Abstract
Background
Gastric adenocarcinoma is known to be the fourth most common cancer type and the second cause of cancer-related deaths. Movement and invasion of cancer cells is one of the major characteristics of the cancer phenotype that various types of network regulate this. Expression levels of slingshot diphosphatase 1 (SSH1) gene has been modulated in this pathway. SSH1 acts as a dephosphorylation and activator of cofilin that this regulating and activating by SSH1 can play a major role in the mobility and migration of the cell. The aim of this study was to compare the expression level of SSH1 genes between tumor and corresponding adjacent non tumor gastric tissues and healthy tissue of gastric adenocarcinoma.
Methods
In this study, mRNA of 40 gastric adenocarcinoma and corresponding adjacent non tumor gastric tissues and 15 healthy biopsy samples was extracted, then after cDNA synthesis, real-time polymerase chain reaction was performed to measure gene expression.
Results
According to REST analysis, the relative expression of SSH1 was significantly increased in gastric cancer tissues compared to the corresponding adjacent non tumor gastric tissue samples and normal tissue. Nevertheless, the result revealed no substantial correlation between the expression levels of SSH1 with clinical features. The biomarker index for SSH1 was obtained as 0.89.
Conclusions
The results obtained from investigating SSH1 expression are indicative of significant changes in the expression of this gene in gastric adenocarcinoma. This gene can also be used as a biomarker for gastric cancer.
Keywords: Slingshot diphosphatase (SSH1), expression, gastric adenocarcinoma, biomarker
Introduction
Gastric cancer, which originates from the cancer of the cells in inner lining of stomach (1), is known to be the fourth most common cancer type and the second cause of cancer-related deaths (2). In terms of anatomy, adenocarcinoma is the most common type of gastric cancer in 90% of cases. Based on the classification made by Lauren system, stomach adenocarcinoma is divided into two types including intestinal and defuse (3). According to some findings, defuse type is more prevalent among women (4), and intestinal type is more associated with intestinal metaplasia and H. pylori infection (5).
The prevalence of gastric cancer among men is twice that of women, and over 70% of cases diagnosed with this cancer have been reported to be in developing countries (6). The incidence and severity of this cancer in the geographical regions of East Asia and Eastern Europe and southern parts of United States are considerably high (7). In general, northern areas of Iran ranging from the northwest to northeast are considered to be high risk areas for this cancer type (8). Gastric cancer is a multifactorial disorder that, unfortunately, is commonly diagnosed when the disease is in its advanced stages and metastatic symptoms are observed (9).
Current treatments for gastric cancer include surgery, chemotherapy, and prosthodontics (10). However, these treatments are often ineffective due to poor prognosis (11), and the overall 5-year survival rate is approximately 25.7%, which has not been changed significantly over the past 30 to 40 years (12).
Since tumor and metastasis destructively affect patients during early stages of development, they are considered to be the main causes of death among cancer patients. Disruption in the regulation of cellular movements in cancer cells is a major contributor to the invasion and metastasis. Actin protein and its regulation protein as basic engines for cellular mobility play a critical role in the development and metastasis of cancer cells (13).
CFL1 is one of the most important actin regulating proteins. By depolymerizing actin, this protein stimulates the mobility and dynamics of cellular skeletons, which is essential for many processes including cytokines and cellular movements (14,15). Through phosphorylation by LIMK, CFL1 is deactivated; it is activated through dephosphorylation by slingshot diphosphatase (SSH) in ser3 (16,17). Thus, regulating and activating the CFL1 by SSH1 can play a major role in the mobility and migration of the cell.
SSH1 is a gene on the long arm of chromosome 12. The protein is encoded by the gene that belongs to SSH phosphate family, which regulates the dynamics of the actin strand by activating CFL1 (18). SSH1 acts as a dephosphorylation and activator of cofilin (19).
Phosphatase domain which belongs to SSH family has an active site motif (HCxxGxxR) found in Dual specificity phosphatase (DSP) and protein tyrosine phosphatases (PTPs). In addition, p domain can identify phosphorylated CFL1 and A domain, and trp 458 to connect to F-actin and to activate SSH1 (20). Protein 14-3-3 is one of the SSH1 inhibitor proteins (21).
