Abstract
Background
Transarterial chemoembolization (TACE) is the standard for unresectable Barcelona Clinic Liver Cancer (BCLC) B hepatocellular carcinoma (HCC) patients but is not an ablative therapy. This study explores stereotactic body radiation therapy (SBRT) as an adjuvant or salvage to drug eluting bead (DEB)-TACE.
Methods
A retrospective review identified patients receiving SBRT within 2 years following DEB-TACE to a target lesion. Primary outcome was objective response (OR) using modified response evaluation criteria in solid tumors (mRECIST). Other outcomes included local control (LC), out of field failures, and overall survival (OS).
Results
One hundred and three patients were identified with median 2 DEB-TACEs prior to SBRT. Fifty-two patients had planned adjuvant SBRT after DEB-TACE and the remainder had salvage SBRT with no statistical differences between groups. Of 95 patients with follow-up imaging, 59 (62.1%) had a complete response and 25 (26.3%) had a partial response (PR). More patients achieved CR (79.6% vs. 43.5%) with planned TACE + SBRT than salvage (P=0.006). LC was 91% and 89% at 1 and 2 years, respectively. One-year survival for planned DEB-TACE SBRT was 70.8% vs. 61.5% for salvage (P=0.052).
Conclusions
Combination TACE + SBRT achieves high OR and LC rates. Adjuvant TACE + SBRT might achieve superior outcomes than salvage. This strategy might be particularly effective as a bridge to transplant.
Keywords: Hepatocellular carcinoma (HCC), transarterial chemoembolization (TACE), stereotactic body radiation therapy (SBRT), transplant
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the second largest contributor to cancer mortality, especially in the developing world (1). While less common in the United States, there has been a consistent increase in prevalence and mortality over the past several decades (2). Among several staging systems, the Barcelona Clinic Liver Cancer (BCLC) is among the most widely accepted, which uses several factors, including tumor size, number of lesions, vascular invasion, and performance status to prognosticate and make treatment recommendations (3). For early stage disease, surgical resection, transplant, or radiofrequency ablation (RFA) are the preferred treatments and can be curative in select patients. For intermediate stage, the only treatment shown to improve survival in a randomized trial is transarterial chemoembolization (TACE) (4). TACE has also produced good outcomes in the BCLC A population via stage migration; however, TACE is generally not considered an ablative treatment.
Stereotactic body radiation therapy (SBRT) has been increasingly incorporated into the management of HCC. By giving larger, more conformal biological doses to tumor, SBRT provides excellent local control (LC) while minimizing damage to adjacent liver and other normal tissue. The most established series with liver SBRT comes from a combination of phase I and II trials from Princess Margaret Hospital (5,6). LC at 1 year in this series was 87%. The population included in these protocols was heterogeneous, with 52% having prior therapies, tumor vascular thrombosis in 55%, multiple lesions in 61%, and even some patients with extra hepatic disease. Multiple studies have been subsequently published showing excellent LC with SBRT, even compared to other treatment modalities such as RFA (7,8).
With safety and efficacy now well established in well compensated liver patients, one of the unanswered questions regarding liver SBRT for HCC remains on how to optimally incorporate this modality into the larger schema of treatment options. Since TACE is the core treatment in BCLC intermediate stage patients but is not a truly “ablative” treatment, SBRT is an appealing adjuvant therapy in this population. The benefit of adjuvant radiation following TACE has been suggested in a meta-analysis, but radiation in most of the included studies predates use of SBRT (9). This study looks at outcomes inpatients that received drug eluting beads (DEB)-TACE followed by SBRT either as a planned or salvage treatment.
