Abstract
Known primarily for its oxygen-sensing capabilities, the carotid body chemoreceptors have recently been implicated, primarily by work in animal models, in the pathophysiology of a number of metabolic conditions. The research presented in this brief review highlights translational work conducted at the Mayo Clinic between 2010 and 2017 in healthy humans and discusses key areas for future work in disease populations.
Keywords: carotid body, chemoreflex, humans, hypoxic ventilatory response
INTRODUCTION
The purpose of this review is to highlight a presentation given on August 4, 2017 at the International Union of Physiological Sciences conference in Rio de Janeiro, Brazil, in a symposium entitled “Polymodal properties of the carotid body chemoreceptors beyond hypoxia.” Known primarily for their oxygen-sensing capabilities, the carotid body chemoreceptors have recently been implicated, primarily by work in animal models, in the pathophysiology of a number of metabolic conditions. The research presented in this review highlights translational work conducted at the Mayo Clinic between 2010 and 2017 in healthy humans (15, 21, 25, 26, 31, 51, 52) and discusses key areas for future work in disease populations.
STUDYING CAROTID BODY CHEMORECEPTOR FUNCTION IN HUMANS
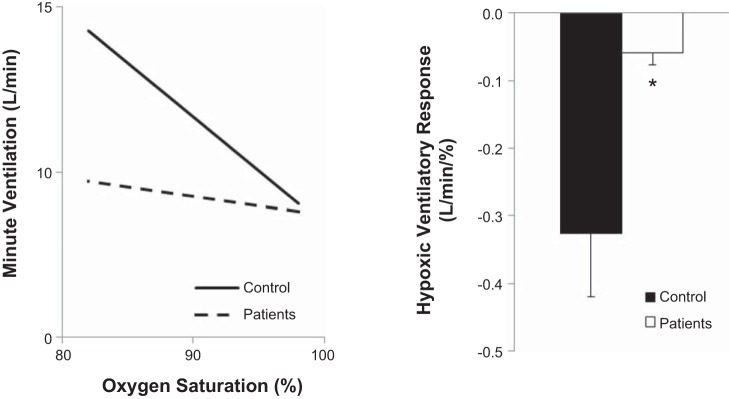
The carotid body chemoreceptors are located bilaterally at the bifurcation of each common carotid artery. The main purpose of the carotid chemoreceptors is to detect chemical changes within the peripheral arterial blood and initiate reflex changes in ventilation and sympathetic nervous system activity, with the primary known stimulus being a reduction in the partial pressure of oxygen (19, 38). Indeed, healthy humans exhibit robust increases in ventilation in response to hypoxia, termed the “hypoxic ventilatory response.” Assessed as the slope of the relationship between oxygen saturation and minute ventilation, the hypoxic ventilatory response is often determined by exposing individuals to a few short breaths of low (0.05 ) or no (0.00 ) oxygen and measuring reflex changes in ventilation (3, 11, 12). Although the method is quite repeatable, our group and others have shown there to be large variability in the hypoxic ventilatory response in healthy humans (23, 33). Despite this, there are clear increases in the hypoxic ventilatory response in human disease states, including those related to metabolic diseases (5, 36). In addition, our group and others have shown individuals who have had their carotid bodies resected have essentially no ventilatory response to hypoxia (21, 26, 45, 46, 52) (Fig. 1, A and B), supporting the effectiveness of testing the hypoxic ventilatory response as a measure of carotid body chemosensitivity.
Fig. 1.
Hypoxic ventilatory response is attenuated in individuals who have undergone bilateral carotid body resection. Carotid body chemosensitivity (hypoxic ventilatory response) is blunted in carotid body resected patients (n = 5) when compared with healthy controls (n = 4). *P < 0.01 vs. Control. Figure originally presented in (52).
