Abstract
We performed a three-locus phylogenetic analysis of Fusarium strains presumably capable of trichothecene production, which were deposited in the Russian national collections. The intra- and interspecific polymorphism of partial sequences of the translation elongation factor 1 alpha (TEF1α) gene and two genes from the trichothecene cluster TRI5 and TRI14 was studied. A study of 60 strains of different origins using DNA markers confirmed, and in the case for several strains, clarified their taxonomic characteristics. As a result, a strain of F. commune (F-900) was identified in Russia for the first time. Furthermore, the strain F-846 proved to be phylogenetically distinct from any of the known Fusarium species. F. equiseti strains from Northwest Russia were found to belong to the North European group (I), whereas a strain from the North Caucasus – to the South European one (II). Partial TRI14 sequences from 9 out of 12 species were determined for the first time. Their comparative analysis demonstrated a relatively high level of intraspecific variability in F. graminearum and F. sporotrichioides, but no correlation between the sequence polymorphism and the geographic origin of the strains or their chemotype was found. Specific chemotypes of trichothecene B producers were characterized using two primer sets. The chemotyping results were verified by HPLC.
Keywords: Fusarium, trichothecene mycotoxins, DNA markers, phylogenetic analysis, identification, chemotype
INTRODUCTION
Fungi of the genus Fusarium from the class Ascomycetes occupy various ecological niches and occur in various climatic zones. In Russia, Fusarium species are ubiquitous in all regions where agricultural crops, primarily cereals, are grown. This fungus causes significant damage to the agricultural and food industries, resulting in several hundred million dollar losses annually. In addition, the mycotoxins produced by members of the genus Fusarium pose a threat to human and animal health and also act as pathogenicity factors for plants [1].
Trichothecene mycotoxins (TrMTs) are the most extensive group of toxic metabolites produced by Fusarium fungi. Trichothecene mycotoxins are produced not only by members of the genus Fusarium, but also by those from the genera Myrothecium, Trichoderma, Cephalosporium, Verticimonosporium, and Stachybotrys [2]. To date, about 200 trichothecene toxins have been identified [3-6]. TrMTs are sesquiterpene compounds consisting of three rings with an epoxide ring at the C-12–C-13 atoms and a double bond at the C-9–C-10 atoms: so, the group is called 12,13-epoxy-trichotec-9-ens. Depending on the side group structure, trichothecene toxins are divided into four types (A–D), with only types A and B produced by Fusarium fungi. More toxic members of type A trichothecenes include diacetoxyscirpenol (DAS), as well as toxins T-2 and HT-2, and the main producers of these toxins are F. sporotrichioides and F. langsethiae. In 2015–2016, a new group of type A trichothecene toxins, named NX, was described [7, 8]. Interestingly, these compounds were identified in cultures of F. graminearum, a traditional producer of B-type TrMTs. Type B is represented by compounds such as nivalenol (NIV), deoxynivalenol (DON), and their acetylated derivatives (3- and 15-ADON and 4,15-ANIV) that are produced by F. graminearum, F. culmorum, F. cerealis, and a group of species known as the F. graminearum species complex (FGSC) [9, 10]. In addition, F. poae, F. venenatum, and F. equiseti are capable of producing both type A and type B toxins [11]. The type of toxins produced by a particular strain is determined by the structure and functions of the genes present in the trichothecene cluster [12, 13]. In most trichothecene-producing Fusarium species, the main cluster comprises 12 genes that encode both of the enzymes responsible for different biosynthesis stages and regulatory factors, some of which control the expression of a large number of the genes associated with various aspects of the fungal metabolism and life activity [14]. Trichothecene toxins inhibit protein synthesis in eukaryotes [15], and trichothecenes such as DON are important aggressiveness factors promoting fungal spread within host plant tissues. Artificial inoculation of cereal ears with mutant F. graminearum strains with abolished DON synthesis has been shown to infect a smaller number of kernels compared to inoculation with wild-type strains [16].
The danger posed by Fusarium fungi and their mycotoxins necessitates the development of methods for a quick and reliable species-specific identification of the strains which will enable a determination of the spectrum of the compounds contained in a culture or batch of grain. At present, DNA polymorphism analysis methods play an important role in taxonomic studies of the genus Fusarium and in the identification of its members. Application of the molecular genetics approach has enabled a clarification of the standards and boundaries of species, as well as the characterization of several new taxa. In particular, a multilocus phylogenetic analysis using sequence-characterized amplified region (SCAR) markers [17] based on 13 housekeeping genes enabled the identification of 9 new species within the FGSC [10], which had been previously considered as a single-species F. graminearum. A little later, species F. vorosii and F. gerlachii were also included in this complex [18]. In total, 16 phylogenetic species can be distinguished in the FGSC [19]. The phylogenetic approach was used to confirm the status of F. pseudograminearum and F. culmorum as separate species [20, 21]. In Russia, an analysis of polymorphic DNA markers enabled the identification of strain groups that were subsequently described as two new species: F. ussurianum, morphologically and phenotypically similar to F. graminearum [22], and F. sibiricum, closely related to F. sporotrichioides [23]. A number of recent phylogenetic studies have determined an intricate structure of the F. equiseti–F. incarnatum species complex (FIESC) and identified several new species within the complex [24]. In addition, investigation of inter- and intraspecific DNA polymorphisms has made possible the development of several highly specific diagnostic and identification systems for the main Fusarium pathogens, which are primarily based on PCR and its modifications [25-29]. The use of modern molecular biological and bioinformatic methods, including whole genome sequencing [30, 31], has significantly accelerated the investigation of the genetic diversity of the genus Fusarium and the functional characterization of genomic elements, but the search for effective methods of molecular typing and informative DNA barcodes still remains topical [32, 33].
The genus Fusarium is different from most other taxa of the kingdom Fungi. The “gold standard” of molecular fungal taxonomy is the ribosomal DNA internal transcribed spacer (ITS) region [34]. However, these markers in the genome of members of the genus Fusarium are represented by two nonorthologous copies and do not possess a sufficient level of interspecific polymorphism [35]. Today, the TEF1α gene is most often used as a marker in phylogenetic and taxonomic studies [36, 37]. It seems promising to use the genes involved in mycotoxin biosynthesis as phylogenetic markers. For example, the trichodiene synthase gene (TRI5) has been used to develop species-specific primer systems [38] and to study intraspecific polymorphism in members of the F. equiseti species complex [39, 40]. However, the phylogenetic characteristics of the TRI5 gene have not been compared with those of “classical” markers, such as TEF1α. Among other genes that comprise the trichothecene cluster and are used in phylogenetic studies, it is necessary to emphasize the role of TRI1 encoding cytochrome P450 monooxygenase and TRI12 encoding a trichothecene efflux pump [41]. A phylogenetic analysis of the TRI1 gene helped to identify a group of F. graminearum strains capable of producing the NX-2 toxin [7]. TRI12 polymorphism was used to design primers for the detection of type B TrMT-producing strains based on their chemotype (3/15-ADON, NIV) [42]. The trichothecene cluster also includes a number of genes that have not been characterized either structurally or functionally; e.g., TRI9 and TRI14 [43].
Fusarium strains from Russian national collections, which are potentially capable of producing TrMTs and represent different climatic and geographic regions of Russia, have not been characterized by molecular genetic methods. Therefore, the main objectives of this study were as follows: (1) a SCAR marker-based analysis of the accuracy of the taxonomic identification of the trichothecene-producing Fusarium strains deposited in Russian national collections; (2) an investigation of the molecular genetic diversity of strains of different geographical origin, isolated in different years and from different sources; (3) determination of the interand intraspecific polymorphism of the TRI14 gene, one of the least studied genes of the trichothecene cluster; and (4) determination of chemotypes of type B TrMT-producing strains using specific PCR primers, with verification of the data by HPLC.
EXPERIMENTAL
Fungal strains
We analyzed 60 strains of 12 Fusarium genus species deposited in Russian national collections and presumably possessing ability for TrMT biosynthesis. The choice of the strains was based on maximum coverage of various natural and geographical zones of Russia. In addition, the study included strains from a number of neighboring countries, as well as from Moldova and Germany. We also studied the F. graminearum strain F-892 (VKPM) deposited in the StrainInfo database (http://www.straininfo.net; ATCC 36015). The list of strains with indication of their geographical origin, host plant species, year of isolation, and the particular collection are given in Table 1. In addition, the morphological features of several strains the initial identification of which had not been confirmed by molecular methods were determined using a MIKMED 6 laboratory microscope (Lomo, Russia). For the microscopic analysis, the fungal strains were grown on carnation leaf agar (CLA) and synthetic nutrient deficient agar (SNA; Nirenberg) for 10–14 days.
Table 1.