Methods
This study was approved by an Institutional Review Board. A chart screen was performed to identify patients with HCC (diagnosed pathologically or by radiologic criteria) treated with SBRT after having had a prior DEB-TACE to the targeted lesion within the prior 2 years. Patients were excluded if they had any prior treatments to the targeted lesion including RFA, ethanol injection, or radioembolization. All patients were reviewed prospectively in a multidisciplinary tumor board. Planned DEB-TACE followed by SBRT was defined as either documentation in the chart showing clinical intent to do combined therapy or no intervening imaging/blood work prompting addition of SBRT following DEB-TACE. Salvage SBRT was performed when post-DEB-TACE imaging showed an incomplete response and additional vascular directed therapy was not felt to be beneficial. The institutional policy is to image all patients with Eovist-MRI unless patient is unable to have an MRI at which point a triple phase CT is performed. Per institutional policy, patients are generally imaged 4–6 weeks following locoregional therapy and every 3–4 months thereafter.
DEB-TACE was performed using either a transradial or transfemoral approach. Sub-selective catheterization was performed using 2.0–2.8 F microcatheters. Catheterization was attempted as close to the tumor vessel as possible to avoid non-target delivery. Treatment targets and coverage zones were confirmed using cone-beam CT (AlluraClarity, Philips Healthcare). Up to two vials of 100–300 microns LB Beads (BTG, Surrey, UK) each loaded with 50 mg Doxorubicin were used for embolization. Stasis in the target hepatic arterial branch was used a treatment endpoint.
For SBRT, patients were simulated in the supine position with a custom alpha cradle, abdominal compression, and IV contrast, when possible. Fiducial markers were not routinely used, but most DEB-TACE was performed with ethiodol staining providing for easy visualization on cone beam computed tomography (CBCT). 4D simulation was used for all patients (Philips, Netherlands). The gross tumor volume (GTV) was identified in all breathing phases to generate and internal target volume (ITV) which was subsequently expanded 3–7 mm for a planned target volume (PTV) at the physician’s discretion. Treatment was delivered with 6 MV photons with daily CBCT guidance (Varian Systems, Palo Alto, USA). Dose was delivered in 3–5 fractions to a total dose of 24–50 Gy, also at the treating physician’s discretion.
Response rate was assessed using modified response evaluation criteria in solid tumors (mRECIST). LC was defined as presence or absence of progression within the radiation treatment target. Additional endpoints included progression in the liver outside of the treated lesion as well overall survival (OS). Comparisons were made using a Student’s t-test. Overall and progression free survival was assessed using the method of Kaplan-Meier. Log rank was used to compare survival curves. Cox-Regression was used for multivariable analysis. All statistics were performed on SSPS version 22 (IBM, Armonk, USA).
Results
From 2011–2015, 103 patients were identified that were treated with SBRT to the liver after a prior DEB-TACE to the target lesion within the past 2 years. Patient characteristics are summarized in Table 1. Median follow up was 15.1 months (2.3–50 months). Prior to SBRT, patients had a median of 2 prior DEB-TACEs (range, 1–7). Fifty-one patients received salvage SBRT after incomplete DEB-TACE, and 52 received a planned SBRT following DEB-TACE. There were no statistically significant differences in baseline characteristics between the planned vs. salvage SBRT groups (Table 1). The median time between DEB-TACE and completion of SBRT was 59 days (range, 10–639 days). For patients with a planned DEB-TACE and SBRT (n=52), the median time from DEB-TACE to completion of SBRT was 18 days.
Table 1. Patient characteristics.
