Abstract
Spinal cord injury (SCI) affects nearly half a million new patients worldwide, with 17,700 in the US each year, and disproportionately impacts young males of reproductive age. Almost every aspect of male reproduction is affected by SCI, resulting in: erectile, endocrine and sexual dysfunction, decreased sperm motility despite an often-normal count, and abnormal semen emission and ejaculation. The aim of this review is to focus on how SCI impacts testicular spermatogenesis, sperm function, semen quality, and overall fecundity while discussing what is not known, and future avenues for research.
Keywords: Spinal cord injury (SCI), male infertility, spermatogenesis
Introduction
The World Health Organization estimates that 250,000 to 500,000 spinal cord injuries (SCI) occur each year worldwide, including an estimated 17,700 new US cases (1,2). The estimated annual incidence of SCI was 54 cases per 1 million US population in 2012, and there are an estimated 276,000 people currently living with SCI (3,4). In young adults (ages 16–30), males are more than 4 times as likely to sustain a SCI as compared to females (5). Almost every aspect of male reproduction is affected by SCI, resulting in: erectile, endocrine and sexual dysfunction, poor spermatogenesis, and abnormal semen emission and ejaculation (6,7). The use of assisted reproductive technologies including vibratory ejaculation, electroejaculation, pharmacologic therapy, and surgical sperm retrieval has vastly improved SCI males’ fertility, and these techniques are adequately discussed in other reviews (6-10). In this review we will briefly describe the pathophysiology of how SCI impacts erection, emission, and ejaculation. The aim of this review is to focus on how SCI impacts spermatogenesis, sperm function, semen quality, and overall fecundity while discussing what is not known, and future avenues for research.
Erection, emission, and ejaculation in SCI
The ability to achieve erection is classically divided into either reflex/tactile or psychogenic, and each is dependent on intact lower motor sacral nerves and spinal cord, respectively (11). A complete injury above T11 usually leads to the inability to achieve psychogenic erection, while sacral nerve root injury deleteriously impacts reflex/tactile erections (12). Men with T11-L2 SCI will often have variable ability to achieve erections by one or both pathways (13). Emission is the complex process of delivering sperm and the plasma proteins/nutrients and ultimately depositing semen in the urethra. This involves afferent signals from penile nerves and sympathetic input from the thoracolumbar (T11-L2) sympathetic nerve roots to induce rhythmic contraction of vas deferens, seminal vesicles, and ejaculatory ducts. Ejaculation in an antegrade fashion for adequate deposit of semen requires a closed bladder neck, and somatically controlled rhythmic contraction of bulbocavernosus and ischiocavernosus muscles with concomitant relaxation of the external sphincter (14). This highly coordinated process of erection, emission, and ejaculation can breakdown in one or multiple steps in the SCI male, and without medication or assisted reproductive technology most often results in infertility. The ability to naturally ejaculate in an antegrade fashion without assistance is dependent on the level and completeness of injury. After complete lower motor neuron (LMN) injury only 18% retain the ability to ejaculate versus 70% with incomplete LMN. With a complete upper motor neuron (UMN) injury, only 11% can ejaculate versus 32% in incomplete UMN (15-17).
Time after injury
Mallidis et al. collected semen samples during the acute phase of spinal shock which were shown to be normal, but after 2 weeks the semen parameters mirrored the poor quality of chronic SCI patients (18). Following this acute phase, the poor semen quality did not change in the long run as a function of time after injury (19). In a dog model of SCI, Ohl et al. demonstrated the onset of significant changes in sperm at 3 weeks after injury, and these changes included: decreased sperm motility (62.9% to 20.1%), and histologically the mean number of spermatids on cross section of the testis decreased compared to controls (13.6 versus 43.9) (20). In contrast, using a rat model of SCI Huang et al. showed that spermatogenesis can improve over time with normal spermatogenesis seen in 30% of rats at three months compared to 47% at 6 months (21).
Seminal plasma
The most consistent finding on SCI patients’ semen analysis is decreased motility and vitality despite frequently normal counts (22,23). There are multiple theories for the abnormal semen motility, which include increased scrotal temperature, leukospermia, antisperm antibodies, and seminal plasma proteins and cytokines. The ejaculate also appears brown or dark colored after SCI (24).
In 1996, Brackett et al. were the first group to show how the seminal plasma in the SCI male impacts motility (25). In this study, seminal plasma from SCI patients was mixed with sperm from normal men, and resulted in impaired sperm motility. Subsequently, they mixed seminal plasma from normal men with sperm from SCI men, resulting in improved motility. A potential reason for these findings includes the fact that seminal plasma in SCI men has a high number of leukocytes, specifically activated T-cells, which can secrete cytotoxic substances and cytokines (IL1-beta, TNFalpha, IL-6) (26,27). The neutralization of these inflammatory substances may improve semen parameters (28). Cohen et al. treated seminal plasma with monoclonal antibodies to these inflammatory cytokines, which resulted in improved sperm motility (29). Brackett et al. showed that in SCI men the motility and viability of aspirated sperm from the vas deferens is significantly improved compared to electroejaculatory samples from the same men, which strongly argues for the deleterious impact of seminal plasma (30). De Silva characterized the seminal plasma proteome, and their work demonstrated that there is prostate gland functional failure in SCI men, representing another possible explanation for the mechanism of decreased sperm motility (31). This group also showed the seminal plasma proteomic signature is impaired in SCI men compared to normal controls. Further, this is independent of whether penile vibratory stimulation or electroejaculation is used to collect semen (32).