Fusarium fungal strains used in the study.
| No. | Accession number | Species | Origin | Source | Year of isolation |
|---|---|---|---|---|---|
| 1 | M-99-43* | F. culmorum | Moscow Region | Wheat | 1999 |
| 2 | 09-1/7* | F. culmorum | Moscow Region | Wheat | 2009 |
| 3 | M-99-9* | F. culmorum | Moscow Region | Wheat | 1999 |
| 4 | M-10-1* | F. culmorum | Moscow Region | Wheat | 2010 |
| 5 | BR-03-19* | F. culmorum | Bryansk Region | Wheat | 2003 |
| 6 | BR-0453* | F. culmorum | Bryansk Region | Wheat | 2004 |
| 7 | OM-0233* | F. culmorum | Omsk Region | Wheat | 2002 |
| 8 | OR-02-37* | F. culmorum | Orel Region | Wheat | 2002 |
| 9 | CM-9864* | F. culmorum | Smolensk region | Wheat | 1998 |
| 10 | KP-1136-66* | F. culmorum | Kirov region | Wheat | 1995 |
| 11 | KP-1599-25/3* | F. culmorum | Kirov Region | Wheat | 1996 |
| 12 | KS-1384-1* | F. culmorum | Kirov Region | Wheat | 2007 |
| 13 | M-05-111* | F. culmorum | Moscow Region | Wheat | 2005 |
| 14 | KC-1716-8* | F. culmorum | Kirov Region | Wheat | 1997 |
| 15 | 58801** | F. culmorum | Moscow Region | Wheat | 2004 |
| 16 | Kz-27* | F. culmorum | Kostanay Region, Kazakhstan | Unknown | 2014 |
| 17 | 74007** | F. culmorum | Arkhangelsk Region | Potato | Unknown |
| 18 | 58030** | F. culmorum | Rostov Region | Cirsium | 2004 |
| 19 | 70505** | F. culmorum | Belarus | Wheat | 2003 |
| 20 | 50106** | F. culmorum | Leningrad Region | Cirsium | 2006 |
| 21 | 64722** | F. cerealis | Khabarovsk Region | Wheat | 2006 |
| 22 | 39295** | F. cerealis | Heilongjiang province, China | Wheat | 2003 |
| 23 | 37032** | F. cerealis | Heilongjiang Province, China | Wheat | 2003 |
| 24 | 39142** | F. cerealis | Heilongjiang Province, China | Wheat | 2003 |
| 25 | 37031** | F. cerealis | Heilongjiang Province, China | Wheat | 2003 |
| 26 | 41727** | F. cerealis | North Ossetia | Cirsium | 2004 |
| 27 | G.8-8** | F. graminearum | Germany | Wheat | 1998 |
| 28 | 41806** | F. graminearum | North Ossetia | Wheat | 2004 |
| 29 | 48702** | F. graminearum | Tula Region | Wheat | Unknown |
| 30 | 58033** | F. graminearum | Leningrad Region | Wheat | 2002 |
| 31 | 70725** | F. graminearum | Orel Region | Wheat | 2006 |
| 32 | 58212** | F. ussurianum1 | Primorsky Krai | Wheat | Unknown |
| 33 | 29813** | F. ussurianum | Jewish Autonomous Oblast | Wheat | 2002 |
| 34 | MM-7* | F. sporotrichioides | Moscow Region | Wheat | 2010 |
| 35 | KG-9744* | F. sporotrichioides | Kirov Region | Wheat | Unknown |
| 36 | 78105** | F. sporotrichioides | Orel Region | Wheat | 2006 |
| 37 | SK-1506* | F. sporotrichioides | North Ossetia | Wheat | 2010 |
| 38 | 64706** | F. sporotrichioides | Primorsky Krai | Barley | 2006 |
| 39 | 33100** | F. sporotrichioides | Primorsky Krai | Wheat | 2003 |
| 40 | 74006** | F. sporotrichioides | Leningrad Region | Barley | 2006 |
| 41 | 11007** | F. sibiricum | Krasnoyarsk Region | Barley | 2000 |
| 42 | 11014** | F. sibiricum | Amur Region | Oat | 2001 |
| 43 | 55201** | F. langsethiae | Kaliningrad Region | Oat | 2005 |
| 44 | 82901** | F. langsethiae | Orel Region | Oat | 2003 |
| 45 | 47401** | F. poae | Moscow Region | Wheat | 2004 |
| 46 | 61701** | F. poae | Saratov Region | Wheat | 2005 |
| 47 | 58242** | F. venenatum | Germany | Unknown | Unknown |
| 48 | 58514** | F. venenatum | Leningrad Region | Oat | 2013 |
| 49 | 58455** | F. venenatum | Novgorod Region | Wheat | 2001 |
| 50 | F-842*** | F. sambucinum | Kiev region, Ukraine | Potato | 1965 |
| 51 | F-3966*** F. sambucinum2 | Tula Region | Soil | 2006 | |
| 52 | F-4360*** | F. sambucinum2 | Buryatia | Wood | 2005 |
| 53 | 64414** | F. equiseti | Kaliningrad Region | Barley | 2006 |
| 54 | 65901** | F. equiseti | Leningrad Region | Barley | 2006 |
| 55 | 97001** | F. equiseti | North Ossetia | Wheat | 2007 |
| 56 | F-3549*** | F. equiseti4 | Negev Desert, Israel | Soil | 1995 |
| 57 | F-2681*** | F. incarnatum | Moscow Region | Unknown | 1966 |
| 58 | F-846*** | F. sp3 | Moldova | Melon | 1958 |
| 59 | F-892 (ATCC36015)**** | F. graminearum | USA | Unknown | 1977 |
| 60 | F-900**** | F. sambucinum2 | Krasnoyarsk Region | Larix sibirica | Unknown |
* – strains from the collection of the All-Russian Research Institute of Phytopathology;
** – strains from the collection of the All-Russian Institute of Plant Protection;
*** – strains from the All-Russian Collection of Microorganisms;
**** – strains from the All-Russian Collection of Industrial Microorganisms.
1 – initially identified as F. graminearum;
2 – initial identification was not confirmed;
3 – initially identified as F. poae.
DNA isolation
Prior to DNA isolation, monospore cultures of fungi were grown on potato sucrose agar (PSA) at room temperature for 10 days, until abundant mycelium was obtained. DNA was isolated from monospore fungal cultures by a method based on the use of cetyltrimethylammonium bromide as a detergent, with allowance for the modifications described earlier [28]. The concentration and purity of DNA samples were evaluated using a NanoVue spectrophotometer (GE HealthCare, USA).
Design of universal primers, PCR, and sequencing
To amplify partial sequences of the TEF1α, TRI5, and TRI14 genes, we constructed the following primer pairs: TEF50F (5’-CGACTCTGGCAAGTCGACCAC-3’) and TEF590R (5’-CTCGGCTTTGAGCTTGTCAAG-3’); TRI5F (5’-ACACTGGTTCTGGACGACAGCA-3’) and TRI5R (5’-CCATCCAGTTCTCCATCTGAG-3’); TRI14F (5’-GAAGCTGCCTCGACATGGCTC-3’) and TRI14R (5’-AATAATATTATGGGGAACAATCAT-3’).
The primers were designed using the ClustalW algorithm [44]. The physicochemical properties of the primers were evaluated using the Oligo 6.71 software.
PCR was performed using the following amplification programs.
Primers TEF50F–590R: 93°C, 90 s; 93°C, 20 s; 64°C, 5 s; 67°C, 5 s (5 cycles); 93°C, 1 s; 64°C, 5 s; 67°C, 5 s (40 cycles).
Primers TRI5F–R and TRI14F–R: 93°C, 90 s; 93°C, 10 s; 55°C, 15 s; 72°C, 10 s (40 cycles).
PCR and electrophoretic analysis were performed according to [27, 28].
PCR products were cloned using an InstA Clone PCR cloning kit (Fermentas, Lithuania) according to the manufacturer’s protocol. DNA was sequenced at the Evrogen JSC using an ABI PRISM BigDye Terminator v.3.1 kit, followed by an analysis of the reaction products on an ABI PRISM 3730 automatic sequencer (Applied Biosystems).
The nucleotide sequences characterized in the present work are deposited in the NCBI GenBank under the accession numbers MG989711-989751 (TEF1α), MH001611-001651 (TRI5), and MH001652-001692 (TRI14).
Phylogenetic analysis
DNA markers with a characterized nucleotide sequence were compared with sequences deposited in the NCBI GenBank and Fusarium MLST databases (http://www.westerdijkinstitute.nl/fusarium/) using the BLAST algorithm. Phylogenetic trees were constructed with the maximum likelihood (ML) method and GTR+G (General Time Reversible) nucleotide substitution model [45] using the MEGA5.1 software [46]. In addition to the studied strains, several sequences of the appropriate genes of typical strains from international collections deposited in databases were used in the phylogenetic analysis. The reliability of phylogenetic tree topologies was confirmed by bootstrap analysis from 1,000 replicates. Insertions and deletions were omitted from the analysis. The number of variable, parsimony informative nucleotides and haplotypes for each marker was calculated with the DnaSP v6 software [47] using a sample of 41 strains.
