Abstract
Elevated evidences show that microRNAs (miRNAs) play vital roles in tumor progression regulation. However, the functional role of let-7b in hepatocellular carcinoma (HCC) is still largely unknown. In this study, we try to investigate the biological activity of let-7b in human HCC cells and try to find the potential regulatory signaling pathway. Our results indicate that let- 7b was remarkably down-regulated in human HCC tissues by qRT-PCR. In addition, let-7b overexpression decreased the expression of β-catenin and c-Myc, while upregulated E-cadherin expression in HCC cells which was verified by quantitative real-time PCR (qRT-PCR) and western blotting. Furthermore, Wnt/β-catenin was involved in let-7b biological activity which was revealed by luciferase assay. Moreover, Wnt/β-catenin signaling inhibitor blocks HCC cell proliferation which is as the same pattern as let-7b overexpression inhibits in HCC cells proliferation. In conclusion, down-regulated let-7b promotes HCC cell proliferation through Wnt/β-catenin signaling in HCC cells. These results suggested that appropriate manipulation of let-7b might be a new treatment of human HCC in the future.
Keywords: Let-7b, Wnt/β-catenin signaling, Cell proliferation
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and has an increasing incidence worldwide (Parkin et al., 2001, Parkin et al., 2005, Llovet and Bruix, 2008). China is one of the areas of the world with high incidence for HCC and contributes to 55% of all HCC cases (Lai et al., 2003). The curative treatments for early-stage HCC are liver transplantation and liver resection. Multistep processes, accumulation of genetic and epigenetic alterations, are included in HCC carcinogenesis. However, the molecular pathogenesis is not well understood (Chu et al., 2014, Hoshida et al., 2009). Thus, it is imperative to identify new possible targets for preventing the initiation and progression of HCC.
MicroRNAs (miRNAs) are non-coding small RNAs (∼21–23nt) that regulate various biological processes by binding to the 3′-untranslated region (3′-UTR) of their target mRNAs, which cause either mRNA degradation or translational repression (Engels and Hutvagner, 2006, Iwakawa and Tomari, 2013). MiRNAs play regulatory roles in multiple cellular processes, including cell proliferation, apoptosis and differentiation (Gibb et al., 2011, Bjorner et al., 2014). Accumulating evidence has shown that both losses and gains of miRNA function are associated with oncogenic transformation. The role of miRNAs in carcinogenesis/cancer has also been discussed. For example, tumor-associated miRNAs have been identified in the serum of patients with diffuse large B-cell lymphoma (Calin et al., 2002, Lawrie et al., 2008). Currently, miRNAs have made them attractive therapeutic targets for cancer molecular therapy. Although much is known about the profiles of miRNAs in many tumors and tissues, the function of miRNAs in HCC has not yet been fully elucidated.
Wnt/β-catenin signaling has been shown to regulate the progression of cancer because it promotes cell proliferation and migration. β-catenin protein is the downstream molecule of Wnt/β-catenin signaling which is accumulated in the nuclear location after WNT1 binds to specific Frizzled (FZD) surface receptors. E-cadherin and c-Myc have been reported to be involved in the β-catenin regulatory network (Thievessen et al., 2003, Zhang et al., 2012, Qualtrough et al., 2015, Su et al., 2015, Xu et al., 2016). The activated Wnt/β-catenin signaling subsequently activates distinct various cellular signaling pathways critical for cancer development (Ito et al., 2000, Masaki et al., 2003). Several clinical studies have reported that abnormal activation of Wnt/β-catenin pathway is contributed to hepatocarcinogenesis (Endo et al., 2000, Inagawa et al., 2002). Thus, it is attractive to further explore the effects of Wnt/β-catenin signaling on HCC.
In conclusion, in this study, we verified that let-7b is down-regulated in HCC. Further investigation showed that Wnt/β-catenin signaling activity was suppressed by let-7b in vitro. Additionally, Wnt/β-catenin signaling inhibition blocks the effects on HCC cell proliferation caused by let-7b knockdown. Our results indicate that let-7b may be a new therapeutic target in HCC and add another layer on miRNA biology.
