Abstract
Alternative splicing of RNA is an underexplored area of transcriptional response. We expect that early changes in alternatively spliced genes may be important for responses to cardiac injury. Hypoxia inducible factor 1 (HIF1) is a key transcription factor that rapidly responds to loss of oxygen through alteration of metabolism and angiogenesis. The goal of this study was to investigate the transcriptional response after myocardial infarction (MI) and to identify novel, hypoxia-driven changes, including alternative splicing. After ligation of the left anterior descending artery in mice, we observed an abrupt loss of cardiac contractility and upregulation of hypoxic signaling. We then performed RNA sequencing on ischemic heart tissue 1 and 3 days after infarct to assess early transcriptional changes and identified 89 transcripts with altered splicing. Of particular interest was the switch in Pkm isoform expression (pyruvate kinase, muscle). The usually predominant Pkm1 isoform was less abundant in ischemic hearts, while Pkm2 and associated splicing factors (hnRNPA1, hnRNPA2B1, Ptbp1) rapidly increased. Despite increased Pkm2 expression, total pyruvate kinase activity remained reduced in ischemic myocardial tissue. We also demonstrated HIF1 binding to PKM by chromatin immunoprecipitation, indicating a direct role for HIF1 in mediating this isoform switch. Our study provides a new, detailed characterization of the early transcriptome after MI. From this analysis, we identified an HIF1-mediated alternative splicing event in the PKM gene. Pkm1 and Pkm2 play distinct roles in glycolytic metabolism and the upregulation of Pkm2 is likely to have important consequences for ATP synthesis in infarcted cardiac muscle.
Keywords: alternative splicing, hypoxia, myocardial infarction, pyruvate kinase, RNA sequencing
INTRODUCTION
Alternative splicing of RNA is increasingly appreciated as an important mechanism by which organisms modulate the functional diversity of proteins. Changes in the expression of specific transcripts are driven by the utilization of alternate promoter and splice site sequences, the availability of transcription factors and RNA binding proteins, and RNA stability. The heart undergoes substantial changes in its splicing networks as the organ develops in utero and postnatally (2). For example, splicing alterations in titin (TTN) can modify sarcomeric stiffness and thereby influence cardiac function. Mutations in RBM20, a splicing factor of TTN, have also been linked to dilated cardiomyopathy (2). While alternative splicing controls a number of physiological processes during development and cell differentiation, it also plays a role in pathological conditions (2). Although the significance of alternative splicing after cardiac injury is not well understood, it is likely to have substantial physiological importance.
The ischemic injury of myocardial infarction (MI) dramatically modifies the tissue environment and subsequent gene expression. Hypoxic signaling is a crucial part of the response to ischemia that includes angiogenesis and enhanced glycolysis along with other oxygen-dependent processes. The principal driver of this signaling is hypoxia inducible factor 1 (HIF1), a transcription factor that is stabilized during low oxygen conditions to orchestrate numerous changes in gene expression (40). HIF1 is also thought to be involved, at least indirectly, in alternative splicing through upregulation of splicing factors. SF3B1, for example, is an HIF1 target gene that mediates an isoform switch in the fructose metabolizing enzyme ketohexokinase related to pathological cardiac hypertrophy (29).
While prior examinations of infarction-related changes in gene expression (typically by microarray analysis) have been productive (13, 46), we focused on examining changes in alternative splicing by high-throughput sequencing. We identified 89 genes that had changes in alternative splicing within the first 3 days after MI. Previous studies have demonstrated that one of these genes, pyruvate kinase, muscle (PKM), is a crucial metabolic enzyme and potentially regulated by HIF1, so we concentrated our efforts on further characterizing this gene in our model.