Molecular typing of type B TrMT producers
To determine the chemotypes of the type B TrMT producers, we analyzed F. graminearum, F. culmorum, F. cerealis, and F. ussurianum strains. Chemotype-specific PCR was performed using three primer sets: two sets of primers for the polymorphic regions of the TRI12 gene [42] (the set is denoted as 12-1), [48 (12-2)], and a pair of primers for the amplification of TRI13 gene fragments with different lengths, depending on the chemotype [49 (13-1)]. The structure of primers and their melting temperatures are presented in Table 2.
Table 2.
Primers used for the chemotyping of type B trichothecene-producing strains.
| Primer set | Sequence | Product length, bp |
Tm, °C | Chemotype | Reference |
|---|---|---|---|---|---|
| 12-1 | 12CON (univ.): 5’-CATGAGCATGGTGATGTC- 3’ | [48] | |||
| 12 NF: 5’-TCTCCTCGTTGTATCTGG-3’ | 840 | 60 | NIV | ||
| 12-15F: 5’-TACAGCGGTCGCAACTTC-3’ | 670 | 60 | 15-ADON | ||
| 12-3F: 5’-CTTTGGCAAGCCCGTGCA-3’ | 410 | 60 | 3-ADON | ||
| 12-2 |
3ADONf: 5’-AACATGATCGGTGAGGTATCGA-3’ 3ADONr: 5’-CCATGGCGCTGGGAGTT-3’ |
60 | 60 | 3-ADON | [42] |
|
15ADONf: 5’-GTTTCGATATTCATTGGAAAGCTAC-3’ 15ADONr: 5’-CAAATAAGTATCGTCTGAAATTGGAAA-5’ |
57 | 60 | 15-ADON | ||
|
NIVf: 5’-GCCCATATTCGCGACAATGT-5’ NIVr: 5’-GGCGAACTGATGAGTAACAAAACC-3’ |
77 | 60 | NIV | ||
| 13-1 |
Tri13P1: 5’-CTCSACCGCATCGAAGASTCTC-3’ Tri13P2: 5’-GAASGTCGCARGACCTTGTTTC-3’ |
859 644 583 |
62 |
15-ADON 3-ADON NIV |
[49] |
un. – universal primer
Tm – melting temperature
In the case of system 12-2, the analysis was performed with each pair of primers separately, not in a multiplex PCR [48] format, to increase analysis specificity and avoid the formation of nonspecific amplicons.
The obtained results were confirmed by quantitative PCR (qPCR) with primer pairs from systems 12-1 and 12-2. In addition to standard components, 1.5 μL of 20× EvaGreen dye (Biotium, USA) was added to the reaction mixture. Amplification and fluorescent signal detection were performed in a DT-96-detecting amplifier (DNA-Technology, Russia). The PCR results were expressed as quantification cycles (Cq, [50]). Each sample was analyzed in two independent replicates.
HPLC analysis of toxin production by type B TrMT-producing strains
To determine the type of TrMTs produced by the studied strains, fungal cultures were grown on a MYRO liquid medium [51] at 25 °C and 220 rpm for 5 days. The ability of isolates to produce DON and its monoacetylated derivatives was determined by reverse phase high pressure liquid chromatography of the culture filtrate supernatant separated from the mycelium by centrifugation [52, 53]. An 8 mL aliquot of the culture liquid supernatant was diluted with a acetonitrile : water (1 : 1) mixture to 10 mL and passed through a 0.22 μm Millipore membrane filter; 10 μL of the sample was introduced into the injector of a Waters 1525 Breeze HPLC system equipped with a Waters 2487 UV detector (Waters, USA). Separation was carried out on a Symmetry C18 (150 × 4.6 mm) column thermostated at 27°C. Mycotoxins were eluted with an acetonitrile : methanol : water (1 : 1 : 8 v/v/v – mobile phase) mixture at a flow rate of 0.5 mL/min and detected at 254 nm. Commercial DON, 3-AcDON, and 15-AcDON (Sigma-Aldrich, USA) were used as standards; as a control, we used the filtrate of the MYRO uninoculated medium that was incubated simultaneously with cultivation of submerged fungal cultures under the above conditions.
RESULTS
Phylogenetic properties of genes, analysis of their partial sequences using the BLAST algorithm, and microscopic analysis of the morphology of strains with controversial identification
The main phylogenetic properties of the analyzed genes are given in Table 3. The DNA of all strains was amplified with a TEF50F–590R primer pair, which resulted in a single 452 to 483 bp amplification product containing two 80 to 100 and 236 to 254 bp introns. Except for the insertions and deletions omitted in the evaluation of the phylogenetic properties, the length of the analyzed sequences was 392 bp, including 129 (32.9%) variable nucleotides. The number of parsimony informative characters was 115 (29.3%), and the number of haplotypes was 17. An analysis of the TEF1α gene sequences using the BLAST algorithm confirmed the initial species identification for 54 strains. Of the six strains without confirmed identification, three were initially classified as F. sambucinum. The TEF1α sequences of strains F-3966 (No. 51, Table 1), NRRL 52726 relating to the F. tricinctum species complex, and NRRL 52727 (F. avenaceum) were shown to be 99.3% similar. Similarity for the strain F-4360 (No. 52) to the F. acuminatum strain, NRRL 52789 was 99.545%. The TEF1α gene sequence from the F-900 strain (No. 60) was 100% similar to a fragment of this gene from the F. commune strain NRRL 52764. The initial morphological species identification of strain F-3549 (No. 56) as F. equiseti was not confirmed: the BLAST analysis revealed 99% similarity to the sequence of strain NRRL 34033 from a relatively rare species, F. brachygibbosum. The strain 58212 (No. 32), initially identified as F. graminearum, had 100% similarity of the TEF1α sequence to that of CBS 123751–123745 strains typical of F. ussurianum. The most interesting result was obtained through a marker sequence analysis of the strain F-846 initially identified as F. poae. Comparison with the TEF1α sequences of typical strains deposited in databases did not reveal 100% similarity to any of them. The closest sequence was that from the F. polyphialidicum strain F-0016 (DQ295144, 97% similarity).
Table 3.
Phylogenetic characteristics of analyzed sequences.
| Locus | SL, bp | GC, % | VS, % | PIS, % | HT | Hd | Pi |
|---|---|---|---|---|---|---|---|
| TEF1α | 392 | 53.2 | 32.9 | 29.3 | 17 | 0.933 | 0.08872 |
| TRI5 | 379 | 48.5 | 37.2 | 36.1 | 13 | 0.907 | 0.13586 |
| TRI14 | 650 | 49.1 | 36.7 | 34.5 | 23 | 0.96 | 0.13510 |
| TEF1α+TRI5+TRI14 | 1421 | 50 | 35.9 | 33.5 | 28 | 0.976 | 0.1233 |
SL – sequence length
VS – variable sites
PIS – parsimony informative sites
HT –haplotypes
Hd – haplotype diversity
Pi – nucleotide diversity
TRI5F-R primer pair provided DNA amplification for all studied strains except for Nos. 51, 52, 56, and 60. An amplification product of the TRI5 gene was 431– 440 bp long and contained one intron of 52–61 bp in length. The length of the analyzed sequences was 379 bp, including 141 (37.2%) variable sites and 137 (36.1%) parsimony informative sites; the number of haplotypes was 13. The BLAST-based sequence analysis confirmed the accuracy of species identification for 54 strains. A DNA amplification product of the strain 58212 had 99% similarity with a TRI5 gene fragment from F. asiaticum (strains NRRL 26156 and 28720). It should be noted that none of the databases contained records of complete or partial structures of this gene in F. ussurianum strains, but given the close relationship between F. asiaticum and F. ussurianum [7], this result seems reliable. A DNA amplification product of the strain F-846 was 98% similar to a partial TRI5 gene sequence from the F. langsethiae strain KF2640 (JF966259).
PCR with the TRI14F-R primer pair revealed no DNA amplification products in samples Nos. 51, 52, 56, and 60 (as in the case of the TRI5F-R pair), as well as No. 58 (F-846). The DNA of the other strains was amplified with the formation of 698 to 705 bp products containing a single 50- to 59-bp intron. The analyzed sequence length (without insertions and deletions) was 650 bp, of which 239 bp (36.8%) were variable, and 224 bp (34.5%) were parsimony informative; the number of haplotypes was 23. The search for similar sequences in the GenBank and Fusarium MLST databases showed that the studied strains of F. graminearum constitute two groups: the first group comprising Nos. 30 and 58 showed 100% similarity with the TRI14 sequence of strain CBS 138562 (KU572434.1), while the second group (Nos. 27–29, 31) was completely identical to the sequence of strain CBS 138561 (KU572429.1), with the sequence similarity in these two groups being 97%.
We performed a microscopic analysis of the main morphological structures of strains whose initial identification was not confirmed by the marker sequence analysis. In strains F-3966 and F-4360 growing on CLA, elongated curved macroconidia with three to four septa typical of F. avenaceum, F. tricinctum, and F. acuminatum [54] were revealed. The strain F-900 also formed curved macroconidia with four septa and oval microconidia about 10 μm in size – features typical of the species F. commune [55]. In the strain F-846, thick-walled microconidia with four to five septa, as well as oval and clavate microconidia, were found. This result confirms the suggestion about erroneous initial identification of the strain as F. poae, because this species is characterized by spherical or spinulose microconidia and rarely forms macroconidia, usually with three septa [54].