2. Material and methods
2.1. Cell culture
Human HCC cell line HepG2 was purchased from American Type Culture Collection (ATCC) and maintained in RPMI-1640 medium (Thermo Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (FBS) and 1X penicillin/streptomycin in a humidified incubator at 37 °C containing 5% CO2.
2.2. RNA isolation and quantitative real-time PCR (qRT-PCR)
Extraction of total RNA from cells was in accordance to the instruction of mirVana™ miRNA Isolation Kit (Thermo Scientific). For isolation of intact RNA from tissues, tissues were minced on ice, frozen in liquid nitrogen immediately and stored at −80 °C until analyzed.
Intact RNA from tissues after homogenization was prepared using mirVana™ miRNA Isolation Kit (Thermo Scientific). Total RNAs were eluted with 100 μl of elution buffer. TaqMan microRNA assay was used to determine levels of mature miRNAs in tissues or cells according to the instruction of TaqMan microRNA reverse transcription kit (Thermofisher).
qRT-PCR is the most sensitive technique for mRNA and miRNA detection. TaqMan qRT-PCR assays were used for the detection of gene/mature miRNA expression in triplicate on an ABI 7500 system (Applied Biosystems, Foster City, CA). All the gene/miRNA-specific probes were bought from Applied Biosystems.
2.3. Overexpression and knockdown of let-7b
To evaluate the functional effect of let-7b, we overexpressed let-7b in HepG2 cell in vitro. We purchased lentiviral let-7b (LV-let-7b) and empty lentiviral vector (LV-VC) from Applied Biological Materials Inc (Richmond, BC). After then, the let-7b and VC overexpression lentiviruses were stored at −80 °C until use. Lentiviral transfection efficiency (>90%) was confirmed by fluorescent microscopy.
To block the signaling regulation caused by let-7b, let-7b inhibitor (GeneCopoeia, Guangzhou, China) assay was performed as previous description (Thomson et al., 2013).
2.4. Wnt/β-catenin activity reporter assay
To monitor Wnt/β-catenin signaling pathway activity in cells, Wnt/β-catenin signaling pathway luciferase reporter assay was performed as previous description (Zhang et al., 2015). In brief, HepG2 cells were seeded overnight in a 96-well plate (2 × 104 cells/well). On the next day, cells were co-transfected with 50 ng let-7b or either a control miRNA (Con-miR) with 100 ng Wnt/β-catenin pathway-luciferase-reporter construct (SABiosciences, Frederick, MD). MiRNA plasmids were constructed as previous description (Zhang et al., 2015). 24 h after transfection, Wnt3a conditioned medium (Wnt3a-CM) were replaced the old medium. After 18 h stimulation, the cells were harvested. Wnt/β-catenin signaling activity was determined by Dual Luciferase Reporter Assay System (Promega, San Luis Obispo, CA). WNT inhibitory factor 1 (WIF1) (Sigma-Aldrich, St Louis, MO) was used to inhibit Wnt/β-catenin signaling, Data was represented as the ratio of firefly luciferase activity to Renilla luciferase activity.
2.5. Western blotting
Western blot is often used to separate and identify proteins. After cells were lysed in lysis buffer (Thermo Scientific, Waltham, MA), protein concentrations were determined using DC Protein Assay (Bio Rad Laboratories, Hercules, CA). Western blot analysis was according to a previous description with 15ug of proteins per well (Zhang et al., 2015). For specific primary antibodies (Ab): anti- β-catenin (1:500; Sigma-Aldrich, St Louis, MO), anti–c-Myc (1:500 dilution; Cell Signaling Technologies, Beverly, MA), anti-E-cadherin (1:1000, Cell signaling) and anti–β-actin (1:500; Sigma-Aldrich) following the addition of the correlated HRP-conjugated secondary Abs (Abcam). In order to avoid incomplete stripping, all of the results are from separate membranes.