Testis biopsy of SCI
In an effort to determine whether there were predictive factors for azoospermia following electroejaculation, Elliott et al. performed 50 testis biopsies in SCI men averaging 8 years following injury. Despite the fact that mature sperm were identified in 43 of 50 testes biopsies, only 28 had normal spermatogenesis, while 15 had hypospermatogenesis, and 7 had maturation arrest (33). Recently, Sánchez-Ramos et al. prospectively studied 28 SCI men at 4 weeks, 3 months, and 6 months following SCI, and at each time interval they evaluated fine needle aspiration biopsy of the testis to histologically characterize spermatogenesis (34). This study demonstrated early recovery of spermatogenesis, where at four weeks post SCI spermatogenesis was normal in only 39% of patient’s testis biopsy. Conversely, this improved to 48% and 80% at 4 and 6 months after injury, respectively. This suggests that spermatogenesis may improve after an initial period of impairment.
Motility: Ca channel ion dependence
Sperm motility is dependent on calcium channels of the sperm (CatSper), located in the sperm flagellum, and they enhance motility through hyperactivation (35-38). These genes are found highly preserved across many mammals including humans, and knockout mice without CatSper 1 and 2 subtypes are infertile because of the decreased motility and hyperactivation (37). In 2008, Rezaian et al. studied the gene expression of CatSper following SCI using a mouse model with 75 mice (25 surgery, 25 sham, 25 control). Animals were sacrificed at the following time periods after T9 SCI was performed: day 1 and weeks 1, 2, 4 and 6. Epididymal aspiration allowed analysis of sperm parameters, and this demonstrated that even as early as 2 weeks post-injury there were significant decreases in motility, morphology, and vitality, and by 4 weeks total count was significantly reduced to almost half. Histology of the SCI testes at weeks 4 and 6 compared to controls demonstrated decreased sperm and significant seminiferous tubule integrity loss. There was significant downregulation of Catsper1 and Catsper2 genes by 4 weeks after injury, thus providing a possible mechanism for the decreased motility seen in the sperm of SCI men (39). These findings also correlate temporally with the other studies discussed above, which found changes in testes/sperm within the first month following injury.
Endocrinopathy following SCI
The literature is mixed regarding the short and long-term hormonal changes seen following SCI in men. A review by Ibrahim et al. graphically represents how studies have examined the associated endocrinopathies seen in spinal cord injured men, and there is little consensus and almost no consistent trend seen regarding changes in testosterone, LH/FSH, or prolactin (6). For instance, four chronic SCI studies showed the prolactin level in SCI men was no different than controls, which is the exact opposite result from the Sanchez-Ramos study which found an elevated and serially increasing prolactin levels at 1, 3, and 6 months following injury (21,34,40-42). There are many promising studies using SCI animal models that have reported neuroprotective effects of exogenous sex steroids, specifically 17β-estradiol (43). This is one avenue of possible early intervention to decrease the deleterious effects during the acute injury phase, however further discussion is beyond the scope of this article.
Testosterone and SCI
There is no published literature that reports the prevalence of testosterone replacement therapy (TRT) in SCI patients. Given the growing body of evidence that men may benefit from TRT, there is a concern that SCI men may be inappropriately prescribed exogenous testosterone. This could potentially lead to medical sterilization a man with a semen profile that is at risk for infertility at baseline. Not all SCI men will have hypogonadism, but the prevalence is estimated to be between 39% and 46% (44,45). There is a higher rate of hypogonadism and an earlier age of onset compared to age matched controls (46). There is also an association between severity of injury (complete SCI) and an increased risk of hypogonadism (44,47). Bauman et al. compared testicular response to human chorionic gonadotropin (hCG) stimulation in SCI men and able bodied men, and found that SCI men had adequate and equal response; thus concluding that the hypogonadal state in SCI is not due to primary testicular failure (48). Naderi et al. also showed that hypogonadism seen in SCI is likely a centrally mediated failure of the hypothalamic-pituitary-testis axis (42). Another study by Sullivan et al. supported this finding by using age matched controls compared to SCI men aged 18–45 years old with chronic SCI (>1 year since injury), and found hypogonadism in 25% versus 6% in SCI versus controls (47). Using this same cohort of men, Sullivan et al. showed that men with low testosterone levels was associated with a higher cardiovascular risk profile (49). There is some evidence that non-hormonal intervention could improve hypogonadism in SCI men. The modifiable risk factors of increased BMI and decreased weekly exercise were associated with hypogonadism in SCI (50). There is an ongoing randomized study through the Veterans Affairs examining the effect of TRT and resistance training versus resistance training alone on body mass index and the metabolic profiles of SCI men (51).
Inflammation and reactive oxygen species
SCI is a highly inflammatory process that affects multiple organs that we do not fully understand, including the testis. Compared to controls, the semen of SCI men have elevated cytokines and caspases. These specifically include apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), caspase-1, Interleukin-1b, and Interleukin-18 (52). This same group later showed that by neutralizing ASC with an antibody they could improve motility in vitro from an average of 13.3% to 23.9% (53). Fortune et al. used a rat SCI model to examine immune cell response in the acute and long-term settings. At 72 hours SCI testes showed increased neutrophil count, and at 1.5 years there was a statistically increased number of T-cells compared to controls (54). This same study performed metabolome analysis, with overall findings of a pro-inflammatory state in the testes starting as early as 24 hours after SCI. They demonstrated increased oxidative stress and decreased testosterone production, and at 72 hours there is already evidence of apoptosis. Compared to controls, white blood cells and reactive oxygen species are higher in SCI men, and there is an inverse correlation between the level of reactive oxygen species and sperm motility (55,56). A known activator of the inflammasome is the pennexin-1 channel, and one study by Ibrahim et al. treated 20 SCI men with probenecid, a common medication for gout that acts as a pennexin-1 inhibitor (57). They found improved motility and rapid linear motility at 4 weeks after initiation of therapy, and the medication was well tolerated.