Pyruvate kinase mediates the conversion of phosphoenolpyruvate to pyruvate in the glycolytic pathway. Two genes, PKLR and PKM, give rise to the four known isoforms (Pkl, Pkr, Pkm1, and Pkm2) through alternate promoter (Pkl and Pkr) and mutually exclusive exon utilization (Pkm1 and Pkm2) (15). Pkl is found in the liver, kidney, and intestine, while Pkr is restricted to erythrocytes (15). Pkm1 is constitutively active and organized as a tetramer in several tissues with high energy demand, including brain and muscle, to promote usage of pyruvate in the tricarboxylic (TCA) cycle and oxidative phosphorylation (5, 8). By contrast, Pkm2 has been found to be expressed more generally throughout the body, though it has been suggested to be particularly important during embryogenesis and in proliferating cells (8, 15). It can form either a dimer or a tetramer, which is modulated through binding of numerous factors including fructose-1,6-bisphosphonate (FBP), phosphotyrosine proteins, and posttranslational modifications (15). As a tetramer Pkm2 activity is similar to Pkm1, but as a dimer it is less efficient, allowing pyruvate to be converted to lactate and upstream metabolites to be utilized in other metabolic pathways (15, 48).
Our data reveal a substantial change in Pkm isoform expression in ischemic heart tissue. Upon injury, constitutively expressed Pkm1 decreased, while Pkm2 expression and its associated splicing factors were substantially increased. These changes corresponded with increased HIF1 expression, which bound to a hypoxia response element in the promoter of PKM, indicating a direct link between HIF1 and Pkm2 expression. Increased Pkm2 expression has been noted in other forms of chronic heart disease and has also been associated with heart failure (25, 35). It appears that enhanced Pkm2 expression during acute MI is an initial response to injury that is sustained for weeks after injury. The early change in metabolism caused by this ischemia-dependent alteration in splicing of PKM mRNA may have lasting effects on the recovery of contractility and heart function.
MATERIALS AND METHODS
Mice and reagents.
C57BL/6 male mice between 8 and 12 wk of age (>25 g) were used for all experiments. All animal protocols and experiments were approved by the Institutional Animal Care and Use Committee of the University of Hawaii at Manoa. Antibodies used in this study were purchased from the following sources: rabbit anti-mouse HIF1α (Novus Biologicals, NB100-479), rabbit anti-human VEGFA (Abcam, ab46154), mouse anti-rabbit GAPDH (Sigma, clone GAPDH-71.1, G8795 or Thermo Fisher, clone 6C5, AM4300), rabbit anti-human Pkm1 (Proteintech, 15821-1-AP), rabbit anti-human Pkm2 (Proteintech, 15822-1-AP), rabbit anti-human hnRNPA1 (Abcam, clone EPR12768, ab177152), mouse anti-human hnRNPA2B1 (Santa Cruz, clone DP3B3, sc-32316), rabbit anti-human Ptbp1 (Cell Signaling, 8776), rabbit anti-human histone H3 (Cell Signaling, 9715), mouse anti-human cardiac troponin T (Genetex, clone 1C11, GTX28295), mouse anti-rabbit sarcomeric alpha-actinin antibody (Genetex, clone EA-53, GTX29465), streptavidin-Alexa Fluor 594 (Thermo Fisher, S32356), and donkey anti-rabbit IgG-Alexa Fluor 488 (Thermo Fisher, A-21206).
Echocardiography.
We used a 38 MHz transducer with a Vevo 2100 system (VisualSonics) to assess left ventricular function by transthoracic echocardiography on sentient mice. B-mode and M-mode imaging were used to obtain left ventricular parasternal short-axis views. M-mode images of at least three heartbeats were then used to measure fractional shortening (FS%) using the following equation: FS% = [(LVEDD − LVESD)/LVEDD] ×100. LVEDD = left ventricular end-diastolic internal diameter, LVESD = left ventricular end-systolic internal diameter.
MI.
To mimic MI we used permanent left anterior descending artery (LAD) ligation as previously described (4). In brief, the chest was depilated, and baseline echo collected. Mice were then intubated for anesthesia with isoflurane. After left-lateral thoracotomy between the third and fourth rib, the pericardial sac was opened, and LAD was ligated with 7.0 silk. Ischemia was confirmed by ST segment elevation on electrocardiogram (Power Laboratory, ADInstruments) and blanching of the tissue observed before incisions were closed. Animals were then extubated and monitored until awake and mobile. Control sham surgeries were performed identically except for ligation of the artery. At designated time points, echocardiograms were performed before mice were euthanized by CO2. Hearts were removed and perfused with PBS. Tissue was then either further dissected into ischemic and remainder fractions, as shown in Fig. 1B, and flash-frozen in liquid nitrogen, or cut coronally at the suture site and placed into 10% neutral-buffered formalin for overnight incubation at 4°C before further processing and paraffin embedding.