Independently of completely removing the carotid bodies, a role for the carotid chemoreceptors under resting/basal conditions and in response to physical and/or environmental stress in conscious humans can be assessed via methodologies such as acute hyperoxia [0.40–1.0 (8, 54)] or intravenous low-dose dopamine (23, 33, 53). Prolonged exposure to high levels of inspired oxygen can elicit reductions in peripheral blood flow, increases in arterial blood pressure, and tachycardia. However, when hyperoxia is employed experimentally in the form of a modified Dejours test (<2 min of 100% oxygen), it has been shown to be a reliable way to assess and/or attenuate peripheral arterial chemosensitivity (7, 8). This is especially true when other variables such as respiratory rate, tidal volume, and/or end-tidal carbon dioxide are also carefully controlled (44). With this, the utility of experimental hyperoxia is limited in situations where long-term changes in blood pressure, blood flow, or responses to hypoxia are of interest. In such cases, low-dose dopamine may be a more useful methodological approach to decrease afferent activity of the carotid body chemoreceptors in humans. Dopamine in low doses (<5 µg/kg/min) is thought to blunt carotid sinus nerve discharge by binding to dopaminergic (primarily D2) receptors on carotid body Type 1 glomus cells to block calcium currents, leading to membrane hyperpolarization, reduced neurotransmitter release, and decreased carotid sinus nerve discharge (1, 2, 4, 13, 20, 27, 42). However, the independent effects of exogenous intravenous dopamine on the carotid body chemoreceptors may be dose dependent, examined in detail by our group (23). Thus a careful dosing scheme may be necessary to reliably use dopamine as an experimental tool to examine carotid body activity in humans. It is also important to acknowledge that exogenous dopamine, even in low doses, could increase circulating glucagon and/or growth hormone (15, 41, 48, 55), both which may confound studies interested in glucose regulation.
GLUCOSE REGULATION AND THE CAROTID CHEMORECEPTORS
Intermittent hypoxia, blood glucose, and contributing mechanisms.
Anecdotal evidence, available for many years, suggests the carotid body chemoreceptors, key oxygen-sensing sensors, may also play a role in glucose regulation (19). For example, 30 min of continuous hypoxia results in glucose intolerance in healthy humans (34). Furthermore, individuals exposed repeatedly to hypoxia during sleep (apnea hypopnea index >10 events per hour) exhibit high fasting glucose and an impaired glucose-insulin ratio (30). Along these lines, blood glucose is increased during intermittent hypoxia in rats (39), and fasting hyperglycemia observed following chronic intermittent hypoxia can be prevented by carotid body denervation (43). Together, these discoveries led our group to examine the effects of experimental intermittent hypoxia on blood glucose levels in humans. Previous work had suggested 8 h of intermittent hypoxia resulted in increases in glucose and impairments in insulin sensitivity (28). However, such prolonged exposures were unlikely to have isolated effects on the carotid chemoreceptors. Therefore, we sought to examine the effect of a shorter hypoxic exposure on blood glucose levels in healthy humans and the potential role of the carotid body chemoreceptors. To our surprise, glucose measured in venous blood was increased after only 30 min of exposure to intermittent hypoxia (~12 events, 8–10% reduction in SpO2 separated by 2 min normoxia) in healthy humans and remained high for 3 h (Fig. 2) (31). Furthermore, this increase in blood glucose occurred independently of any changes in insulin sensitivity (31). We also a observed a positive relationship, albeit weak, between glucose and the hypoxic ventilatory response, suggesting individuals with the greatest increase in carotid chemosensitivity following intermittent hypoxia had the highest blood glucose (32). Although intriguing, this study leaves more questions to be answered, such as: 1) where is this glucose coming from (changes in peripheral blood flow and glucose distribution? changes in insulin secretion? increased gluconeogenesis?), 2) are these effects exacerbated in individuals with sleep apnea and/or diabetes, 3) can this increase in glucose with intermittent hypoxia be prevented (exercise? medication?), and lastly, 4) what is the role of the carotid chemoreceptors in this response?
Fig. 2.
