Abstract
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the fifth leading cause of cancer-related death worldwide. Novel prognostic biomarkers are urgently needed for patients with HCC. Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) overexpression may promote tumor metastasis in HCC. However, few studies investigate the prognosis predictive role of LGR5 in patients with HCC. Herein, we aimed to examine the expression level of LGR5 in tumors and its correlation with clinical characteristics and survivals of patients with HCC. LGR5 expression in tumor specimens and adjacent tissue resected from 66 patients were detected by immunohistochemistry. The results showed that the expression of LGR5 was markedly higher in HCC than in normal adjacent tissues (P = .006). High expression of LGR5 was significantly correlated with later disease stage (P = .009). In addition, high LGR5 expression was remarkably correlated with short overall survival than those with low LGR5 expression (P < .05). The median overall survival of patients with high LGR5 expression was 12 months, whereas that of patients with low LGR5 expression was still not reached (longer than 70 months). Notably, in our limited cases, we did not detect any difference in tumor size, lymphatic invasion, or metastasis in patients with high or low expression of LGR5. In conclusion, high protein level of LGR5 was associated with poor prognosis of these patients. LGR5 appears to be a valuable prognostic predictor clinically and a potential target in HCC therapy.
Keywords: Liver cancer, LGR5, Immunohistochemistry, Clinicopathology, Overall survival
Abbreviations: HBs, hepatitis B virus; EMT, epithelial-to-mesenchymal transition; Flot2, flotillin-2; GPCR, G-protein-coupled receptor; GPR49, G-protein coupled receptor 49; GRP78, glucose-regulated protein 78; HCC, hepatocellular carcinoma; LGR5, leucine-rich repeat-containing G protein-coupled receptor; PD-L1, programmed death ligand 1
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the fifth leading cause of cancer-related deaths worldwide. Few patients are suitable for surgery and liver transplantation, which are believed to be effective treatments for HCC. Currently, there exist no effective drugs to treat HCC (Bertuccio et al., 2017, Siegel et al., 2017), and the general prognosis of these patients remains poor with a 5-year overall survival rate of approximately 10% (Yang and Roberts, 2010). To predict prognosis and to guide treatment in HCC patients, many biomarkers have been explored. These include glucose-regulated protein 78 (GRP 78) (Ying et al., 2017), programmed death ligand 1 (PD-L1) (Dai et al., 2017), and flotillin-2 (Flot2) (Wang et al., 2017). However, the sensitivity and specificity of these biomarkers are not optimal. Therefore, novel prognostic biomarkers are urgently needed for HCC.
Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5), also known as G-protein coupled receptor 49 (GPR 49) and G-protein coupled receptor 67 (GPR 67), is a member of the G-protein-coupled receptor (GPCR) family. The GPCR class of proteins have 7 transmembrane domains and can transduce extracellular signals into the cells (Carmon et al., 2011, de Lau et al., 2011). LGR5 has been identified as a stem cell marker in the colon, stomach, hair follicles, kidney, and mammary glands (Gil-Sanchis et al., 2013). Moreover, LGR5-positive stem cells drive malignant progression in both small intestine and colon (Barker et al., 2009). LGR5 is also found overexpressed in many types of cancer including breast cancer (Yang et al., 2015), basal cell carcinoma (Tanese et al., 2008), and HCC (Liu et al., 2017, Yamamoto et al., 2003). As for its clinical relevance, LGR5 overexpression is reported to be correlated with lymphatic invasion in gastric cancer (Yamanoi et al., 2013). In addition, LGR5 overexpression also indicates poor prognosis in breast patients (Yang et al., 2015). In HCC, LGR5 is closely associated with Wnt/β-Catenin signaling (Effendi et al., 2014, Lei et al., 2015) and mediates epithelial-to-mesenchymal transition (EMT) (Liu et al., 2017). Since EMT leads to stronger invasive capacity (Zhang et al., 2013), LGR5 overexpression may promote tumor metastasis in HCC. However, few studies have investigated the predictive value of LGR5 in HCC prognosis (Lei et al., 2015).
In this study, we examined the expression of LGR5 in 66 HCC tissues and matched normal specimens that were acquired from surgical resection. We analyzed the association between LGR5 expression and clinical parameters, and explored the prognostic role LGR5 in patients with HCC.