Fecundity
In a meta-analysis that examined SCI couple’s fertility, which included various sperm retrieval processes, Deforge et al. found a 51% pregnancy rate and 40% live birth rate from 1993 to 2003 (58). With the addition of assisted reproductive technology live birth rates approach 70% (59,60). In the general population, pregnancy rates depend on female age, declining in an almost linear fashion with increasing female age. SCI couple pregnancy rates are still lower than the non-SCI general population with female partners 25–27 years old with estimated 79% pregnancy rates after 12 months (61).
Sexual satisfaction and quality of life
There are a multiple survey-based studies that examine quality of life measures in regards to sexual activity in SCI men, it is estimated that 30–70% of SCI men are sexually inactive (62-64). Factors that positively impacted sexual satisfaction include time since injury and younger age. Negative predictors for sexual satisfaction include ejaculatory dysfunction, chronic pain, and bowel and bladder incontinence (65-67). Gomes et al. found that erectile function was the number one predictor of sexual satisfaction in SCI men (64). Abramson et al. used a systematic review to demonstrate that there was no consensus regarding the sexual function questionnaires used across studies of SCI and sexual function/quality of life (68). Furthermore, another systematic review found that many of the patient related outcome measure instruments that evaluate sexual function in SCI are not methodologically valid (69). There are many limitations to the current literature on sexual satisfaction and quality of life, most importantly is the lack of prospectively collected data and questionnaires that follow patients from time of injury going forward. We lack understanding of how each factor of sexual satisfaction can impact a SCI man’s quality of life. Limitations include the lack of adequate data regarding how medical or surgical interventions impact satisfaction, and how men change with time in regards to function and their perception of their sexual quality of life.
Genetics
There is a paucity of genetic studies of the infertile male with SCI. There are no genome-wide association studies (GWAS), and only a few of the studies discussed in this review examined genetic changes (38,39,54). One recent study by Shi et al. used a rat model with RNA-sequencing to identify genes and pathways of SCI, and found differential expression involving genes related to the immune response, cytokines, and ion channels (70). This is a good first step to identify novel genes and pathways to further evaluate in human studies of SCI. There is an argument against using GWAS studies in spinal cord injuries because it is an isolated event, compared to something like cancer with heritable somatic genes to study. We do not know why there is such a broad spectrum in the presentation and severity of infertility in men with SCI. Could there be somatic genes that are more susceptible to the insults of SCI, or do some men with SCI have genes that promote resilience and DNA repair? We also don’t know how SCI impacts germline DNA.
Epigenetics
The study of epigenetics focuses on changes to the genome other than the primary sequence that can alter gene expression. Two commonly studied epigenetic changes are DNA methylation and histone modification (71). Multiple studies have implicated epigenetic changes that affect spermatogenesis, fertilization, and early embryogenesis (72-75). An example of a recent study by Jenkins et al. identified DNA methylation differences in two groups of non-SCI men with equal semen parameters, but vastly different fecundity (76). Impaired fertility is also seen in men that smoke compared to non-smokers; and a recent study demonstrated broad DNA methylation changes in smokers (77,78). One could argue that SCI is in some ways similar to smoking in that they both are highly inflammatory and increase reactive oxygen species. We need the same type of study comparing DNA methylation patterns in sperm from SCI males compared to controls. To date there have been no published data exploring epigenetic changes in the sperm of SCI men. This is an unexplored area that could shed light on why many SCI couples need to ultimately use assisted reproductive technology.
Need for national database and repository
The SCI Model Systems database is a powerful multi-institutional repository, from 29 medical centers, with over 40 years worthy of patient demographic data paired with psychosocial, medical, employment, and survival outcomes (2). Such a network of institutions that specialize in SCI research and treatment would be a prime source of collaboration with urology and infertility research. Many centers have already set up data and semen repositories, but to date there have been no published multi-institutional studies looking at the impact of SCI on spermatogenesis. The National Cancer Institute’s cancer genome atlas is an example of how multiple institutions and scientists have contributed tissue samples to a central repository, which then shares a common database for cancer research. Many breakthroughs in diagnosis and treatment have been made with this powerful and collaborative tool. SCI research needs to emulate this example so we can more readily collaborate to identify new genes and pathways that lead to impaired erections, ejaculation, spermatogenesis, and ultimately fertility. SCI males without antegrade ejaculation seeking reproductive assistance will likely undergo a procedure (penile vibratory stimulation, electroejaculation, or surgical sperm retrieval) to obtain a semen sample. This is a simple, but potentially missed opportunity, for academic centers to biobank sperm and research samples. Ideally urologists and reproductive endocrinologists would collaborate with physical medicine and rehabilitation physicians to enroll SCI participants interested in optimizing their fertility treatment options.