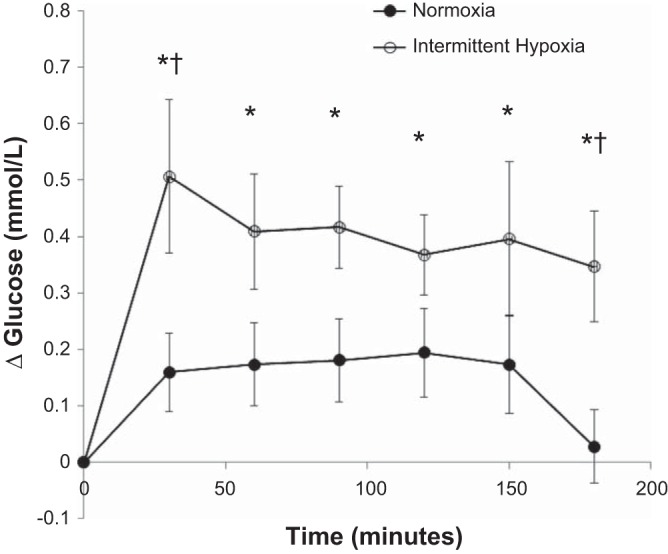
Venous blood glucose during 3 h of intermittent hypoxia or continuous normoxia in healthy humans. Glucose levels remained unchanged during the control visit (P > 0.05 for all). During the intermittent hypoxia visit, glucose levels were greater than T0 at all time points (*P < 0.01 for all). When data were compared between visits, there was a significant difference at T30 (†P = 0.02) and T180 (†P = 0.03). Figure originally presented in (31).
Hypoglycemia counterregulation: acute responses vs. long-term adaptations.
In contrast to increases blood glucose with hypoxia, others have found oxygen therapy improve glucose tolerance and/or insulin sensitivity in patients with diabetes and chronic obstructive pulmonary disease (10, 14, 49). Although contributing mechanisms are unclear, some have suggested that hyperoxia works by attenuating the ability of key peripheral receptors (i.e., carotid chemoreceptors) to sense and/or respond to alterations in blood glucose (49). In line with this idea, Pardal and Lopez-Barneo (35) showed that the carotid bodies exhibit a secretory response during exposure to low glucose. Furthermore, removal of the carotid body chemoreceptors in dogs impaired their ability to respond to hypoglycemia and initiate appropriate counterregulatory responses (18). With this information in mind, we sought to examine whether decreases in carotid chemoreceptor activity would decrease mobilization of key glucoregulatory (e.g., glucagon, growth hormone, epinephrine, cortisol) hormones and impair normal glucose control in healthy humans.
Using systemic hyperoxia to attenuate carotid body afferent activity, Wehrwein and colleagues (51) showed the normal rise in glucoregulatory hormones (e.g., epinephrine, cortisol, glucagon, growth hormone) during a hyperinsulinemic-hypoglycemic clamp was impaired in healthy adult humans. These data (51) were some of the first in humans to show by acutely reducing carotid body chemoreceptor activity the counterregulatory hormone response to hypoglycemia is attenuated. However, the chronic effect of carotid chemoreceptor “desensitization” on glucose control was unclear. For this reason, we recruited five individuals who had their carotid bodies resected bilaterally (for the removal of glomus tumors) to complete the same experiment (52). These individuals underwent two hyperinsulinemic-hypoglycemic clamps during normoxia and hyperoxia, completed on separate days. As we expected, there was no observable effect of hyperoxia on hypoglycemia counterregulation in individuals who had their carotid body chemoreceptors removed, suggesting any effect of hyperoxia on glucose regulation in healthy adults was via the carotid body chemoreceptors (52).
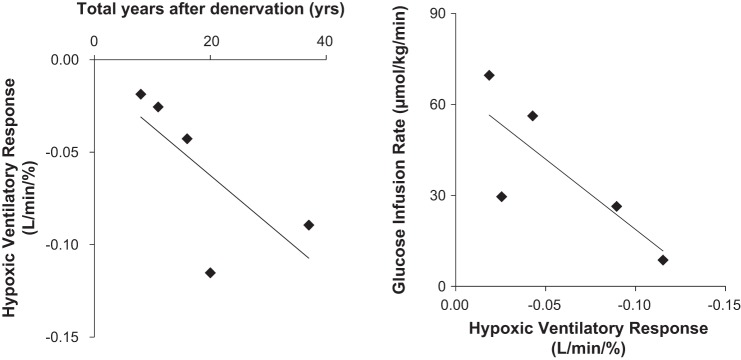
We also found, quite unexpectedly, individuals who had their carotid bodies resected exhibited essentially normal glucoregulatory responses to hypoglycemia under normoxic conditions (52). At first glance these data would suggest long-term removal of the carotid body chemoreceptors does not impact normal glucose control. However, after closer examination of the data we observed two key relationships in the individuals studied. First, there was an association between the time from bilateral resection (years) and the presence of carotid chemoreceptor sensitivity (hypoxic ventilatory response) such that those individuals that underwent surgery most recently exhibited the greatest impairments in chemoreceptor sensitivity to hypoxia (Fig. 3A). Second, those patients with the lowest level of chemoreceptor sensitivity tended to require the greatest amount of glucose infused during the hyperinsulinemic-hypoglycemic clamp (Fig. 3B). In other words, the level of impairment in the counterregulatory response to hypoglycemia was related to the level of residual carotid body chemosensitivity. Together these data suggest the carotid body chemoreceptors play an important role in the counterregulatory response to hypoglycemia when examined acutely in healthy humans and/or shortly after bilateral carotid body resection. However, long-term adaptations likely occur following carotid body resection in humans such that redundant mechanisms are able to recover any previous role of the carotid body chemoreceptors in glucose regulation.