Analysis of phylogenetic tree topology
The phylogenetic trees generated on the basis of the structures of the three studied genes were characterized by both similarity and several significant topological differences. The TEF1α gene dendrogram (Fig. 1) comprises four large clusters supported by high bootstrap values (98 to 99%). Each cluster includes strains of species characterized by similar spectra of the produced mycotoxins. Cluster I (bootstrap support of 98%) is represented by type B TrMT-producing species (including F. pseudograminearum CBS109954, No. KM434220); cluster II (99%) is formed by type A TrMT producers; cluster III (98%) is represented by species producing both TrMT types; and cluster IV (99%) is represented by the species F. equiseti and F. incarnatum that are also capable of producing both type A and B toxins; however, unlike F. poae and closely related species, they also possess the ability to produce zearalenone and some other mycotoxins. It should be noted that clusters I–III and IV form separate groups supported by high bootstrap values (99%), and “F. sp.” (Fusarium sp. strain F-846) located in an intermediate position between these two groups. Clusters I and II include two subgroups: the first subgroup of cluster I (support 99%) comprises strains of the species F. culmorum and F. cerealis, and the second subgroup (98%) contains F. graminearum and F. ussurianum. Subgroups of cluster II are represented by the species F. langsethiae and F. sporotrichioides/F. sibiricum (bootstrap support of 99% each). Cluster IV is the most heterogeneous and represented by F. equiseti and F. incarnatum species. Two typical strains of F. incarnatum (NRRL 22244 and NRRL 34059) form a separate group supported by a bootstrap value of 59%, which, however, does not include the F-2681 strain investigated in this study. The F. equiseti strain 97001, together with strains H2-2-5B (JF496575) and 10393 (LN901566), forms a subgroup (90%), and strains 65901 and 64414 are included in another subgroup (99%) that also comprises strains VI01095 (AJ543560), VI01070 (AJ543562), and 10675 (LN901573).
Fig. 1.
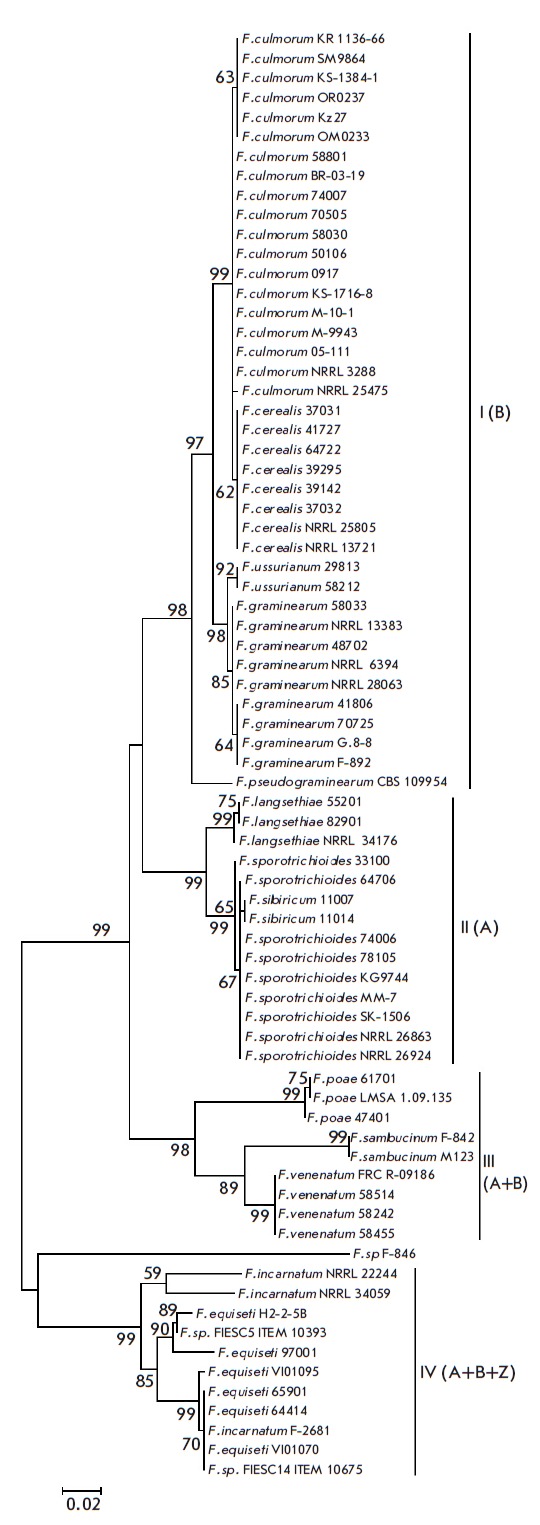
Phylogenetic tree constructed based on the alignment of partial TEF1α gene sequences of trichothecene-producing species using the maximum likelihood method (74 sequences). Only bootstrap values higher than 50% from 1,000 replicates are shown. 21 sequences from the NCBI GenBank are also included.
On the TRI5 gene dendrogram (Fig. 2), the bootstrap support of the main clusters corresponding to the toxigenic profiles of the studied species ranges from 94 (cluster III) to 100% (clusters I, II, and IV). In contrast to the TEF1α gene dendrogram, “F. sp.” is located in an intermediate position between clusters II and III. In addition, F. sporotrichioides strains do not form a single group but are distributed within cluster II; in particular, the strain 33100 belongs to the same subgroup as F. sibiricum strains (support of 98%). Cluster I lacks the F. graminearum-ussurianum subgroup characteristic of the TEF1α dendrogram. On the TRI14 gene phylogenetic tree (Fig. 3), F. ussurianum strains, together with F. cerealis strains, form a subgroup (bootstrap support of 95%), while F. graminearum strains are divided into two subgroups supported by bootstrap values of 99 and 100%, respectively. Cluster II includes the subgroups F. langsethiae (98%) and F. sporotrichioides/ sibiricum (91%); therefore, the topology of this cluster on the TRI14 dendrogram corresponds to the topology of TEF1α rather than the TRI5 dendrogram. It should be noted that in cluster IV, the F. equiseti strains 64414 and 65901 and F. incarnatum strain F-2681, on the one hand, and the F. equiseti strain 97001, on the other, form separate branches on the dendrograms of both TRI genes.
Fig. 2.
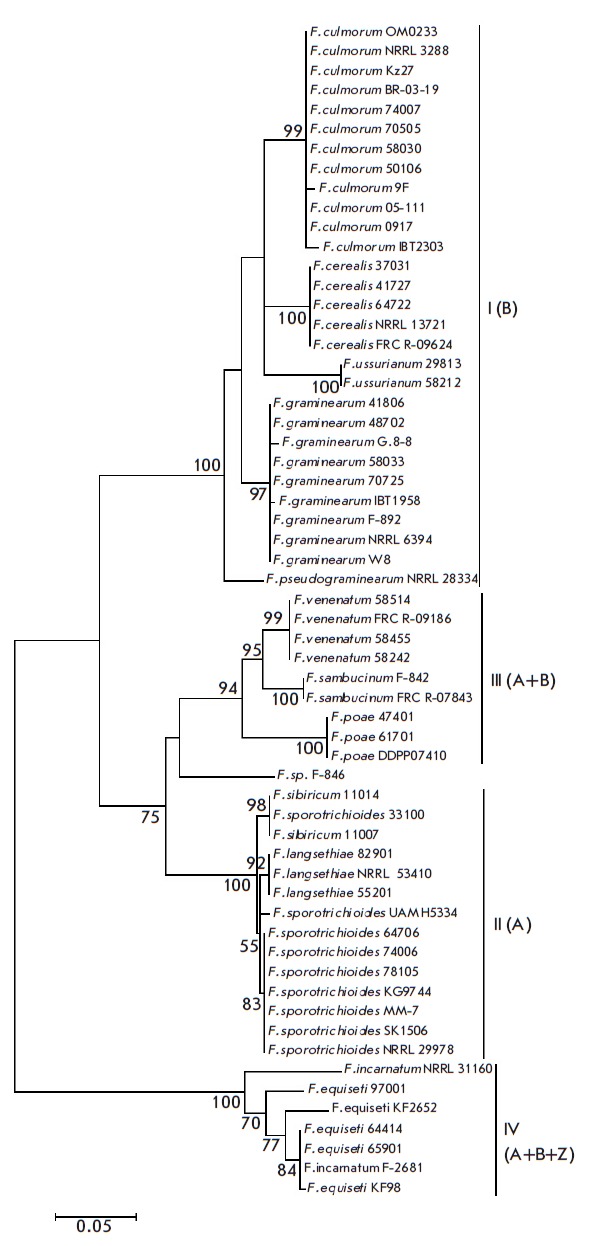
Phylogenetic tree constructed based on the alignment of partial TRI5 gene sequences of trichothecene-producing species using the maximum likelihood method (60 sequences). Only bootstrap values higher than 50% from 1,000 replicates are shown. 18 sequences from the NCBI GenBank are also included.
