Abstract
Although many strategies have been developed for non-small cell lung cancer (NSCLC), more secondary and further treatments are needed due to drug resistance or tumor recurrence. Apatinib is a novel oral antiangiogenic agent and in this study, we aim to investigate the clinical value of apatinib in heavily pretreated NSCLC. Here, we reported the characteristics, efficacy and adverse events of three patients treated with apatinib (500 mg/day). We also summarized the currently available evidence and ongoing clinical trials regarding the use of apatinib in NSCLC. Two cases of adenocarcinoma and one case of squamous cell carcinoma were treated with apatinib due to disease progression after previous treatments of chemotherapy and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI). All patients responded to apatinib rapidly and underwent drug resistance shortly afterwards. The patient with squamous cell carcinoma died of hemoptysis. Other adverse events were acceptable. All previous relevant studies were compared and showed similar results but a longer progression-free survival. Additionally, ongoing clinical trials were systematically searched and listed. In conclusion, apatinib shows some efficacy in heavily treated NSCLC and generally tolerable toxicity in non-squamous NSCLC. More solid evidence will be accessible in near future.
Keywords: NSCLC, Antiangiogenesis, Adverse effect, Efficacy, Clinical trial
Abbreviations: ALK, anaplastic lymphoma kinase; CT, computed tomography; EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; OS, overall survival; ROS1, ROS proto-oncogene 1; PD, progression disease; PET, proton emission tomography; PFS, progression-free survival; PR, partial response; SD, stable disease; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor
1. Introduction
Non-small cell lung cancer (NSCLC) accounts for nearly 70% of lung malignancy, which is the leading cause of cancer-related death worldwide and in China as well (Chen et al., 2016, Siegel et al., 2017). The silver lining is that precision medicine advances quite well in NSCLC (Politi and Herbst, 2015), with many targeted therapies currently available for NSCLC harboring specific mutations and rearrangements of oncogenes including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), RET and ROS proto-oncogene 1 (ROS1) (Chan and Hughes, 2015). For those without targetable mutations, platinum-based chemotherapy is the standard first-line treatment for NSCLC (Rossi and Di Maio, 2016). However, second-line and further treatments are usually needed for NSCLC due to frequent tumor resistance or recurrence (Weiss and Stinchcombe, 2013). Thus, other strategies such as antiangiogenesis that can be jointly or solely used for better disease control are needed (Villaruz and Socinski, 2015).
Given that most solid tumors are highly dependent on angiogenesis for nutrients and oxygen supply to support their growth, antiangiogenic therapy has been proven valuable in various scenarios (Vasudev and Reynolds, 2014). Several strategies aiming to cut off the blood supply of NSCLC have been designed and antitumoral activity has been observed in some NSCLC patients; however, the efficacy were controversial and could be dependent on the antiangiogenic agents. For example, sorafenib is believed to be valuable in a subset of NSCLC patients (Zhang et al., 2012a), while sunitinib failed to improve overall survival (OS) when combined with erlotinib in a phase III trial (Scagliotti et al., 2012). With more and more clinical trials of antiangiogenic agents being conducted, the benefits of a range of antiangiogenic agents have been clinically proved in NSCLC (Kurzrock and Stewart, 2017). For instance, bevacizumab, a humanized anti-vascular endothelial growth factor (VEGF) monoclonal antibody, showed OS benefit in patients with NSCLC when combined with carboplatin/paclitaxel (ECOG 4599 trial) or cisplatin/gemcitabine (AVAil trial) (Reck et al., 2009, Sandler et al., 2006). Ramucirumab, an anti-VEGF receptor (VEGFR)-2 antibody, showed better prognosis in patients with advanced NSCLC when combined with docetaxel (REVEL trial) (Garon et al., 2014). Similarly, nintedanib, an oral triple angiokinase inhibitor, was also reported to significantly improve both progression-free survival (PFS) and OS in NSCLC patients (LUME-Lung 1 trial) (Reck et al., 2014).