2.6. Cell viability assay
Cell viability of HepG2 cell was determined by Cell Counting Kit-8 (CCK8) method according to the manufacture's protocol (Promega). Brief, cells were seeded in 96-well plates at densities of 5000 cells/ well and transfected with 50 nM let-7b or con-miR for 48 h. At the end of transfection, 100 μl fresh medium was added to replace the medium used for transfection. Cells were treated with CCK8 dye at days 0–7 of culture. The absorbance at 450 nm was then measured in a microplate reader. Cell viability was calculated by comparing with blank control (BC) cells. BC cells are cells without miRNA transfection. Experiments were repeated for 3 independent experiments.
2.7. Colony formation assay
500 of let-7b or con-miR transfected HepG2 and BC cells were grown in a 6-well plate. After 10 days culture in complete medium, colonies were fixed with 70% ethanol and then stained with 15% crystal violet solution.
2.8. Statistics
SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) for Windows was used for statistical analyses. Data are expressed as mean ± standard deviation (n = 3). Unpaired student t-test was performed to determine whether the difference between two groups is significant. P (probability) less than 0.05 was accepted as significant difference.
3. Results
3.1. Let-7b is down-regulated in HCC tissues
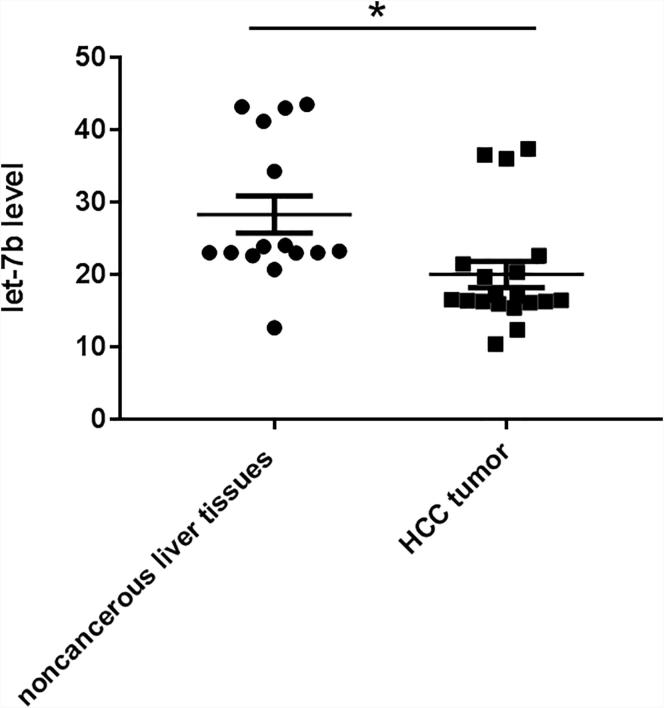
Of our microRNA microarray preliminary studies from 19 HCC tissue samples and 15 noncancerous liver tissue samples, let-7b was found to have 2.1-fold significantly downregulated in HCC tissues (data not shown). qRT-PCR was used to verify let-7b expression level in HCC tissues and noncancerous tissues. As shown in Fig. 1, the expression level of let-7b was remarkably decreased compared with that of noncancerous tissues (P < 0.05). Our results suggested the importance of let-7b down-regulation in HCC progression.
Fig. 1.
Let-7b expression level in HCC tumor and noncancerous liver tissues. Let-7b expression level was determined by qRT-PCR. U6 was used as an endogenous control for the quantification. Results are mean ± SD. *p < 0.05 vs HCC tumor.