Fig. 3.
Relationship between carotid body chemosensitivity and time from surgery (A), hypoglycemia counterregulation (B). A: those patients that underwent carotid body resection most recently exhibited the greatest impairments in chemoreceptor sensitivity to hypoxia (r = −0.835, P = 0.08). B: those patients with the lowest level of chemoreceptor sensitivity (hypoxic ventilatory response) required the greatest amount of glucose infused during the hyperinsulinemic-hypoglycemic clamp (r = 0.798, P = 0.11). Figure originally presented in (52).
POTENTIALLY CONFOUNDING EFFECTS OF INSULIN
Now until this time, the majority of our experiments designed to access a role for the carotid chemoreceptors in glucose regulation in humans occurred in the setting of high insulin (e.g., hyperinsulinemic clamp). However, in the midst of our work Ribeiro and colleagues (40) found insulin receptors on the carotid bodies and showed insulin increases carotid body neurosecretion and elicits hyperventilation in rats. Because high levels of insulin may have confounded our previous findings (22), we followed up our previous work by asking two important questions: 1) Can we experimentally decrease blood glucose in humans independently of insulin? and 2) Do the carotid bodies sense and/or respond to insulin in humans?
Decreasing blood glucose independently of insulin.
Koyama and colleagues (17) showed in dogs that prolonged high-intensity exercise could elicit a significant reduction in glucose independently of insulin (50% V̇o2max for 150 min, 6 mg/dl reduction in glucose by ~90 min). Furthermore, in the same model of exercise, carotid body resection resulted in fall in blood glucose that was more severe (~11 mg/dl), occurred much earlier (within 30 min), and glucose remained low throughout the entire exercise protocol (17). Thus, to further our understanding of the role for the carotid bodies in glucose regulation and translate these findings to humans, our group, led by Blair Johnson (15), recruited elite cyclists known for their ability to exercise at a high intensity for a long period of time. These individuals completed an upright cycling protocol at 65% peak oxygen consumption for up to 120 min under a control (saline) condition and during intravenous infusion of low-dose dopamine. Similar to what was seen in dogs, decreasing carotid body afferent activity with low-dose dopamine resulted in a greater fall in blood glucose during the exercise protocol in the elite cyclists studied. As a result, exercise duration was also significantly reduced during dopamine infusion when compared with control (15). These data support a role for the carotid chemoreceptors in normal glucose control during prolonged exercise in healthy exercise-trained individuals and are agreement with our earlier work using hyperinsulinemic-hypoglycemic clamps. Thus, using an insulin-independent model of hypoglycemia, we were able to confirm previous work suggesting the carotid chemoreceptors play a role in normal glucose control.
Insulin-sensing capabilities of the carotid chemoreceptors in humans.