| Characteristic | Total (n=103), n (% or range) | Salvage-SBRT (n=51), n (% or range) | Adjuvant-SBRT (n=52), n (% or range) | P |
|---|---|---|---|---|
| Male | 73 (70.9) | 39 (76.5) | 33 (63.5) | 0.15 |
| Median age (year) | 62 [42–83] | 63 [42–82] | 61.5 [42–83] | 0.519 |
| Performance status | ||||
| ECOG 0 | 48 (46.6) | 25 (49.0) | 23 (44.2) | 0.743 |
| ECOG 1 | 50 (48.5) | 23 (45.1) | 27 (51.9) | |
| ECOG 2 | 5 (4.9) | 3 (5.9) | 2 (3.8) | |
| Etiology | ||||
| HBV | 8 (7.8) | 4 (7.8) | 4 (7.7) | 0.427 |
| HCV | 68 (66.0) | 34 (66.7) | 34 (65.4) | |
| Alcohol | 9 (8.7) | 5 (9.8) | 4 (7.7) | |
| NASH | 6 (5.8) | 5 (9.8) | 1 (1.9) | |
| Mixed/other | 13 (12.6) | 3 (5.9) | 10 (19.2) | |
| Median size (cm) | 3 (1.2–9.4) | 3.4 (1.4–9.4) | 2.7 (1.2–8) | 0.912 |
| Median AFP (ng/mL) | 15.4 (1.6–12,976) | 15.9 (2.4–12,796) | 14.4 (1.6–7,765) | 0.305 |
| Child-Turcotte-Pugh score | ||||
| A | 61 (59.2) | 25 (49.0) | 32 (61.5) | 0.456 |
| B | 39 (37.9) | 23 (45.1) | 18 (34.6) | |
| C | 2 (1.9) | 0 (0) | 2 (3.8) | |
| AJCC tumor stage | ||||
| T1 | 42 (40.8) | 17 (33.3) | 25 (48.1) | 0.365 |
| T2 | 54 (52.4) | 27 (52.9) | 25 (48.1) | |
| T3 | 6 (5.8) | 4 (7.8) | 2 (3.8) | |
| T4 | 1 (1.0) | 1 (2.0) | 0 (0) | |
| BCLC stage | ||||
| A | 31 (30.1) | 12 (23.5) | 19 (36.5) | 0.325 |
| B | 63 (61.2) | 34 (66.7) | 29 (55.8) | |
| C | 9 (8.7) | 5 (9.8) | 4 (7.7) | |
| Median number of TACEs to target lesion | 2 [1–7] | 2 [1–4] | 1 [1–7] | 0.104 |
| Median SBRT dose (Gy) | 40 [24–50] | 40 [24–50] | 40 [30–50] | 0.855 |
| Transplant | 32 (31.1) | 14 (27.5) | 18 (34.6) | 0.432 |
SBRT, stereotactic body radiation therapy; TACE, transarterial chemoembolization; BCLC, Barcelona Clinic Liver Cancer.
Of the 103 treated lesions, 95 had at least one post-treatment image to assess response via mRECIST. Results are summarized in Table 2. Overall, 59 (62.1%) had a complete response (CR) and 25 (26.3%) had a partial response (PR) for an objective response (OR) rate of 88.43%. Of patients who achieved a complete response, the median time to achieve a complete response was 3.2 months (range, 0.5–15.1 months). When compared, significantly more patients achieved a CR (79.6% vs. 43.5%) with planned DEB-TACE + SBRT vs. salvage SBRT after incomplete DEB-TACE (P=0.006).
Table 2. Response rate via mRECIST.
| Response | Total (n=95) | Salvage-SBRT (n=46) | Adjuvant-SBRT (n=49) | P |
|---|---|---|---|---|
| Complete response | 59 (62.1) | 20 (43.5) | 39 (79.6) | 0.006 |
| Partial response | 25 (26.3) | 17 (37.0) | 8 (16.3) | |
| Stable disease | 6 (6.3) | 5 (10.9) | 1 (2.0) | |
| Progression of disease | 5 (5.3) | 4 (8.7) | 1 (2.0) |
mRECIST, modified response evaluation criteria in solid tumors; SBRT, stereotactic body radiation therapy.
LC, as defined as no radiographic progression in the targeted lesion, was 91% and 89% at 1 and 2 years, respectively. The 1- and 2-year rates of out of field progression free survival were 68.2% and 60.4%, respectively. There was a trend towards improved LC at 1-year with planned DEB-TACE + SBRT (95.4%) vs. salvage SBRT after DEB-TACE (86.3%) (P=0.052) and significantly improved LC in patients with just one DEB-TACE prior to SBRT vs. greater than one (P=0.035) (Table 3). There was significantly lower out of field progression in patients undergoing planned DEB-TACE + SBRT (80.9%) vs. salvage SBRT (54.3%) (P=0.008) on univariable analysis. Radiation dose greater than vs. less than 40 Gy did not affect LC. On multivariable analysis, no factor was found to independently predict for LC (Table 3).
