Abstract
Background
Neoadjuvant conventionally fractionated radiotherapy (CFRT) versus hypofractionated radiotherapy (HFRT) for rectal cancer (RC) is among the most controversial and debatable areas of radiotherapeutic management. This is the only known study evaluating the utilization of neoadjuvant HFRT for RC in the United States, and focuses on trends and health disparities.
Methods
The National Cancer Data Base was queried [2004–2015] for newly-diagnosed cT3–T4 Nany or cTany N1–2 M0 rectal adenocarcinoma undergoing neoadjuvant RT, with or without chemotherapy, followed by resection. Following analysis based on temporal trends, multivariate logistic regression determined factors associated with receipt of HFRT.
Results
Altogether, 29,994 patients met study criteria: 29,724 (99%) were treated with CFRT, and 270 (1%) with HFRT. Temporally, utilization of HFRT rose significantly, from 0.2% in 2004 to 2.0% in 2015, with the steepest slope at most recent time periods. HFRT was more likely administered to older patients, those with more comorbidities, and node-positive disease (P<0.05 for all). There were racial differences, as African-Americans were independently less likely to receive HFRT (P=0.043). The two strongest predictors of HFRT administration (by odds ratio) were time period and therapy at academic centers (P<0.05 for all).
Conclusions
Although HFRT is underutilized in the US, its use is rising and has increased nearly tenfold over the last decade. Disparities in HFRT delivery are emphasized, especially concerning disease-/patient-specific factors, socioeconomic status, and race. These data may serve as a benchmark for future investigation as well as for health disparities in the radiotherapeutic treatment of RC.
Keywords: Rectal cancer (RC), radiotherapy, short course, hypofractionation, conventional fractionation, long course
Introduction
Although the management of locally advanced rectal cancer (RC) continues to evolve, the current standard of care is neoadjuvant chemoradiotherapy (nCRT) followed by total mesorectal excision (TME) (1). Neoadjuvant radiotherapy (nRT) to the pelvis is primarily delivered using a hypofractionated approach (HFRT, most commonly 25 Gy in 5 Gy fractions) or with conventional fractionation (CFRT, 45–54 Gy in 1.8–2.0 Gy fractions). The choice of radiation therapy fractionation is highly clinician-dependent and among the most controversial realms of radiotherapeutic management.
Factors supporting HFRT include the lower costs, increased patient convenience, and the numerous phase III trials that have utilized this regimen (2). Moreover, phase III trials comparing HFRT to CFRT have shown no differences in local or distant relapses, overall survival, sphincter preservation, and late toxicities, with fewer acute toxicities (in part owing to the lack of concurrent chemotherapy) (3-5). On the other hand, more randomized trials have utilized CFRT, which could also allow the safer delivery of concurrent chemotherapy; this may in turn be associated with an increased pathologic complete response (pCR) rate (6). It has also been postulated that the aforementioned phase III comparisons of HFRT and CFRT were underpowered to show many of the aforementioned endpoints (7).
Amidst these highly debatable issues, to date there has been no study evaluating the utilization of neoadjuvant HFRT for RC in the United States. Given that both HFRT and CFRT are appropriate options per the National Comprehensive Cancer Network (1) and the American Society for Radiation Oncology (8), this novel study of the National Cancer Data Base [NCDB, estimated to capture 70% of US cancer diagnoses (9)], highlights trends and disparities associated with delivery of HFRT versus CFRT in the United States.
Methods
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society and consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population (9-12). The NCDB contains information not included in the Surveillance, Epidemiology, and End Results database, including details regarding use of systemic therapy. The data used in the study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
The most recently released NCDB dataset corresponded to the years 2004–2015. Inclusion criteria for this study involved patients age ≥18 with newly-diagnosed cT3–T4 Nany or cTany N1–2 M0 RC comprising histologic codes of adenocarcinoma [International Classification of Disease for Oncology (ICD-O-3) codes 8140, 8141, 8143, 8144, 8145, 8147, 8255, 8260, 8310, 8340, 8480, 8481]. For inclusion, patients required histological diagnostic confirmation and neoadjuvant radiation therapy followed by definitive surgery. The use of neoadjuvant chemotherapy was not required for inclusion, because this is largely not utilized with neoadjuvant HFRT (2), and because the purpose of the study was to specifically evaluate nRT regimens. HFRT was defined as a dose of 25 Gy in 5 fractions, and CFRT of 45–50.4 Gy in 25–28 fractions (1). Using a classification scheme from other published studies utilizing the NCDB, an academic facility was an institution with both an accession of more than 500 newly diagnosed cancer cases per year and one that provided postgraduate medical education in at least four program areas, including internal medicine and general surgery (13). All other facilities, including Comprehensive Community Cancer Programs, Community Cancer Programs, and Integrated Network Programs, were categorized as non-academic, as none of these institutions require graduate medical education.