The foundation for potential research across institutions exists, but we must prioritize and fund SCI research. An example of multi-institutional research in SCI is the Neurogenic Bladder Research Group (www.NBRG.org), which began as collaboration between Universities of Michigan, Minnesota, and Utah. Their initial study, funded by the Patient Centered Outcomes Research Institute (PCORI) aimed to study quality of life outcomes related to bladder management after SCI (79). This group has subsequently grown to 7 institutions and the lessons learned could be applied to the study of infertility in SCI patients. With a multi-institutional collaboration of SCI researchers focused on infertility there would be the infrastructure and relationships set up for future clinic trials. First, we must identify novel targets for therapeutics to explore in future clinical trials. An example of an ongoing multi-institutional clinic trial in non-SCI male infertility is the FAZST trial, which will assess the effects of folic acid and zinc dietary supplementation in males on semen quality and fertility rates among couples.
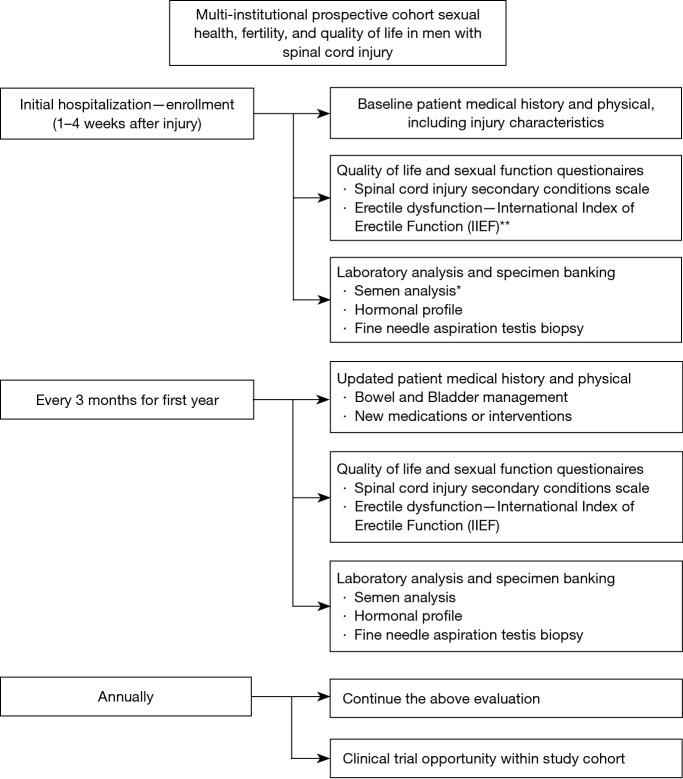
Figure 1 is a proposed study design to assess sexual satisfaction, infertility, hormonal profile, and quality of life in spinal cord injured men. The specific tests and questionnaires would need to be chosen as a group, but the more important features of this study would be consistency between centers, building a biobank of testis tissue, semen, and blood for hormonal studies with an emphasis on studying early SCI men to help identify new mechanisms to target future therapy.
Figure 1.
A proposed multi-Institutional prospective cohort study of fertility in spinal cord injury patients. *, In the first year after injury, semen analysis may be difficult to obtain because the ejaculatory reflex is one of the last reflexes to return, which make methods of semen retrieval inconsistent in the first year after injury; **, the IIEF must be interpreted carefully because, studies attribute improvement in patients whose scores on a given question go from 0 to 1. This is a misconception on questions in which “0” denotes no opportunity for sexual activity. IIEF, International Index of Erectile Function.
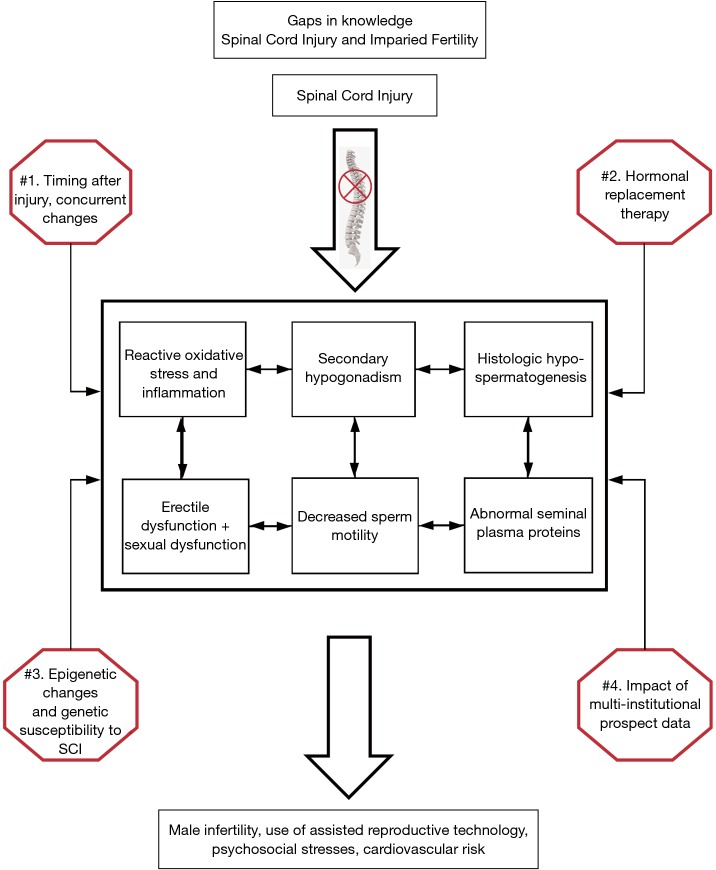
Figure 2 summarizes the known mechanisms discussed in this review that contribute to male infertility in SCI men, and highlights knowledge gaps in the current literature. First, we know that SCI is an enormous stress to the human body with many downstream effects. The six boxes in the middle represent some of the main findings in SCI men that contribute to male infertility. Most studies thus far have only investigated one facet, for example reactive oxygen species. There is little known about if there is a single early injury pathway, or how they are interconnected mechanistically or temporally, represented by the arrows. Second, there is conflicting evidence regarding hormonal changes in SCI men following the injury. We think that a large number of young men have secondary hypogonadism following SCI, which is likely centrally mediated. We do not know the prevalence of testosterone or gonadotropin use in this population. There is a dearth of literature regarding the impact of gonadotropin use on the semen parameters or SCI fertility. Third, there is a growing body of literature supporting epigenetic changes in the sperm of men that leads to impaired fertility. In SCI men we see histologic changes with decreased spermatogenesis as well as an immune response with leukocytes and inflammation, which may be a gross reflection of epigenetic insults to the spermatogonial stem cells. Epigenetic changes in SCI sperm have yet to be studied. Lastly, we have discussed how almost all studies regarding the mechanism of infertility in SCI are from single institutions, with small study sizes, and rarely collect data in a prospective manner. There are emerging study groups in North America focused on the urologic needs of SCI patients that would be a potential avenue to expand into a multi-institutional group to study infertility in SCI.