Apatinib is a novel oral VEGFR-2 tyrosine kinase inhibitor (TKI) and has been shown to improve prognosis in gastric cancer (Li et al., 2013, Li et al., 2016, 2016). Beside its antiangiogenic effect, it is reported that apatinib can enhance chemosensitization by reversing multidrug resistance (Mi et al., 2010). Presently, many clinical trials are conducted to evaluate the antitumoral effects of apatinib (Zhang, 2015). Limited evidence also showed that apatinib provided some clinical benefits for patients with NSCLC (Ding et al., 2016, Fang et al., 2017, Song et al., 2017). Unfortunately, only 48 cases in total have been formally reported (Ding et al., 2016, Fang et al., 2017, Peng et al., 2017, Song et al., 2017). Therefore, the efficacy and toxicity of apatinib in NSCLC are far from understood and need further evaluation. Here, we reported the clinical use of apatinib in three cases of heavily treated NSCLC in our institute. Moreover, we reviewed currently available studies and registered clinical trials regarding the use of apatinib in NSCLC.
2. Case report
2.1. Patient 1
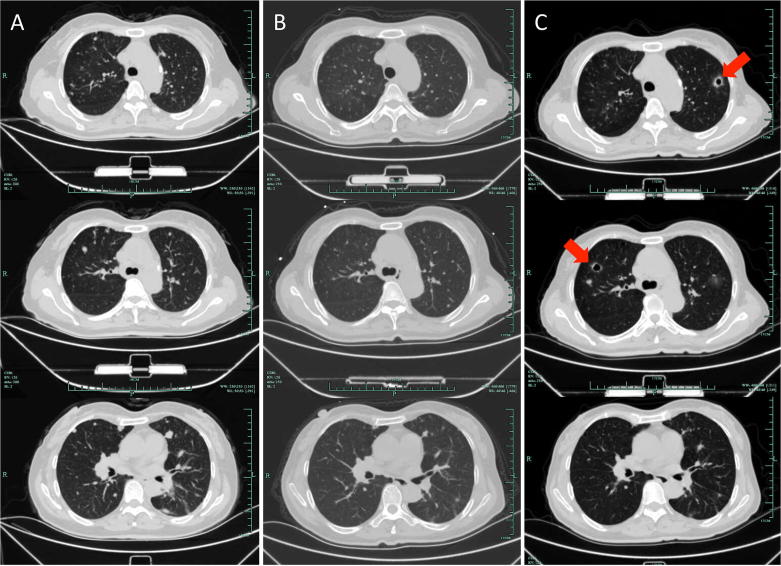
A 61-year-old male was admitted to our hospital because of chest tightness, cough, and right chest pain for four months. Computed tomography (CT) scan showed a lesion in his right lower lung. Proton emission tomography (PET)-CT confirmed the lesion with abnormally enhanced signal, and additionally revealed metastases in the hilar lymph nodes and possible metastasis in the 4th lumbar vertebral arch. Percutaneous pulmonary biopsy indicated pulmonary adenocarcinoma. The patient received pemetrexed/cisplatin doublet chemotherapy as first-line treatment and had a partial response (PR). Gefitinib was used at the 8th month of initial treatment since an EGFR mutation (L858R) was reported in the tumor tissue (Table 1). The mass was found enlarged by CT scan 22 months later. After another seven months of pemetrexed/cisplatin treatment and a single dose of intrathoracic chemotherapy of cisplatin, the therapeutic evaluation was progression disease (PD). Docetaxel and pemetrexed were then sequentially administered until one year later we found more metastases in his left lung. The patient then tried AZD9291 for 4 months, erlotinib for 1 month, and AZD3759 for 4 months. Finally, apatinib (500 mg/day) was prescribed because of PD was considered following the treatment of up-mentioned drugs. The primary tumor and metastases in the left lung were significantly shrunk after a 3-week treatment of apatinib (Fig. 1A–C). Unfortunately, CT scan showed a response of PD merely another three weeks later. The patient died a few days later due to respiratory and circulatory failure. During the use of apatinib, the patients suffered from hand-foot syndrome (grade 3), mild diarrhea (grade 1), and controllable hypertension (grade 1) (Table 2).
Table 1.
Clinical characteristics of the patients reported.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Age, year | 61 | 59 | 73 |
| Gender | Male | Female | Male |
| Pathology | Adenocarcinoma | Adenocarcinoma | Squamous carcinoma |
| Smoking | |||
| Stage | IV (T4N0M1) | IV (T4N0M1) | IV (T3N0M1) |
| Mutation | EGFR L858R | None | EGFR L858R |
| Therapy (response) | |||
| First line | Pemetrexed/cisplatin (PR) | Docetaxel/cisplatin (SD) | Gemcitabine/cisplatin (PR) |
| Second line | Gefitinib (SD) | Pemetrexed/cisplatin (PD) | Icotinib (PD) |
| Third line | Gefitinib | ||
| Forth line | Pemetrexed/carboplatin (SD) | ||
Abbreviations: EGFR, epidermal growth factor receptor; PD, progression disease; PFS, progression-free survival; PR, partial response; SD, stable disease.