Table 3. Univariable and multivariable analysis of factors affecting local control and overall survival.
| Variable | Local control | Overall survival | |||
|---|---|---|---|---|---|
| Univariable (P) | Multivariable HR (range); P | Univariable (P) | Multivariable HR (range); P | ||
| Sex | <0.001 | 5.7 (0.43–72.4); 0.19 | 0.1 | 0.94 (0.39–2.22); 0.88 | |
| Age ≥65 | 0.32 | 0.8 (0.15–4.39); 0.80 | 0.33 | 0.82 (0.39–1.69); 0.59 | |
| ECOG performance status (0 vs. ≥1) | 0.88 | 2.2 (0.24–19.2); 0.49 | 0.085 | 2.41 (1.01–5.78); 0.041 | |
| CTP score | 0.76 | 1.4 (0.21–9.09); 0.74 | 0.25 | 3.08 (1.51–6.26); 0.002 | |
| BCLC stage | 0.44 | 0.25 (0.02–3.00); 0.27 | 0.13 | 0.64 (0.30–1.35); 0.24 | |
| Pre-SBRT AFP (≥200 ng/mL) | 0.65 | 0.98 (0.16–5.98); 0.98 | 0.002 | 1.34 (0.62–2.91); 0.46 | |
| Pre-SBRT tumor size (≥3 cm) | 0.39 | 0.17 (0.02–1.14); 0.07 | 0.65 | 0.75 (0.37–1.52); 0.42 | |
| Number of prior DEB-TACE to target lesion (1 vs. ≥2) | 0.035 | 0.67 (0.58–104); 0.061 | 0.62 | 0.81 (0.39–1.66); 0.56 | |
| Planned vs. salvage SBRT | 0.052 | 0.17 (0.02–1.67); 0.13 | 0.023 | 0.56 (0.29–1.11); 0.10 | |
| SBRT dose (≥4,000 cGy) | 0.53 | 0.88 (0.15–5.29); 0.75 | 0.42 | 0.45 (0.42–1.89); 0.76 | |
| Transplant (yes vs. no) | 0.015 | 0.01 (0.001–>10); 0.96 | <0.0001 | 0.05 (0.01–0.18); <0.01 | |
SBRT, stereotactic body radiation therapy; DEB, drug eluting bead; TACE, transarterial chemoembolization.
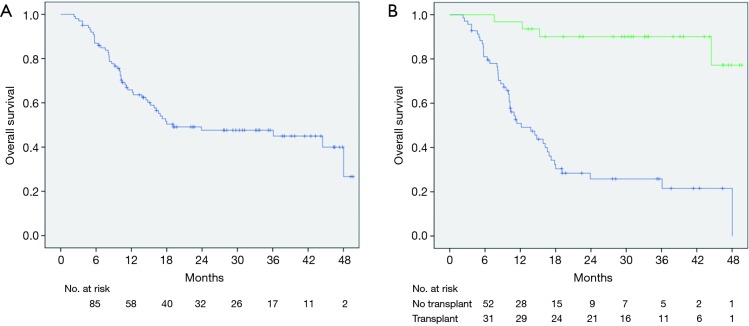
Median OS for this cohort was 23.9 months (range, 3.5–44 months) (Figure 1A). There was a dramatic difference in median survival between patients who bridged to transplant vs. no transplant (Figure 1B). Median survival without transplant was 13.8 months (range, 8.9–18.6 months) and was not reached in transplanted patients (P<0.001). When comparing OS between patients who had a planned DEB-TACE+SBRT vs. SBRT used as a salvage treatment, there was improved survival in patients with planned treatments (P=0.023) (Figure 2). One-year survival for planned DEB-TACE and SBRT was 70.8% vs. 61.5% when salvage SBRT was used. Other variables affecting OS are shown in Table 3. On multivariable analysis, ECOG performance status (HR 2.41, 95% CI: 1.01–5.78; P=0.041), receipt of transplant (HR 0.05, 95% CI: 0.01–0.18; P<0.01), and CTP score (HR 3.08, 95% CI: 1.51–6.26; P=0.002) were the only variables shown to improve survival.