Figure 2.
Gaps in knowledge in spinal cord injury and impaired fertility research.
Conclusions
SCI is an incredibly destructive event that sets off a cascade of events in the human body that affect a man’s fertility long after the acute injury. Multiple groups are investigating this from many different angles, from proteomics to genetics. There are dog, mouse, and rat models for SCI and we also highlighted a few small prospectively collected human studies. A common theme in this body of work is trying to explain the mechanism behind the impaired motility and overall decreased fertility despite the often-normal sperm counts. There are expression changes in the calcium ion channel proteins responsible for hyperactivation of sperm flagellae, while reactive oxygen species increase and cytokines are released by white blood cells, which makes the environment within the testis hostile to spermatogenesis. Seminal plasma proteins in SCI are also implicated in the decreased motility and overall fertility. Future research efforts in the field would benefit from multi-institutional collaboration with sharing ideas, biobanked specimens, and patients in clinical trials. Male factor infertility research is rapidly changing and the same advancing genetic/epigenetic techniques need to be applied to the large population of SCI men seeking fertility assistance.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.World Health Organization. Spinal Cord Injury. 2013. Available online: http://www.who.int/mediacentre/factsheets/fs384/en/
- 2.Database TNSCIMSS. The National Spinal Cord Injury Model Systems (SCIMS) Database Data Sheet. 2017. Available online: https://www.nscisc.uab.edu/Public_Pages/Database_files/What_Is_The_National_SCIMS_Database.pdf
- 3.Jain NB, Ayers GD, Peterson EN, et al. Traumatic Spinal Cord Injury in the United States, 1993–2012. JAMA 2015;313:2236-43. 10.1001/jama.2015.6250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Spinal Cord Injury Statistical Center. Spinal Cord Injury (SCI) Facts and Figures at a Glance. 2014. Available online: https://www.nscisc.uab.edu/PublicDocuments/fact_figures_docs/Facts%202014.pdf
- 5.Chamberlain JD, Deriaz O, Hund-Georgiadis M, et al. Epidemiology and contemporary risk profile of traumatic spinal cord injury in Switzerland. Inj Epidemiol 2015;2:28. 10.1186/s40621-015-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibrahim E, Lynne CM, Brackett NL. Male fertility following spinal cord injury: an update. Andrology 2016;4:13-26. 10.1111/andr.12119 [DOI] [PubMed] [Google Scholar]
- 7.Trofimenko V, Hotaling JM. Fertility treatment in spinal cord injury and other neurologic disease. Transl Androl Urol 2016;5:102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fode M, Ohl DA, Sønksen J. A step-wise approach to sperm retrieval in men with neurogenic anejaculation. Nat Rev Urol 2015;12:607. 10.1038/nrurol.2015.241 [DOI] [PubMed] [Google Scholar]
- 9.Brackett NL, Lynne CM, Ibrahim E, et al. Treatment of infertility in men with spinal cord injury. Nat Rev Urol 2010;7:162. 10.1038/nrurol.2010.7 [DOI] [PubMed] [Google Scholar]
- 10.Courtois F, Charvier K. Sexual dysfunction in patients with spinal cord lesions. Handb Clin Neurol 2015;130:225-45. 10.1016/B978-0-444-63247-0.00013-4 [DOI] [PubMed] [Google Scholar]
- 11.Giuliano F, Clement P. Neuroanatomy and physiology of ejaculation. Annu Rev Sex Res 2005;16:190-216. [PubMed] [Google Scholar]
- 12.Courtois FJ, Goulet MC, Charvier KF, et al. Posttraumatic erectile potential of spinal cord injured men: How physiologic recordings supplement subjective reports. Arch Phys Med Rehabil 1999;80:1268-72. 10.1016/S0003-9993(99)90028-X [DOI] [PubMed] [Google Scholar]
- 13.Dean RC, Lue TF. Physiology of Penile Erection and Pathophysiology of Erectile Dysfunction. Urol Clin North Am 2005;32:379-95, v. 10.1016/j.ucl.2005.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clement P, Giuliano F. Physiology and Pharmacology of Ejaculation. Basic Clin Pharmacol Toxicol 2016;119:18-25. 10.1111/bcpt.12546 [DOI] [PubMed] [Google Scholar]
- 15.Brackett NL, Ibrahim E, Iremashvili V, et al. Treatment for Ejaculatory Dysfunction in Men With Spinal Cord Injury: An 18-Year Single Center Experience. J Urol 2010;183:2304-8. 10.1016/j.juro.2010.02.018 [DOI] [PubMed] [Google Scholar]
- 16.Chéhensse C, Bahrami S, Denys P, et al. The spinal control of ejaculation revisited: a systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum Reprod Update 2013;19:507-26. 10.1093/humupd/dmt029 [DOI] [PubMed] [Google Scholar]
- 17.Ohl DA, Bennett CJ, McCabe M, et al. Predictors of Success in Electroejaculation of Spinal Cord Injured Men. J Urol 1989;142:1483-6. 10.1016/S0022-5347(17)39132-2 [DOI] [PubMed] [Google Scholar]
