Abstract
Malignant glioma is the most common and lethal type of primary tumor of the central nervous system. The incidence of glioma is increasing year by year. In recent years, a variety of new treatment methods have emerged, among which gene therapy has become a hotspot. MicroRNAs (miRNAs) are a class of small non-coding single-strand RNAs that negatively regulate gene expression at the post-transcriptional and/or translational level by binding loosely complimentary sequences in the 3′ untranslated regions (UTRs) of target mRNAs. Several miRNAs have been reported to modulate glioma progression. This study aimed to determine the function of miR-30b-5p in glioma and its underlying molecular mechanism. miR-30b-5p expression was significantly lower in gliomas than the normal brain tissues. Overexpression of miR-30b-5p was found to significantly inhibit glioma cell proliferation in vitro. Further, MTDH expression was significantly higher in the gliomas compared with the normal brain tissues. In addition, MTDH was validated as direct target of miR-30b-5p. Moreover, cellular proliferation was increased after MTDH overexpression in the glioma cells, which reversed the effects of miR-30b-5p. Taken together, these results reveal miR-30b-5p impacts glioma cell proliferation via direct targeting MTDH and could be a potential novel therapeutic target for the treatment of glioma.
Keywords: miR-30b-5p, MTDH, Proliferation
1. Introduction
Malignant glioma (MG; World Health Organization [WHO] grade III or IV) is a devastating neuro-oncologic disease with almost invariably poor prognosis, which is leading to progressive functional decline, cognitive impairment, and almost invariably death (Diamond et al., 2017). Gliomas are characterized by high proliferation, migration, and invasion abilities (Peng et al., 2014). Because of its invasive growth, difficulty to completely remove and susceptibility to relapse, it has a poor prognosis and a high mortality. The median survival period of patients is approximately one year with the most malignant histological subtype of glioma (Lacroix et al., 2001, Zhou et al., 2011, Wang et al., 2012). Comprehensive therapies such as surgical resection, radiation and chemotherapy are effective therapies for gliomas. Despite these treatments, the prognosis of glioma remains extremely bad (Wen and Kesari (2008)). Multiple factors affect the effectiveness of glioma therapies including rapid tumor growth. Therefore, therapies targeting rapid tumor growth would be a novel therapeutic strategy.
MicroRNAs (miRNAs) are endogenous small noncoding RNAs. They regulate gene expression through antisense completely or incompletely binding to the 3′ UTR (untranslated regions) of specific mRNAs. Mounting evidence has confirmed that miRNAs play pivotal roles in cell proliferation, migration, invasion, apoptosis, and so on (Hwang and Mendell, 2006, Kloosterman and Plasterk, 2006, Gabriely et al., 2008, Yang et al., 2012, Bhardwaj et al., 2017, Masood and Yasmin, 2017, Wang et al., 2017). Many miRNAs have been shown as significantly attractive diagnostic biomarkers in glioma development, such miR-320c, miR-124, 137, 10b and 218 (Godlewski et al., 2008, Silber et al., 2008, Lin et al., 2012, Tu et al., 2013, Lv et al., 2018). Recently, the expression level of miR-30b-5p has been reported to be reduced in multiple types of cancers, such as hepatocellular carcinoma, gastric cancer and renal cell carcinoma (Qiao et al., 2014, Liu et al., 2017, Qin et al., 2017). However, the molecular mechanism of miR-30b-5p regulatory network in the glioma development remains elusive. In this study, miR-30b-5p was found to dramatically downregulated in the gliomas. The cellular proliferation of glioma cells was significantly inhibited after miR-30b-5p overexpression.
Matadherin (MTDH), also known as astrocyte elevated gene-1 (AEG-1) and LYRIC, is an important regulatory gene in the initiation and progression of most malignant tumors (Sarkar and Fisher, 2013, Mizrak Kaya et al., 2017). MTDH was also found to highly expressed in glioma and could interact with NF-κB component P65 to promote straphyococcal nuclease domain containing 1 (SND1) (Tong et al., 2016). MTDH serves as an important regulatory molecule in many oncogenic signaling pathways. However, the roles of MTDH involved in the miRNA regulatory network is still poorly understood in glioma. In this report, we found MTDH was dramatically upregulated in gliomas. Furthermore, MTDH was verified as the direct target of miR-30b-5p. Overexpression of MTDH reversed the effects of miR-30b-5p on glioma cells. Collectively, our data indicates that miR-30b-5p inhibits glioma cell proliferation by modulating MTDH.