On the basis of the data presented above, it appears the carotid body chemoreceptors play at least a minor role in glucose regulation in healthy humans. However, in day-to-day situations, it is rare to observe changes in glucose and/or insulin independent of the other. With this, we continued to ask the question: What is the role of the carotid body chemoreceptors in the reflex response to insulin? As noted above, Ribeiro and colleagues (40) found insulin receptors on the carotid bodies and showed insulin increases carotid body neurosecretion and elicits hyperventilation in rats. Furthermore, in humans, the hypoxic ventilatory response has been shown to be increased in the presence of insulin in the form of a hyperinsulinemic clamp (50) and in response to a meal (56). It is commonly accepted that even small increases in plasma insulin concentrations have marked sympathoexcitatory effects, and data from animals have shown injection of insulin into the carotid artery increases blood pressure through a sympathetic mechanism (37). Therefore, we sought to explore a potential role for the carotid body chemoreceptors in insulin-mediated sympathoexcitation. To do this, we completed hyperinsulinemic, euglycemic clamps in healthy humans and measured reflex changes in ventilation (tidal volume, respiratory rate) and sympathetic nervous system activity [muscle sympathetic nerve activity via microneurography (47)]. Healthy individuals completed experiments under normoxic and hyperoxic conditions, in addition to saline and low-dose dopamine infusions. As expected, both muscle sympathetic nerve activity and minute ventilation significantly increased in the presence of increases in systemic insulin; however, any effect of acute hyperoxia did not differ between baseline and hyperinsulinemia (25). These data suggest the carotid chemoreceptors do not contribute acutely to insulin-mediated increases in sympathetic nervous system activity in healthy humans (25). However, when low-dose dopamine was given before the hyperinsulinemic clamp, the rise in sympathetic activity with insulin was attenuated (25). Although we observed potentially confounding changes in basal sympathetic activity with exogenous dopamine administration, these data may support a role for the carotid chemoreceptors in more long-term exposure to insulin (25). With this, it would be interesting to see whether similar conclusions would be made if the individuals studied were insulin resistant (5, 6, 22).
NEW QUESTIONS AND FUTURE DIRECTIONS
In summary, we have been able to translate a number of key studies in animals to humans and have expanded upon our understanding of the role for the carotid body chemoreceptors in glucose regulation. By manipulating the level of carotid body activation in healthy humans, we can significantly increase fasting glucose (intermittent hypoxia) and impair normal glucose regulation (acute hyperoxia, low-dose dopamine). Much of the observed changes in glucose control appear to occur independently of changes in insulin; however, a limitation of the current body of knowledge is the completion of studies in only relatively young, healthy adults. Whether similar findings can be translated to patient populations known for exhibiting impairments in glucose control (e.g., insulin resistance, diabetes) has yet to be thoroughly addressed; however, there are strong data to suggest, in disease populations, the role for the carotid bodies in glucose regulation (and/or dysregulation) may be significant (36, 40, 49). With this, there are clear opportunities for long-term adaptation in glucose control following carotid body resection (52). Thus, for carotid body-mediated therapies to become a viable avenue for treatment, it becomes increasingly important to build a better understanding of: 1) what are the carotid bodies sensing (16), 2) who would benefit from such therapies and how can they be identified (24), 3) are there nonpharmacological and/or nonsurgical methods that may be beneficial (9, 29)?
GRANTS
Funding for the work described in this paper came from: DK-090541 (Joyner); HL-83947 (Joyner); HL-130339 (Limberg); AHA15SDG25080095 (Limberg); DK-084624 (Wehrwein).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
J.K.L. conceived and designed research, prepared figures, drafted manuscript, edited and revised manuscript, approved final version of manuscript.
ACKNOWLEDGMENTS
The majority of the data presented were collected in the laboratory of Michael Joyner at the Mayo Clinic, in collaboration with Timothy Curry, Robert Rizza, Rita Basu, Ananda Basu, William Young, and Adrian Vella. Key contributors include Erica Wehrwein, Blair Johnson, Sushant Ranadive, and Walter Holbein. The experiments could not have been completed without support from Shelly Roberts, Sarah Wolhart, Christopher Johnson, Nancy Meyer, Jennifer Taylor, Michael Mozer, and Lauren Newhouse.