3.2. Let-7b suppresses proliferation of HepG2 cells in vitro
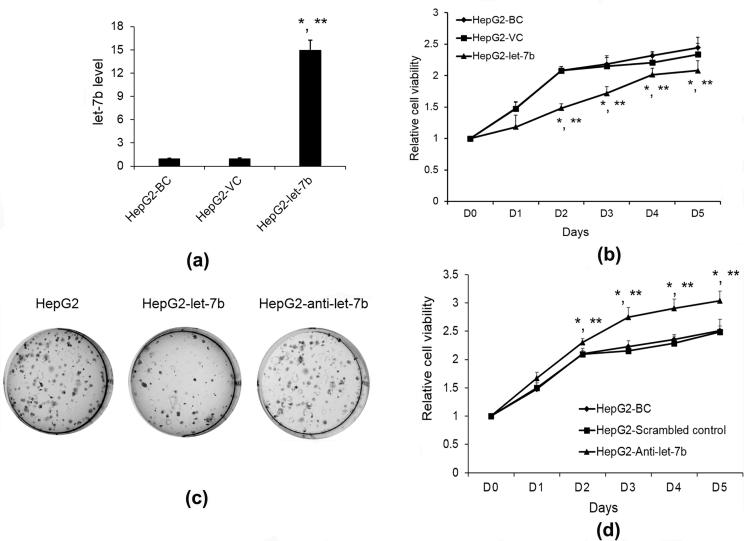
Cell proliferation is the process that results in an increase of the number of cells over time. We subsequently evaluate the functional role of let-7b in HCC cells by monitoring cell proliferation in HepG2 cell. We first determined let-7b expression level after infecting HepG2 cells at multiplicity of infection 50 (MOI = 50) of LV-let-7b or LV-VC (Fig. 2A). The blank control (BC) condition was HepG2 cell without virus infection. CCK-8 proliferation assay was employed to monitor the proliferation of HepG2 cells. As shown in Fig. 2B, cell proliferation was significantly suppressed by let-7b overexpression from day 2 to day 5 than LV-VC and BC groups. In addition, we observed that let-7b overexpression also remarkably reduced the colony numbers of HepG2 cells in comparison with BC and LV-VC groups (Fig. 2C).
Fig. 2.
Effect of let-7b on HCC cell growth. (A) HepG2 were infected with MOIs let-7b lentivirus or VC at MOI 50. Let-7b expression level was determined by qRT-PCR. Data are expressed as the fold change relative to HepG2 cells without infection. *p < 0.05 vs BC; **p < 0.05 vs VC; (B) The cell growth curves showed the let-7b significantly inhibits HepG2 cell growth after day 2. *p < 0.05 vs BC; **p < 0.05 vs VC; (C) let-7b reduced the number of HepG2 colonies. However, anti-let-7b increased the number of HepG2 colonies; (D) Loss of let-7b promoted the HepG2 cell growth, especially after day 2. Results are mean ± SD (n = 3); *p < 0.05 vs BC; **p < 0.05 vs VC.
3.3. Let-7b down-regulates Wnt/β-catenin signaling pathway in vitro
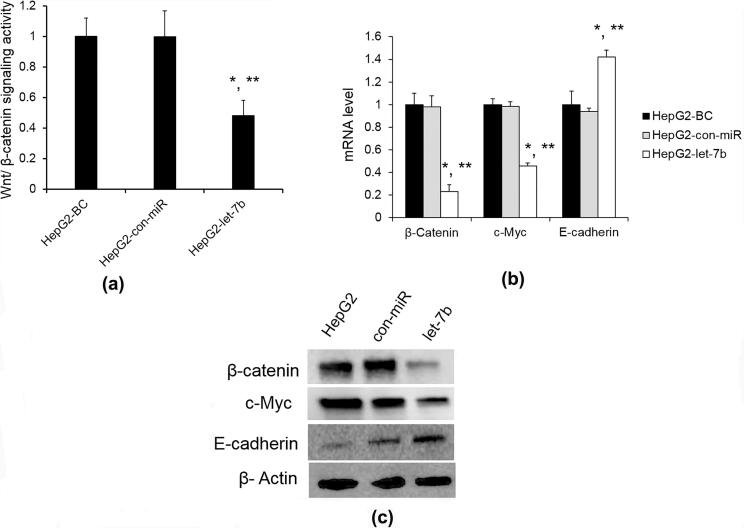
Let-7 families were reported to inhibit Wnt/β-catenin signaling pathway (Vinas et al., 2013, Sun et al., 2016). Moreover, Wnt signaling blockage was known to inhibit cell growth (Bilir et al., 2013). To explore whether the responsible mechanism of let-7b is also related to Wnt/β-catenin signaling in HCC progression, we first overexpressed let-7b or con-miR in HepG2 cells and tested Wnt / β-catenin signaling activity by Wnt/β-catenin reporter assay. As shown in Fig. 3A, we found that let-7b downregulated the activity of the Wnt/β-catenin luciferase reporter construct after Wnt3a conditional medium stimulation in comparison with con-miR. After then, we monitored the effect of let-7b on the components included in Wnt/β-catenin signaling pathway. Let-7b overexpression significantly down-regulated β-catenin, c-Myc but up-regulated E-cadherin mRNA and protein levels compared with con-miR (Fig. 3B, C).