Consequently, the SSH1 connection to cofilin and its dephosphorylation in SER3 can trigger the activation of cofilin and increase polymerization and de-polymerization of actin, thus result in cellular mobility (22). On the other hand, the SSH1 causes dephosphorylation and deactivation of LIMK 1, which inhibits CFL1. As a result, the SSH1 plays a twofold role in the regulation and activation of CFL1 and actin (20).
Due to the destructive effects of tumor metastasis in the early stages of development, which is the main cause of the death in cancer patients, the SSH1 gene (as an active agent in regulating CFL1 and actin, and cellular and metastatic mobility) was used in the current research to enable the researchers to examine the changes in this gene in the tumor samples compared to healthy ones.
Accordingly, by examining the results obtained, probable determination of these genes as tumor markers was taken into account.
Methods
Study population
Forty pairs of advanced gastric cancer tissues (T1–T40) and corresponding adjacent non tumor gastric tissues(marginal samples) (N1–N40) (in proximal gastric tumor, the samples were taken from gastric distal healthy tissues, and in distal gastric tumor, the samples were taken from gastric proximal healthy tissues) as well as 15 healthy samples were randomly obtained from healthy participants with the permission of the Research Ethics Committee, who underwent endoscopy at Madani and Imam Reza Hospital (Azarbayjan, Iran).
None of the samples from patient who underwent chemotherapy or radiotherapy were transferred into liquid nitrogen after removal from the body. The adjacent non-tumorous tissues were taken from the farthest distance from tumor (more than 4 cm). A pathologist determined the type of cancer. The average age of patients with gastric cancer was 66 years, and 67.5% of the participants were males and the rest were females. Pathological examinations and the confirmation of tumorigenic results and the type and stage of tumor were done by a pathologist. The consent was received from all patients. The clinicopathological characteristics of all patients are shown in Table 1.
Table 1. Slingshot diphosphatase 1 (SSH1) expression and clinicopathological characteristics of patients with gastric cancer.
| Variable | N | SSH1 relevant expression (2−ÄÄCt) | P value |
|---|---|---|---|
| Sample type | 0.001 | ||
| Tumor | 40 | 0.161±0.027 | |
| Adjacent non-tumor | 40 | 0.024±0.004 | |
| Normal | 15 | 0.035±0.007 | |
| Age | 0.267 | ||
| ≤66 years | 20 | 0.186±0.046 | |
| >66 years | 20 | 0.135±0.028 | |
| Gender | 0.892 | ||
| Male | 27 | 0.160±0.0369 | |
| Female | 13 | 0.163±0.0362 | |
| Histological grade | 0.759 | ||
| Well differentiated | 15 | 0.188±0.063 | |
| Moderate differentiated | 4 | 0.104±0.048 | |
| Poorly differentiated | 21 | 0.152±0.024 |
RNA isolation and complementary DNA synthesis
Total RNA isolation from tumor tissues and the adjacent non tumorous and healthy tissues was done by Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The quality and quantity of isolated RNAs were analyzed respectively by 1% agarose gel electrophoresis and picodrop at the 260/280 ratio of absorbance [picodrop, Bob Batty International (BBI), UK]. For the degradation of any DNA contamination in extracted RNAs, the researchers used Dnase1 enzyme in accordance with the company’s instruction. Subsequently, complementary DNA (cDNA) was obtained according to the manufacturer’s instruction (Fermentas, Canada) in a final 20 µL reaction volume.
Real-time PCR
Primer sequences for β2m (as an internal control) and SSH1 were designed from published sequences in the GeneBank nucleic acid database and synthesized by Macrogen, Korea. The β2m expression level was used as an endogenous normalization factor.
Real-time PCR reactions were performed using SYBR® Green (purchased from Amplicon) by Rotor-gene detection system and the following primer pairs:
β2m forward primer: 5'-CTACTCTCTCTTTCTGGCCTG-3'; β2m reverse primer: 5'-GACAAGTCTGAATGCTCCAC-3';
SSH1 forward primer: 5'-GATGGAGATGGTGGGTTCAG-3'; SSH1 reverse primer: 5'-GGGGAAGTAGTTGTGCCTC-3'.
The experiments were done in triplicate for each sample with a control without a template in each experiment. The protocol used for Real-time PCR is as follows: A denaturation program, 95 °C for 15 min; and an amplification and a quantification program, 40 cycles of 95 °C for 30 s, 63 °C for 35 s, 72 °C for 30 s with final extension, 72 °C for 5 min.