Collectively, we made a deep learning of the affection and molecular mechanism of miR-30b-5p in the cell proliferation of glioma. MiR-30b-5p expression is significantly lower in glioma compared with the noncancerous tissues. Also, miR-30b-5p overexpression dramatically inhibits glioma cell proliferation in vitro. In addition, we found MTDH expression is significantly higher in glioma than the noncancerous tissues. Further investigation revealed that MTDH was directly targeted by miR-30b-5p and MTDH overexpression reversed the effects of miR-30b-5p on the cell proliferation. Our present findings suggest a novel therapeutic strategy for treatment of glioma.
2. Material and methods
2.1. Cell culture
The human glioma cell line, SHG44, was commercially available at the Chinese Academy of Sciences (Shanghai, China). SHG44 cells were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) and 1% penicillin-streptomycin (Invitrogen). The cells were maintained in the humidified chamber with 5% CO2 at 37 °C.
2.2. MiR-30b-5p overexpression
We overexpressed miR-30b-5p in the SHG44 cells by transiently transfecting with has-miR-30b-5p mimic (life technologies, Carlsbad, CA) with RANiMAX reagent (life technologies). The miRNA vector control (miR-NC) was also purchased from life technologies. Briefly, SHG44 cells were seeded into a 6 well plate (20,000 cells/well). After 24 h culture, SHG44 cells were transfected with 10 nM miR-30b-5p mimic or miR-NC using with RANiMAX reagent in antibiotic-free Opti-MEN medium (Gibco). DMEM complete medium replace the medium after 6 h transfection.
2.3. MTDH overexpression
To overexpress MTDH in SHG44 cells, we used the lentiviral system (Applied Biological Materials, Canada) to stably overexpress MTDH (Lenti-MTDH). The overexpression was following the manufacturer’s instructions. The lentiviral system for negative control vector (Lenti-VC) overexpression was also commercially available from Applied Biological Materials. Briefly, SHG44 cells were seeded into a 6 well plate (10,000 cells/well). After cell confluency reached 75%, lentiviruses were added to cells in the growth media with 2 μg/ml polybrene (Sigma-Aldrich, St Louis, MO). The SHG44 cells were infected with the lentiviruses Lenti-MTDH or Lenti-VC at a multiplicity of infection of 10. After 48 h, fresh growth media replaced the media containing lentiviruses.
2.4. RNA isolation and qRT-PCR
The total RNA of the tissue samples and cells were extracted by the mirVanaTM isolation kit (Ambion, Austin, TX) according to the manufacturer’s instruction. For the detection of gene expression, RNA was transcribed to cDNA by RNA-to-cDNA kit (Thermo Fisher Scientific, Waltham, MA) after removal of residual DNA by DNAase I (Invitrogen). The Taqman quantitative real-time (qRT-PCR) was employed to determine MTDH mRNA expression. GAPDH was used as an internal reference. The MTDH and GAPDH Taqman primers are commercially available at Applied Biosystem.
For the determination of the mature miR-30b-5p, total RNA was reverse-transcribed by the Taqman advanced miRNA cDNA synthesis kit following the manufacturer’s protocols (Applied Biosystems). The small nuclear RNA (U6) was used as the internal control. U6 was reverse-transcribed by the TaqmanTM microRNA reverse transcription kit (Applied Biosystem). MiR-30b-5p qRT-PCR primer was purchased from Applied Biosystem.
2.5. Luciferase assay
SHG44 cells were seeded in a 6-well plate (BD Biosciences, Bedford, MA) (0.25 M cells/well). After being in culture for 24hrs, the cells were co-transfected with 1 μg of MTDH 3′UTR luciferase reporter construct (GeneCopoeia, Rockville, MD) with 20 nM miR-30-5p or miR-NC by lipofectamine 2000 (Invitrogen) with Opti-MEN (Gibco). After 48hrs, the cells were collected to perform the dual-luciferase reporter assay using the dual-luciferase reporter assay reagent from GeneCopeia. Data was normalized by the normalization of firefly luciferase activity to Renilla luciferase activity.
2.6. Proliferation assay
The effect of miR-30b-5p or MTDH on the growth of SHG44 cells were monitored by Cyquant assay (Thermo Fisher Scientific). The cell suspension was seeded in the 96-well plate at a density of 5000 cells per well. The plates were frozen at the indicated incubation time. Subsequently, plates were read at excitation at 497 nm and emission at 520 nm. All studies were performed in 3 independent cell preparations and the data were presented as mean ± SE.
2.7. 2D colony formation assay
SHG44 Cells were trypsinized and counted. The suspended cells were seeded in the 6-well plate (1000 cells/well). After 10 days, the cells were fixed with 80% ethanol after removal of growth media and dyed with crystal violet solution (Millipore).