REFERENCES
- 1.Bisgard GE, Forster HV, Klein JP, Manohar M, Bullard VA. Depression of ventilation by dopamine in goats–effects of carotid body excision. Respir Physiol 40: 379–392, 1980. doi: 10.1016/0034-5687(80)90036-5. [DOI] [PubMed] [Google Scholar]
- 2.Black AM, Comroe JH Jr, Jacobs L. Species difference in carotid body response of cat and dog to dopamine and serotonin. Am J Physiol 223: 1097–1102, 1972. [DOI] [PubMed] [Google Scholar]
- 3.Chua TP, Coats AJ. The reproducibility and comparability of tests of the peripheral chemoreflex: comparing the transient hypoxic ventilatory drive test and the single-breath carbon dioxide response test in healthy subjects. Eur J Clin Invest 25: 887–892, 1995. doi: 10.1111/j.1365-2362.1995.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 4.Ciarka A, Vincent JL, van de Borne P. The effects of dopamine on the respiratory system: friend or foe? Pulm Pharmacol Ther 20: 607–615, 2007. doi: 10.1016/j.pupt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Conde SV, Ribeiro MJ, Melo BF, Guarino MP, Sacramento JF. Insulin resistance: a new consequence of altered carotid body chemoreflex? J Physiol 595: 31–41, 2017. doi: 10.1113/JP271684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conde SV, Sacramento JF, Guarino MP, Gonzalez C, Obeso A, Diogo LN, Monteiro EC, Ribeiro MJ. Carotid body, insulin, and metabolic diseases: unraveling the links. Front Physiol 5: 418, 2014. doi: 10.3389/fphys.2014.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejours P. Chemoreflexes in breathing. Physiol Rev 42: 335–358, 1962. doi: 10.1152/physrev.1962.42.3.335. [DOI] [PubMed] [Google Scholar]
- 8.Dejours P. Control of respiration by arterial chemoreceptors. Ann N Y Acad Sci 109: 682–695, 1963. doi: 10.1111/j.1749-6632.1963.tb13497.x. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio R, Marcus NJ, Schultz HD. Calorie restriction as a new approach for resetting carotid body chemoreflex control in heart failure. FASEB J 28, 1 Suppl: 873.11, 2014. [Google Scholar]
- 10.Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 134: 686–692, 2008. doi: 10.1378/chest.08-0556. [DOI] [PubMed] [Google Scholar]
- 11.Duffin J. Measuring the ventilatory response to hypoxia. J Physiol 584: 285–293, 2007. doi: 10.1113/jphysiol.2007.138883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabel RA, Kronenberg RS, Severinghaus JW. Vital capacity breaths of 5 percent or 15 percent CO 2 in N 2 or O 2 to test carotid chemosensitivity. Respir Physiol 17: 195–208, 1973. doi: 10.1016/0034-5687(73)90061-3. [DOI] [PubMed] [Google Scholar]
- 13.Ide T, Shirahata M, Chou CL, Fitzgerald RS. Effects of a continuous infusion of dopamine on the ventilatory and carotid body responses to hypoxia in cats. Clin Exp Pharmacol Physiol 22: 658–664, 1995. doi: 10.1111/j.1440-1681.1995.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsson P, Jorfeldt L. Oxygen supplementation increases glucose tolerance during euglycaemic hyperinsulinaemic glucose clamp procedure in patients with severe COPD and chronic hypoxaemia. Clin Physiol Funct Imaging 26: 271–274, 2006. doi: 10.1111/j.1475-097X.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson BD, Peinado AB, Ranadive SM, Curry TB, Joyner MJ. Effects of intravenous low-dose dopamine infusion on glucose regulation during prolonged aerobic exercise. Am J Physiol Regul Integr Comp Physiol 314: R49–R57, 2018. doi: 10.1152/ajpregu.00030.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joyner MJ, Limberg JK. Hitting the wall: glycogen, glucose and the carotid bodies. J Physiol 592: 4413–4414, 2014. doi: 10.1113/jphysiol.2014.281790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab 281: E742–E748, 2001. doi: 10.1152/ajpendo.2001.281.4.E742. [DOI] [PubMed] [Google Scholar]
- 18.Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49: 1434–1442, 2000. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Bin-Jaliah I. Adequate stimuli of the carotid body: more than an oxygen sensor? Respir Physiol Neurobiol 157: 12–21, 2007. doi: 10.1016/j.resp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Lahiri S, Nishino T. Inhibitory and excitatory effects of dopamine on carotid chemoreceptors. Neurosci Lett 20: 313–318, 1980. doi: 10.1016/0304-3940(80)90166-4. [DOI] [PubMed] [Google Scholar]