Fig. 3.
Let-7b inhibit Wnt/β-catenin signaling. (A) HepG2 HCC cells were co-transferred with Wnt/β-catenin luciferase-reporter construct and let-7b or con-miR. Wnt/β-catenin activity was normalized to Renilla luciferase activity. (B) β-catenin, c-Myc and E-cadherin mRNA level in HepG2 HCC cells after let-7b overexpression; (C) β-catenin, c-Myc and E-cadherin protein expression was determined by WB. β-Actin was used as an internal control. Results are mean ± SD (n = 3). *p < 0.05 vs BC; **p < 0.05 vs con-miR.
3.4. Wnt/β-catenin signaling pathway is crucial to HepG2 cell proliferation
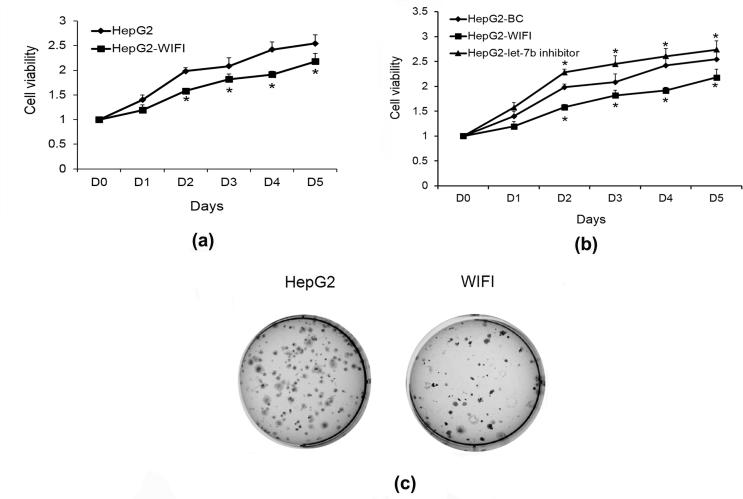
The next step, we validated that Wnt/β-catenin signaling is crucial to HCC cell proliferation. We first inhibited let-7b in HepG2 cells, and then WIFI was added to block Wnt/β-catenin activity. WIFI is ligand special and inhibit Wnt signaling by directly sequestering WNT ligands, WIFI inhibits both canonical and noncanonical WNT signaling (Baron and Kneissel, 2013). After adding WIFI, cell proliferation was remarkedly suppressed from day 2 to day 5 which is similar to the pattern of let-7b overexpression (Fig. 4A). In addition, WIFI also remarkably blocked the increased proliferation of HepG2 caused by let-7b inhibitor (Fig. 4B). Moreover, the colony counts of HepG2 cells were decreased after Wnt/β-catenin inhibition by WIFI (Fig. 4C). These results infer that Wnt/β-catenin signaling is essential to HCC progression.
Fig. 4.
Effect of WIFI on HCC cell growth. (A) The cell growth curves showed the WIFI significantly suppress HepG2 cell growth after day 2. Results are mean ± SD (n = 3); *p < 0.05 vs BC; (B) WIFI remarkably decreased the cell proliferation of HepG2 which had an opposite effect caused by let-7b inhibitor. Results are mean ± SD (n = 3); *p < 0.05 vs BC; (C) WIFI reduced the number of HepG2 colonies.