2.8. Western blotting
The protein was isolated from SHG44 cells using RIPA lysis buffer (Millipore, Temecula, CA) and separated on the commercially available 4–15% precast gels (Bio-Rad, Richmond, CA). After transferred to the nitrocellulose membranes, the membranes were incubated with anti-MTDH antibody (Santa Cruz, Santa Cruz, CA) overnight, followed by incubation with HRP conjugated secondary antibodies. GAPDH (ZSGG-Bio, Beijing, China) was used as a loading control. The proteins were visualized by ECL chemiluminescence and exposed to X-ray film. All the studies were performed in triplicate.
2.9. Statistical analysis
Statistical analyses were performed by the SPSS version 13 (SPSS Inc, Chicago, IL). The data was presented as mean ± SE from different samples from 3 independent preparations. The expression differences were analyzed with two-tailed unpaired student’s t-test. A value of P < 0.05 was considered as the statistical significance.
3. Results
3.1. MiR-30b-5p is downregulated in gliomas
In this study, we determined miR-30b-5p expression levels in 13 pairs of normal brain tissues and gliomas. MiR-30b-5p expression was found to be significantly downregulating in gliomas compared with normal brain tissues (p < 0.05) with Taqman qRT-PCR (Fig. 1).
Fig. 1.
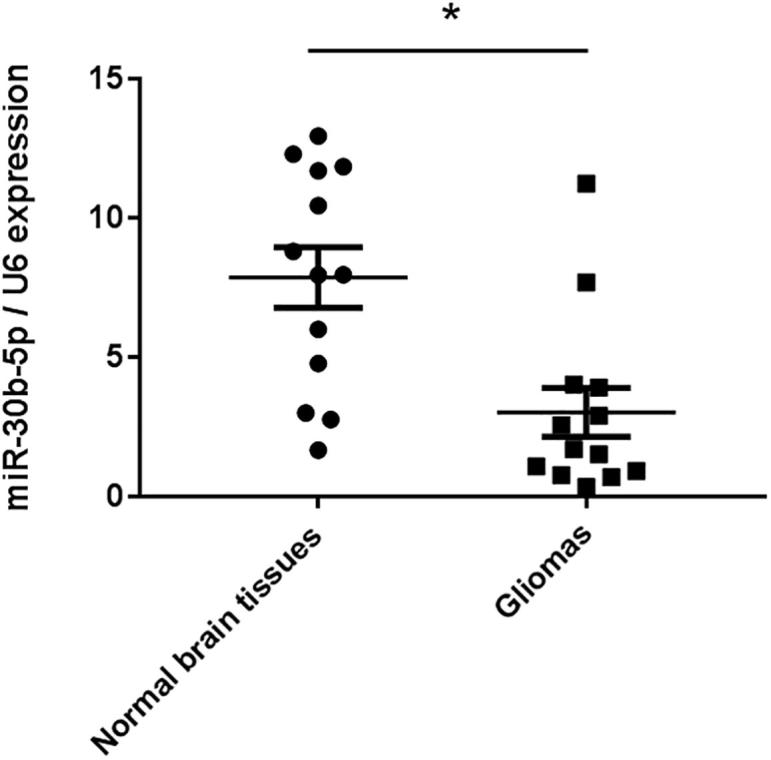
miR-30b-5p expression in the gliomas. Taqman qRT-PCR analysis of miR-30b-5p expression in 13 cases of gliomas and paired normal brain tissues. U6 was used as an internal reference. P < 0.05 vs. normal brain tissues.
3.2. Overexpression of miR-30b-5p inhibits glioma cell proliferation
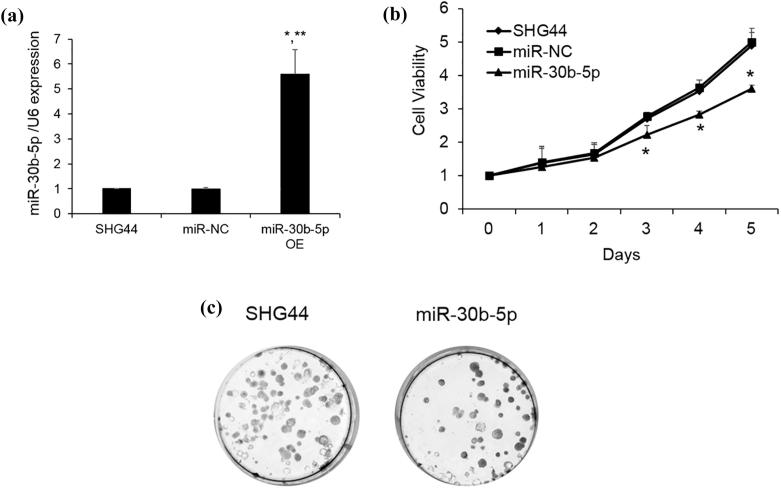
After SHG44 cells were transfected with the mature sequence of miR-30b-5p, the cells produced high levels of miR-30b-5p (Fig. 2A). First, we monitored the effect of miR-30b-5p on the glioma cells proliferation by Cyquant assay. As shown in Fig. 2B, the miR-30b-5p overexpressing cells showed considerably slower cell growth compared with the miR-NC cells at day 3, 4 and 5 (p < 0.05). Furthermore, the 2D colony formation assay also revealed that miR-30b-5p overexpression dramatically suppressed glioma cell growth (Fig. 2C). Our data indicated that miR-30b-5p could significantly inhibit the proliferation of glioma cells.