Fig. 3.
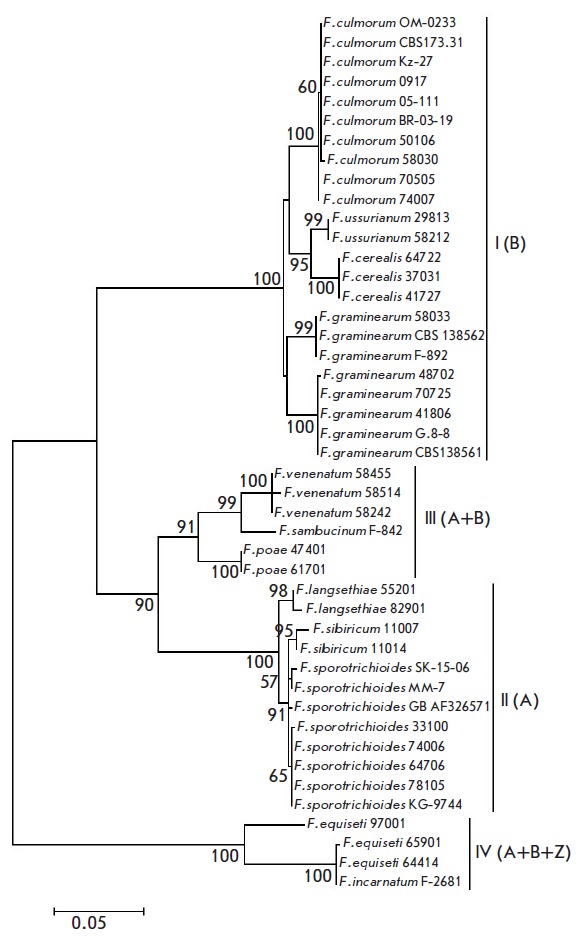
Phylogenetic tree constructed based on the alignment of partial TRI14 gene sequences of trichothecene-producing species using the maximum likelihood method (45 sequences). Only bootstrap values higher than 50% from 1,000 replicates are shown. 4 sequences from the NCBI GenBank are also included.
The structure of a phylogenetic tree generated based on the analysis of the combined sequence of the genes TEF1α, TRI5, and TRI14 (Fig. 4) involves four large clusters supported by 100% bootstrap values. Of particular interest is a common group comprising clusters II and III (bootstrap support of 96%).
Fig. 4.
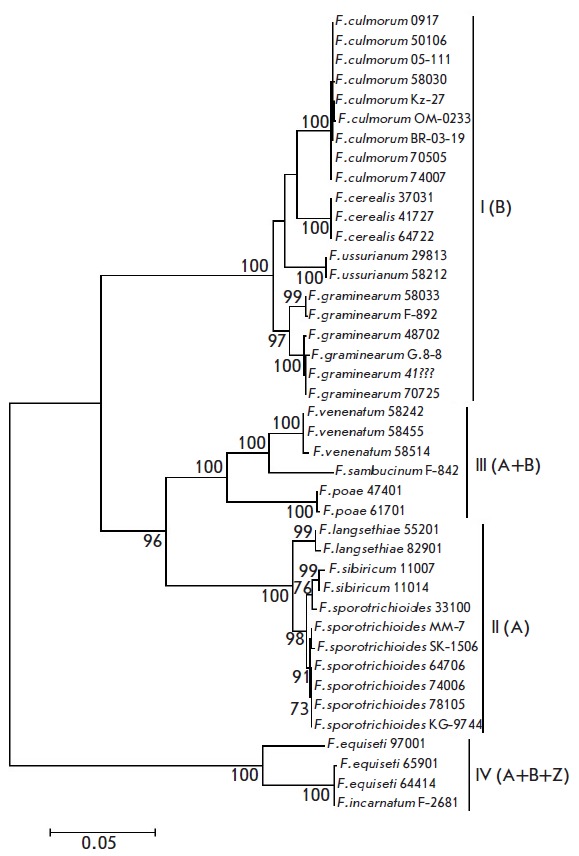
Phylogenetic tree constructed based on the alignment of combined (TEF1α+TRI5+TRI14) sequences of trichothecene-producing species using the maximum likelihood method (41 sequences). Only bootstrap values higher than 50% from 1,000 replicates are shown.
Chemotyping of type B TrMT-producing strains and determination of mycotoxins by HPLC
Table 4 presents the results of the analysis of 33 type B TrMT-producing strains using qPCR with the primer sets 12-1 and 12-2. During the study, we decided to exclude set 13-1 from the use because an electrophoretic analysis of amplification products revealed two specific bands in all samples (data not shown): i.e., it was not possible to separate 3-ADON and 15-ADON chemotypes in F. graminearum. According to the chemotyping results, all analyzed F. culmorum and F. ussurianum strains belong to the 3-ADON chemotype, and the F. graminearum strains 58033 and 70725 also belong to it. The DNA of the F. graminearum strains G.8-8, 41806, and 48702 was amplified using a pair of 12CON– 12-15F primers, indicating that they belong to the 15- ADON chemotype (however, minor bands were also detected in samples of F. graminearum 14-17, which was probably related to PCR conditions).
Table 4.
Results of qPCR with primer sets 12-1 and 12-2 for 33 strains of trichothecene B-producing Fusarium species. «?» - minor bands on electrophoresis gel or minor amplification on last cycles are detected; «n/a» - not analyzed.
| Strain | 3-ADON | 15-ADON | NIV | HPLC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primer set | 12-1 | 12-2 | 12-1 | 12-2 | 12-1 | 12-2 | ||||
| F. culmorum M-99-43 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum 09-1/7 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum M-99-9 | + | + | - | ? | - | - | 3-ADON | |||
| F. culmorum M-10-1 | + | + | - | - | - | - | n/a | |||
| F. culmorum BR-03-19 | + | + | - | - | - | - | n/a | |||
| F. culmorum BR-0453 | + | + | - | - | - | - | n/a | |||
| F. culmorum OM-0233 | + | + | - | - | - | - | n/a | |||
| F. culmorum OR-02-37 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum CM-9864 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum KP-1136-66 | + | + | - | - | - | - | n/a | |||
| F. culmorum KP-1599-25/3 | + | + | - | - | - | - | DON | |||
| F. culmorum KS-1384-1 | + | + | - | - | - | - | DON | |||
| F. culmorum M-05-111 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum KC-1716-8 | + | + | - | - | - | - | n/a | |||
| F. culmorum 58801 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum Kz-27 | + | + | - | - | - | - | n/a | |||
| F. culmorum 74007 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum 58030 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum 70505 | + | + | - | - | - | - | 3-ADON | |||
| F. culmorum 50106 | + | + | - | - | - | - | 3-ADON | |||
| F. cerealis 64722 | - | - | - | - | + | + | n/a | |||
| F. cerealis 39295 | - | - | - | - | + | + | n/a | |||
| F. cerealis 37032 | - | - | - | - | + | + | n/a | |||
| F. cerealis 39142 | - | - | - | - | + | + | n/a | |||
| F. cerealis 37031 | - | - | - | - | + | + | n/a | |||
| F. cerealis 41727 | - | - | - | - | + | + | n/a | |||
| F. graminearum G.8-8 | - | - | + | + | - | - | DON | |||
| F. graminearum 41806 | ? | ? | + | + | - | - | 15-ADON | |||
| F. graminearum 48702 | - | ? | + | + | - | - | 15-ADON | |||
| F. graminearum 58033 | + | + | - | - | - | - | 3-ADON | |||
| F. graminearum 70725 | + | + | - | - | - | - | 3-ADON | |||
| F. ussurianum 58212 | + | + | - | - | - | - | DON | |||
| F. ussurianum 29813 | + | + | - | - | - | - | 3-ADON | |||
An HPLC-based analysis of strains for the toxin-forming ability confirmed the molecular typing data for most samples (Table 4). In most cultures, DON quantitatively predominated over acetylated derivatives; no derivatives were detected in strains F. graminearum G.8-8, F. culmorum KP-1599-25/3 and KS-1384-1, and F. ussurianum 58212. Figure 5 shows an example chromatogram of the culture liquid of the F. ussurianum strain 29813, with peaks corresponding to DON (retention time, 4.453 min) and 3-ADON (retention time, 5.483 min).
Fig. 5.