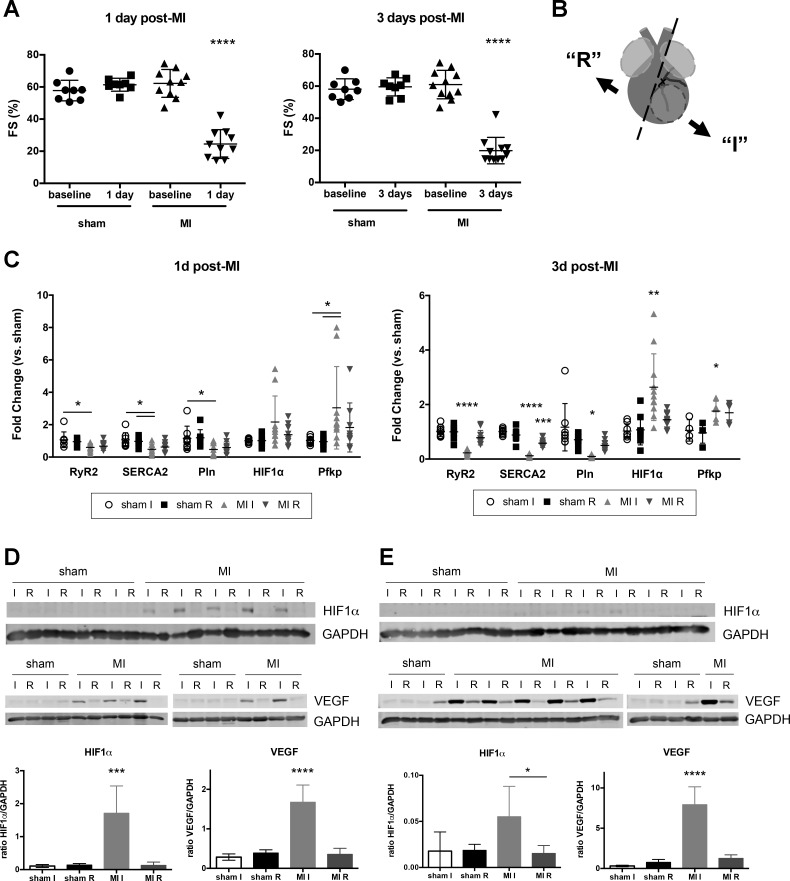
Fig. 1.
Early response to myocardial infarction (MI) injury included upregulation of hypoxic signaling and decreased cardiac contractility. A: fractional shortening (FS) in sham and MI mice at baseline, 1 and 3 days after MI. B: diagram of tissue dissection after MI. At designated time point, hearts were collected and atria were removed (light gray). Ventricular tissue (dark gray) was then separated into ischemic free wall (I, infarct + border regions of left ventricle) and remainder (R, includes some left ventricle, septum, and right ventricle). Ischemic tissue was identified by tissue blanching, and the surrounding border region was included in this fraction. Sham animals were dissected in a similar manner with the free wall considered the “ischemic” fraction and remaining tissue as “remainder.” Circled area indicates infarcted region of left ventricle, below the suture ligating the left anterior descending artery. C: semiquantitative PCR data for 1 and 3 days (d) after MI. Data shown with means ± SD. For A and C, n = 8–11 mice for each time point except Pfkp at 3 days after MI (n = 4–5). Western blotting and quantification for HIF1α and VEGF in heart tissue lysate at 1 day (D) and 3 days post-MI (E). GAPDH used for loading control and normalization. All analyses were performed with one-way ANOVA with Tukey’s multiple comparisons or Kruskal-Wallis with Dunn’s multiple comparisons. All significant differences indicate comparison to all other fractions unless otherwise denoted. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
RNA isolation and semiquantitative PCR.