Fig. 2.
Effect of miR-30b-5p on cell proliferation and migration of glioma cells. (A) Glioma cells were overexpressed miR-30b-5p or vector control (miR-NC). The expression level was detected by qRT-PCR. Data are expressed as fold change (mean ± SE). *P < 0.05 vs SHG44; **P < 0.05 vs miR-NC (n = 3); (B) the cellular proliferation of glioma cells overexpressing miR-30b-5p or miR-NC was monitored by Cyquant assay after 5 days incubation compared with the non-treatment cells. Data are expressed as fold change (mean ± SE) compared with day 0. *P < 0.05, one-way ANOVA followed by Tukey’s multiple comparison; (C) representative images of the colony formation potential of wild type SHG44 cells or cells overexpressing miR-30b-5p.
3.3. MTDH is upregulated in gliomas
Predicted miRNA targets by public computational algorithms were retrieved from the respective public websites, such as TargetScan 7.0 (Agarwal et al., 2015) and http://microrna.org. MTDH was predicted as one of the direct targets of miR-30b-5p. MTDH has been reported to play important roles in various cancers (Shi and Wang, 2015). Therefore, MTDH was chosen for the further analysis. We next determined MTDH mRNA expression in gliomas and normal brain tissues. MTDH was observed to be upregulated in the gliomas compared to the normal brain tissues (Fig. 3).
Fig. 3.
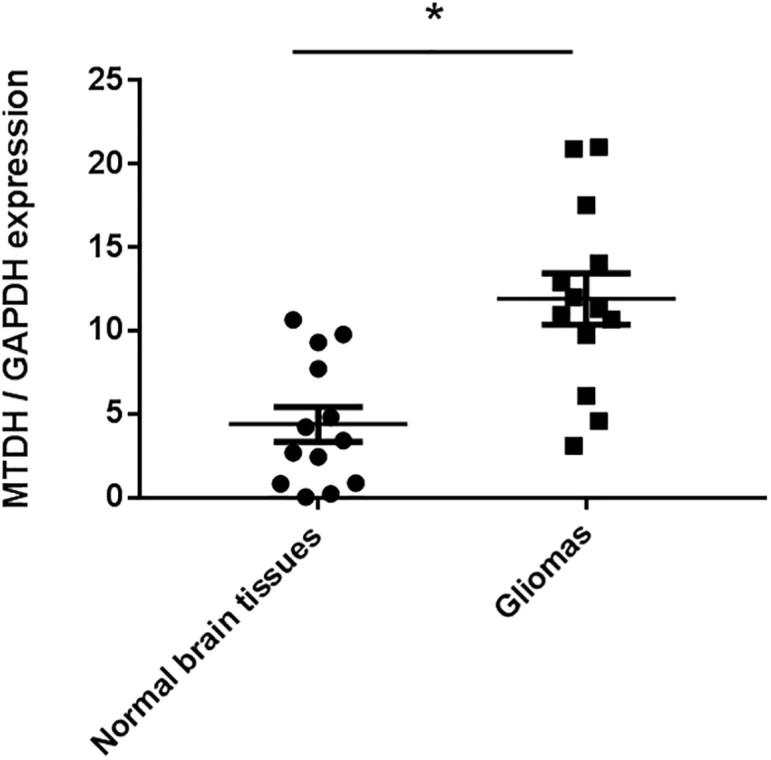
MTDH expression in the gliomas. Taqman qRT-PCR analysis of MTDH expression in 13 pairs of gliomas and normal brain tissues. GAPDH was used as an internal control. P < 0.05 vs. normal brain tissues.