4. Discussion
The expression and functions of miRNAs are cell and tissue specific. The frequent aberrant expression of miRNAs implies that they may function as either oncogenes or tumor suppressors under certain conditions. The expression and roles of miRNAs in the progression of HCC are not yet fully understood. In our study, let-7b was found to be down-regulated in HCC in comparison with noncancerous liver tissue samples. Overexpression of let-7b decreased the activity of Wnt/β-catenin signaling pathway in HCC cell. Moreover, overexpression of let-7b suppresses HCC cell proliferation. Additionally, inhibition of Wnt/β-catenin signaling pathway suppresses HCC cell proliferation which is similar to the pattern of let-7b. Taken together; these data indicated that let-7b suppresses HCC progression through Wnt/β-catenin pathway. Our results suggest that let-7b might be useful biomarker and raise the possibility of development of new therapeutic strategy for patients with HCC.
Recently, it has been noted that the expression profiles of miRNAs can be used for diagnosis and prognosis of human malignancies (Slaby et al., 2007, Dyrskjot et al., 2009). In this study, let-7b was down-regulated in HCC tissues and verified qRT- PCR. It is widely believed that let-7b is involved in many biological processes in cancers. Let-7b has been reported to reduce cancer cell growth and induce apoptosis by directly downregulating CYP2J2 (Chen et al., 2012). Decreased expression of let-7b was also found in tumor tissue and correlated with microvessel density and survival outcomes in non-small-cell lung cancer (Jusufovic et al., 2012). In addition, let-7b suppresses human gastric cancer malignancy via targeting ING1 (Han et al., 2015). However, despite all these studies, there are very few in vitro functional studies of let-7b related to HCC progression. In the present study, we identified that let-7b suppressed HCC progression through Wnt/β-catenin pathway.
It is clear that Wnt/β-catenin signaling cooperates with half a dozen other signaling systems and plays a prevalent theme in the regulation of cell growth and survival of a variety of cancer cells. For example, activation of Wnt/β-catenin signaling promotes bladder cell proliferation (Guo et al., 2013). In human breast cancer stem cells (BCSCs), classic Wnt signaling was significantly enhanced by miR-142 up-regulation (Isobe et al., 2014). Moreover, overexpression of HOTAIR promotes cell proliferation by activating Wnt/β-catenin signaling pathway in human ovarian cancer (Li et al., 2016). In this study, we demonstrated that decreased expression of let-7b up-regulates Wnt/β-catenin signaling pathway in HCC cells. Furthermore, we also confirmed that the protein levels of down-stream components in Wnt/β-catenin signaling pathway changed after let-7b treatment. Moreover, Wnt signaling inhibitor blocks cell proliferation caused by let-7b. These data suggest that let-7b can regulate Wnt/β-catenin signaling as a tumor suppressor in HCC progression. And, it would be interesting to figure out the direct target of let-7b associated with Wnt/β-catenin signaling.
In conclusion, this present study revealed that the deregulated expression of let-7b contributes to the HCC development through Wnt/β-catenin signaling pathway. The effects of let-7b on HCC cell proliferation may provide a novel target for HCC cancer therapy and adds another layer of complexity to miRNA regulation in cancer.
Conflicts of interest