To isolate total RNA, we pulverized frozen heart tissue and processed it with a Qiagen miRNeasy mini kit according to manufacturer’s instructions. RNA (1 μg) was then reverse-transcribed and assessed with FastStart Universal SYBR Green Master Mix (Roche) or QuantiTect SYBR Green PCR Kit (Qiagen) and primers as described. Primers were either purchased from Qiagen [QuantiTect Primer Assays; HIF1a, Pfkp, RyR2, Atp2a2 (SERCA2), Pln] or designed (Pkm1, Pkm2, Ptbp1, hnRNPA1, hnRNPA2B1) with sequences listed in Table 1. When possible, primers were designed to span exon-exon junctions. Samples were run on a 7900HT Fast Real-Time System or QuantStudio 12K Flex Real-Time PCR System (Applied Biosystems), and relative expression was calculated by ΔΔCt according to standard methods. Tangerin (EHBP1L1) transcript was used for normalization as its abundance is similar to many of the targets, and its expression has previously been shown to be unaffected by hypoxia (3). Expression was then further normalized to the mean value of “sham I” heart fractions with data displayed as fraction thereof (“sham I” set to a fold change value of 1.0).
Table 1.
Primer sequences designed for semiquantitative PCR
| Transcript | Forward Sequence | Reverse Sequence |
|---|---|---|
| Pkm1 | CACCGTCTGCTGTTTGAAGA | AGCACTCCTGCCAGACT |
| Pkm2 | CATCTACCACTTGCAGCTATTC | GAGCACTCCTGCCAGACT |
| hnRNPA1 | ACTCTACCGTCATGTCTAAGT | GACTCTCGTCGGTTGTTTC |
| hnRNPA2B1 | GGACCAGGAAGCAACTTTAG | GTCCTCCTCCATACCCATTA |
| Ptbp1 | GGGTCGGTTTCTGCTATTC | CTTTGTACCGACTGCTATGT |
RNA sequencing and analysis.
Total RNA was isolated as described above, and quality was assessed by an Agilent 2100 Bioanalyzer. Only samples with an RNA integrity number value of ≥ 7.5 were used for sequence analysis. Samples were then sent to Genewiz (South Plainfield, NJ) for rRNA depletion and paired-end sequencing on an Illumina HiSeq2500. We explored FASTQ files with FastQC (Babraham Institute, Cambridge, UK) and cleaned them by filtering out reads having mean Phred quality score < 20 (99% accurate) with Prinseq, a perl script (37). The Mus musculus UCSC mm10 reference genome was indexed by Bowtie2 v2.2.5 (23). High quality reads were mapped against the reference genome with Tophat v2.0.14 (20). Resulting mapped binary alignment/map (BAM) files for sham and MI samples were sorted and converted to sequence alignment map (SAM) files with SAMtools v0.1.2 and then used to generate count reads mapped to mouse gene models with htseq-count script on HTSeq package (1). Differential gene expression from the count data was identified with the DESeq2 Bioconductor package (26). CLC Genomic Workbench v9 software (CLC Bio), along with empirical analysis of DGE algorithm implemented in it, was used for the transcript-level expression analysis. The genes and transcripts with Benjamini and Hochberg q value <0.05 were called differentially expressed between sham and MI. Both the differentially expressed genes and transcripts with a log2 (fold change) ≥2 or ≤−2 (i.e., fold change ≥4) and false discovery rate (FDR) <0.05 were analyzed by the functional annotation tool in the Database for Annotation, Visualization and Integrated Discovery (DAVID, v.6.8) program to search for enriched gene ontology (GO) terms. To determine which genes had transcripts that were both up- and downregulated at a given time point, all transcripts with a log2 (fold change) ≥1 or ≤−1 (i.e., fold change ≥2) and FDR <0.05 were analyzed with the IsoProfiler tool in Ingenuity Pathway Analysis (Qiagen). Mapped BAM files for sham and MI samples were used with Mixture of Isoforms software (17) to determine the alternative splicing events and to generate Sashimi plots. Resulting BAM files were also uploaded to Integrative Genomics Viewer (IGV v.2.3) to visualize sequence reads. All primary RNA sequencing (RNA-Seq) data are available on Gene Expression Omnibus under accession number GSE104187.
Western blotting.