3.4. MTDH is directly repressed by miR-30b-5p
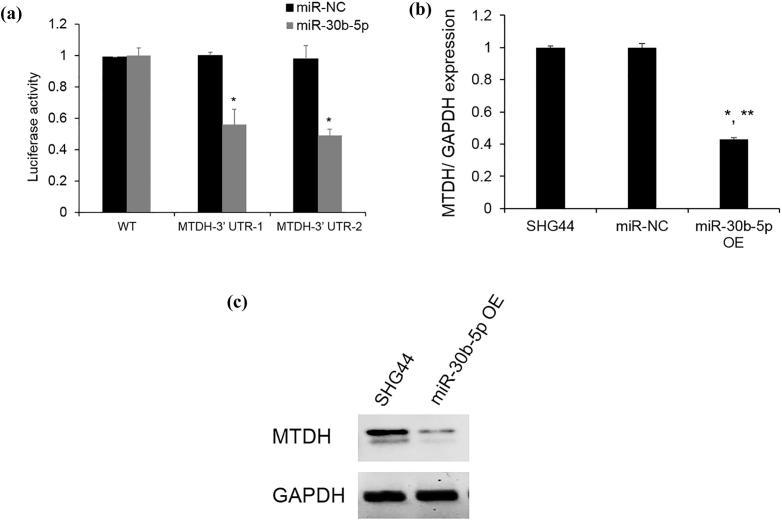
To validate whether miR-30b-5p directly target MTDH, SHG44 cells were co-transfected with the MTDH 3′ UTR and miR-30b-5p or miR-NC. Because of the length of the MTDH 3′ UTR, the binding sites 1 and 2 were cloned into 2 different reporter vectors (MTDH-3′ UTR-1 and MTDH-3′ UTR-2). The dual luciferase assay showed that miR-30b-5p directly target MTDH (Fig. 4A). MTDH mRNA and protein levels were also found to be significantly decreased after miR-30b-5p overexpression (Fig. 4B, C).
Fig. 4.
MTDH is a direct target of miR-30b-5p (A) 3′ UTR luciferase reporter assay. SHG44 cells were co-transfected with MTDH 3′ UTR-1 or MTDH 3′ UTR-2 or miR-NC or miR-30b-5p for 48hrs. Firefly activity was normalized with Renilla luciferase activity. *p < 0.05 vs miR-NC (n = 3); (B) MTDH mRNA level was detected by qRT-PCR. *p < 0.05 vs SHG44, **p < 0.05 vs miR-NC (n = 3); (C) MTDH protein expression after miR-30b-5p overexpression was determined by western blot.
3.5. MTDH reversed miR-30b-5p effect on glioma cells
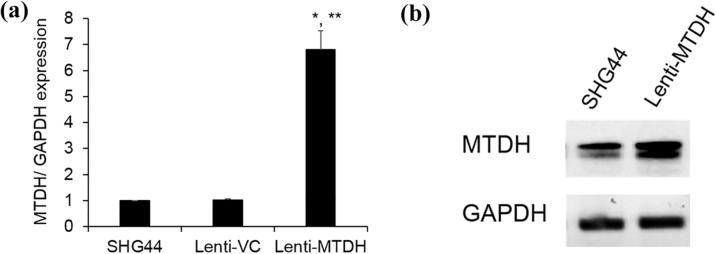
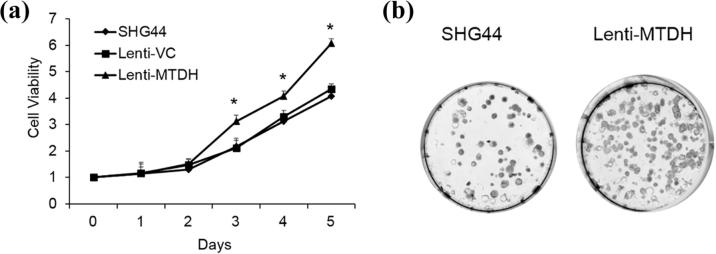
To assess the role of MTDH involved in the miR-30b-5p modulation of glioma cell proliferation, we overexpressed MTDH in the SHG44 cells. As seen in Fig. 5A, B, the MTDH mRNA and protein levels were significantly upregulated compared to the parental cells. As shown in Fig. 6A, B, the cellular proliferation was significantly increased in the SHG44 cells. These results indicate that miR-30b-5p regulate the cell proliferation via MTDH.
Fig. 5.
MTDH were overexpressed in SHG44 cells. (A) MTDH mRNA expression level was detected by qRT-PCR. Data are expressed as fold change (mean ± SE). *P < 0.05 vs SHG44; **P < 0.05 vs Lenti-VC (n = 3); (B) MTDH protein expression was determined by western blot.
Fig. 6.