- 18.Mallidis C, Baker HWG, Johnston WIH, et al. Collection of semen from men in acute phase of spinal cord injury. Lancet 1994;343:1072-3. 10.1016/S0140-6736(94)90183-X [DOI] [PubMed] [Google Scholar]
- 19.Brackett NL, Ferrell SM, Aballa TC, et al. Semen quality in spinal cord injured men: Does it progressively decline postinjury? Arch Phys Med Rehabil 1998;79:625-8. 10.1016/S0003-9993(98)90034-X [DOI] [PubMed] [Google Scholar]
- 20.Ohl DA, SØNksen J, Wedemeyer G, et al. Canine model of infertility after spinal cord injury: time course of acute changes in semen quality and spermatogenesis. J Urol 2001;166:1181-4. 10.1016/S0022-5347(05)65942-3 [DOI] [PubMed] [Google Scholar]
- 21.Huang HFS, Linsenmeyer TA, Anesetti R, et al. Suppression and Recovery of Spermatogenesis Following Spinal Cord Injury in the Rat. J Androl 1998;19:72-80. [PubMed] [Google Scholar]
- 22.Utida C, Truzzi JC, Bruschini H, et al. Male infertility in spinal cord trauma. Int Braz J Urol 2005;31:375-83. 10.1590/S1677-55382005000400013 [DOI] [PubMed] [Google Scholar]
- 23.Momen MN, Fahmy I, Amer M, et al. Semen parameters in men with spinal cord injury: changes and aetiology. Asian J Androl 2007;9:684-9. 10.1111/j.1745-7262.2007.00277.x [DOI] [PubMed] [Google Scholar]
- 24.Wieder JA, Lynne CM, Ferrell SM, et al. Brown-Colored Semen in Men With Spinal Cord Injury. J Androl 1999;20:594-600. [PubMed] [Google Scholar]
- 25.Brackett NL, Davi RC, Padron OF, et al. Seminal Plasma of Spinal Cord Injured Men Inhibits Sperm Motility of Normal Men. J Urol 1996;155:1632-5. 10.1016/S0022-5347(01)66148-2 [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Aballa TC, Ferrell SM, et al. Inflammatory Cytokine Concentrations Are Elevated in Seminal Plasma of Men With Spinal Cord Injuries. J Androl 2004;25:250-4. 10.1002/j.1939-4640.2004.tb02785.x [DOI] [PubMed] [Google Scholar]
- 27.Trabulsi EJ, Shupp-Byrne D, Sedor J, et al. Leukocyte subtypes in electroejaculates of spinal cord injured men. Arch Phys Med Rehabil 2002;83:31-4. 10.1053/apmr.2002.26250 [DOI] [PubMed] [Google Scholar]
- 28.Brackett NL, Cohen DR, Ibrahim E, et al. Neutralization of Cytokine Activity at the Receptor Level Improves Sperm Motility in Men With Spinal Cord Injuries. J Androl 2007;28:717-21. 10.2164/jandrol.106.002022 [DOI] [PubMed] [Google Scholar]
- 29.Cohen DR, Basu S, Randall JM, et al. Sperm Motility in Men With Spinal Cord Injuries Is Enhanced by Inactivating Cytokines in the Seminal Plasma. J Androl 2004;25:922-5. 10.1002/j.1939-4640.2004.tb03162.x [DOI] [PubMed] [Google Scholar]
- 30.Brackett NL, Lynne CM, Aballa TC, et al. Sperm motility from the vas deferens of spinal cord injured men is higher than from the ejaculate. J Urol 2000;164:712-5. 10.1016/S0022-5347(05)67287-4 [DOI] [PubMed] [Google Scholar]
- 31.da Silva BF, Meng C, Helm D, et al. Towards Understanding Male Infertility After Spinal Cord Injury Using Quantitative Proteomics. Mol Cell Proteomics 2016;15:1424-34. 10.1074/mcp.M115.052175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva BF, Souza GHMF, Turco EGl, et al. Differential seminal plasma proteome according to semen retrieval in men with spinal cord injury. Fertil Steril 2013;100:959-69.e3. 10.1016/j.fertnstert.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Elliott SP, Orejuela F, Hirsch IH, et al. Testis biopsy findings in the spinal cord injured patient. J Urol 2000;163:792-5. 10.1016/S0022-5347(05)67806-8 [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Ramos A, Vargas-Baquero E, Martin-de Francisco FJ, et al. Early spermatogenesis changes in traumatic complete spinal cord-injured adult patients. Spinal Cord 2017;55:570. 10.1038/sc.2016.184 [DOI] [PubMed] [Google Scholar]
- 35.Quill TA, Sugden SA, Rossi KL, et al. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci U S A 2003;100:14869-74. 10.1073/pnas.2136654100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren D, Navarro B, Perez G, et al. A sperm ion channel required for sperm motility and male fertility. Nature 2001;413:603. 10.1038/35098027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobley A, Pierron V, Reynolds L, et al. Identification of human and mouse CatSper3 and CatSper4 genes: Characterisation of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol 2003;1:53. 10.1186/1477-7827-1-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh AP, Rajender S. CatSper channel, sperm function and male fertility. Reprod Biomed Online 2015;30:28-38. 10.1016/j.rbmo.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 39.Rezaian J, Movahedin M, Mowla SJ. CatSper genes expression, semen characteristics and histology of testes in the contusive spinal cord-injured mice model. Spinal Cord 2009;47:76. 10.1038/sc.2008.81 [DOI] [PubMed] [Google Scholar]