To prepare protein lysates, frozen whole heart tissue was pulverized before incubation with standard radioimmunoprecipitation assay (RIPA) buffer plus protease inhibitors (complete mini protease inhibitor cocktail tablets, Roche). BCA assays were used to quantify protein concentrations according to manufacturer’s protocols (Pierce). Equal amounts of lysate (10–20 μg) were run on 8% SDS-PAGE gels (Tris·HCl) under reducing conditions before transfer to PVDF membrane (Immobilon-FL, EMD Millipore). Membranes were then blocked with Odyssey blocking buffer (LI-COR) and probed with primary antibodies at room temperature for 1 h or 4°C overnight. All primary antibodies were used at a 1:1,000 dilution except GAPDH (Sigma, 1:20,000 or Thermo Fisher, 1:4,000) and hnRNPA2B1 (1:500). Blots were then washed in PBS + 0.1% Tween 20 before incubation with appropriate secondary antibodies (IRDye 800CW Donkey anti-Goat IgG, IRDye 800CW Donkey anti-Rabbit IgG, or IRDye 680RD Donkey anti-Mouse IgG) diluted at 1:10,000, for 1 h at room temperature, washed, and scanned with a Li-Cor Odyssey IR Imaging System and quantified with Image Studio densitometry analysis software.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded hearts were cut into 5 μm sections for histological analysis. For Pkm1 staining, sections were deparaffinized and antigen retrieval was performed with trypsin digest. In brief, slides were incubated in 0.05% trypsin (MP Biomedicals, 02101179.1) + 0.1% CaCl2 solution (both wt/vol), pH 7.8 for 20 min at 37°C before being rinsed in running tap water for 3 min. Slides were then incubated with Tris-buffered saline (TBS) buffer before being stained for Pkm1 and sarcomeric α-actinin with a mouse-on-mouse basic kit (M.O.M., Vector Laboratories) according to manufacturer’s instructions with 3% donkey serum added at each step. Slides were blocked for 1 h before incubation overnight at 4°C with primary antibodies, both at a 1:100 dilution. We then incubated slides with biotin anti-mouse IgG for 10 min before adding streptavidin-Alexa Fluor 594 and donkey anti-rabbit IgG-Alexa Fluor 488 (both diluted 1:500) for 1 h. All staining and washing buffers were made in TBS + 0.025% Triton X-100, and all incubations performed at room temperature unless otherwise stated. Slides were mounted with ProLong diamond antifade mountant with DAPI (Thermo Fisher, P36962) and imaged on a Leica TCS SP5 confocal microscope. Images were then processed with ImageJ software.
For Pkm2 staining, slides were deparaffinized, and heat-mediated antigen retrieval was performed. Slides were heated in 10 mM sodium citrate, pH 6.0, in a scientific microwave at 94°C for 15 min twice. Buffer was replaced between incubations. Slides were cooled and washed twice in distilled water before incubation with 3% H2O2 for 10 min. Slides were again washed twice with distilled water and washed once with PBS before being stained with a M.O.M. kit with 1% BSA at all steps. All incubation buffers and washes were performed with PBS. Slides were blocked for 1 h before incubation overnight at 4°C with Pkm2 and troponin T antibodies at a 1:100 dilution. A tyramide signal amplification kit was then used to amplify Pkm2 staining according to manufacturer’s instructions (Thermo Fisher, T20922). Slides were incubated with goat anti-rabbit IgG-HRP for 30 min before incubation with directly conjugated tyramide-Alexa Fluor 488 for 10 min in tyramide working solution. Slides were then washed with PBS before incubation with biotin-anti-mouse IgG for 10 min and streptavidin-Alexa Fluor 594 for 1 h at a 1:500 dilution. Slides were then mounted and imaged as described for Pkm1 staining.
Nuclear fractionation.