Effect of MTDH on cell proliferation and migration of glioma cells. (A) the cellular proliferation of glioma cells overexpressing MTDH or the vector control (Lent-VC) or wild type was analyzed by Cyquant assay after 5 days incubation. Data are expressed as fold change (mean ± SE) compared with day 0. *P < 0.05, one-way ANOVA followed by Tukey’s multiple comparison; (B) representative images of the colony formation potential of wild type SHG44 cells or cells overexpressing MTDH.
4. Discussion
The complexity of transcription and posttranscriptional regulation of gene expression is a highly-orchestrated process in the cancer development (Ying et al., 2013). MiRNAs have emerged as one of the crucial regulators that plays important roles in cancer (Zhang et al., 2012, Colden et al., 2017, Devi et al., 2017). A growing body of evidence suggests the importance of miRNAs in glioma (Hummel et al., 2011, Palumbo et al., 2014). However, the precise function of dysregulated miRNAs in glioma progression and development is still poorly understood. In this study, miR-30b-5p was shown to be significantly downregulated in the gliomas compared with the normal brain tissues. Furthermore, miR-30b-5p overexpression was found to inhibit the glioma cells proliferation. We also observed that MTDH was dramatically upregulated in the gliomas. Thus, we next validated that MTDH is a direct functional target of miR-30b-5p. MTDH overexpression reversed the effect of miR-30b-5p on the glioma cells. Therefore, our results suggest miR-30b-5p inhibits cellular proliferation of glioma cells via MTDH, which raises the possibility of a potential therapeutic target for the glioma.
Recently, miRNAs are the most studied class of small non-coding RNAs. The roles of miRNAs in carcinogenesis/cancer have been discussed in many studies (Reddy, 2015, Colden et al., 2017, Devi et al., 2017). However, little is known about the extent of the involvement of miR-30b-5p and its underlying mechanism in the regulation of glioma. miR-30b-5p has been proved to participate in multiple biological behaviors of tumor. MiR-30b-5p repressed cell proliferation and cell cycle in human hepatocellular carcinoma (Qin et al., 2017). Moreover, miR-30b-5p expression involved in the gastric cancer metastasis by the regulation of DNMT1 methylation (Qiao et al., 2014). Those studies have suggested miR-30b-5p could be an attractive biomarker for glioma diagnosis and therapeutic. In this study, we observed that miR-30b-5p was downregulated in the gliomas and miR-30b-5p inhibit the cellular proliferation of glioma cells. However, larger number of samples will be required to be proceeded in clinic so as to verify the actual function of miR-30b-5p. This study provides a hint for the profound learning of miR-30b-5p.
It has been shown that the alteration of MTDH expression interacted with a variety of biological processes in tumors. In breast cancer, overexpression of MTDH has been shown to be associated with an aggressive phenotype and a poor prognosis (Tokunaga et al., 2014). In the lung squamous cell carcinoma, miR-145 was found to significantly contributed the pathogenesis by coordinately regulating MTDH (Mataki et al., 2016). Moreover, MTDH bases DNA vaccine suppressed metastasis and enhances the chemosensitivity to paclitaxel in pelvic lymph node metastasis (Agarwal et al., 2015). However, it is still unclear about how MTDH could contribute to the glioma progression via the miR-30b-5p regulation network. In this study, we showed that MTDH is upregulated in the gliomas and revealed that MTDH is the direct target of miR-30b-5p. In addition, MTDH overexpression has opposite the effect of miR-30b-5p on cell proliferation in the glioma cells.
Intriguingly, we revealed miR-30b-5p can inhibit glioma cell proliferation via regulation of MTDH. So far, there is a report revealed that miR-30a-5p inhibited liver cancer cell proliferation and induces apoptosis by targeting MTDH (Li et al., 2016). To our knowledge, this is the first study to investigate the role of miR-30b-5p in inhibiting glioma cell proliferation via MTDH.
In summary, our results reveal a new mechanism by which miR-30b-5p regulates glioma proliferation through MTDH. Consequently, this study provides the basis for a future research strategy in glioma progression. Therefore, it broadens our understanding of miR-30b-5p function in cancer research and further confirm that miR-30b-5p is a promising target for cancer prevention and therapy.