Information collected on each patient broadly included demographic data, comorbidity information, clinicopathologic tumor parameters, and treatment facility characteristics. All statistical tests were two-sided, with a threshold of <0.05 for statistical significance, and were performed using STATA (version 14, College Station, TX, USA). Fisher’s exact or χ2 test analyzed categorical proportions between groups in the non-parametric and parametric settings, respectively. Because the primary goal herein was to evaluate temporal trends and predictors of HFRT use, multivariable logistic regression modeling was utilized to determine characteristics that were predictive for receipt of HFRT.
Results
A complete flow diagram of patient selection is given in Figure 1. In total, 29,994 patients met study criteria (Table 1). Of these, 270 (1%) were treated with HFRT and 29,724 (99%) with CFRT. It is noteworthy that the pCR rate was higher with CFRT (10.4% vs. 5.2%, P<0.001), but the rate of positive margins was similar between groups (5.3% vs. 5.9%, P=0.104) (Table 1).
Figure 1.
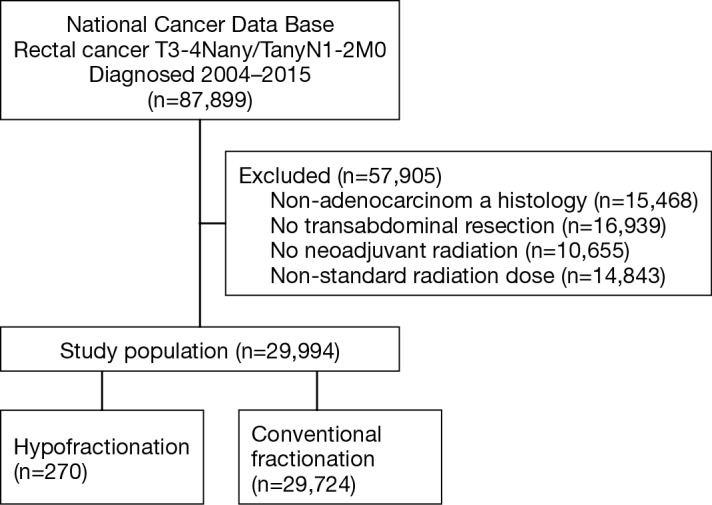
Patient selection diagram.
Table 1. Demographic characteristics.
| Characteristic | Conventional fractionation n=29,724 (%) | Hypofractionation n=270 (%) | P value |
|---|---|---|---|
| Age (years) | <0.001 | ||
| <65 | 18,974 (63.8) | 116 (43.0) | |
| 65–74 | 7,001 (23.6) | 67 (24.8) | |
| 75+ | 3,749 (12.6) | 87 (32.2) | |
| Sex | 0.472 | ||
| Male | 18,577 (62.5) | 163 (60.4) | |
| Female | 11,147 (37.5) | 107 (39.6) | |
| Race | 0.180 | ||
| White | 25,588 (86.1) | 243 (90.0) | |
| African American | 2,426 (8.2) | 16 (5.9) | |
| Other/not recorded | 1,710 (5.8) | 11 (4.1) | |
| T stage | 0.679 | ||
| T1 | 219 (0.7) | 1 (0.4) | |
| T2 | 1,405 (4.7) | 16 (5.9) | |
| T3 | 26,006 (87.5) | 236 (87.4) | |
| T4 | 2,094 (7.0) | 17 (6.3) | |
| N stage | 0.002 | ||
| N0 | 13,578 (45.7) | 149 (55.2) | |
| N+ | 16,146 (54.3) | 121 (44.8) | |
| Charlson Deyo score | <0.001 | ||
| 0 | 23,712 (79.8) | 190 (70.4) | |
| 1 | 4,843 (16.3) | 61 (22.6) | |
| 2 | 885 (3.0) | 11 (4.1) | |