All the authors had no conflicts of interest to declare in relation to this article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- Bilir B., Kucuk O., Moreno C.S. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J. Transl. Med. 2013;11:280. doi: 10.1186/1479-5876-11-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorner S., Fitzpatrick P.A., Li Y., Allred C., Howell A., Ringberg A., Olsson H., Miller C.J., Axelson H., Landberg G. Epithelial and stromal microRNA signatures of columnar cell hyperplasia linking Let-7c to precancerous and cancerous breast cancer cell proliferation. PLoS One. 2014;9:e105099. doi: 10.1371/journal.pone.0105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., Rassenti L., Kipps T., Negrini M., Bullrich F., Croce C.M. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Chen C., Yang S., Gong W., Wang Y., Cianflone K., Tang J., Wang D.W. Let-7b inhibits human cancer phenotype by targeting cytochrome P450 epoxygenase 2J2. PLoS One. 2012;7:e39197. doi: 10.1371/journal.pone.0039197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu R., Mo G., Duan Z., Huang M., Chang J., Li X., Liu P. miRNAs affect the development of hepatocellular carcinoma via dysregulation of their biogenesis and expression. CellCommun Signal. 2014;12:45. doi: 10.1186/s12964-014-0045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyrskjot L., Ostenfeld M.S., Bramsen J.B., Silahtaroglu A.N., Lamy P., Ramanathan R., Fristrup N., Jensen J.L., Andersen C.L., Zieger K., Kauppinen S., Ulhoi B.P., Kjems J., Borre M., Orntoft T.F. Genomic profiling of microRNAs in bladder cancer: miR11 129 is associated with poor outcome and promotes cell death in vitro. Cancer Res. 2009;69:4851–4860. doi: 10.1158/0008-5472.CAN-08-4043. [DOI] [PubMed] [Google Scholar]
- Endo K., Ueda T., Ueyama J., Ohta T., Terada T. Immunoreactive E-cadherin, alphacatenin, beta-catenin, and gamma-catenin proteins in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, and patients' survival. Hum. Pathol. 2000;31:558–565. doi: 10.1053/hp.2000.6683. [DOI] [PubMed] [Google Scholar]
- Engels B.M., Hutvagner G. Principles and effects of microRNA-mediated posttranscriptional gene regulation. Oncogene. 2006;25:6163–6169. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- Gibb E.A., Brown C.J., Lam W.L. The functional role of long non-coding RNA in human carcinomas. Mol. Cancer. 2011;10:38. doi: 10.1186/1476-4598-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Ying L., Tian Y., Yang P., Zhu Y., Wang Z., Qiu F., Lin J. miR-144 downregulation increases bladder cancer cell proliferation by targeting EZH2 and regulating Wnt signaling. FEBS J. 2013;280:4531–4538. doi: 10.1111/febs.12417. [DOI] [PubMed] [Google Scholar]
- Han X., Chen Y., Yao N., Liu H., Wang Z. MicroRNA let-7b suppresses human gastric cancer malignancy by targeting ING1. Cancer Gene. Ther. 2015;22:122–129. doi: 10.1038/cgt.2014.75. [DOI] [PubMed] [Google Scholar]
- Hoshida Y., Nijman S.M., Kobayashi M., Chan J.A., Brunet J.P., Chiang D.Y., Villanueva A., Newell P., Ikeda K., Hashimoto M., Watanabe G., Gabriel S., Friedman S.L., Kumada H., Llovet J.M., Golub T.R. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagawa S., Itabashi M., Adachi S., Kawamoto T., Hori M., Shimazaki J., Yoshimi F., Fukao K. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin. Cancer Res. 2002;8:450–456. [PubMed] [Google Scholar]
- Isobe T., Hisamori S., Hogan D.J., Zabala M., Hendrickson D.G., Dalerba P., Cai S., Scheeren F., Kuo A.H., Sikandar S.S., Lam J.S., Qian D., Dirbas F.M., Somlo G., Lao K., Brown P.O., Clarke M.F., Shimono Y. miR-142 regulates the tumorigenicity of human breast cancer stem cells through the canonical WNT signaling pathway. Elife. 2014;3 doi: 10.7554/eLife.01977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Shiraki K., Sugimoto K., Yamanaka T., Fujikawa K., Ito M., Takase K., Moriyama M., Kawano H., Hayashida M., Nakano T., Suzuki A. Survivin promotes cell proliferation in human hepatocellular carcinoma. Hepatology. 2000;31:1080–1085. doi: 10.1053/he.2000.6496. [DOI] [PubMed] [Google Scholar]
- Iwakawa H.O., Tomari Y. Molecular insights into microRNA-mediated translational repression in plants. Mol. Cell. 2013;52:591–601. doi: 10.1016/j.molcel.2013.10.033. [DOI] [PubMed] [Google Scholar]