Conflicts of interest
None declared.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agarwal V., Bell G.W., Nam J.W., Bartel D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj A., Singh H., Rajapakshe K., Tachibana K., Ganesan N., Pan Y., Gunaratne P.H., Coarfa C., Bedrosian I. Regulation of miRNA-29c and its downstream pathways in preneoplastic progression of triple-negative breast cancer. Oncotarget. 2017;8:19645–19660. doi: 10.18632/oncotarget.14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colden M., Dar A.A., Saini S., Dahiya P.V., Shahryari V., Yamamura S., Tanaka Y., Stein G., Dahiya R., Majid S. MicroRNA-466 inhibits tumor growth and bone metastasis in prostate cancer by direct regulation of osteogenic transcription factor RUNX2. Cell Death Dis. 2017;8:e2572. doi: 10.1038/cddis.2017.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond E.L., Prigerson H.G., Correa D.C., Reiner A., Panageas K., Kryza-Lacombe M., Buthorn J., Neil E.C., Miller A.M., DeAngelis L.M., Applebaum A.J. Prognostic awareness, prognostic communication, and cognitive function in patients with malignant glioma. Neuro Oncol. 2017;19:1532–1541. doi: 10.1093/neuonc/nox117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely G., Wurdinger T., Kesari S., Esau C.C., Burchard J., Linsley P.S., Krichevsky A.M. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 2008;28(17):5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski J., Nowicki M.O., Bronisz A., Williams S., Otsuki A., Nuovo G., RayChaudhury A., Newton H.B., Chiocca E.A., Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128 inhibits glioma proliferation and self-renewal. Cancer Res. 2008;68(22):9125–9130. doi: 10.1158/0008-5472.CAN-08-2629. [DOI] [PubMed] [Google Scholar]
- Hummel R., Maurer J., Haier J. MicroRNAs in brain tumors: a new diagnostic and therapeutic perspective? Mol. Neurobiol. 2011;44:223–234. doi: 10.1007/s12035-011-8197-x. [DOI] [PubMed] [Google Scholar]
- Hwang H.W., Mendell J.T. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br. J. Cancer. 2006;94(6):776–780. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterman W.P., Plasterk R.H. The diverse functions of microRNAs in animal development and disease. Devel. Cell. 2006;11(4):441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Lacroix M., Abi-Said D., Fourney D.R., Gokaslan Z.L., Shi W., DeMonte F., Sawaya R. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J. Neurosurg. 2001;95(2):190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- Li H.W., Dai H., Qu Q., Zuo G.Q., Liu C.A. Overexpression of microRNA-30a-5p inhibits liver cancer cell proliferation and induces apoptosis by targeting MTDH/PTEN/AKT pathway. Tumour Biol. 2016;37(5):5885–5895. doi: 10.1007/s13277-015-4456-1. [DOI] [PubMed] [Google Scholar]
- Lin J., Teo S., Lam D.H., Jeyaseelan K., Wang S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012;3:e398. doi: 10.1038/cddis.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Li H., Wang Y., Zhao X., Guo Y., Jin J., Chi R. MiR-30b-5p functions as a tumor suppressor in cell proliferation, metastasis and epithelial-to-mesenchymal transition by targeting G-protein subunit alpha-13 in renal cell carcinoma. Gene. 2017;626:275–281. doi: 10.1016/j.gene.2017.05.040. [DOI] [PubMed] [Google Scholar]
- Lv Q., Zhu H., Cheng X., Li H., Zhou H., Chen S. Down-regulation of miRNA-320c promotes tumor growth and metastasis and predicts poor prognosis in human glioma. Brain Res. Bull. 2018 doi: 10.1016/j.brainresbull.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Mataki H., Seki N., Mizuno K., Nohata N., Kamikawaji K., Kumamoto T., Koshizuka K., Goto Y., Inoue H. Dual-strand tumor-suppressor microRNA-145 (miR-145-5p and miR-145-3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget. 2016;7(44):72084–72098. doi: 10.18632/oncotarget.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood N., Yasmin A. Entangling relation of micro RNA-let7, miRNA-200 and miRNA-125 with various cancers. Pathol. Oncol. Res. 2017;23:707–715. doi: 10.1007/s12253-016-0184-0. [DOI] [PubMed] [Google Scholar]
- Mizrak Kaya D., Dong X., Nogueras-Gonzalez G.M., Xu Y., Estrella J.S., Harada K., Lopez A., Amlashi F.G., Hofstetter W.L., Maru D.M., Nguyen Q.N., Lee J.H., Weston B., Bhutani M.S., Erasmus J.J., Thomas I., Rogers J.E., Song S., Ajani J.A. Post-trimodality expression levels of metadherin (MTDH) as a prognostic biomarker for esophageal adenocarcinoma patients. Med. Oncol. 2017;34:135. doi: 10.1007/s12032-017-0994-2. [DOI] [PubMed] [Google Scholar]