2. Methods
2.1. Patients and specimens
The formalin-fixed, paraffin-embedded tissues used for the study were collected from 66 HCC patients who underwent curative surgery at Zhejiang Provincial People’s Hospital from 2008 to 2015. These specimens were grouped as tumor tissues and matched adjacent normal tissues. Overall survival was evaluated from the date of surgical resection of the primary tumor to the date of death or the last follow-up (March, 2016). This study was approved by the Ethics Committee of the Zhejiang Provincial People’s Hospital. All patients provided written informed consent before any study-related procedures.
2.2. Immunohistochemistry (IHC)
IHC was performed by a standard method as previously described (Bai et al., 2015). Briefly, 5-μm sections from tissue microarrays were baked at 70 °C for 2 h. The sections were then removed from paraffin in xylene solution, rehydrated using a gradient of ethanol concentrations, boiled in 1 mM Tris-EDTA buffer with a high-pressure cooker for 3 min to retrieve antigens, blocked with 3% hydrogen peroxide for 15 min to inhibit activities of endogenous peroxidases and incubated with 10% goat non-immune serum for 20 min to reduce non-specific staining. Then, the sections were incubated with rabbit anti-LGR5 monoclonal antibody (1:500 dilution; Abcam, Cambridge, UK) at 4 °C overnight, then incubated with biotin-labeled secondary antibody (Invitrogen, Carlsbad, CA, USA) at room temperature for 15 min, followed by incubation with HRP-conjugated streptavidin (Invitrogen) at room temperature for another 15 min. Color development was performed with DAB Substrate Kit (Dako, Glostrup, Denmark). Finally, the sections were counterstained with hematoxylin, dehydrated, cleared, and mounted.
2.3. Evaluation of IHC staining
The IHC staining was independently scored by two experienced pathologists based on the intensity and the proportion of positively stained cells. Stain intensity was evaluated with a 4-point grading system: 0 = negative, 1 = weak, 2 = moderate and 3 = strong. The percentage of positive cells were scored as follows: 0 for no cells stained, 1 for 1–25% of cells stained, 2 for 26–50% of cells stained, 3 for 51–75% of cells stained and 4 for more than 75% of cells stained. Scores for intensity and percentage of positive cells were multiplied. Scores ≤2 was used to define tumors with low LGR5 expression and scores >2 with high LGR5 expression.
2.4. Statistical analysis
Statistical analyses were performed using SPSS Statistics version 24.0 (IBM, Armonk, NY, USA) and Prism version 6.0 (GraphPad, San Diego, CA, USA). The correlations between LGR5 and clinical parameters were tested using Fisher’s exact test or Pearson’s chi-square test, as appropriate. Overall survival was evaluated using the Kaplan-Meier method, and log-rank test was used to compare the difference between groups. Cox regression analysis of survival was performed through the SPSS software, version 24 (SPSS Inc., Chicago, IL, USA). All results were considered significant when P < .05.
3. Results
3.1. Expression of LGR5 in HCC tissues
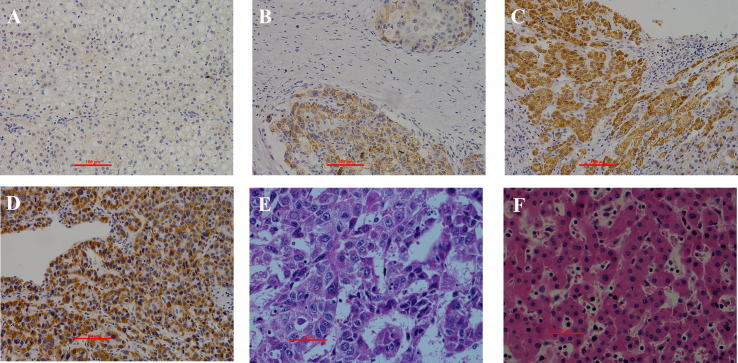
The protein levels of LGR5 in tumor tissues and matched adjacent normal tissues from 66 HCC patients were detected by IHC staining (Fig. 1). High LGR5 expression was observed in 25 out of 66 (38%) cases of tumor tissues and 11 out of 66 (17%) cases in adjacent normal tissues. Comparison between the expression levels of LGR5 in HCC tissue and adjacent normal tissues showed that LGR5 (P = .006) was significantly increased in HCC (Table 1).