- 21.Larson KF, Limberg JK, Baker SE, Joyner MJ, Curry TB. Intact blood pressure, but not sympathetic, responsiveness to sympathoexcitatory stimuli in a patient with unilateral carotid body resection. Physiol Rep 5: e13212, 2017. doi: 10.14814/phy2.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Limberg JK, Curry TB, Prabhakar NR, Joyner MJ. Is insulin the new intermittent hypoxia? Med Hypotheses 82: 730–735, 2014. doi: 10.1016/j.mehy.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limberg JK, Johnson BD, Holbein WW, Ranadive SM, Mozer MT, Joyner MJ. Interindividual variability in the dose-specific effect of dopamine on carotid chemoreceptor sensitivity to hypoxia. J Appl Physiol (1985) 120: 138–147, 2016. doi: 10.1152/japplphysiol.00723.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Limberg JK, Joyner MJ. Should we be ‘doping’ the peripheral chemoreceptors? J Physiol 592: 1177, 2014. doi: 10.1113/jphysiol.2014.271023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limberg JK, Mozer MT, Holbein WW, Johnson BD, Prabhakar NR, Curry TB, Joyner MJ. Low-dose dopamine, but not acute hyperoxia, attenuates the sympathoexcitatory response to hyperinsulinemia in healthy humans. FASEB J 31 1 Suppl: lb1-1091.2, 2017. [Google Scholar]
- 26.Limberg JK, Taylor JL, Mozer MT, Dube S, Basu A, Basu R, Rizza RA, Curry TB, Joyner MJ, Wehrwein EA. Effect of bilateral carotid body resection on cardiac baroreflex control of blood pressure during hypoglycemia. Hypertension 65: 1365–1371, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llados F, Zapata P. Effects of dopamine analogues and antagonists on carotid body chemosensors in situ. J Physiol 274: 487–499, 1978. doi: 10.1113/jphysiol.1978.sp012162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis M, Punjabi NM. Effects of acute intermittent hypoxia on glucose metabolism in awake healthy volunteers. J Appl Physiol (1985) 106: 1538–1544, 2009. doi: 10.1152/japplphysiol.91523.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcus NJ, Pügge C, Mediratta J, Schiller AM, Del Rio R, Zucker IH, Schultz HD. Exercise training attenuates chemoreflex-mediated reductions of renal blood flow in heart failure. Am J Physiol Heart Circ Physiol 309: H259–H266, 2015. doi: 10.1152/ajpheart.00268.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meslier N, Gagnadoux F, Giraud P, Person C, Ouksel H, Urban T, Racineux JL. Impaired glucose-insulin metabolism in males with obstructive sleep apnoea syndrome. Eur Respir J 22: 156–160, 2003. doi: 10.1183/09031936.03.00089902. [DOI] [PubMed] [Google Scholar]
- 31.Newhouse LP, Joyner MJ, Curry TB, Laurenti MC, Man CD, Cobelli C, Vella A, Limberg JK. Three hours of intermittent hypoxia increases circulating glucose levels in healthy adults. Physiol Rep 5: e13106, 2017. doi: 10.14814/phy2.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newhouse LP, Joyner MJ, Curry TB, Limberg JK. 3 hours of intermittent hypoxia increases circulating glucose levels in healthy adults. FASEB J 30, 1 Suppl: 1001, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niewinski P, Tubek S, Banasiak W, Paton JF, Ponikowski P. Consequences of peripheral chemoreflex inhibition with low-dose dopamine in humans. J Physiol 592: 1295–1308, 2014. doi: 10.1113/jphysiol.2013.266858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oltmanns KM, Gehring H, Rudolf S, Schultes B, Rook S, Schweiger U, Born J, Fehm HL, Peters A. Hypoxia causes glucose intolerance in humans. Am J Respir Crit Care Med 169: 1231–1237, 2004. doi: 10.1164/rccm.200308-1200OC. [DOI] [PubMed] [Google Scholar]
- 35.Pardal R, López-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci 5: 197–198, 2002. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- 36.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension 61: 5–13, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 37.Pereda SA, Eckstein JW, Abboud FM. Cardiovascular responses to insulin in the absence of hypoglycemia. Am J Physiol 202: 249–252, 1962. doi: 10.1152/ajplegacy.1962.202.2.249.14485087 [DOI] [Google Scholar]