- Palumbo S., Miracco C., Pirtoli L., Comincini S. Emerging roles of microRNA in modulating cell-death processes in malignant glioma. J. Cell Physiol. 2014;229:277–286. doi: 10.1002/jcp.24446. [DOI] [PubMed] [Google Scholar]
- Devi K.Pandima, Rajavel T., Daglia M., Nabavi S.F., Bishayee A., Nabavi S.M. Targeting miRNAs by polyphenols: novel therapeutic strategy for cancer. Semin. Cancer Biol. 2017;46:146–157. doi: 10.1016/j.semcancer.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Peng B., Li D., Qin M., Luo D., Zhang X., Zhao H., Hu S. MicroRNA218 inhibits glioma migration and invasion via inhibiting glioma-associated oncogene homolog 1 expression at N terminus. Tumour Biol. 2014;35(4):3831–3837. doi: 10.1007/s13277-013-1507-3. [DOI] [PubMed] [Google Scholar]
- Qiao F., Zhang K., Gong P., Wang L., Hu J., Lu S., Fan H. Decreased miR-30b-5p expression by DNMT1 methylation regulation involved in gastric cancer metastasis. Mol. Biol. Rep. 2014;41(9):5693–5700. doi: 10.1007/s11033-014-3439-4. [DOI] [PubMed] [Google Scholar]
- Qin X., Chen J., Wu L., Liu Z. MiR-30b-5p acts as a tumor suppressor, repressing cell proliferation and cell cycle in human hepatocellular carcinoma. Biomed. Pharmacother. 2017;89:742–750. doi: 10.1016/j.biopha.2017.02.062. [DOI] [PubMed] [Google Scholar]
- Reddy K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38. doi: 10.1186/s12935-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar D., Fisher P.B. AEG-1/MTDH/LYRIC: clinical significance. Adv. Cancer Res. 2013;120:39–74. doi: 10.1016/B978-0-12-401676-7.00002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X., Wang X. The role of MTDH/AEG-1 in the progression of cancer. Int. J. Clin. Exp. Med. 2015;8(4):4795–4807. [PMC free article] [PubMed] [Google Scholar]
- Silber J., Lim D.A., Petritsch C., Persson A.I., Maunakea A.K., Yu M., Hodgson J.G. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC Med. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga E., Nakashima Y., Yamashita N., Hisamatsu Y., Okada S., Akiyoshi S., Maehara Y. Overexpression of metadherin/MTDH is associated with an aggressive phenotype and a poor prognosis in invasive breast cancer. Breast Cancer. 2014;21(3):341–349. doi: 10.1007/s12282-012-0398-2. [DOI] [PubMed] [Google Scholar]
- Tong L., Wang C., Hu X., Pang B., Yang Z., He Z., Chu M. Correlated overexpression of metadherin and SND1 in glioma cells. Biol. Chem. 2016;397(1):57–65. doi: 10.1515/hsz-2015-0174. [DOI] [PubMed] [Google Scholar]
- Tu Y., Gao X., Li G., Fu H., Cui D., Liu H., Jin W., Zhang Y. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73(19):6046–6055. doi: 10.1158/0008-5472.CAN-13-0358. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li P., Li A., Jiang W., Wang H., Wang J., Xie K. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J. Exp. Clin. Cancer Res. 2012;31:97. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Guo D., Li C. Downregulation of miRNA-26b inhibits cancer proliferation of laryngeal carcinoma through autophagy by targeting ULK2 and inactivation of the PTEN/AKT pathway. Oncol. Rep. 2017;38:1679–1687. doi: 10.3892/or.2017.5804. [DOI] [PubMed] [Google Scholar]
- Wen P.Y., Kesari S. Malignant gliomas in adults. New Engl. J. Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- Yang L., Li Q., Wang Q., Jiang Z., Zhang L. Silencing of miRNA-218 promotes migration and invasion of breast cancer via Slit2-Robo1 pathway. Biomed. Pharmacother. 2012;66(7):535–540. doi: 10.1016/j.biopha.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Ying Z., Li Y., Wu J., Zhu X., Yang Y., Tian H., Li M. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013;73(2):990–999. doi: 10.1158/0008-5472.CAN-12-2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Dutta A., Abounader R. The role of microRNAs in glioma initiation and progression. Front. Biosci. (Landmark Ed) 2012;17:700–712. doi: 10.2741/3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Su Z., Huang Y., Sun T., Chen S., Wu T., Du Z. The Zfx gene is expressed in human gliomas and is important in the proliferation and apoptosis of the human malignant glioma cell line U251. J. Exp. Clin. Cancer Res. 2011;30:114. doi: 10.1186/1756-9966-30-114. [DOI] [PMC free article] [PubMed] [Google Scholar]