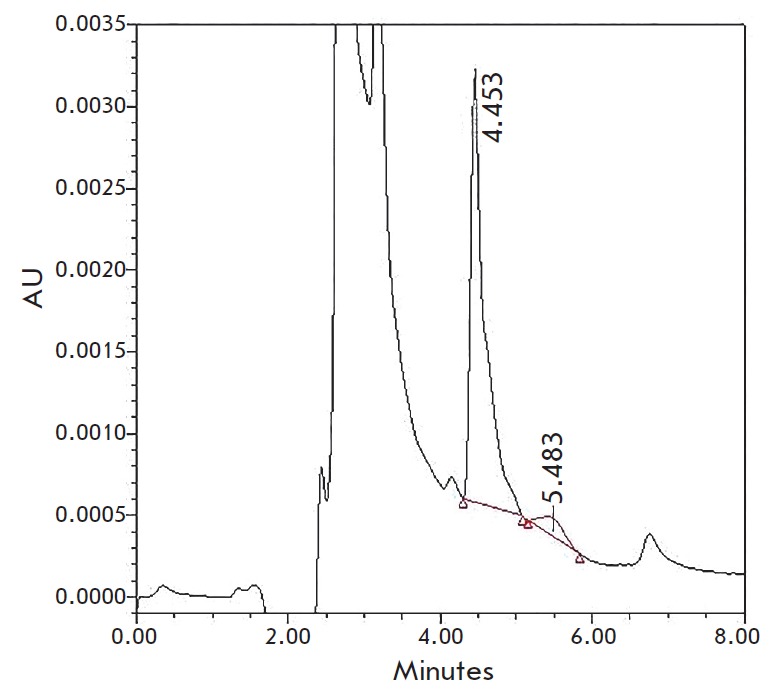
An HPLC chromatogram of a liquid culture medium of F. ussurianum 29813. DON has a retention time of 4.453 min; 3-ADON has a retention time of 5.483 min.
DISCUSSION
The main objective of this work was to study the genetic diversity and toxigenic characteristics of Fusarium fungal strains potentially capable of producing TrMTs, which were isolated in different regions of Russia and deposited in Russian national collections. Another important aspect of the study was to extend information about the structural features of the trichothecene cluster genes associated with the synthesis of the toxins and pathogenic properties of fungi.
Because the TEF1α gene is now considered to be the most studied and phylogenetically informative SCAR marker of genus Fusarium members, an analysis of its sequences has become the basis for verification of the taxonomic status of collection strains. The initial identification of six out of the 60 samples was not confirmed. The use of a combination of molecular genetic and morphological approaches enabled a highly reliable species identification for “controversial” strains. The strain 58212 (initially F. graminearum) was identified as F. ussurianum, and this fact correlates well with its geographic origin (Primorye region) and with the fact that species F. graminearum and F. ussurianum are almost indistinguishable by morphological features. Strains F-3966 and F-4360 deposited in collections as F. sambucinum were identified as F. avenaceum and F. acuminatum, respectively. Species F. acuminatum and F. avenaceum do not produce trichothecene mycotoxins; however, according to their ecological niches and growth pattern on potato sucrose agar, they are similar to F. sambucinum. There are no data on similar errors, but it is argued that F. torulosum, closely related to these two species, has been misidentified as F. sambucinum [54]. The strain F-900 isolated from forest tree nursery soil in the Krasnoyarsk region was identified as F. commune based on the results of a comprehensive study. The species F. commune described in 2003 [55] has not been detected to date in Russia. According to the available data, F. commune can be both a soil saprophyte and a pathogen of various plants, including economically important crops. F. commune is considered to be taxonomically close to the F. oxysporum species complex, but it is incapable of producing TrMTs [56], which is confirmed by the absence of PCR amplification products with primer pairs to the TRI5 and TRI14 genes. Based on an analysis of TEF1α and TRI5 gene sequences, the strain F-846 isolated from melon (Moldova, 1958), which was referred to as F. poae according to the Russian National Collection of Microorganisms, had no 100% similarity with any of the sequences deposited in databases. According to its marker gene structure, the strain was most close to the strains F. polyphialidicum F-0016 (TEF1α) and F. langsethiae F2640 (TRI5). The relationship between F-846 and F. polyphialidicum was not confirmed by an analysis of their morphological structures. Regarding the strain F2640 deposited in the GenBank, there are doubts about the accuracy of its identification as F. langsethiae, which are confirmed by significant differences in the TRI5 gene structure between this strain and typical strains of F. langsethiae. On dendrograms of the TEF1α and TRI5 genes, F-846 was located in an intermediate position and was not assigned to any of the large clusters. We may tentatively suggest that the strain F-846 is a separate phylogenetic species; however, confirmation of this suggestion requires additional analysis using a broader spectrum of DNA markers, investigation of its toxin-producing ability, and additional morphological and physiological data.
In recent years, the species F. equiseti, together with the closely related F. incarnatum, has been shown to have a complex phylogenetic structure, forming a heterogeneous group called the F. incarnatum-equiseti species complex (FIESC). F. equiseti strains from Northern and Southern Europe form two different groups named type I and II [57]. In the present study, marker sequences of the TEF1α gene of strains from different regions of Russia were compared with sequences of this gene from strains VI01070 and VI01095 belonging to type I, strain H2-2-5B belonging to type II, and strains 10675 and 10393 analyzed in [40]. Sequence comparison and phylogenetic analysis of TEF1α demonstrated that the strain 97001 appears to belong to type II (Southern Europe), which correlates with its geographic origin (North Ossetia), while strains 64414 and 65901 (Kaliningrad and Leningrad Region, respectively) belong to the North European type I. An exception to this rule was the strain 10675 (LN901573) isolated from wheat in Spain in 2009, assigned, however, according to the constructed tree topology, to type I. The F. incarnatum strain F-2681 also belonged to type I.
The use of structures of the genes responsible for various stages of mycotoxin biosynthesis in phylogenetic studies has been a controversial issue, because most of these genes have been acquired through horizontal transfer. Usually, a comparative analysis of these markers does not correctly reflect the evolutionary relationships among taxa, but it may be useful in identifying the features of toxin formation or the pathogenic properties of the strains. In this study, we investigated the partial structures of two trichothecene cluster genes: TRI5 encoding trichodiene synthase responsible for the first stage of TrMT biosynthesis, and TRI14 the exact function of which is unknown but is presumably associated with the regulation of DON biosynthesis or its transport beyond the cell during the development of the fungus in infected plant tissues [16]. The information on the sequences of the trichothecene cluster genes presented in databases is rather limited. For example, one TRI5 gene sequence from F. venenatum and F. incarnatum and two gene copies from F. sambucinum are deposited. There are only three TRI14 gene annotations for F. sporotrichioides, one annotation for F. pseudograminearum, and seven and four annotations among complete sequences of the trichothecene cluster of F. graminearum and F. culmorum, respectively. Therefore, for the first time, partial sequences of the TRI14 gene for 9 of 12 examined species were determined and deposited in the GenBank database. A characteristic feature of the TRI5 and TRI14 genes is the fact that a significant portion of the variable sites in the genes is located in the coding part, which is reflected in the differences in the amino acid sequences of appropriate proteins among and within species. The reverse is observed for the TEF1α gene, in which all variable sites occur in introns, and the coding part is highly conserved. We believe that these differences may reflect the different evolutionary history of these genes and also the fact that the translation elongation factor does not belong to proteins specific to the genus Fusarium, while the polymorphism of the amino acid sequences of the proteins encoded by the TRI genes has an important adaptive value. This may also explain the differences in topology of phylogenetic trees constructed based on TEF1α, TRI5, and TRI14. One of the differences is common branches formed by clusters II (type A TrMT producers) and III (TrMT A+B) on the dendrograms of the TRI5 and TRI14 genes, respectively, and supported by high bootstrap values (90 and 75%). In turn, on the TEF1α gene dendrogram, cluster II, along with cluster I (type B TrMT producers), forms a common branch, although the bootstrap support of this branch is low (less than 50%). Also, on dendrograms of the TRI5 and TRI14 genes, F. ussurianum strains form common clusters with strains of the species F. culmorum and F. cerealis; this contradicts the evolutionary data according to which F. ussurianum is most close to the species F. graminearum [22], which is confirmed by a phylogenetic analysis of TEF1α. On the TRI14 gene dendrogram, the studied strains of F. graminearum were divided into two clusters with high bootstrap support, but we could not refer this grouping either to the chemotype of the strains or to their geographical origin. We could assume a relationship between the TRI14 gene polymorphism and differences in the aggressiveness of different strains with respect to the host plant. However, similar studies were not performed in the present work. For the TRI5 gene, no correlation between F. graminearum chemotypes (3/15-ADON) and the species’ phylogenetic structure was found. In addition, none of the analyzed F. graminearum and F. culmorum strains contained an 8-nucleotide deletion (TGGAACAA), a marker for weakly toxigenic strains of these species [58].
Despite an approximately identical content of variable and phylogenetically informative sites, as well as haplotypic diversity of the three genes, an analysis of TEF1α more accurately reflected the phylogenetic structure and presumable evolutionary development of a group of Fusarium genus species TrMT producers. This result agrees with the data in an earlier study of the polymorphism of other genes involved in mycotoxin biosynthesis, e.g. TRI1 [7], or the gene of enniatin synthetase (Esyn1), a key enzyme of enniatin biosynthesis in F. avenaceum and closely related species [28, 59].