Fig. 1.
Differential expression of leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) in hepatocellular carcinoma in human HCC specimens and adjacent tissues. (A) Normal HCC specimen Negative (−); stain intensity = 0, percentage of positive cell = 0 (B) Weak (+), stain intensity = 1, percentage of positive cell = 1; (C) Moderate (++), stain intensity = 2, percentage of positive cell = 3; (D) Strong (+++), stain intensity = 3, percentage of positive cell = 4;(E) H&E stain of HCC; (F) H&E stain of normal liver tissue.
Table 1.
Overexpression of LGR5 in HCC.
| Total | LGR5 expression |
P value | ||
|---|---|---|---|---|
| High | Low | |||
| Hepatocellular carcinoma | 66 | 25 | 41 | .006* |
| Adjacent tissue | 66 | 11 | 55 | |
P < .05, statistically significant, Pearson’s χ2 test.
3.2. LGR5 expression is associated with disease stage of HCC
We then investigated the correlation between LGR5 and clinical features of the 66 HCC patients. According to the IHC staining of tumor tissue, we classified all patients into LGR5-low and LGR5-high groups. Intriguingly, LGR5 expression in tumors was positively correlated with disease stage (P = .009), but no other clinical parameters including age, sex, tumor size, tumor number, Edmondson grade, metastasis, vessel invasion, hepatitis B virus, hepatitis C virus, cirrhosis, AFP levels, satellite nodule or Child-Pugh scores (Table 2). This finding suggested that LGR5 was more likely to be a good indicator of disease progression rather than merely tumor biology in HCC.
Table 2.
Association between LGR5 expression and the clinical characteristics.
| Clinical parameters | Total | LGR5 expression |
P value | |
|---|---|---|---|---|
| high (25) | Low (41) | |||
| Age (years) | .839 | |||
| <60 | 38 | 14 | 24 | |
| ≥60 | 28 | 11 | 17 | |
| Gender | .662 | |||
| Male | 57 | 21 | 36 | |
| Female | 9 | 4 | 5 | |
| Size | .631 | |||
| <5 | 42 | 15 | 27 | |
| ≥5 | 24 | 10 | 14 | |
| Tumor number | .919 | |||
| Single | 61 | 23 | 38 | |
| Multiple | 5 | 2 | 3 | |
| Edmondson grade | .450 | |||
| I + II | 58 | 21 | 37 | |
| III | 8 | 4 | 4 | |
| Metastasis | .139 | |||
| M0 | 64 | 23 | 41 | |
| M1 | 2 | 2 | 0 | |
| Vessel invasion | .180 | |||
| Absence | 38 | 17 | 21 | |
| Presence | 28 | 8 | 20 | |
| HBs antigen | .679 | |||
| Negative | 15 | 5 | 10 | |
| Positive | 51 | 20 | 31 | |
| HCV infection | 1.000 | |||
| Yes | 0 | 0 | 0 | |
| No | 66 | 25 | 41 | |
| TNM stage | .009* | |||
| I + II | 31 | 17 | 38 | |
| III + IV | 24 | 8 | 3 | |
| Cirrhosis | .980 | |||
| Yes | 21 | 8 | 13 | |
| No | 45 | 17 | 28 | |
| AFP levels | .665 | |||
| ≧400 μg/L | 6 | 3 | 3 | |
| <400 μg/L | 60 | 22 | 38 | |
| Satellite nodule | .642 | |||
| Yes | 5 | 1 | 4 | |
| No | 61 | 24 | 37 | |
| Child-Pugh scores | .868 | |||
| A | 63 | 24 | 39 | |
| B | 3 | 1 | 2 | |
| C | 0 | 0 | 0 | |
aAmerican Joint Committee on Cancer Staging (2010).
HBs: hepatitis B virus.
HCV: hepatitis C virus.
P < .05, statistically significant, Pearson’s χ2 test was applied to analyze the relationship between expression of LGR5 and various clinical parameters except for metastasis, HCV infection, satellite nodule and Child-Pugh scores by which were used Fisher’s exact test.