| ≥3 | 284 (1.0) | 8 (3.0) | |
| Surgical margins | 0.104 | ||
| Negative | 27,464 (92.4) | 253 (93.7) | |
| Positive | 1,584 (5.3) | 16 (5.9) | |
| Not recorded | 676 (2.3) | 1 (0.4) | |
| Pathologic complete response | <0.001 | ||
| No | 20,788 (69.9) | 244 (90.4) | |
| Yes | 3,085 (10.4) | 14 (5.2) | |
| Not recorded | 5,851 (19.7) | 12 (4.4) | |
| Facility type | <0.001 | ||
| Non academic | 18,562 (62.5) | 911 (33.7) | |
| Academic | 9,775 (32.9) | 172 (63.7) | |
| Not recorded | 1,387 (4.7) | 7 (2.6) | |
| Income | 0.080 | ||
| ≤ $62,999 | 20,145 (67.8) | 200 (74.1) | |
| $63,000+ | 9,383 (31.6) | 68 (25.2) | |
| Not recorded | 196 (0.7) | 2 (0.7) | |
| Year of diagnosis | <0.001 | ||
| 2004–2009 | 10,597 (35.7) | 37 (13.7) | |
| 2010–2015 | 19,127 (64.4) | 233 (86.3) | |
| Payer status | <0.001 | ||
| Medicaid | 1,980 (6.7) | 27 (10.0) | |
| Medicare | 10,198 (34.3) | 147 (54.4) | |
| Private | 15,386 (51.8) | 80 (29.6) | |
| Uninsured | 1,395 (4.7) | 7 (2.6) | |
| Other | 765 (2.6) | 9 (3.3) | |
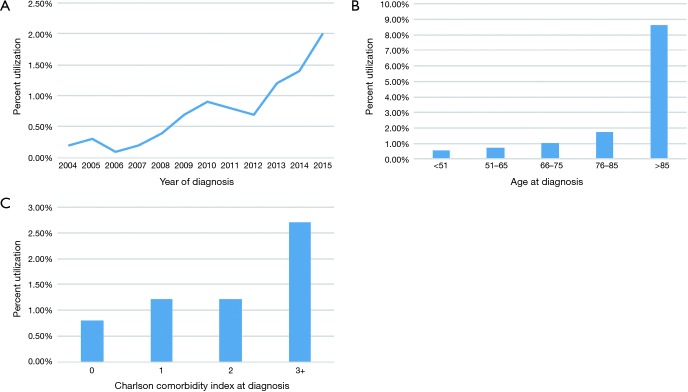
Despite the clear imbalances in sample size between groups, analysis of temporal trends revealed a steady rise in HFRT from 0.2% in 2004 to 2.0% in 2015 (Figure 2A). The slope seemed to be the steepest at most recent time periods.
Figure 2.
Temporal trends in HFRT utilization (A), distribution of HFRT by age (B), distribution of HFRT by comorbidity index (C).
Numerically, HFRT seemed to be administered more to older patients and those with increasing comorbidities (Figure 2B,C). This was corroborated by multivariable logistic regression analysis (Table 2). Node-positive disease was also independently associated with less HFRT delivery (P<0.001). There were also racial differences, as African-Americans were independently less likely to receive HFRT (P=0.043). Socioeconomic differences also existed, as patients with higher incomes (P=0.019) and private insurance (P=0.001) were less likely to undergo HFRT. The two strongest predictors of HFRT administration (by odds ratio) were time period and therapy at academic centers (P<0.05 for all).