Figure 1.
Kaplan-Meier estimates of overall survival (blue) for all patients (A) and stratified for transplant (green) (B).
Figure 2.
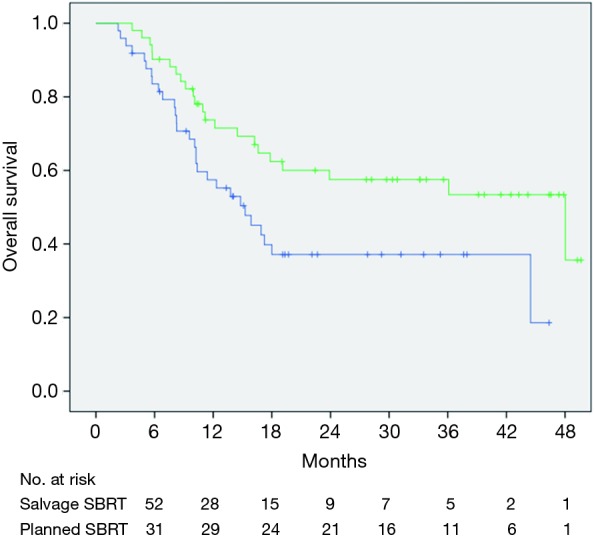
Kaplan-Meier estimates of overall survival with planned TACE + SBRT (green) vs. salvage SBRT (blue). TACE, transarterial chemoembolization; SBRT, stereotactic body radiation therapy.
Discussion
This study shows that combination of DEB-TACE followed by SBRT for HCC achieves high OR rates via mRECIST and excellent rates of LC. While the population in this study was heterogeneous, 70.2% were BCLC B or C and over half of the patients had tumors greater than ≥3 cm. These results compare favorably with OR rates for DEB-TACE alone in this population which range from 51–73% (10-13). More encouraging is the complete response of 62.1% in this series compared to the 5–26.8% reported with DEB-TACE alone. This number is even higher with adjuvant SBRT following DEB-TACE.
With a statistically improved OR of planned DEB-TACE and SBRT vs. salvage SBRT after DEB-TACE, the data suggests a possible synergistic effect by performing SBRT immediately following DEB-TACE. Improved OR rates had been shown in smaller series and confirmed a meta-analysis showing improved response rates with TACE + radiation (not necessarily SBRT) (9). This would theoretically provide maximum treatment to the target lesion with optimal tumor kill. The groups were well matched with regard to baseline characteristics. However, the selection bias in using salvage SBRT only after incomplete response to DEB-TACE or progression obscures the potential synergistic effect. Only a prospective randomized study would eliminate this bias.
Multiple studies have shown SBRT LC rates of 87–100% at one year, especially for tumors less than 3 cm (6,8,14,15). Despite these very high rates of control, many of these studies performed SBRT in a heavily pre-treated population, making determination of the appropriate tumors for SBRT difficult to discern. Several groups have therefore specifically incorporated SBRT following TACE or DEB-TACE. A pilot study of 28 patients with up to 3 HCCs, each less than 3 cm, had a 1 year LC of 96.3% (16). More recently, a larger phase II study tested combination TACE + SBRT (although TACE was only performed in 64% of patients) for single HCC ≤4 cm in treatment naïve patients. In this series, an impressive 3-year LC rate of 96.3% was achieved (17). Interestingly, there was no improvement in cause-specific or OS when TACE was performed prior before SBRT vs. SBRT alone.
A major advantage to the current study is that half of the treated tumors were greater than 3 cm, some even up to 9 cm, a population where TACE and/or RFA loses efficacy (7,18,19). This is a particularly appealing target population since the current standard of practice does not effectively address this group, and SBRT might provide a significant contribution. Another advantage to this analysis is that it confirms that SBRT can possibly serve as an effective bridge to transplant (14). While it is impossible to know how many patients remained eligible and received a transplant as a result of SBRT, it is likely some of these patients were able to be successfully bridged to transplant as result of using this combination modality over TACE alone. A recent analysis showed that SBRT compared favorably to other bridging therapies prior to liver transplant (20).