Statistical analyses
The alteration of SSH1 gene expression was analyzed by ΔΔCt method, and SPSS software (with P<0.05) was used for statistical analyses. Normalization was done by using β2m as an endogenous reference gene which is essential for the compensation of intra- and inter-kinetic RT-PCR variations. Independent t-test and One-way ANOVA LSD test and post-hoc multiple comparison test were used for comparisons between two groups and among three groups, respectively. P<0.05 was considered to be statistically significant and data were shown as mean ± SD.
The relative expression analysis of SSH1 was performed by a randomization test using the Relative Expression Software Tool (REST) 2009. Receiver operating characteristic (ROC) curve was used to evaluate the specificity and sensitivity of predicting gastric cancer and normal tissues by SSH1 expression levels. Moreover, the sensitivity/specificity at various cut off values was analyzed using Sigma Plot 12.5. P values <0.05 were considered to indicate a statistically significant difference.
Results
Expression levels of SSH1 in three types of gastric tissues
The relative expression of SSH1 was significantly increased in gastric cancer tissues compared to the paired adjacent non tumor ones (according to REST analysis). The results obtained from checking the expression of SSH1 by normalizing the expression using internal control of β2m showed significant changes in the expression of this gene with a 6.044 times increase risk of gastric cancer in gastric adenocarcinoma samples compared to healthy tumor adjacencies (P=0.001).
Expression of SHH1 in three types of gastric tissues (i.e., tumoral tissue, adjacent non tumor, and normal tissues) was analyzed by One-way ANOVA LSD test and post-hoc multiple comparison test. However, a review of SSH1 gene expression in adjacent non tumor samples and healthy samples did not show a significant increase in SSH1 expression (P=0.757>0.05) (Figure 1).
Figure 1.
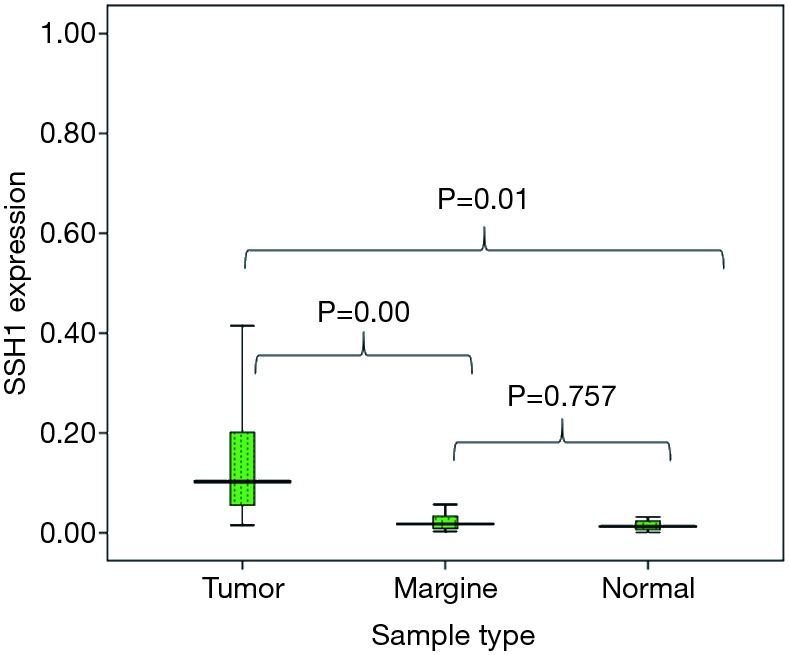
Relative expression of slingshot diphosphatase 1 (SSH1) in gastric cancer compared with its expression in marginal and normal tissues.
Association between overexpression of SSH1 and clinicopathological features
The association of SSH1 expression with clinicopathological features such as age (P=0.267), gender (P=0.892), and differentiation (P=0.759) was analysed by using SPSS 16. The results indicated that the upregulation of SSH1 did not significantly correlate with clinicopathological features in Gastric adenocarcinoma patients. These results are shown in Table 1.