Table 2. Multivariable logistic regression for factors predictive of hypofractionated radiation therapy.
| Characteristic | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Age (years) | |||
| <65 | 1 (reference) | ||
| 65–74 | 1.195 | 0.785–1.820 | 0.406 |
| 75+ | 2.97 | 1.944–4.538 | <0.001 |
| Sex | |||
| Male | 1 (reference) | ||
| Female | 1.089 | 0.849–1.397 | 0.500 |
| Race | |||
| White | 1 (reference) | ||
| African American | 0.586 | 0.349–0.983 | 0.043 |
| Other/not recorded | 0.609 | 0.330–1.126 | 0.114 |
| T stage | |||
| T1 | 1 (reference) | ||
| T2 | 2.165 | 0.283–16.574 | 0.457 |
| T3 | 1.283 | 0.177–9.324 | 0.806 |
| T4 | 1.003 | 0.131–7.691 | 0.998 |
| N stage | |||
| N0 | 1 (reference) | ||
| N+ | 0.601 | −0.465–0.779 | <0.001 |
| Charlson Deyo score | |||
| 0 | 1 (reference) | ||
| 1 | 1.314 | 0.977–1.767 | 0.071 |
| 2 | 1.028 | 0.552–1.917 | 0.930 |
| ≥3 | 2.260 | 1.081–4.728 | 0.030 |
| Facility type | |||
| Non academic | 1 (reference) | ||
| Academic | 4.155 | 3.203–5.390 | <0.001 |
| Not recorded | 1.968 | 0.892–4.344 | 0.094 |
| Income | |||
| ≤ $62,999 | 1 (reference) | ||
| $63,000+ | 0.712 | 0.536–0.945 | 0.019 |
| Not recorded | 1.752 | 0.417–7.352 | 0.444 |
| Year of diagnosis | |||
| 2004 | 1 (reference) | ||
| 2005 | 1.247 | 0.278–5.602 | 0.773 |
| 2006 | 0.570 | 0.095–3.428 | 0.540 |
| 2007 | 0.784 | 0.157–3.901 | 0.766 |
| 2008 | 2.073 | 0.558–7.697 | 0.276 |
| 2009 | 3.261 | 0.945–11.253 | 0.061 |
| 2010 | 4.352 | 1.306–14.500 | 0.017 |
| 2011 | 3.901 | 1.161–13.109 | 0.028 |
| 2012 | 3.300 | 0.979–11.126 | 0.054 |
| 2013 | 6.008 | 1.848–19.537 | 0.003 |
| 2014 | 7.278 | 2.260–23.437 | 0.001 |
| 2015 | 10.171 | 3.184–32.486 | <0.001 |
| Payer status | |||
| Medicaid | 1 (reference) | ||
| Medicare | 0.802 | 0.475–1.355 | 0.410 |
| Private | 0.474 | 0.302–0.744 | 0.001 |
| Uninsured | 0.451 | 0.195–1.044 | 0.063 |
| Other | 0.740 | 0.341–1.609 | 0.448 |
Discussion
This is the only known study evaluating the utilization of neoadjuvant HFRT for RC in the United States. Although HFRT is highly underutilized in the US, its use is rising and has increased nearly tenfold over the last decade. Disparities in HFRT delivery are emphasized, namely that HFRT was more likely given at academic institutions and to older patients with more comorbidities. HFRT was less likely in patients with node-positive disease, higher income, private insurance, and amongst African-Americans. These data may serve as a benchmark for future investigation following the implementation of bundled payments as well as for health disparities in the radiotherapeutic treatment of RC.
There are several reflections meriting further discussion. First, although it is intuitive that higher-risk, node-positive disease is more often treated with CFRT, it was curious that higher T stage disease did not independently associate with a decreased likelihood of HFRT delivery. This could be owing to low numbers, or because these concerns have been partially attenuated in the TME era. Indeed, it is noteworthy that both groups displayed similar rates of positive margins, which is important to establish (Table 1). It is also interesting and noteworthy that older patients with more comorbidities were more likely to receive HFRT, because if one presumes that these patients are more likely to have CRT-related toxicities and/or surgical complications that may be impacted by neoadjuvant therapy, there is in fact less reason to deliver HFRT. Rather, these results may reflect the understanding that acute toxicities are reduced with HFRT and/or that HFRT is less likely delivered with concurrent chemotherapy. Additionally, the increased utilization of HFRT by older, sicker patients may be due to its rapid course, and the perception that these patients may be more likely to complete a shorter course of neoadjuvant treatment when compared to the 5-week long CFRT regimens. Although the effect of post-nRT delay (time to surgery) was not evaluated in this study because it was uncertain prior to the recent findings of the Stockholm III trial, the data further serve the point that HFRT with appropriate delay decreases postoperative complications (over immediate surgery), and thereby potentially alleviates some toxicity-related concerns (14).