There are numerous limitations to this study. To start, it is retrospective and treatment decisions were biased by multiple uncontrolled factors. Secondly, the inclusion population was very heterogeneous, some patients having had other prior treatments to other lesions in the liver including TACE, RFA, ethanol ablation, and even radioembolization. This can certainly influence OS and progression outside of the target lesion. Also, by including transplanted patients, a significant number of patients did not have long term follow up or even imaging to accurately assess LC.
Overall, this study supports the growing evidence that addition of SBRT to DEB-TACE can increase OR rates in the treatment of HCC over DEB-TACE alone. The optimal combination might be immediate SBRT following DEB-TACE as opposed to using SBRT as a salvage treatment for incomplete DEB-TACE. Prospective studies are needed to further validate this methodology and identify a group of HCC patients that most benefit from this approach. Our institution is currently conducting a prospective trial of combined DEB-TACE followed by planned SBRT for ≥4 cm HCC.
Acknowledgements
None.
Ethical statement: The study was approved by the institutional review board of Mt. Sinai Medical Center (No. HS# 13-00891) and was deemed IRB exempt from consenting patients.
Footnotes
Conflicts of Interest: E Kim—Speaking and Advisory Board, BTG; A Fischman—Consultant, Advisory Board for Terumo, Advisory Board for Embolx, Consultant for Surefire Medical, Research Grant, Consultant for Merit Medical; S Blacksburg—Speaker’s Bureau for Bayer Pharmaceutical, Consultant for Accuracy; M Schwartz—Consultant for Bayer Pharmaceuticals, Celsion, ArQule, H3 Biomedicine and Merck. The other authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787-94. 10.1200/JCO.2015.64.7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734-9. 10.1016/S0140-6736(02)08649-X [DOI] [PubMed] [Google Scholar]
- 5.Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2008;26:657-64. 10.1200/JCO.2007.14.3529 [DOI] [PubMed] [Google Scholar]
- 6.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. 10.1200/JCO.2012.44.1659 [DOI] [PubMed] [Google Scholar]
- 7.Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 2016;34:452-9. 10.1200/JCO.2015.61.4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huertas A, Baumann AS, Saunier-Kubs F, et al. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol 2015;115:211-6. 10.1016/j.radonc.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 9.Meng MB, Cui YL, Lu Y, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 2009;92:184-94. 10.1016/j.radonc.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 10.Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. 10.1007/s00270-009-9711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malagari K, Pomoni M, Kelekis A, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol 2010;33:541-51. 10.1007/s00270-009-9750-0 [DOI] [PubMed] [Google Scholar]
- 12.Carr BI, Kondragunta V, Buch SC, et al. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer 2010;116:1305-14. 10.1002/cncr.24884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Luna LE, Yang JD, Sanchez W, et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol 2013;36:714-23. 10.1007/s00270-012-0481-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-53. 10.1016/j.ijrobp.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Aikata H, Takahashi S, et al. Stereotactic body radiotherapy for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Hepatol Res 2015;45:378-86. 10.1111/hepr.12359 [DOI] [PubMed] [Google Scholar]
- 16.Honda Y, Kimura T, Aikata H, et al. Pilot study of stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatogastroenterology 2014;61:31-6. [PubMed] [Google Scholar]
- 17.Takeda A, Sanuki N, Tsurugai Y, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 2016;122:2041-9. 10.1002/cncr.30008 [DOI] [PubMed] [Google Scholar]
- 18.Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology 2005;234:954-60. 10.1148/radiol.2343040153 [DOI] [PubMed] [Google Scholar]
- 19.Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32. 10.1200/JCO.2012.42.9936 [DOI] [PubMed] [Google Scholar]
- 20.Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9. 10.1016/j.jhep.2017.02.022 [DOI] [PubMed] [Google Scholar]