The belonging to a particular chemotype is a specific feature of a B-trichothecene producing strain. In recent years, studies have been published on the occurrence of chemotypes of B-trichothecene producers (primarily F. graminearum) in various regions of the world, e.g., in South America [60], Africa [61], and Europe [62, 63]. In 2016, the results of an extensive study conducted by experts from 17 European countries, including Russia, were published [64], which led to the creation of a European database (www.catalogueeu.luxmcc.lu). This database contains information on 187 strains isolated on the territory of Russia; however, it lacks information about strains from several regions, such as Western Siberia and the Volga-Vyatka Region, as well as information on the chemotypes of collection samples isolated in previous years. In the present study, the TrMT type was analyzed using a combined approach involving chemotyping with the chemotype-specific primers described earlier (see Molecular typing of type B TrMT producers) and an analysis of the culture fluids of some strains by HPLC. By using sets of primers (12-1 and 12-2) specific to the polymorphic regions of the TRI12 gene, we identified the chemotypes of the strains of four type BTrMT-producing species. It was demonstrated that the F. culmorum and F. ussurianum strains belong to the 3-ADON chemotypes, the F. cerealis strains belong to the NIV chemotype, and that there were both 3-ADON and 15-ADON producers among F. graminearum strains. It should be taken into account that PCR analysis data in some cases may not coincide with the actual toxigenic profile of a strain, which emphasizes the need to confirm genetic data by chemical methods [65]. HPLC confirmed the results of chemotyping for 16 out of 20 strains selected for chromatographic analysis. The culture liquids of four strains contained only DON, and acetylated derivatives were absent. The data published in recent years have demonstrated that 3-ADON producers predominate among F. graminearum strains of different geographical origins, which is mainly related to their higher aggressiveness compared to that of 15-ADON and NIV producers [66, 67]. An analysis of European strains of F. graminearum has demonstrated that the 3-ADON chemotype is common in Northern Europe, while the 15-ADON chemotype is more common in Central and Southern Europe [62-64]. Of the three studied F. graminearum strains with the 15-ADON chemotype, two may be assigned to the Central European group (G.8- 8 – Germany, 48702 – Tula Region), and one may be assigned to the South European group (41806 – North Ossetia). 3-ADON chemotype strains may be assigned to the North European (58033 – Leningrad Region) and Central European (70725 – Orel Region) groups. All the studied cultures of the species F. culmorum were assigned to the 3-ADON chemotype. It is believed that only two of the three known B chemotypes, 3-ADON and NIV, are characteristic of F. culmorum, with 3-ADON being much more ubiquitous [61, 64], which was confirmed in the present study. The F. ussurianum strain 29813 representing the 3-ADON chemotype produced the largest amount of DON among all the analyzed cultures. The relation of F. cerealis strains to the NIV chemotype is also in line with the data in [68]. We believe that the results obtained in the present study can partially compensate for information absent in the European database on the chemotypes of strains from certain regions of Russia. For example, strains from the Volga-Vyatka Region (No. 10–12), as well as Western Siberia and its neighboring Kostanay Region of Kazakhstan (No. 7, 16), were characterized for the first time.
CONCLUSION
The findings of this study have both a basic and applied significance. The fundamental significance is associated with the great expansion of information on the molecular genetic diversity of trichothecene-producing strains of the genus Fusarium, which have different origins and represent different regions of Russia. The use of a complex approach combining the classical study of morphological structures with the analysis of highly informative DNA markers made it possible to verify and clarify species identification for a number of collection samples, in particular, to discover the first F. commune strain on the territory of Russia. For the first time, the TRI14 gene was used for phylogenetic studies; the gene analysis revealed, on the one hand, a high level of interand intraspecific polymorphism and, on the other hand, the need for further investigations of its structure and functions, which would provide better understanding of its role in pathogenesis and mycotoxin biosynthesis. The applied significance of this study is related to the possibility of using the studied markers for developing monitoring systems for food contamination by TrMT producers. A combined study of the relation of type B TrMT producers to the main chemotypes using specific PCR and HPLC enabled an evaluation of their occurrence in Russia, including regions where TrMT producers had not been previously found.
Acknowledgments
The authors are grateful to T.Yu. Gagkaeva (AllRussian Research Institute of Plant Protection) for valuable comments on morphological characterization of studied strains.
The study was supported by the Russian Foundation for Basic Research, grants No. 15-29-02527 (L.S., S.Z.; selection of collection samples, phylogenetic analysis of TEF1α and TRI5 genes, and microscopy) and 16-34-01369 (A.S.; structural and phylogenetic analysis of the TRI14 gene and analysis of the toxinproducing ability of strains by HPLC).
Glossary
Abbreviations
- PCR
polymerase chain reaction
- TrMT
trichothecene mycotoxin
- TEF1α
translation elongation factor 1-α gene
- TRI5
trichodiene synthase gene
- bp
base pairs
References
- 1.Moretti A., Susca A., Mule G., Logrieco A.F., Proctor R.H.. Int. J. Food Microbiol. 2013;167:57–66. doi: 10.1016/j.ijfoodmicro.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Ueno Y., Adv. Food Nutr. Res. 1989;3:301–305. [Google Scholar]
- 3.Desjardins A.E. Fusarium mycotoxins: chemistry, genetics and biology. APS Press, St. Paul, Minnesota, 2006. 2006. [Google Scholar]
- 4.Grovey J.F. The trichothecenes and their biosynthesis. Progress in the Chemistry of Organic Natural Products. EdsHerz W., Falk H., Kirby G.W. Vienna: Springer-Verlag, 2007. 2007. pp. 63–130. [PubMed] [Google Scholar]
- 5.McCormick S.P., Stanley A.M., Stover N.A., Alexander N.J.. Toxins. 2011;3:802–814. doi: 10.3390/toxins3070802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nesic K., Ivanovic S., Nesic V.. Rev. Environ. Contam. Toxicol. 2014;228:101–120. doi: 10.1007/978-3-319-01619-1_5. [DOI] [PubMed] [Google Scholar]
- 7.Kelly A., Proctor R.H., Belzile F., Chulze S.N., Clear R.M., Cowger C., Elmer W., Lee T., Obanor F., Waalwijk C., Ward T.J.. Fungal Genet. Biol. 2016;95:39–48. doi: 10.1016/j.fgb.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Varga E., Wiesenberger G., Hametner C., Ward T.J., Dong Y., Schöfbeck D., McCormick S., Broz K., Stückler R., Schuhmacher R.. Environ. Microbiol. 2015;17(8):2588–2600. doi: 10.1111/1462-2920.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell K., Kistler H.C., Tacke B.K., Casper H.H.. Proc. Natl. Acad. Sci. USA. 2000;97:7905–7910. doi: 10.1073/pnas.130193297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell K., Ward T.J., Geiser D.M., Kistler H.C., Aoki T.. Fungal Genet. Biol. 2004;41:600–623. doi: 10.1016/j.fgb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Moss M.O., Thrane U.. Toxicol. Lett. 2004;153(1):23–28. doi: 10.1016/j.toxlet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Brown D.W., Dyer R.B., McCormick S.P., Kendra D.F., Plattner R.D.. Fungal Genet. Biol. 2004;41:454–462. doi: 10.1016/j.fgb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Alexander N.J., McCormick S.P., Waalwijk C., van der Lee T., Proctor R.H.. Fungal Genet. Biol. 2011;48(5):485–495. doi: 10.1016/j.fgb.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Nasmith C.G., Walkowiak S., Wang L., Leung W.W.Y., Gong Y., Johnston A., Harris L.J., Guttman D.S., Subramaniam R.. PLoS Pathog. 2011;7(9):e1002266. doi: 10.1371/journal.ppat.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cundliffe E., Cannon M., Davies J.. Proc. Natl. Acad. Sci. USA. 1974;71(1):30–34. doi: 10.1073/pnas.71.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer R.B., Plattner R.D., Kendra D.F., Brown D.R.. J. Agric. Food Chem. 2005;53:9281–9287. doi: 10.1021/jf051441a. [DOI] [PubMed] [Google Scholar]
- 17.Paran I., Michelmore R.W.. Theor. Appl. Genet. 1993;85(8):985–993. doi: 10.1007/BF00215038. [DOI] [PubMed] [Google Scholar]