Fig. 1.
Patient 1: Computed tomography showed the primary tumor in the right lung and metastases in the left lung (A) before apatinib treatment, (B) three weeks after apatinib treatment, and (C) six weeks after apatinib treatment. Arrows indicate tumoral cavitation.
Table 2.
Therapeutic results of apatinib in the patients.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| EGFR-TKI co-therapy | AZD3759 | Gefitinib | AZD9291 |
| Duration of apatinib treatment, days | 42 | 46 | 22 |
| Withdrawal reason | PD | PD | Hemoptysis |
| PFS, months | 1.4 | 1.5 | – |
| Adverse events | |||
| Hypertension | Grade 1 | Grade 3 | – |
| Hand-foot syndrome | Grade 3 | – | – |
| Hemorrhage | – | – | Grade 5 |
| Diarrhea | Grade 1 | – | – |
| Cardiac toxicity | – | – | – |
| Bone marrow suppression | – | – | – |
Abbreviations: PD, progression disease; PFS, progression-free survival; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor.
2.2. Patient 2
A 59-year-old female found an elevated level of carcinoembronic antigen during her regular physical examination. PET-CT showed a mass in the left lower lung and multiple metastases in both lungs. Lymphadenectasis of hilus and mediastinum was also suggested by imaging. Percutaneous biopsy indicated pulmonary adenocarcinoma, and no gene mutation was detected. The patient was firstly treated by docetaxel/cisplatin doublet chemotherapy for six months and had a response of stable disease (SD). The patient then tried pemetrexed monotherapy for four months and tried combination of pemetrexed and cisplatin for another five months. The therapeutic evaluation was PR. Gefitinib was then prescribed and a PFS of 15 months was achieved. The patient then underwent pemetrexed/cisplatin doublet chemotherapy again for three months and the response was still SD. After that, the patient received apatinib (500 mg/day) for three weeks, and CT scan suggested reduced volume of primary and metastatic lesions (Fig. 2A–C). However, new metastases were identified in the lungs 25 days later, and apatinib was ceased, with a monotherapy of gefitinib. The patient had significantly increased blood pressure that was up to 175/110 mmHg during apatinib treatment.
Fig. 2.
Patient 2: Computed tomography showed primary tumor the right lung and metastases in the left lung (A) before apatinib treatment, (B) three weeks after apatinib treatment, and (C) 46 days after apatinib treatment. Arrows indicate tumoral cavitation.
2.3. Patient 3
A 73-year-old male was admitted due to cough and pulmonary tightness. Chest CT scan presented with consolidation and bronchial obstruction of left upper lobe, and left pleural effusion was observed. Electronic bronchoscopy showed a neoplastic lesion in the left upper lobe, and squamous carcinoma was confirmed by pathology. An EGFR (L858R) mutation was reported (Table 1). Doublet chemotherapy of gemcitabine/carboplatin was prescribed for two months and then icotinib was used as second-line therapy for another month. Apatinib (500 mg/day) combined with AZD9291 was administered as the third-line therapy. The patient was treated with apatinib for a total of three weeks and his clinical symptoms were relieved and partial re-expansion of the left lung was noticed (Fig. 3A and B). Unfortunately, the patient died of massive hemoptysis suddenly one week later.
Fig. 3.
Patient 3: Computed tomography showed primary tumor the right lung (A) before and (B) two weeks after apatinib treatment.
3. Discussion
According to the previous reports, the median PFS in NSCLC patients with apatinib treatment was around five months (Table 3) (Fang et al., 2017, Peng et al., 2017, Song et al., 2017, Zhang et al., 2012b), which was longer than that in patients with gastric cancer (Li et al., 2013, Li et al., 2016, 2016). The disease control rate was approximately 60–85% (Song et al., 2017, Ya, 2016, Zhang et al., 2012b), and the OS was 4–6 months for NSCLC patients (Table 3) (Song et al., 2017, Ya, 2016). However, since apatinib was mostly used in NSCLC as a third- or forth-line therapy, PFS rather than OS may be a more suitable indicator to reflect the efficacy of apatinib. Although our cases responded to apatinib rapidly, their PFS was short (Table 2). For the first case, the bad pulmonary function at the beginning of apatinib treatment might limit the efficacy of apatinib. The second case had been heavily treated before apatinib was prescribed, and the tumor might have already become extremely malignant because of the selection by different drugs. The third patient died unexpectedly and we could not evaluate the actual efficacy of apatinib. Although only a few publications regarding apatinib for lung cancer are available now, dozens of clinical trials are ongoing or in preparation (Table 4). We can expect more evidence in near future.