- 38.Prabhakar NR, Peng YJ. Oxygen sensing by the carotid body: past and present. Adv Exp Med Biol 977: 3–8, 2017. doi: 10.1007/978-3-319-55231-6_1. [DOI] [PubMed] [Google Scholar]
- 39.Rafacho A, Gonçalves-Neto LM, Ferreira FB, Protzek AO, Boschero AC, Nunes EA, Zoccal DB. Glucose homoeostasis in rats exposed to acute intermittent hypoxia. Acta Physiol (Oxf) 209: 77–89, 2013. doi: 10.1111/apha.12118. [DOI] [PubMed] [Google Scholar]
- 40.Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes 62: 2905–2916, 2013. doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubí B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, Maechler P. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem 280: 36824–36832, 2005. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- 42.Sampson SR. Mechanism of efferent inhibition of carotid body chemoreceptors in the cat. Brain Res 45: 266–270, 1972. doi: 10.1016/0006-8993(72)90236-3. [DOI] [PubMed] [Google Scholar]
- 43.Shin MK, Yao Q, Jun JC, Bevans-Fonti S, Yoo DY, Han W, Mesarwi O, Richardson R, Fu YY, Pasricha PJ, Schwartz AR, Shirahata M, Polotsky VY. Carotid body denervation prevents fasting hyperglycemia during chronic intermittent hypoxia. J Appl Physiol (1985) 117: 765–776, 2014. doi: 10.1152/japplphysiol.01133.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol 586: 1743–1754, 2008. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmers HJ, Karemaker JM, Wieling W, Marres HA, Folgering HT, Lenders JW. Baroreflex and chemoreflex function after bilateral carotid body tumor resection. J Hypertens 21: 591–599, 2003. doi: 10.1097/00004872-200303000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Timmers HJ, Wieling W, Karemaker JM, Lenders JW. Denervation of carotid baro- and chemoreceptors in humans. J Physiol 553: 3–11, 2003. doi: 10.1113/jphysiol.2003.052415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallbo AB, Hagbarth KE, Torebjörk HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev 59: 919–957, 1979. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- 48.Vance ML, Kaiser DL, Frohman LA, Rivier J, Vale WW, Thorner MO. Role of dopamine in the regulation of growth hormone secretion: dopamine and bromocriptine augment growth hormone (GH)-releasing hormone-stimulated GH secretion in normal man. J Clin Endocrinol Metab 64: 1136–1141, 1987. doi: 10.1210/jcem-64-6-1136. [DOI] [PubMed] [Google Scholar]
- 49.Vera-Cruz P, Guerreiro F, Ribeiro MJ, Guarino MP, Conde SV. hyperbaric oxygen therapy improves glucose homeostasis in type 2 diabetes patients: a likely involvement of the carotid bodies. Adv Exp Med Biol 860: 221–225, 2015. doi: 10.1007/978-3-319-18440-1_24. [DOI] [PubMed] [Google Scholar]
- 50.Ward DS, Voter WA, Karan S. The effects of hypo- and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol 582: 859–869, 2007. doi: 10.1113/jphysiol.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol 588: 4593–4601, 2010. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wehrwein EA, Limberg JK, Taylor JL, Dube S, Basu A, Basu R, Rizza RA, Curry TB, Joyner MJ. Effect of bilateral carotid body resection on the counterregulatory response to hypoglycaemia in humans. Exp Physiol 100: 69–78, 2015. doi: 10.1113/expphysiol.2014.083154. [DOI] [PubMed] [Google Scholar]
- 53.Welsh MJ, Heistad DD, Abboud FM. Depression of ventilation by dopamine in man. Evidence for an effect on the chemoreceptor reflex. J Clin Invest 61: 708–713, 1978. doi: 10.1172/JCI108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whipp BJ, Wasserman K. Carotid bodies and ventilatory control dynamics in man. Fed Proc 39: 2668–2673, 1980. [PubMed] [Google Scholar]
- 55.Zhang Y, Zheng R, Meng X, Wang L, Liu L, Gao Y. Pancreatic endocrine effects of dopamine receptors in human islet cells. Pancreas 44: 925–929, 2015. doi: 10.1097/MPA.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 56.Zwillich CW, Sahn SA, Weil JV. Effects of hypermetabolism on ventilation and chemosensitivity. J Clin Invest 60: 900–906, 1977. doi: 10.1172/JCI108844. [DOI] [PMC free article] [PubMed] [Google Scholar]