- 18.Starkey D.E., Ward T.J., Aoki T., Gale L.R., Kistler H.C., Geiser D.M., Suga H., Toth B., Varga J., O’Donnell K.. Fungal Genet. Biol. 2007;44:1191–1204. doi: 10.1016/j.fgb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Aoki T., Ward T.J., Kistler H.C., O’Donnell K., JSM Mycotoxins. 2012;62:91–102. [Google Scholar]
- 20.Obanor F., Erginbas-Orakci G., Tunali B., Nicol J.M., Chakraborty S.. Fungal Biol. 2010;114:753–765. doi: 10.1016/j.funbio.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Scott J.B., Chakraborty S.. Mycol. Res. 2006;110:1413–1425. doi: 10.1016/j.mycres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Yli-Mattila T., Gagkaeva T., Ward T.J., Aoki T., Kistler H.C., O’Donnell K.. Mycologia. 2009;101(6):841–852. doi: 10.3852/08-217. [DOI] [PubMed] [Google Scholar]
- 23.Yli-Mattila T., Ward T.J., O’Donnell K., Proctor R.H., Burkin A.A., Kononenko G.P., Gavrilova O.P., Aoki T., McCormick S.P., Gagkaeva T.Yu.. Int. J. Food Microbiol. 2011;147:58–68. doi: 10.1016/j.ijfoodmicro.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell K., Sutton D.A., Rinaldi M.G., Gueidan C., Crous P.W., Geiser D.M.. J. Clin. Microbiol. 2009;47:3851–3861. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez-Ortuno D., Waalwijk C., van der Lee T., Fan J., Atkins S., West J.S., Fraaije B.A.. Int. J. Food Microbiol. 2013;166:148–154. doi: 10.1016/j.ijfoodmicro.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Nicolaisen M., Surponiene S., Nielsen L.K., Lazzaro I., Spliid N.H., Justensen A.F.. J. Microbiol. Meth. 2009;76:234–240. doi: 10.1016/j.mimet.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Stakheev A.A., Ryazantsev D.Yu., Gagkaeva T.Yu., Zavriev S.K., Food Control. 2011;22:462–468. [Google Scholar]
- 28.Stakheev A.A., Khairulina D.R., Zavriev S.K.. Int. J. Food Microbiol. 2016;225:27–37. doi: 10.1016/j.ijfoodmicro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Yli-Mattila T., Nayaka S.C., Venkataramana M., Yörük E.. Methods Mol. Biol. 2017;1542:269–291. doi: 10.1007/978-1-4939-6707-0_18. [DOI] [PubMed] [Google Scholar]
- 30.Walkowiak S., Rowland O., Rodrigue N., Subramaniam R.. BMC Genomics. 2016;17:1014. doi: 10.1186/s12864-016-3371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lysøe E., Frandsen R.J.N., Divon H.H., Terzi V., Orru L., Lamontanara A., Kolseth A.K., Nielsen K.F., Thrane U.. Int. J. Food Microbiol. 2016;221:29–36. doi: 10.1016/j.ijfoodmicro.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hatmi A.M.S., van den Ende A.H.G.G., Stielow J.B., van Diepeningen A.D., Seifert K.A., McCormick W., Assabgui R., Gräfenhan T., de Hoog G.S., Levesque C.A.. Fungal Biol. 2016;120:231–245. doi: 10.1016/j.funbio.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Nirmaladevi D., Venkataramana M., Srivastava R.K., Uppalapati S.R., Gupta V.K., Yli-Mattila T., Clement Tsui K.M., Srinivas C., Niranjana S.R., Chandra N.S.. Sci. Rep. 2016;6:21367. doi: 10.1038/srep21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoch C.L., Seifert K.A., Huhndorf S., Robert V., Spouge J.L., Levesque C.A., Chen W.. Proc. Natl. Acad. Sci. USA. 2012;109:6241–6246. doi: 10.1073/pnas.1117018109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell K., Cigelnik E.. Mol. Phylogenet. Evol. 1997;7(1):103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- 36.Kristensen R., Torp M., Kosiak B., Holst-Jensen A.. Mycol. Res. 2005;109(2):173–186. doi: 10.1017/s0953756204002114. [DOI] [PubMed] [Google Scholar]
- 37.Geiser D.M., Jimenez-Case M., Kang S., Makalowska I., Veerararaghavan N., Ward T., Zhang N., Kuldau G.A., O’Donnell K., Eur. J. Plant Pathol. 2004;110:473–479. [Google Scholar]
- 38.Niessen L., Schmidt H., Vogel R.F.. Int. J. Food Microbiol. 2004;95:305–319. doi: 10.1016/j.ijfoodmicro.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Stępień L., Gromadzka K., Chelkowski J.. J. Appl. Genet. 2012;53:227–236. doi: 10.1007/s13353-012-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villani A., Moretti A., De Saeger S., Han Z., Di Mavungu J.D., Soares C.M.G., Proctor R.H., Venancio A., Lima N., Stea G.. Int. J. Food Microbiol. 2016;234:24–35. doi: 10.1016/j.ijfoodmicro.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Menke J., Dong Y., Kistler H.C.. Mol. Plant Microbe In. 2012;25(11):1408–1418. doi: 10.1094/MPMI-04-12-0081-R. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen L.K., Jensen J.D., Rodriguez A., Jorgensen L.N., Justensen A.F.. Int. J. Food Microbiol. 2012;157:384–392. doi: 10.1016/j.ijfoodmicro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Wachowska U., Packa D., Wiwart M.. Toxins. 2017;9(12):408. doi: 10.3390/toxins9120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson J.D., Higgins D.G., Gibson T.J.. Nucleic Acids Research. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nei M., Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press, 2000. 2000. [Google Scholar]
- 46.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S.. Mol. Biol. Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., GuiraoRico S., Librado P., Ramos-Onsins S.E., Sánchez-Gracia A.. Mol. Biol. Evol. 2017;34(12):3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 48.Ward T.J., Bielawski J.P., Kistler H.C., Sullivan E., O’Donnell K.. Proc. Natl. Acad. Sci. USA. 2002;99:9278–9283. doi: 10.1073/pnas.142307199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J.H., Li H.P., Qu B., Zhang J.B., Huang T., Chen F.F., Liao Y.C.. Int. J. Mol. Sci. 2008;9:2495–2504. doi: 10.3390/ijms9122495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., Vandesompele J., Wittwer C.T.. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 51.Farber J.M., Sanders G.W.. Appl. Environ. Microbiol. 1986;51(2):381–384. doi: 10.1128/aem.51.2.381-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta V.K., Chattopadhyay P., Kalita M.C., Chaurasia A.K., Gogoi H.K., Singh L.. Pharm. Methods. 2011;2(1):25–29. doi: 10.4103/2229-4708.81087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han Z., Tangni E.K., Huybrechts B., Munaut F., Scauflaire J., Wu A., Callebaut A.. Mycotoxin Res. 2014;30:231–240. doi: 10.1007/s12550-014-0207-1. [DOI] [PubMed] [Google Scholar]
- 54.Leslie J.F., Summerell B.A., The Fusarium laboratory manual. Blackwell Publ., 2006. 2006:266–267.
- 55.Skovgaard K., Rosendahl S., O’Donnell K., Hirenberh H.I.. Mycologia. 2003;95:630–636. [PubMed] [Google Scholar]
- 56.Zhu Z., Zheng L., Pan L.. Plant Disease. 2014;98(7):977–987. doi: 10.1094/PDIS-08-13-0805-RE. [DOI] [PubMed] [Google Scholar]
- 57.Marin P., Moretti A., Ritieni A., Jurado M., Vazquez C., Teresa Gonzalez-Jaen M.. Food Microbiol. 2012;31:229–237. doi: 10.1016/j.fm.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 58.Bakan B., Giraud-Delville C., Pinson L., Richard-Molard D., Fournier E., Brygoo Y.. Appl. Environ. Microbiol. 2002;68(11):5472–5479. doi: 10.1128/AEM.68.11.5472-5479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulik T., Pszczolkowska A., Lojko M.. Int. J. Mol. Sci. 2011;12:5626–5640. doi: 10.3390/ijms12095626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan D., Mionetto A., Calero N., Reynoso M.M., Torres A., Bettucci L.. Gen. Mol. Res. 2016;15(1):15017270. doi: 10.4238/gmr.15017270. [DOI] [PubMed] [Google Scholar]
- 61.Laraba I., Boureghda H., Abdallah N., Bouaicha O., Obanor F., Moretti A., Geiser D.M., Kim H.-S., McCormick S.P., Proctor R.H.. Fungal Genet. Biol. 2017;103:34–41. doi: 10.1016/j.fgb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 62.Yli-Mattila T., Rämö S., Hietaniemi V., Hussien T., Carlobos-Lopez A.L., Cumagun C.J.R.. Microorganisms. 2013;1:162–174. doi: 10.3390/microorganisms1010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surponiene S., Sakalauskas S., Mankeviciene A., Barcauskaite K., Jonaviciene A., Zemdirbyste-Agriculture. 2016;103(3):281–287. [Google Scholar]
- 64.Pasquali M., Beyer M., Logrieco A., Audenaert K., Balmas V., Basler R., Boutigny A.L., Chrpova J., Czembor E., Gagkaeva T.. Front. Microbiol. 2016;7:406. doi: 10.3389/fmicb.2016.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulik T., Busko M., Bilska K., Ostrowska-Kolodziejczak A., van Diepeningen A.D., Perkowski J., Steinglein S.. Toxins. 2016;8(11):330. doi: 10.3390/toxins8110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Covarelli L., Beccari G., Prodi A., Generotti S., Etruschi F., Juan C., Ferrer E., Manes J.. J. Sci. Food Agric. 2014;95:540–551. doi: 10.1002/jsfa.6772. [DOI] [PubMed] [Google Scholar]
- 67.Pasquali M., Giraud F., Brochot C., Cocco E., Hoffmann L., Bohn T.. Int. J. Food Microbiol. 2010;137:246–253. doi: 10.1016/j.ijfoodmicro.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Amarasinghe C.C., Tittlemier S.A., Fernando W.G.D., Plant Pathol. 2014;64(4):988–995. [Google Scholar]


