical carcinoma cells through PTNP9. It also raises the possibility of the potential of miR-96 as a therapeutic target of human cervical cancer.
MiRNAs are well known as a class of small regulatory RNA molecules that can regulate gene expression in a sequence-specific manner. Recently, miRNAs have also been reported to play critical roles in various biological processes including cellular proliferation, oncogenesis, invasion and metastasis (Calin and Croce, 2006, Esquela-Kerscher and Slack, 2006, Gregory and Shiekhattar, 2005). MiR-96 has been reported to upregulated and contribute to many biological processes (Agirre et al., 2008, Bandres et al., 2006, Pineau et al., 2010). It has been shown that upregulated miR-96 induces cell proliferation in human breast cancer through negatively regulating transcriptional factor FOXO3a (Lin et al., 2010).
In breast cancer, miR-96 was also found to promote cell proliferation, migration and invasion by downregulating PTPN9 (Hong et al., 2016). MiR-96 had been identified to suppress KRAS which functions as a tumor suppressor gene in pancreatic cancer (Yu et al., 2010). In the non-small cell lung cancer (NSCLC) cells, miR-96a downregulated RECK to promote cell growth and motility (Guo et al., 2014). Despite all of these studies, there are few functional studies of miR-96 in the human cervical cancer. We observed that miR-96 was upregulated in human cervical cancer and miR-96 can enhance the cellular proliferation of human cervical carcinoma cells. Our findings add to the growing body of work demonstrating cell- and tissue-specific functions of miR-96.
PTPN9 is a cytoplasmic phosphatase belonging to the classic tyrosine-specific protein tyrosine phosphatases (PTPs), which are key regulators in multiple cellular functions (He et al., 2014, Su et al., 2012). It has been shown that downregulated expression of PTPN9 contributes to human hepatocellular carcinoma growth and progression (Hu et al., 2016).
Moreover, PTPN9 has also been reported to promote cell proliferation and invasion in esophageal squamous cell carcinoma (ESCC) cells (Zhu et al., 2017). PTPN9 has also been observed to contribute to the invasion and metastasis in breast carcinoma (Du et al., 2013). In this study, we showed that PTPN9 was downregulated in human cervical cancer tissues and directly repressed by miR-96. More importantly, PTPN9 overexpression has opposite effect of miR-96 on cell proliferation and tumorigenicity ability of human cervical cells. Therefore, we speculate that miR-96 modulation of PTPN9 can promote the human cervical cell proliferation and tumorigenicity ability.
In conclusion, this study demonstrated an important link between miR-96-mediated proliferation of cervical cancer cells and downregulation of PTPN9. More importantly, this current study also provides a new candidate for the therapeutic targeting in human cervical carcinoma. Further research on miR-96 and PTPN9 may also reveal a new avenue for treatment of cervical cancer.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agirre X., Jimenez-Velasco A., San Jose-Eneriz E. Down-regulation of hsamiR- 10a in chronic myeloid leukemia CD34+ cells increases USF2-mediated cell growth. Mol. Cancer Res. 2008;6:1830–1840. doi: 10.1158/1541-7786.MCR-08-0167. [DOI] [PubMed] [Google Scholar]
- Bandres E., Cubedo E., Agirre X. Identification by Real-time PCR of 13 mature microRNAs differentially expressed in colorectal cancer and non-tumoral tissues. Mol. Cancer. 2006;5:29. doi: 10.1186/1476-4598-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Carroll A.P., Goodall G.J., Liu B. Understanding principles of miRNA target recognition and function through integrated biological and bioinformatics approaches. Wiley Interdiscip. Rev. RNA. 2014;5:361–379. doi: 10.1002/wrna.1217. [DOI] [PubMed] [Google Scholar]
- Du W.W., Fang L., Li M. MicroRNA miR-24 enhances tumor invasion and metastasis by targeting PTPN9 and PTPRF to promote EGF signaling. J. Cell Sci. 2013;126:1440–1453. doi: 10.1242/jcs.118299. [DOI] [PubMed] [Google Scholar]
- Duenas-Gonzalez A., Campbell S. Global strategies for the treatment of early-stage and advanced cervical cancer. Curr. Opin. Obstet. Gynecol. 2016;28:11–17. doi: 10.1097/GCO.0000000000000234. [DOI] [PubMed] [Google Scholar]
- Ebert M.S., Sharp P.A. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquela-Kerscher A., Slack F.J. Oncomirs - microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- Gregory R.I., Shiekhattar R. MicroRNA biogenesis and cancer. Cancer Res. 2005;65:3509–3512. doi: 10.1158/0008-5472.CAN-05-0298. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S., Grocock R.J., van Dongen S., Bateman A., Enright A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H., Li Q., Li W., Zheng T., Zhao S., Liu Z. MiR-96 downregulates RECK to promote growth and motility of non-small cell lung cancer cells. Mol. Cell. Biochem. 2014;390:155–160. doi: 10.1007/s11010-014-1966-x. [DOI] [PubMed] [Google Scholar]