Table 3.
Clinical data of previously reported study.
| Fang et al. (2017) | Ding et al. (2016) | Peng et al. (2017) | Song et al. (2017) | Ya (2016)* | Zhang et al., 2012a, Zhang et al., 2012b# | |
|---|---|---|---|---|---|---|
| Institute | The First Affiliated Hospital of Nanjing Medical University, Nanjing, China | Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Guangzhou, China | China-Japan Friendship Hospital, Beijing University of Chinese Medicine, Beijing, China | Zhejiang Cancer Hospital, Hangzhou, China | Si Chuan Cancer Hospital, Chengdu, China | Twenty centers in China |
| Pathology | NSCLC, EGFR(–), ALK(–) | Poor differentiated adenocarcinoma, squamous carcinoma | NSCLC | NSCLC | Metastatic NSCLC, EGFR(–) | Non-squamous NSCLC |
| No. of patients | 3 | 2 | 1 | 42 | 20 | 90 |
| Line of treatment | Post third line | Second and forth line | Third line | Post second line | Post third line | Post third line |
| Dosage, mg/day | 500 | 850 | 250 or 500 | 500 | 500 | 750 |
| ORR | 100% | 50% | 100% | 9.5% | 30% | 20% |
| DCR | 100% | 100% | 100% | 61.9% | 85% | 68.9% |
| PR | 3/3 | 1/2 | 1/1 | 4/42 | NR | NR |
| SD | 0/3 | 1/2 | 0/1 | 22/42 | NR | 51/90 |
| PFS, months | 2.8–6.0 | 4.6, 6.0 | 5.1 | 4.2 | NR | 4.7 |
| OS, months | NR | NR | NR | 6.0 | 4 | NR |
| Adverse events (Grade 3/4) | 2/3 | NR | 1/1 | 21/42 | Hypertension (5%), HFS (5%), hemoptysis (5%) | NR |
Abbreviations: ALK, anaplastic lymphoma kinase; DCR, disease control rate; EGFR, epidermal growth factor receptor; HFS, hand-foot syndrome; NR, not reported; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease.
Presented at ESMO Asia 2016 Congress, only abstract available.
Presented at 2012 ASCO Annual Meeting, only abstract available.
Table 4.
Clinical trials for the use of apatinib in lung cancer.
| Registration No. | Disease | Treatment | Sample size | Primary outcome | Status | Institute | Study type |
|---|---|---|---|---|---|---|---|
| ChiCTR-OPC-17011039* | NSCLC, stage IIIB/IV, IIIA (not suitable for surgery) | Apatinib + RFA | 30 | PFS | Recruiting | Sir Run Run Shaw Hospital, Hangzhou, China | Observational |
| ChiCTR-OPC-17011020* | Advanced refractory NSCLC, VEGFR-2 positive | Apatinib | 30 | DCR, ORR, MST | Recruiting | 101th Hospital of PLA, Wuxi, China | Observational |
| ChiCTR-OIC-1701101* | Recurrent and metastatic small cell lung cancer | Apatinib + etoposide | 60 | PFS | Pending | The Affiliated Tumor Hospital Of General Hospital Of Ningxia Medical University, Yinchuan, China | Observational, Phase II |
| ChiCTR-IPR-17010776* | Advanced non-squamous NSCLC harboring EGFR mutations, stage IIIB/IV | Gefitinib + apatinib | 30 | PFS | Pending | The First Affiliated Hospital of the Third Military Medical University, Chongqing, China | First-line RCT |
| ChiCTR-IPR-17010361* | Small cell lung cancer, stage IV | EP regimen + apatinib | 60 | PFS | Pending | The North Hospital of the Affiliated Hospital of Qingdao University, Qingdao, China | First-line RCT |
| ChiCTR-OPC-16009894* | Non-squamous NSCLC without EGFR mutation, stage IIIB/IV | Apatinib + cisplatin/docetaxel | 40 | PFS | Recruiting | The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China | Observational |
| ChiCTR-OPC-16009048* | Advanced squamous cell lung cancer | Apatinib + S-1 | 30 | PFS | Recruiting | Anhui Chest Hospital, Hefei, China | Second-line Observational |
| ChiCTR-OPN-16008694* NCT02852798# |
Advanced non-squamous NSCLC with wild-type EGFR, stage III/IV | Apatinib + docetaxel | 60 | PFS | Pending | Dongguan People’s Hospital, Dongguan, China | Second-line Observational |