Capability of SSH1 to be a candidate biomarker for the detection of gastric cancer
ROC curves were used to analyze the sensitivity and specificity of SSH1. The findings showed a ROC area (AROC) of 0.89 biomarker index for this gene. ROC curve data are shown in Figure 2.
Figure 2.
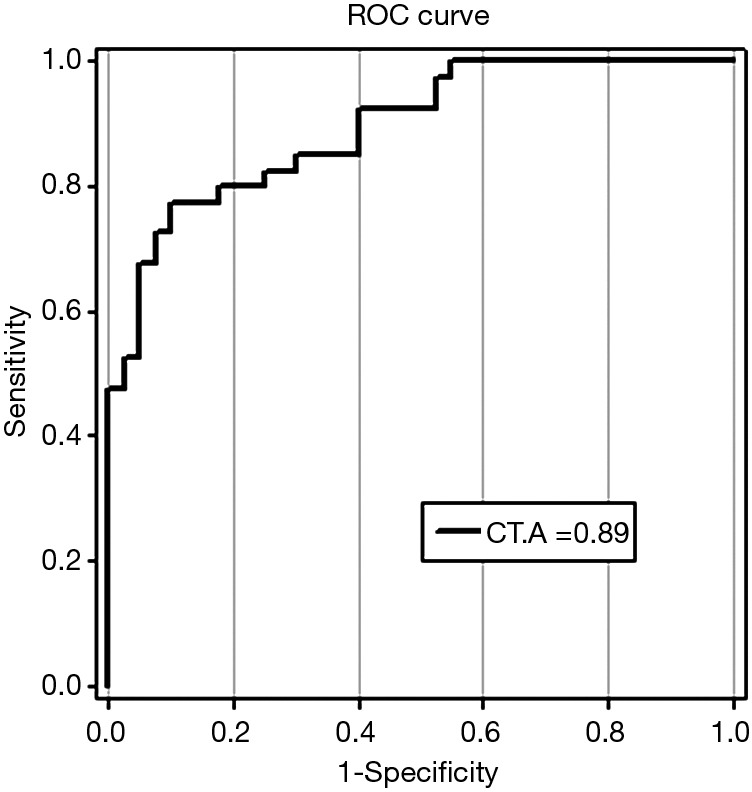
Receiver operating characteristic (ROC) curve for SSH1 which distinguishes gastric cancer from benign disease. The area under the ROC is 0.89 out of 1. ROC is a graph which plots tpr (true positive rate) vs. FPR (false positive rate) for different descriptors threshold. Since the vertical axis(y) in ROC curve indicates true positive rate and hypothetical x-value indicates false positive. The bigger y-value for a hypothetical x-value shows the better performance of a descriptor. CT, cycle threshold; A, area under the ROC.
Discussion
Nowadays, cancer is a common cause of death all over the world (23). In recent years, the researchers suggest that molecular biological features play an important role in the diagnosis and treatment of gastric cancer. Therefore, the identification of strong biomarkers is essential for early diagnosis of the disease. The mobility and invasion of cancer cells is one of the essential properties of malignant phenotype. Dynamic regulation of actin strand is essential for cell migration and invasion. CFL1 can be dephosphorylated and activated by SSH1. Furthermore, the activity of cofilin phosphatase (SSH1) is significantly increased by binding it to F-actin (24).
By regulating CFL1 and actin, SSH1 plays an important role in the activation and invasion of cells. Therefore, in the current research, changes in the expression of SSH1 in the gastric adenocarcinoma were evaluated by measuring the expression of SSH1 in gastric tumor samples, adjacent non-tumor tissues, and healthy samples from healthy people. The ability of SSH1 to act as a diagnostic marker for gastric adenocarcinoma was also studied.
The results obtained from investigating SSH1 expression with normalization of expression by β2m internal control is indicative of significant changes in the expression of this gene in gastric adenocarcinoma. In addition, the findings indicate that this gene with a biomarker indicator of 0.89 can act as a relatively suitable biomarker, which is consistent with the findings reported in the previous studies. In the current article, also, changes in the expression of healthy samples were compared with those of tumor samples, which showed a significant increase in the expression of tumor marginal samples.
In the meantime, an analysis of expression of SSH1 in the adjacent tumor tissues and healthy tissues did not indicate any significant increase. Therefore, adjacent non-tumor tissues did not differ from healthy tissues in this regard, which is indicative of the low number of tumor cells and the difference in the areas adjacent to tumor; thereafter, it would be possible to discriminate tumor areas from non-tumor ones.