- Guo Y., Liu H., Zhang H., Shang C., Song Y. miR-96 regulates FOXO1-mediated cell apoptosis in bladder cancer. Oncol. Lett. 2012;4:561–565. doi: 10.3892/ol.2012.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttilla I.K., White B.A. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J. Biol. Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haflidadottir B.S., Larne O., Martin M. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS One. 2013;8:e72400. doi: 10.1371/journal.pone.0072400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R.J., Yu Z.H., Zhang R.Y., Zhang Z.Y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 2014;35:1227–1246. doi: 10.1038/aps.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y., Liang H., Uzair Ur R. miR-96 promotes cell proliferation, migration and invasion by targeting PTPN9 in breast cancer. Sci. Rep. 2016;6:37421. doi: 10.1038/srep37421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- How C., Pintilie M., Bruce J.P. Developing a prognostic micro-RNA signature for human cervical carcinoma. PLoS One. 2015;10:e0123946. doi: 10.1371/journal.pone.0123946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Yan X., Liu F. Downregulated expression of PTPN9 contributes to human hepatocellular carcinoma growth and progression. Pathol. Oncol. Res. 2016;22:555–565. doi: 10.1007/s12253-015-0038-1. [DOI] [PubMed] [Google Scholar]
- Hu X., Schwarz J.K., Lewis J.S. A microRNA expression signature for cervical cancer prognosis. Cancer Res. 2010;70:1441–1448. doi: 10.1158/0008-5472.CAN-09-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A., Siegel R., Ward E. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Krutzfeldt J., Poy M.N., Stoffel M. Strategies to determine the biological function of microRNAs. Nat. Genet. 2006;38(Suppl):S14–19. doi: 10.1038/ng1799. [DOI] [PubMed] [Google Scholar]
- Li H., Sheng Y., Zhang Y., Gao N., Deng X., Sheng X. MicroRNA-138 is a potential biomarker and tumor suppressor in human cervical carcinoma by reversely correlated with TCF3 gene. Gynecol. Oncol. 2017;145:569–576. doi: 10.1016/j.ygyno.2017.01.018. [DOI] [PubMed] [Google Scholar]
- Lin H., Dai T., Xiong H. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS One. 2010;5:e15797. doi: 10.1371/journal.pone.0015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M., Shen D., Zhou X., Chen X., Wang W. MicroRNA-497 is a potential prognostic marker in human cervical cancer and functions as a tumor suppressor by targeting the insulin-like growth factor 1 receptor. Surgery. 2013;153:836–847. doi: 10.1016/j.surg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Pineau P., Volinia S., McJunkin K. miR-221 overexpression contributes to liver tumorigenesis. Proc. Natl. Acad. Sci. USA. 2010;107:264–269. doi: 10.1073/pnas.0907904107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapti S.M., Kontos C.K., Papadopoulos I.N., Scorilas A. High miR-96 levels in colorectal adenocarcinoma predict poor prognosis, particularly in patients without distant metastasis at the time of initial diagnosis. Tumour Biol. 2016;37:11815–11824. doi: 10.1007/s13277-016-5023-0. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Su F., Ren F., Rong Y. Protein tyrosine phosphatase Meg2 dephosphorylates signal transducer and activator of transcription 3 and suppresses tumor growth in breast cancer. Breast Cancer Res. 2012;14:R38. doi: 10.1186/bcr3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Li B., Xie X. The roles and clinical significance of microRNAs in cervical cancer. Histol. Histopathol. 2016;31:131–139. doi: 10.14670/HH-11-666. [DOI] [PubMed] [Google Scholar]
- Wang T.H., Yeh C.T., Ho J.Y., Ng K.F., Chen T.C. OncomiR miR-96 and miR-182 promote cell proliferation and invasion through targeting ephrinA5 in hepatocellular carcinoma. Mol. Carcinog. 2016;55:366–375. doi: 10.1002/mc.22286. [DOI] [PubMed] [Google Scholar]
- Xia H., Chen S., Chen K., Huang H., Ma H. MiR-96 promotes proliferation and chemo- or radioresistance by down-regulating RECK in esophageal cancer. Biomed. Pharmacother. 2014;68:951–958. doi: 10.1016/j.biopha.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Xu D., He X., Chang Y. Inhibition of miR-96 expression reduces cell proliferation and clonogenicity of HepG2 hepatoma cells. Oncol. Rep. 2013;29:653–661. doi: 10.3892/or.2012.2138. [DOI] [PubMed] [Google Scholar]
- Yu S., Lu Z., Liu C. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- Yuan T., Wang Y., Zhao Z.J., Gu H. Protein-tyrosine phosphatase PTPN9 negatively regulates ErbB2 and epidermal growth factor receptor signaling in breast cancer cells. J. Biol. Chem. 2010;285:14861–14870. doi: 10.1074/jbc.M109.099879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Li H., Ma J., Huang H., Qin J., Li Y. PTPN9 promotes cell proliferation and invasion in Eca109 cells and is negatively regulated by microRNA-126. Oncol. Lett. 2017;14:1419–1426. doi: 10.3892/ol.2017.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]