| ChiCTR-OOC-16008238* | Advanced non-squamous NSCLC | Apatinib + Xiaoyan Tang | 30 | PFS, adverse effect | Recruiting | The First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China | Observational |
| ChiCTR-OPN-15007443* | Advanced squamous cell lung cancer | Apatinib + docetaxel | 60 | PFS | Recruiting | The Second People’s Hospital of Lianyungang, Lianyungang, China | Observational |
| NCT02515435# | Advanced non-squamous NSCLC | Apatinib | 40 | ORR | Recruiting | Shanghai Pulmonary Hospital, Tongji University, Shanghai, China | Phase II, post third line |
| NCT02540824# | Advanced non-squamous NSCLC with positive RET fusion | Apatinib | 40 | ORR | Recruiting | Shanghai Pulmonary Hospital, Tongji University, Shanghai, China | Phase II, post first line |
| NCT02493582# | Advanced non-squamous NSCLC with wild-type EGFR, stage IIIB/IV | Apatinib + CIK | 400 | OS | Ongoing | The First People’s Hospital of Changzhou, Changzhou, China | Phase II, third line, RCT |
| NCT03129256# | NSCLC | Apatinib + S-1 | 52 | PFS | Recruiting | Changzhou Cancer Hospital of Soochow University, Soochow, China | Phase II, post first line |
| NCT02974933# | Advance non-squamous NSCLC, stage IIIB/IV | Apatinib + pemetrexed | 48 | PFS | Recruiting | Hubei Cancer Hospital, Wuhan, China | Phase II |
| NCT02875457# | Small cell lung cancer, extensive stage | Apatinib + etoposideand cisplatin | 100 | PFS | Recruiting | Daping Hospital, The Third Military Medical University, Chongqing, China | Phase III |
| NCT02733107# | Advanced NSCLC with wild-type EGFR | Apatinib + etoposide | 25 | PFS | Recruiting | Daping Hospital, The Third Military Medical University, Chongqing, China | Phase II, post first line |
| NCT03083041# | Advanced NSCLC | Apatinib + SHR-1210 | 118 | Adverse events, ORR | Recruiting | Shanghai Pulmonary Hospital, Tongji University, Shanghai, China | Phase II, post first/s line |
| NCT03050411# | Advanced EGFR-TKI resistant NSCLC | Apatinib + EGFR-TKIs | 30 | Optimal dosage, PFS | Recruiting | Peking University Third Hospital, Beijing, China | Phase I |
| NCT02332512# | Advanced non-squamous NSCLC harboring wild-type EGFR | Apatinib | 417 | OS | Recruiting | Sun Yet-sen University, Guangzhou, China; Shanghai Pulmonary Hospital, Shanghai, China | Phase III, RCT, post second line |
| NCT01270386# | Advanced non-squamous NSCLC | Apatinib | 136 | PFS | Completed | Sun Yat-sen University, Guangzhou, China | Phase II, RCT, post second line |
| NCT02945852# | Refractory small cell lung cancer | Apatinib | 30 | PFS | Recruiting | Zhejiang Cancer Hospital, Hangzhou, China | Phase II, post second line |
| NCT02691871# | Advanced lung cancer harboring wild-type EGFR, stage IV | Apatinib + docetaxel | 20 | MTD, DLT | Pending | Cancer Institute and Hospital, Chinese Academy of Medical Sciences, Beijing, China | Phase I, second line |
| NCT02780778# | Advanced non-squamous NSCLC harboring wild-type EGFR | Apatinib + docetaxel | 20 | PFS | Recruiting | Tianjin Medical University Cancer Institute and Hospital, Tianjin, China | Phase II/III, second line |
| NCT02980809# | Small cell lung cancer, limited or extensive stage | Apatinib + topotecan vs. topotecan | 60 | PFS | Pending | First Hospitals affiliated to the China PLA General Hospital, Beijing, China | Phase II, second line |
| NCT03127319# | Advanced non-squamous NSCLC harboring wild-type EGFR with bone metastases | Apatinib + docetaxel/zoledronic | 40 | PFS | Pending | Affiliated Hospital of Hebei University, Baoding, China | Phase II, post first line |
| NCT02995187# | Small cell lung cancer | Apatinib | 25 | PFS | Pending | Chinese Academy of Medical Sciences, Beijing, China | Phase II, post second line |