- 40.Huang HFS, Linsenmeyer TA, Li MT, et al. Acute Effects of Spinal Cord Injury on the Pituitary-Testicular Hormone Axis and Sertoli Cell Functions: A Time Course Study. J Androl 1995;16:148-57. [PubMed] [Google Scholar]
- 41.Brackett NL, Lynne CM, Weizman MS, et al. Endocrine Profiles and Semen Quality of Spinal Cord Injured Men. J Urol 1994;151:114-9. 10.1016/S0022-5347(17)34885-1 [DOI] [PubMed] [Google Scholar]
- 42.Naderi AR, Safarinejad MR. Endocrine profiles and semen quality in spinal cord injured men. Clin Endocrinol (Oxf) 2003;58:177-84. 10.1046/j.1365-2265.2003.01693.x [DOI] [PubMed] [Google Scholar]
- 43.Elkabes S, Nicot AB. Sex steroids and neuroprotection in spinal cord injury: A review of preclinical investigations. Exp Neurol 2014;259:28-37. 10.1016/j.expneurol.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 44.Durga A, Sepahpanah F, Regozzi M, et al. Prevalence of Testosterone Deficiency After Spinal Cord Injury. PM R 2011;3:929-32. 10.1016/j.pmrj.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 45.Sinha V, Elliott S, Ibrahim E, et al. Reproductive Health of Men with Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2017;23:31-41. 10.1310/sci2301-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bauman WA, La Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med 2014;37:32-9. 10.1179/2045772313Y.0000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sullivan SD, Nash MS, Tefera E, et al. Prevalence and Etiology of Hypogonadism in Young Men With Chronic Spinal Cord Injury: A Cross-Sectional Analysis From Two University-Based Rehabilitation Centers. PM R 2017;9:751-60. 10.1016/j.pmrj.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauman WA, La Fountaine MF, Cirnigliaro CM, et al. Testicular Responses to hCG Stimulation at Varying Doses in Men with Spinal Cord Injury. Spinal Cord 2017;55:659-63. 10.1038/sc.2017.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan SD, Nash MS, Tefara E, et al. Relationship Between Gonadal Function and Cardiometabolic Risk in Young Men With Chronic Spinal Cord Injury. PM R 2018;10:373-81. 10.1016/j.pmrj.2017.08.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barbonetti A, Vassallo MRC, Pacca F, et al. Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2014;2:721-8. 10.1111/j.2047-2927.2014.00235.x [DOI] [PubMed] [Google Scholar]
- 51.Gorgey AS, Khalil RE, Gill R, et al. Effects of Testosterone and Evoked Resistance Exercise after Spinal Cord Injury (TEREX-SCI): study protocol for a randomised controlled trial. BMJ Open 2017;7:e014125. 10.1136/bmjopen-2016-014125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Ibrahim E, de Rivero Vaccari JP, et al. Involvement of the inflammasome in abnormal semen quality of men with spinal cord injury. Fertil Steril 2013;99:118-24.e2. 10.1016/j.fertnstert.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 53.Ibrahim E, Castle SM, Aballa TC, et al. Neutralization of ASC improves sperm motility in men with spinal cord injury. Hum Reprod 2014;29:2368-73. 10.1093/humrep/deu230 [DOI] [PubMed] [Google Scholar]
- 54.Fortune RD, Grill RJ, Beeton C, et al. Changes in Gene Expression and Metabolism in the Testes of the Rat following Spinal Cord Injury. J Neurotrauma 2017;34:1175-86. 10.1089/neu.2016.4641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Padron OF, Brackett NL, Sharma RK, et al. Seminal reactive oxygen species and sperm motility and morphology in men with spinal cord injury. Fertil Steril 1997;67:1115-20. 10.1016/S0015-0282(97)81448-3 [DOI] [PubMed] [Google Scholar]
- 56.de Lamirande E, Leduc BE, Iwasaki A, et al. Increased reactive oxygen species formation in semen of patients with spinal cord injury**Supported by a grant from the Medical Research Council of Canada, Ottawa, Ontario, Canada to C.G.††Presented in part at the 1992 Annual Meeting of the American Urological Association, Washington, D.C., May 10 to 14, 1992. Fertil Steril 1995;63:637-42. 10.1016/S0015-0282(16)57438-X [DOI] [PubMed] [Google Scholar]
- 57.Ibrahim E, Aballa TC, Lynne CM, et al. Oral probenecid improves sperm motility in men with spinal cord injury. J Spinal Cord Med 2017:1-4. 10.1080/10790268.2017.1320875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeForge D, Blackmer J, Garritty C, et al. Fertility following spinal cord injury: a systematic review. Spinal Cord 2005;43:693-703. 10.1038/sj.sc.3101769 [DOI] [PubMed] [Google Scholar]
- 59.Nehra A, Werner MA, Bastuba M, et al. Vibratory Stimulation and Rectal Probe Electroejaculation as Therapy for Patients with Spinal Cord Injury: Semen Parameters and Pregnancy Rates. J Urol 1996;155:554-9. 10.1016/S0022-5347(01)66448-6 [DOI] [PubMed] [Google Scholar]