Monetary and socioeconomic aspects of oncologic and radiotherapeutic care are easily apparent from these data, which are crucial to consider in the pursuit of equal-opportunity care in the United States going forward. Owing to monetary/billing issues, community and private practices are well-documented to offer conventional fractionation for many neoplasms for which there is level I evidence for hypofractionation (15-17), and herein we demonstrate a similar situation for RC cases. Further proving these points were the findings that patients with private insurance and higher incomes were more likely to receive CFRT, likely related to the “fee-for-service” billing model widely in effect in the United States. Lastly, the racial disparity observed in this work, with African-Americans being less likely to receive HFRT, could be because colorectal cancer in this cohort is often diagnosed at advanced stages from a relative lack of colonoscopic and/or sigmoidoscopic surveillance as compared to Caucasian patients (18). Taken together, the findings of HFRT utilization in this investigation will be therefore noteworthy as a benchmark, given the future implementation of bundled payments (19,20). This very issue was addressed in a recent survey of 182 US radiation oncologists regarding HFRT for RC, 20% of which indicated that instituting alternative payment models may impact consideration for HFRT (21). Therefore, with the clear rise in HFRT utilization in recent years, there are many reasons to believe that the rate could increase even more dramatically in the future, especially following the implementation of so-called “lump-sum” payments.
In summary, neoadjuvant therapy for locally advanced RC is continuing to evolve. As mentioned before, the Stockholm III trial has now shown an enhanced safety profile if a 4–8 weeks delay is provided between nRT and surgery (14). Moreover, phase II trials have evaluated delivering nCRT in the form of HFRT followed by chemotherapy, which has shown promise (22). The accruing PROSPECT trial is also assessing the selective omission of nRT in responders to 6 cycles of chemotherapy (23), although this is likely a surrogate for favorable biology rather than true de-escalation of therapy for all patients.
Although the NCDB provides a unique platform with which to study this novel clinical question, limitations must be acknowledged. Aside from the retrospective nature and its associated caveats, a major piece of information missing in the NCDB that may be highly applicable to the issue at hand is whether the disease was located in the high or low rectum. This is important because it has been historically thought that there may be less likelihood for sphincter preservation when delivering HFRT to lower tumors (7), although data have not corroborated this notion (4). Second, the rationale for deliberately not analyzing chemotherapy is presented above. Additionally, we intentionally chose not to perform survival analysis in this paper owing to the numerous known and unforeseen biases in large datasets, even when utilizing techniques such as propensity matching, and because nRT has not been shown to improve overall survival (the only survival endpoint in the NCDB) in the TME era (1). Moreover, multiple randomized trials have failed to demonstrate any difference in either local control or overall survival between neoadjuvant HFRT and CFRT regimens (3-5), and the purpose of this trial was primarily to determine current practice patterns and trends in HFRT utilization within the U.S. Third, although the NCDB encompasses roughly 70% of the US population, only CoC-accredited centers contribute data. Thus, the findings may not necessarily be representative of the entire US. Lastly, the NCDB does not keep track of several noteworthy variables, such as radiotherapy field design/volumes/techniques, specific chemotherapy type, or other endpoints such as tolerance of therapy (including premature cessation of chemotherapy and/or RT).
Conclusions
This is the only known study evaluating utilization of neoadjuvant HFRT for RC in the United States. Although HFRT is highly underutilized in the US, it is rising and has increased nearly fivefold over the last decade. Disparities in HFRT delivery are emphasized, namely that HFRT was more likely given at academic institutions and to older patients with more comorbidities. HFRT was less likely in patients with node-positive disease, higher income, private insurance, and African-Americans. These data may serve as a benchmark for future investigation following the implementation of bundled payments as well as for health disparities in the radiotherapeutic treatment of RC.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.National Comprehensive Cancer Network. Rectal Cancer. Version 4.2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed December 12, 2017.