By studying pancreatic cancer samples, Wang et al. reported the progression of SSH1 expression in tumor cells among these patients in their final stages. The authors also showed that SSH1 is an important regulator of CFL1. In addition, by silencing the gene in the pancreatic cancer cell line by the siRNA SSH1, they indicated that this gene also plays an important role in the progression of cancer and metastasis, and it may be a potential target for the prevention or treatment of invasive and metastatic pancreatic cancer (25). The findings are consistent with the ones obtained from the current research.
In another research performed on the inhibition of mobility and cell invasion of pancreatic cancer using sennoside A, it was shown that cellular mobility inhibition by sennoside A has been occurred by suppressing SSH1; as a result, regulating the activity of SSH1 can be a new therapeutic approach for patients with pancreatic cancer (26).
By silencing the CFL1 gene through CFL1 siRNA in the prostate cancer cell line, it was shown that silencing of the CFL1 gene, metastasis and cellular movement in cell line can reduce the prostate cancer (27). This finding is consistent with the results obtained from the study performed on VSMC. In this study, by examining the function of cofilin and SSH1 and their relationship with VSMC and silencing cofilin by siRNA, Rebecca et al. showed that cofilin is of high significance for regulating the VSMC mobility and migration. Moreover, regulation of VMSC migration by cofilin totally depends on SSH1. Furthermore, by inhibiting SSH1 by siRNA, the authors reported some results which were similar to those obtained from the inhibition of cofilin, and suggested that SSH1 plays an important role in cellular invasion and mobility of the VSMC (17).
In the leading study of SSH1 gene expression in tumor samples, considering the pathological characteristics of individuals such as age (above and below 66), sex (i.e., being male or female) and degree of tumor differentiation, it was shown that increased expression of this gene was independent of sex, age, and the degree of tumor differentiation in patients.
Overall, the findings of this study showed that the expression of SSH1 has a significant relationship with the cancerous nature of the tumors, which means that SSH1 would have a high potential to be a marker molecule in the diagnosis and treatment of gastric tumors.
Acknowledgements
We would like to thank the patients, staff, and nurses in the Endoscopy and the Pathology Department of Tabriz Madani and Imam Reza Hospital, who sincerely helped conduct this project.
Ethical Statement: The study was approved by ethics board of Tabriz University of Medical Sciences (No. 1396-911) and informed consent was taken from all the patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Taylor VM, Ko LK, Hwang JH, et al. Gastric cancer in asian american populations: a neglected health disparity. Asian Pac J Cancer Prev 2014;15:10565-71. 10.7314/APJCP.2014.15.24.10565 [DOI] [PubMed] [Google Scholar]
- 2.Mohammadi M, Zarghami N, Hedayati M, et al. Visfatin effects on telomerase gene expression in AGS gastric cancer cell line. Indian J Cancer 2015;52:32-5. 10.4103/0019-509X.175567 [DOI] [PubMed] [Google Scholar]
- 3.Hwang SW, Lee DH, Lee SH, et al. Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 2010;25:512-8. 10.1111/j.1440-1746.2009.06106.x [DOI] [PubMed] [Google Scholar]
- 4.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. 10.1111/apm.1965.64.1.31 [DOI] [PubMed] [Google Scholar]
- 5.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999;36:873-80. [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman R, Asombang AW, Ibdah JA. Characteristics of gastric cancer in Asia. World J Gastroenterol 2014;20:4483-90. 10.3748/wjg.v20.i16.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radmard AR. Five common cancers in Iran. Arch Iran Med 2010;13:143-6. [PubMed] [Google Scholar]
- 8.Azarbarzin S, Feizi MAH, Safaralizadeh R, et al. The Value of miR-299-5p in Diagnosis and Prognosis of Intestinal-Type Gastric Adenocarcinoma. Biochem Genet 2016;54:413-20. 10.1007/s10528-016-9728-y [DOI] [PubMed] [Google Scholar]