- 60.Kathiresan ASQ, Ibrahim E, Aballa TC, et al. Comparison of in vitro fertilization/intracytoplasmic sperm injection outcomes in male factor infertility patients with and without spinal cord injuries. Fertil Steril 2011;96:562-6. 10.1016/j.fertnstert.2011.06.078 [DOI] [PubMed] [Google Scholar]
- 61.Wesselink AK, Rothman KJ, Hatch EE, et al. Age and fecundability in a North American preconception cohort study. Am J Obstet Gynecol 2017;217:667.e1-.e8. [DOI] [PMC free article] [PubMed]
- 62.Ku JH, Oh SJ, Jeon HG, et al. Sexual activity in Korean male patients on clean intermittent catheterization with neurogenic bladder due to spinal cord injury. Int J Urol 2006;13:42-6. 10.1111/j.1442-2042.2006.01226.x [DOI] [PubMed] [Google Scholar]
- 63.Anderson KD, Borisoff JF, Johnson RD, et al. The impact of spinal cord injury on sexual function: concerns of the general population. Spinal Cord 2007;45:328. 10.1038/sj.sc.3101977 [DOI] [PubMed] [Google Scholar]
- 64.Gomes CM, Miranda EP, de Bessa J, et al. Erectile Function Predicts Sexual Satisfaction in Men With Spinal Cord Injury. Sex Med 2017;5:e148-e55. 10.1016/j.esxm.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valtonen K, Karlsson AK, Siösteen A, et al. Satisfaction with sexual life among persons with traumatic spinal cord injury and meningomyelocele. Disabil Rehabil 2006;28:965-76. 10.1080/09638280500404362 [DOI] [PubMed] [Google Scholar]
- 66.Fisher TL, Laud PW, Byfield MG, et al. Sexual health after spinal cord injury: A longitudinal study. Arch Phys Med Rehabil 2002;83:1043-51. 10.1053/apmr.2002.33654 [DOI] [PubMed] [Google Scholar]
- 67.Cobo Cuenca AI, Sampietro‐ampipo A, Virseda‐irseda A, Sampietroetro after spinal cord injury: A longitudinal study. study. longitudinal study. yelocgoJ Sex Med 2015;12:436-44. 10.1111/jsm.12741 [DOI] [Google Scholar]
- 68.Abramson CE, McBride KE, Konnyu KJ, et al. Sexual health outcome measures for individuals with a spinal cord injury: a systematic review. Spinal Cord 2008;46:320. 10.1038/sj.sc.3102136 [DOI] [PubMed] [Google Scholar]
- 69.‘t Hoen LA, Groen J, Scheepe JR, et al. A Quality Assessment of Patient-Reported Outcome Measures for Sexual Function in Neurologic Patients Using the Consensus-based Standards for the Selection of Health Measurement Instruments Checklist: A Systematic Review. Eur Urol Focus 2017;3:444-56. 10.1016/j.euf.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 70.Shi LL, Zhang N, Xie XM, et al. Transcriptome profile of rat genes in injured spinal cord at different stages by RNA-sequencing. BMC Genomics 2017;18:173. 10.1186/s12864-017-3532-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrell DT, Aston KI. The search for SNPs, CNVs, and epigenetic variants associated with the complex disease of male infertility. Syst Biol Reprod Med 2011;57:17-26. 10.3109/19396368.2010.521615 [DOI] [PubMed] [Google Scholar]
- 72.Brykczynska U, Hisano M, Erkek S, et al. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat Struct Mol Biol 2010;17:679-87. 10.1038/nsmb.1821 [DOI] [PubMed] [Google Scholar]
- 73.Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010;139:287-301. 10.1530/REP-09-0281 [DOI] [PubMed] [Google Scholar]
- 74.Aston KI, Punj V, Liu L, et al. Genome-wide sperm deoxyribonucleic acid methylation is altered in some men with abnormal chromatin packaging or poor in vitro fertilization embryogenesis. Fertil Steril 2012;97:285-92 e4. [DOI] [PubMed]
- 75.Nanassy L, Carrell DT. Abnormal methylation of the promoter of CREM is broadly associated with male factor infertility and poor sperm quality but is improved in sperm selected by density gradient centrifugation. Fertil Steril 2011;95:2310-4. 10.1016/j.fertnstert.2011.03.096 [DOI] [PubMed] [Google Scholar]
- 76.Jenkins TG, Aston KI, Meyer TD, et al. Decreased fecundity and sperm DNA methylation patterns. Fertil Steril 2016;105:51-7.e3. 10.1016/j.fertnstert.2015.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jenkins TG, James ER, Alonso DF, et al. Cigarette smoking significantly alters sperm DNA methylation patterns. Andrology 2017;5:1089-99. 10.1111/andr.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiziler AR, Aydemir B, Onaran I, et al. High levels of cadmium and lead in seminal fluid and blood of smoking men are associated with high oxidative stress and damage in infertile subjects. Biol Trace Elem Res 2007;120:82-91. 10.1007/s12011-007-8020-8 [DOI] [PubMed] [Google Scholar]
- 79.Patel DP, Lenherr SM, Stoffel JT, et al. Study protocol: patient reported outcomes for bladder management strategies in spinal cord injury. BMC Urology 2017;17:95. 10.1186/s12894-017-0286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]