3.3. High LGR5 expression in tumor tissue is associated with short survival
The Kaplan-Meier curves indicated that patients with high LGR5 expression were remarkably correlated with short overall survival than those with low LGR5 expression (P < .05, Fig. 2). The median overall survival of patients with high LGR5 expression was 12 months, whereas that of patients with low LGR5 expression was still not reached (longer than 70 months).
Fig. 2.

Kaplan-Meier survival analysis in patients with HCC. Overall survival curves for patients with HCC according to the low and high expression levels of LGR5 of IHC variables in tumor samples.
Cox regression analysis revealed that high LGR5 expression was an independent predictor of short OS (Table 3). As expected, other independent parameters including Edmondson grade, vessel invasion, and metastasis were also identified as independent predictors of OS in HCC patients. This finding suggested that LGR5 expression in HCC could be a promising tool for predict overall survival of HCC patients.
Table 3.
Cox-regression analysis of the clinicopathological parameters in HCC patients.
| Parameters | Univariate analyses |
Multivariate analyses |
||
|---|---|---|---|---|
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | |
| LGR5 expression | 0.181 (0.078–0.416) | .000* | 0.112 (0.043–0.293) | .000* |
| Edmondson Grade | 2.730 (1.097–6.793) | .031* | 3.795 (1.353–10.647) | .011* |
| Vessel invasion | 0.576 (0.276–1.201) | .141 | 0.337 (0.144–0.787) | .012* |
| Metastasis | 0.032 (0.004–0.225) | .001* | 0.044 (0.004–0.535) | .014* |
CI, confidence interval.
p < .05 statistically significant.
4. Discussion
It has been reported that overexpression of LGR5 could promote HCC cell viability and enhance colony formation (Fukuma et al., 2013). Overexpression of LGR5 resulted in enhanced proliferation and resistance to chemotherapy (Hsu et al., 2013). In addition, a meta-analysis showed that LGR5 could be a valuable and reliable prognostic factor of colorectal cancer progression (Jiang et al., 2016). Furthermore, it was recently reported that the expression level of LGR5 was correlated with E-cadherin and N-cadherin (Liu et al., 2017), indicating that LGR5 promoted HCC metastasis through inducing EMT. Evidence also showed that LGR5 could be regarded as a candidate biomarker for prognosis and as a target in therapy (Liu et al., 2017). Moreover, there are some studies concerning the prognostic values of LGR5 in several types of cancer such as gastric carcinoma (Jiang et al., 2016, Yamanoi et al., 2013). However, there are still few studies that show the relationship of LGR5 expression and survival in HCC patients, about which we care much as clinicians. Here, we investigated the correlation between LGR5 expression and cancer progression in 66 HCC patients to evaluate its clinical value in HCC. The present study revealed that the expression of LGR5 was markedly higher in HCC than in normal adjacent tissues. High expression of LGR5 was significantly correlated with later disease stage. In addition, HCC patients with high expressions of LGR5 showed short overall survival. Meanwhile, Cox regression analysis showed that LGR5 expression was an independent indicator for the OS. Notably, in our limited cases, we did not detect any difference in tumor size, lymphatic invasion, metastasis, hepatitis C virus, cirrhosis, AFP levels, satellite nodule or Child-Pugh scores in patients with high or low expression of LGR5. This discrepancy implicated that LGR5 probably influences HCC progression in a more general way, rather than merely EMT or proliferation. On the contrary, Liu and colleagues showed that LGR5 expression was significantly associated with tumor size, pathological grade, early recurrence and metastasis using a similar IHC scoring system with different cutoff criteria from ours (Liu et al., 2017). Evaluation of IHC staining can vary greatly, and relies highly on the processors who conduct the procedures and pathologists who interpret the results. Thus, only when more reports from different centers are available, should we make a convincing judgment in the clinical value of LGR5 as a prognostic predictor.