| NCT02704767# | Lung cancer with mutant EGFR | Apatinib + erlotinib vs. erlotinib | 60 | PFS | Pending | The First Affiliated Hospital of Kunming Medical College, Kunming, China | Phase II |
| NCT03100955# | Small cell lung cancer, extensive stage | Apatinib + cisplatin/etoposide | 120 | PFS | Recruiting | Affiliated Hospital of Qingdao University, Qingdao, China | Phase III, RCT |
| NCT03129698# | Small cell lung cancer, extensive stage | Apatinib | 52 | PFS | Recruiting | Peking Union Medical College, Beijing, China | Phase II |
| NCT02824458# | Advanced non-squamous NSCLC harboring EGFR mutations, stage IIIB/IV | Apatinib + gefitinib vs. Gefitinib | 246 | DLT, MTD, PFS | Recruiting | Sun Yat-sen University, Guangzhou, China | Phase III, RCT |
Abbreviations: CIK, cytokine-induced killer cell; DCR, disease control rate; DLT, dose limited toxicity; EGFR, epidermal growth factor receptor; MST, median survival time; MTD, maximum tolerated dose; NSCLC, non-small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PLA, People’s liberty army; RCT, randomized controlled trial; RFA: radiofrequency ablation; TKI, tyrosine kinase inhibitor.
Note: EP regimen: EP, 80 mg/m2, D1; cisplatin, etoposide 100 mg/m2, D1-3.
Registered at Chinese Clinical Trial Registry.
Registered at ClinicalTrial.gov.
The well-known adverse events of apatinib are hand-foot syndrome, proteinuria, and hypertension, because of the antiangiogenetic effects of apatinib in other organs (Li et al., 2013, Li et al., 2016, 2016). At a dose of 500 mg/day, the adverse events of apatinib are acceptable, with a grade-3 toxicity rate of 50% and no grade 4 toxicity (Song et al., 2017). No grade 4 adverse events were found in our patients, but unfortunately, the third case died of sudden hemoptysis (grade 5). Although we have no definite conclusion regarding the causality between hemoptysis and apatinib treatment, a potentially increased risk of squamous cell lung cancer should be pay more attention to when apatinib is considered to be used in NSCLC patients.
With more data acquired from gastric cancer, the dose of apatinib was recommended as 850 mg/day by a phase II trial (Li et al., 2013). At this dosage, the incidences of severe adverse events were similar between apatinib- and placebo-treated groups, except for a slightly increased incidence of hypertension (around 10%). However, in previous reports of apatinib for NSCLC, a dose of 500 mg/day was more commonly adopted (Table 3) (Fang et al., 2017, Song et al., 2017). A patient received 250 mg/day of apatinib could still have a PR as shown in a case report (Peng et al., 2017). In addition, the case showed a dynamic change of tumor volume according to the use and dose adjustment of apatinib, suggesting that the efficacy of apatinib was dose-dependent. Thus, for NSCLC patients with normal weights, the dose of apatinib is recommended to be initiated at 500 mg/per and can be further adjusted in case of severe adverse events.
Since EGFR-TKI is frequently used in NSCLC, the combination of apatinib and EGFR-TKI is common. Actually, EGFR-TKI was continuously used when our patients were prescribed with apatinib (Table 2). In Song’s cohort, the two patients with apatinib and EGFR-TKI co-therapy gained a much longer PFS (11.0 and 9.0 months, respectively) than the median PFS of their whole cohort (4.2 months) (Song et al., 2017). In another case report, apatinib was believed to overcome EGFR-TKI resistance of NSCLC and obtained another 5.1-month PFS for the patient (Peng et al., 2017). These findings shed a light on the combination therapy of EGFR-TKI and apatinib in NSCLC.