- 2.Bujko K, Bujko M. Point: Short-course radiation therapy is preferable in the neoadjuvant treatment of rectal cancer. Semin Radiat Oncol 2011;21:220-7. 10.1016/j.semradonc.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 3.Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 x 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 2016;27:834-42. 10.1093/annonc/mdw062 [DOI] [PubMed] [Google Scholar]
- 4.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. 10.1002/bjs.5506 [DOI] [PubMed] [Google Scholar]
- 5.Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol 2012;30:3827-33. 10.1200/JCO.2012.42.9597 [DOI] [PubMed] [Google Scholar]
- 6.Minsky BD. Counterpoint: Long-Course Chemoradiation Is Preferable in the Neoadjuvant Treatment of Rectal Cancer. Semin Radiat Oncol 2011;21:228-33. 10.1016/j.semradonc.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 7.Minsky BD. Short-Course Radiation Versus Long-Course Chemoradiation for Rectal Cancer: Making Progress. J Clin Oncol 2012;30:3777-8. 10.1200/JCO.2012.45.0551 [DOI] [PubMed] [Google Scholar]
- 8.Goodman KA, Patton CE, Fisher GA, et al. Appropriate Customization of Radiation Therapy for Stage II and III Rectal Cancer: An ASTRO Clinical Practice Statement Using the RAND/UCLA Appropriateness Method. Available online: http://www.practicalradonc.org/cms/attachment/2077798173/2070877868/mmc1.pdf [DOI] [PubMed]
- 9.Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. 10.1245/s10434-007-9747-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol 2017;133:369-75. 10.1007/s11060-017-2443-7 [DOI] [PubMed] [Google Scholar]
- 11.Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33. 10.1016/j.ygyno.2016.10.033 [DOI] [PubMed] [Google Scholar]
- 12.Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. 10.1016/j.ijrobp.2016.09.036 [DOI] [PubMed] [Google Scholar]
- 13.Brower JV, Chen S, Bassetti MF, et al. Radiation dose escalation in esophageal cancer revisited: a contemporary analysis of the National Cancer Data Base. Int J Radiat Oncol Biol Phys 2016;96:985-93. 10.1016/j.ijrobp.2016.08.016 [DOI] [PubMed] [Google Scholar]
- 14.Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336-46. 10.1016/S1470-2045(17)30086-4 [DOI] [PubMed] [Google Scholar]
- 15.Mak KS, Agarwal A, Qureshi MM, et al. Hypofractionated short-course radiotherapy in elderly patients with glioblastoma multiforme: an analysis of the National Cancer Database. Cancer Med 2017;6:1192-200. 10.1002/cam4.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stokes WA, Kavanagh BD, Raben D, et al. Implementation of hypofractionated prostate radiation therapy in the United States: A National Cancer Database analysis. Pract Radiat Oncol 2017;7:270-8. 10.1016/j.prro.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 17.Rice SR, Molitoris JK, Bentzen SM, et al. Trends in Utilization of Hypofractionated Whole Breast Irradiation in Triple Negative Breast Cancer: A National Cancer Database Analysis. Int J Radiat Oncol Biol Phys 2017;99:E43-4. 10.1016/j.ijrobp.2017.06.696 [DOI] [Google Scholar]
- 18.Williams R, White P, Nieto J, et al. Colorectal Cancer in African Americans: An Update. Clin Transl Gastroenterol 2016;7:e185. 10.1038/ctg.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekelman JE, Epstein AJ, Emanuel EJ. Getting the next version of payment policy "right" on the road toward accountable cancer care. Int J Radiat Oncol Biol Phys 2014;89:954-7. 10.1016/j.ijrobp.2014.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Society for Radiation Oncology. Radiation Oncology Alternative Payment Model (RO-APM). Available online: https://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Medicare_Payment_Initiatives/Alternative_Payment_Model_Program/Content_Pieces/ROAPM_Description.pdf
- 21.Mowery YM, Salama JK, Zafar SY, et al. Neoadjuvant long-course chemoradiation remains strongly favored over short-course radiotherapy by radiation oncologists in the United States. Cancer 2017;123:1434-41. 10.1002/cncr.30461 [DOI] [PubMed] [Google Scholar]
- 22.van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762-9. 10.1093/annonc/mdt124 [DOI] [PubMed] [Google Scholar]
- 23.Clinicaltrials.gov. PROSPECT: Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT01515787