Many studies have explored the mechanisms by which LGR5 promotes HCC. Yamamoto et al. (2003) found that LGR5 might be associated with Wnt/β-catenin pathway and play a critical role in the development of HCC (Yamamoto et al., 2003). It was reported that LGR5 was overexpressed in HCCs with nuclear accumulation of β-catenin and was down-regulated as a result of Wnt signaling suppression (Yamanoi et al., 2013). Therefore, we think LGR5 might be a downstream target of Wnt signaling and be associated with β-catenin that induces EMT process through the Wnt/β-catenin signaling pathway. However, this hypothesis needs further investigation. Several studies revealed that overexpression of LGR5 conferred HCC cells with some stem cell-like properties including sphere formation and enhanced survival (Fukuma et al., 2013). In whole, LGR5 expression was closely related to the key markers of EMT and cancer stemness. Such properties of LGR5 may partially explain why high expression of LGR5 in HCC correlates with late disease stage and poor prognosis of patients. However, an in vivo study also showed that LGR5 transformed tumors from a diffused phenotype to a more nodular one, and from a metastatic phenotype to a less metastatic one (Fukuma et al., 2013). The contradictory results suggest that the roles of LGR5 in HCC is complicated, and warrant further investigations especially in vivo and ex vivo.
In conclusion, we identified the expression of LGR5 in 66 HCC patients and found that a high protein level of LGR5 was associated with poor prognosis in HCC patients. LGR5 appears to be a valuable prognostic predictor clinically and a potential target in HCC therapy. However, more large scale studies are necessary to confirm these findings.
Declarations of interest
None.
Acknowledgments
Acknowledgements
We show our respect and express our gratitude to the patients who participate in our study.
Acknowledgments
Funding
This study was financially supported by the Natural Science Foundation of Hangzhou Medical College (HZYXY2016B0), and the Medical Science & Technology Program of Zhejiang Province (2015KYA114).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Qi Zhang, Email: qi.zhang@nih.gov.
Yupeng Hong, Email: hypbdn@zju.edu.cn.
References
- Bai X.L., Zhang Q., Ye L.Y., Liang F., Sun X., Chen Y., Hu Q.D., Fu Q.H., Su W., Chen Z., Zhuang Z.P., Liang T.B. Myocyte enhancer factor 2C regulation of hepatocellular carcinoma via vascular endothelial growth factor and Wnt/beta-catenin signaling. Oncogene. 2015;34:4089–4097. doi: 10.1038/onc.2014.337. [DOI] [PubMed] [Google Scholar]
- Barker N., Ridgway R.A., van Es J.H., van de Wetering M., Begthel H., van den Born M., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Bertuccio P., Turati F., Carioli G., Rodriguez T., La Vecchia C., Malvezzi M., Negri E. Global trends and predictions in hepatocellular carcinoma mortality. J. Hepatol. 2017;67:302–309. doi: 10.1016/j.jhep.2017.03.011. [DOI] [PubMed] [Google Scholar]
- Carmon K.S., Gong X., Lin Q., Thomas A., Liu Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Xue J., Hu J., Yang S.L., Chen G.G., Lai P.B.S., Yu C., Zeng C., Fang X., Pan X., Zhang T. Positive expression of programmed death ligand 1 in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Transl. Oncol. 2017;10:511–517. doi: 10.1016/j.tranon.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T.Y., Koo B.K., Li V.S., Teunissen H., Kujala P., Haegebarth A., Peters P.J., van de Wetering M., Stange D.E., van Es J.E., Guardavaccaro D., Schasfoort R.B., Mohri Y., Nishimori K., Mohammed S., Heck A.J., Clevers H. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- Effendi K., Yamazaki K., Fukuma M., Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) represents a typical Wnt/beta-catenin pathway-activated hepatocellular carcinoma. Liver Cancer. 2014;3:451–457. doi: 10.1159/000343873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuma M., Tanese K., Effendi K., Yamazaki K., Masugi Y., Suda M., Sakamoto M. Leucine-rich repeat-containing G protein-coupled receptor 5 regulates epithelial cell phenotype and survival of hepatocellular carcinoma cells. Exp. Cell Res. 2013;319:113–121. doi: 10.1016/j.yexcr.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Gil-Sanchis C., Cervello I., Mas A., Faus A., Pellicer A., Simon C. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol. Hum. Reprod. 2013;19:407–414. doi: 10.1093/molehr/gat014. [DOI] [PubMed] [Google Scholar]