- 9.Layke JC, Lopez PP. Gastric cancer: diagnosis and treatment options. Am Fam Physician 2004;69:1133-40. [PubMed] [Google Scholar]
- 10.Watanabe T, Kume K, Taip M, et al. Gastric mucosal cancer smaller than 7mm can be treated with conventional endoscopic mucosal resection as effectively as with endoscopic submucosal dissection. Hepatogastroenterology 2010;57:668-73. [PubMed] [Google Scholar]
- 11.Hoshyar R, Bathaie SZ, Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, cells. DNA Cell Biol 2013;32:50-7. 10.1089/dna.2012.1866 [DOI] [PubMed] [Google Scholar]
- 12.Kumar RK, Raj SS, Shankar EM, et al. Gastric carcinoma: a review on epidemiology, current surgical and chemotherapeutic options. Gastric Carcinoma-New Insights into Current Management. Intech; 2013. [Google Scholar]
- 13.Otterbein LR, Graceffa P, Dominguez R. The crystal structure of uncomplexed actin in the ADP state. Science 2001;293:708-11. 10.1126/science.1059700 [DOI] [PubMed] [Google Scholar]
- 14.Bamburg JR, McGough A, Ono S. Putting a new twist on actin: ADF/cofilins modulate actin dynamics. Trends Cell Biol 1999;9:364-70. 10.1016/S0962-8924(99)01619-0 [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Eddy R, Condeelis J. The cofilin pathway in breast cancer invasion and metastasis. Nat Rev Cancer 2007;7:429-40. 10.1038/nrc2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Mouneimne G, Sidani M, et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol 2006;173:395-404. 10.1083/jcb.200510115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres RA, Drake DA, Solodushko V, et al. Slingshot isoform-specific regulation of cofilin-mediated vascular smooth muscle cell migration and neointima formation. Arterioscler Thromb Vasc Biol 2011;31:2424-31. 10.1161/ATVBAHA.111.232769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishita M, Wang Y, Tomizawa C, et al. Phosphoinositide 3-kinase-mediated activation of cofilin phosphatase Slingshot and its role for insulin-induced membrane protrusion. J Biol Chem 2004;279:7193-8. 10.1074/jbc.M312591200 [DOI] [PubMed] [Google Scholar]
- 19.Ohta Y, Kousaka K, Nagata-Ohashi K, et al. Differential activities, subcellular distribution and tissue expression patterns of three members of Slingshot family phosphatases that dephosphorylate cofilin. Genes Cells 2003;8:811-24. 10.1046/j.1365-2443.2003.00678.x [DOI] [PubMed] [Google Scholar]
- 20.Mizuno K. Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cell Signal 2013;25:457-69. 10.1016/j.cellsig.2012.11.001 [DOI] [PubMed] [Google Scholar]
- 21.Huang TY, DerMardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol 2006;18:26-31. 10.1016/j.ceb.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 22.Kaji N, Ohashi K, Shuin M, et al. Cell cycle-associated changes in Slingshot phosphatase activity and roles in cytokinesis in animal cells. J Biol Chem 2003;278:33450-5. 10.1074/jbc.M305802200 [DOI] [PubMed] [Google Scholar]
- 23.Soltanzad F, Samadishams S, Barar J, et al. Evaluation of cytotoxicity and anti-cancer effect of Ferula szowitsiana methanolic extract on lung cancer A549 cell-lines. Res Pharm Sci 2012;7:103.23181087 [Google Scholar]
- 24.Takahashi K, Kanno S-i, Mizuno K. Activation of cytosolic Slingshot-1 phosphatase by gelsolin-generated soluble actin filaments. Biochem Biophys Res Commun 2014;454:471-7. 10.1016/j.bbrc.2014.10.108 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Kuramitsu Y, Kitagawa T, et al. Cofilin-phosphatase slingshot-1L (SSH1L) is over-expressed in pancreatic cancer (PC) and contributes to tumor cell migration. Cancer Lett 2015;360:171-6. 10.1016/j.canlet.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Kim W, Lee YG, et al. Identification of sennoside A as a novel inhibitor of the slingshot (SSH) family proteins related to cancer metastasis. Pharmacol Res 2017;119:422-30. 10.1016/j.phrs.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 27.Nowak D, Mazur A, Popow-Woźniak A, et al. Subcellular distribution and expression of cofilin and ezrin in human colon adenocarcinoma cell lines with different metastatic potential. Eur J Histochem 2010;54:e14. 10.4081/ejh.2010.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]