- Jusufovic E., Rijavec M., Keser D., Korosec P., Sodja E., Iljazovic E., Radojevic Z., Kosnik M. let-7b and miR-126 are down-regulated in tumor tissue and correlate with microvessel density and survival outcomes in non-small-cell lung cancer. PLoS One. 2012;7:e45577. doi: 10.1371/journal.pone.0045577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.L., Ratziu V., Yuen M.F., Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- Lawrie C.H., Gal S., Dunlop H.M., Pushkaran B., Liggins A.P., Pulford K., Banham A.H., Pezzella F., Boultwood J., Wainscoat J.S., Hatton C.S., Harris A.L. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large Bcell lymphoma. Br. J. Haematol. 2008;141:672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- Li J., Yang S., Su N., Wang Y., Yu J., Qiu H., He X. Overexpression of long noncoding RNA HOTAIR leads to chemoresistance by activating the Wnt/beta-catenin pathway in human ovarian cancer. Tumour Biol. 2016;37:2057–2065. doi: 10.1007/s13277-015-3998-6. [DOI] [PubMed] [Google Scholar]
- Llovet J.M., Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masaki T., Shiratori Y., Rengifo W., Igarashi K., Yamagata M., Kurokohchi K., Uchida N., Miyauchi Y., Yoshiji H., Watanabe S., Omata M., Kuriyama S. Cyclins and cyclindependent kinases: comparative study of hepatocellular carcinoma versus cirrhosis. Hepatology. 2003;37:534–543. doi: 10.1053/jhep.2003.50112. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Qualtrough D., Rees P., Speight B., Williams A.C., Paraskeva C. The hedgehog inhibitor cyclopamine reduces beta-catenin-Tcf transcriptional activity, induces E-Cadherin expression, and reduces invasion in colorectal cancer cells. Cancers (Basel) 2015;7:1885–1899. doi: 10.3390/cancers7030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaby O., Svoboda M., Fabian P., Smerdova T., Knoflickova D., Bednarikova M., Nenutil R., Vyzula R. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology. 2007;72:397–402. doi: 10.1159/000113489. [DOI] [PubMed] [Google Scholar]
- Su Y.J., Chang Y.W., Lin W.H., Liang C.L., Lee J.L. An aberrant nuclear localization of E-cadherin is a potent inhibitor of Wnt/beta-catenin-elicited promotion of the cancer stem cell phenotype. Oncogenesis. 2015;4:e157. doi: 10.1038/oncsis.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Xu C., Tang S.C., Wang J., Wang H., Wang P., Du N., Qin S., Li G., Xu S., Tao Z., Liu D., Ren H. Let-7c blocks estrogen-activated Wnt signaling in induction of selfrenewal of breast cancer stem cells. Cancer Gene Ther. 2016;23:83–89. doi: 10.1038/cgt.2016.3. [DOI] [PubMed] [Google Scholar]
- Thievessen I., Seifert H.H., Swiatkowski S., Florl A.R., Schulz W.A. E-cadherin involved in inactivation of WNT/beta-catenin signalling in urothelial carcinoma and normal urothelial cells. Br. J. Cancer. 2003;88:1932–1938. doi: 10.1038/sj.bjc.6601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D.W., Bracken C.P., Szubert J.M., Goodall G.J. On measuring miRNAs after transient transfection of mimics or antisense inhibitors. PLoS One. 2013;8:e55214. doi: 10.1371/journal.pone.0055214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinas J.L., Ventayol M., Brune B., Jung M., Sola A., Pi F., Mastora C., Hotter G. miRNA let-7e modulates the Wnt pathway and early nephrogenic markers in mouse embryonic stem cell differentiation. PLoS One. 2013;8:e60937. doi: 10.1371/journal.pone.0060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Chen Y., Huo D., Khramtsov A., Khramtsova G., Zhang C., Goss K.H., Olopade O.I. beta-catenin regulates c-Myc and CDKN1A expression in breast cancer cells. Mol. Carcinog. 2016;55:431–439. doi: 10.1002/mc.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Huang C., Guo Y., Gou X., Hinsdale M., Lloyd P., Liu L. MicroRNA-26b Modulates the NF-kappaB Pathway in Alveolar Macrophages by Regulating PTEN. J. Immunol. 2015;195:5404–5414. doi: 10.4049/jimmunol.1402933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Li Y., Wu Y., Shi K., Bing L., Hao J. Wnt/beta-catenin signaling pathway upregulates c-Myc expression to promote cell proliferation of P19 teratocarcinoma cells. Anat Rec (Hoboken) 2012;295:2104–2113. doi: 10.1002/ar.22592. [DOI] [PubMed] [Google Scholar]