Tumoral cavitation in CT scan is frequently observed during apatinib treatment. Two out of three patients in our case series displayed cavitation formation. This phenomenon was also observed in previous studies, in which some but not all patients displayed tumoral cavication after apatinib treatment (Ding et al., 2016, Fang et al., 2017, Zhang et al., 2012b). Similarly, tumoral cavication was reported in patients receiving bevacizumab treatment. In patients with NSCLC, 19% cases were reported to develop cavitation during bevacizumab treatment (Nishino et al., 2012). However, no difference in terms of PFS or OS was found between patients with or without tumoral cavitation. Cavication reduces tumor volume, thus can be a sign of tumor response to the treatment. However, whether tumoral cavication correlates with patients’ prognosis needs further investigation. Tumoral cavitation is probably due to necrosis of tumor cells in tumor core where blood supply is extensively affected during antiangiogenic therapy.
Since a high rate of hemoptysis was associated with squamous NSCLC in patients treated with bevacizumab in a phase II trial (Johnson et al., 2004), squamous NSCLC is usually a contraindication for bevacizumab. One of our patients with squamous NSCLC died of hemoptysis during apatinib treatment. However, in Song’s cohort, nearly 30% of the included patients had squamous NSCLC and no hemoptysis was reported after apatinib treatment (Song et al., 2017). Therefore, the risk of hemoptysis in squamous NSCLC patients is inconclusive and current limited data suggest that squamous NSCLC may be not a contraindication of apatinib. Currently, at least two ongoing clinical trials of apatinib include patients with squamous NSCLC, and will provide more evidence to answer this question (Table 4).
4. Conclusion
With the report of three cases and literature review, we conclude that apatinib can be used in heavily treated NSCLC patients. Apatinib can induce some responses in NSCLC patients with tolerable toxicity. However, more evidence is urgently needed.
Conflict of interests
None.
Acknowledgements
This study is financially supported by the General Medical Association of Anhui Province (No. 2016QK069).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Qi Zhang, Email: qi.zhang@nih.gov.
Ping Fang, Email: fangping1964@126.com.
References
- Chan B.A., Hughes B.G. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl. Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., Jemal A., Yu X.Q., He J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Ding L., Li Q.J., You K.Y., Jiang Z.M., Yao H.R. The use of apatinib in treating nonsmall-cell lung cancer: case report and review of literature. Medicine (Baltimore) 2016;95:e3598. doi: 10.1097/MD.0000000000003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S.C., Zhang H.T., Zhang Y.M., Xie W.P. Apatinib as post second-line therapy in EGFR wild-type and ALK-negative advanced lung adenocarcinoma. OncoTargets Ther. 2017;10:447–452. doi: 10.2147/OTT.S126613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon E.B., Ciuleanu T.E., Arrieta O., Prabhash K., Syrigos K.N., Goksel T., Park K., Gorbunova V., Kowalyszyn R.D., Pikiel J., Czyzewicz G., Orlov S.V., Lewanski C.R., Thomas M., Bidoli P., Dakhil S., Gans S., Kim J.H., Grigorescu A., Karaseva N., Reck M., Cappuzzo F., Alexandris E., Sashegyi A., Yurasov S., Perol M. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- Johnson D.H., Fehrenbacher L., Novotny W.F., Herbst R.S., Nemunaitis J.J., Jablons D.M., Langer C.J., DeVore R.F., 3rd, Gaudreault J., Damico L.A., Holmgren E., Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Kurzrock R., Stewart D.J. Exploring the benefit/risk associated with antiangiogenic agents for the treatment of non-small cell lung cancer patients. Clin. Cancer Res. 2017;23:1137–1148. doi: 10.1158/1078-0432.CCR-16-1968. [DOI] [PubMed] [Google Scholar]