- Hsu H.C., Liu Y.S., Tseng K.C., Hsu C.L., Liang Y., Yang T.S., Chen J.S., Tang R.P., Chen S.J., Chen H.C. Overexpression of Lgr5 correlates with resistance to 5-FU-based chemotherapy in colorectal cancer. Int. J. Colorectal Dis. 2013;28:1535–1546. doi: 10.1007/s00384-013-1721-x. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Li W., He X., Zhang H., Jiang F., Chen Z. Lgr5 expression is a valuable prognostic factor for colorectal cancer: evidence from a meta-analysis. BMC Cancer. 2016;16:12–20. doi: 10.1186/s12885-015-1986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Z.J., Wang J., Xiao H.L., Guo Y., Wang T., Li Q., Liu L., Luo X., Fan L.L., Lin L., Mao C.Y., Wang S.N., Wei Y.L., Lan C.H., Jiang J., Yang X.J., Liu P.D., Chen D.F., Wang B. Lysine-specific demethylase 1 promotes the stemness and chemoresistance of Lgr5+ liver cancer initiating cells by suppressing negative regulators of beta-catenin signaling. Oncogene. 2015;34:3188–3198. doi: 10.1038/onc.2015.129. [DOI] [PubMed] [Google Scholar]
- Liu J., Yu G.Z., Cheng X.K., Li X.D., Zeng X.T., Ren X.Q. LGR5 promotes hepatocellular carcinoma metastasis through inducting epithelial-mesenchymal transition. Oncotarget. 2017;8:50896–50903. doi: 10.18632/oncotarget.15143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Tanese K., Fukuma M., Yamada T., Mori T., Yoshikawa T., Watanabe W., Ishiko A., Amagai M., Nishikawa T., Sakamoto M. G-protein-coupled receptor GPR49 is up-regulated in basal cell carcinoma and promotes cell proliferation and tumor formation. Am. J. Pathol. 2008;173:835–843. doi: 10.2353/ajpath.2008.071091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.H., Zhu X.D., Ma D.N., Sun H.C., Gao D.M., Zhang N., Qin C.D., Zhang Y.Y., Ye B.G., Cai H., Shi W.K., Cao M.Q., Tang Z.Y. Flot2 promotes tumor growth and metastasis through modulating cell cycle and inducing epithelial-mesenchymal transition of hepatocellular carcinoma. Am. J. Cancer. Res. 2017;7:1068–1083. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Sakamoto M., Fujii G., Tsuiji H., Kenetaka K., Asaka M., Hirohashi S. Overexpression of orphan G-protein-coupled receptor, Gpr49, in human hepatocellular carcinomas with beta-catenin mutations. Hepatology. 2003;37:528–533. doi: 10.1053/jhep.2003.50029. [DOI] [PubMed] [Google Scholar]
- Yamanoi K., Fukuma M., Uchida H., Kushima R., Yamazaki K., Katai H., Kanai Y., Sakamoto M. Overexpression of leucine-rich repeat-containing G protein-coupled receptor 5 in gastric cancer. Pathol. Int. 2013;63:13–19. doi: 10.1111/pin.12013. [DOI] [PubMed] [Google Scholar]
- Yang J.D., Roberts L.R. Epidemiology and management of hepatocellular carcinoma. Infect. Dis. Clin. North Am. 2010;24:899–919. doi: 10.1016/j.idc.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tang H., Kong Y., Xie X., Chen J., Song C., Liu X., Ye F., Li N., Wang N., Xie X. LGR5 promotes breast cancer progression and maintains stem-like cells through activation of Wnt/beta-catenin signaling. Stem Cells. 2015;33:2913–2924. doi: 10.1002/stem.2083. [DOI] [PubMed] [Google Scholar]
- Ying X., Han S.X., He C.C., Zhou C.Y., Dong Y.P., Cai M.J., Sui X., Ma C.X., Sun X., Zhang Y.Y., Gou W.L., Mason C., Zhu Q. Autoantibodies against glucose-regulated protein 78 as serological biomarkers in metastatic and recurrent hepatocellular carcinoma. Oncotarget. 2017;8:24828–24839. doi: 10.18632/oncotarget.15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Bai X., Chen W., Ma T., Hu Q., Liang C., Xie S., Chen C., Hu L., Xu S., Liang T. Wnt/beta-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1alpha signaling. Carcinogenesis. 2013;34:962–973. doi: 10.1093/carcin/bgt027. [DOI] [PubMed] [Google Scholar]