- Li J., Qin S., Xu J., Guo W., Xiong J., Bai Y., Sun G., Yang Y., Wang L., Xu N., Cheng Y., Wang Z., Zheng L., Tao M., Zhu X., Ji D., Liu X., Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- Li J., Qin S., Xu J., Xiong J., Wu C., Bai Y., Liu W., Tong J., Liu Y., Xu R., Wang Z., Wang Q., Ouyang X., Yang Y., Ba Y., Liang J., Lin X., Luo D., Zheng R., Wang X., Sun G., Wang L., Zheng L., Guo H., Wu J., Xu N., Yang J., Zhang H., Cheng Y., Wang N., Chen L., Fan Z., Sun P., Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J. Clin. Oncol. 2016;34:1448–1454. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- Mi Y.J., Liang Y.J., Huang H.B., Zhao H.Y., Wu C.P., Wang F., Tao L.Y., Zhang C.Z., Dai C.L., Tiwari A.K., Ma X.X., To K.K., Ambudkar S.V., Chen Z.S., Fu L.W. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70:7981–7991. doi: 10.1158/0008-5472.CAN-10-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M., Cryer S.K., Okajima Y., Sholl L.M., Hatabu H., Rabin M.S., Jackman D.M., Johnson B.E. Tumoral cavitation in patients with non-small-cell lung cancer treated with antiangiogenic therapy using bevacizumab. Cancer Imaging. 2012;12:225–235. doi: 10.1102/1470-7330.2012.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Cui H., Liu Z., Liu D., Liu F., Song Y., Duan H., Qiu Y., Li Q. Apatinib to combat EGFR-TKI resistance in an advanced non-small cell lung cancer patient with unknown EGFR status: a case report. OncoTargets Ther. 2017;10:10. doi: 10.2147/OTT.S130990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi K., Herbst R.S. Lung cancer in the era of precision medicine. Clin. Cancer Res. 2015;21:2213–2220. doi: 10.1158/1078-0432.CCR-14-2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M., Kaiser R., Mellemgaard A., Douillard J.Y., Orlov S., Krzakowski M., von Pawel J., Gottfried M., Bondarenko I., Liao M., Gann C.N., Barrueco J., Gaschler-Markefski B., Novello S., Group LU-LS Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15:143–155. doi: 10.1016/S1470-2045(13)70586-2. [DOI] [PubMed] [Google Scholar]
- Reck M., von Pawel J., Zatloukal P., Ramlau R., Gorbounova V., Hirsh V., Leighl N., Mezger J., Archer V., Moore N., Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J. Clin. Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- Rossi A., Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev. Anticancer Ther.. 2016;16:653–660. doi: 10.1586/14737140.2016.1170596. [DOI] [PubMed] [Google Scholar]
- Sandler A., Gray R., Perry M.C., Brahmer J., Schiller J.H., Dowlati A., Lilenbaum R., Johnson D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Scagliotti G.V., Krzakowski M., Szczesna A., Strausz J., Makhson A., Reck M., Wierzbicki R.F., Albert I., Thomas M., Miziara J.E., Papai Z.S., Karaseva N., Thongprasert S., Portulas E.D., von Pawel J., Zhang K., Selaru P., Tye L., Chao R.C., Govindan R. Sunitinib plus erlotinib versus placebo plus erlotinib in patients with previously treated advanced non-small-cell lung cancer: a phase III trial. J. Clin. Oncol. 2012;30:2070–2078. doi: 10.1200/JCO.2011.39.2993. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Song Z., Yu X., Lou G., Shi X., Zhang Y. Salvage treatment with apatinib for advanced non-small-cell lung cancer. OncoTargets Ther. 2017;10:1821–1825. doi: 10.2147/OTT.S113435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudev N.S., Reynolds A.R. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17:471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villaruz L.C., Socinski M.A. The role of anti-angiogenesis in non-small-cell lung cancer: an update. Curr. Oncol. Rep. 2015;17:26. doi: 10.1007/s11912-015-0448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J.M., Stinchcombe T.E. Second-line therapy for advanced NSCLC. Oncologist. 2013;18:947–953. doi: 10.1634/theoncologist.2013-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ya Z.C. Apatinib (YN968D1) in performance status 2 or 3 patients with EGFR wild-type metastatic non-small cell lung cancer (NSCLC): A single-arm phase II study. Ann. Oncol. 2016;27:18. [Google Scholar]
- Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des. Devel. Ther. 2015;9:6075–6081. doi: 10.2147/DDDT.S97235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Gold K.A., Kim E. Sorafenib in non-small cell lung cancer. Expert Opin. Investig. Drugs. 2012;21:1417–1426. doi: 10.1517/13543784.2012.699039. [DOI] [PubMed] [Google Scholar]
- Zhang L., Shi M.Q., Huang C., Liu X.Q., Xiong J.P., Chen G.Y., Liu W., Liu W.C., Zhang Y.P., Li K., Yu H., Jiang H.Y. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. J. Clin. Oncol. 2012;30(abstr 7548) [Google Scholar]