Subcellular fractionation was performed as described previously with minor modifications (9). Frozen, pulverized heart tissue was resuspended in 500 μl sucrose-Tris·HCl-MgCl2 (STM) buffer each and incubated on ice for 30 min. Samples were then vortexed for 15 s before centrifugation at 800 g for 15 min at 4°C. Supernatant was collected and used as cytoplasmic fraction sample in subsequent analysis. The pellet was resuspended in another 500 μl STM buffer, vortexed for 15 s, and spun again at 800 g for 15 min at 4°C. The remaining pellet was resuspended in 500 μl NET buffer, vortexed for 15 s and then incubated on ice for 30 min. Samples were then sonicated with a Fisher Scientific Sonic Dismembrator, model 100, once for 5–10 s at a power of 5 before centrifugation at 9,000 g for 30 min at 4°C. Supernatant from this spin was collected and considered nuclear fraction for later experiments. Protein was quantified by Bradford or BCA assay (Bio-Rad or Pierce, respectively), and equal amounts were loaded on 8% SDS-PAGE gels for Western blotting as described above.
Pyruvate kinase activity.
Total pyruvate kinase enzymatic activity was assessed using a pyruvate kinase activity assay kit according to manufacturer’s instructions (Sigma Aldrich, MAK072). Lysate from heart tissue was diluted 1:2,000, and fluorometric products were measured from 0 to 5 min at room temperature. Quantification of protein concentration was assessed by BCA assay as described above.
Chromatin immunoprecipitation sequencing analysis.
Raw ChIP-seq (ChIP-seq) data sets from Guimarães-Camboa et al. (GSE61247) (12) were mapped to mm10 with ArrayStudio software, version 10 (OmicSoft, Cary, NC) (14). Coverage was calculated with BEDTools genomecov (34). Raw coverage plots were generated in UCSC Genome Browser (https://genome.ucsc.edu) (18). Statistically significant peaks were determined by Guimarães-Camboa et al. (12) as described.
ChIP semiquantitative PCR.
Preparation of chromatin was performed as described by van den Boogaard et al. (47) with modifications. Heart tissue was isolated from naïve and 1 day post-MI mice. Tissue was perfused with PBS, and ischemic (or free wall) portion of heart was dissected and weighed before being flash-frozen in liquid nitrogen. Hearts were then pulverized before incubation with 1% formaldehyde in PBS for 30 min at room temperature. Cross-linking was stopped by addition of 1.25 M glycine to a final concentration of 0.125 M and incubated for 5 min. Samples were then centrifuged for 200 g for 10 min at 4°C. and washed with cold PBS three times. Tissue was then briefly homogenized in cold PBS with a PowerGen 125 homogenizer (Fisher Scientific) at medium power. Samples were spun down at 2,300 g for 5 min at 4°C and resuspended in RIPA buffer containing protease inhibitors. Tissue was then incubated at 4°C for 1 h before Dounce homogenization to release nuclei. Samples were then sonicated with a EpiShear Probe Sonicator (Active Motif) for 20 pulses (30 s on/30 s off) to produce 200–500 bp fragments of sheared DNA. MAGnify Chromatin Immunoprecipitation System (Life Technologies) was used then to isolate and purify DNA according to manufacturer’s instructions. Rabbit IgG and anti-HIF1α antibodies were used for immunoprecipitation. Purified DNA samples were run on a QuantStudio 12K Flex Real-Time PCR System (Applied Biosystems) with primers listed in Table 2 and Mouse Negative Control Primer Set 1 (Active Motif). Percent of total input DNA was calculated according to standard methods.
Table 2.
Primer sequences designed for ChIP PCR
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| Pkm HRE #1 | CAGCATCCTTTCAACCTCCATA | CGCGGACGTGACTAGAATG |
| Pkm HRE #2 | TACTCAGGTTTGGAGGTGGT | GATGCTCAGAGCTCCCTAGA |
| Ptbp1 HRE #1 | CAGTGCCTGCTTGCAATATG | CAATGAGCGAGCCTTTGTTC |
| Ptbp1 HRE #2 | CGTTTCCGTCTTCCGTCTT | CAGGGCCTTCTATTGGTCAAA |
| hnRNPA2B1 | GACCTAGTTCCGGACGTAGTAA | TACACTCTCCTTACGCCAAGTA |
| Hmox1 | TGCTCCAGACTGAACCCTTA | GGGCTGTATCTGAACCTAAACTATC |
ChIP, chromatin immunoprecipitation sequencing.
Statistics.
Data were analyzed with GraphPad software. For normally distributed data, Student’s t-test or one-way ANOVA with Tukey test for multiple comparisons were used. Kruskal-Wallis test with Dunn’s multiple comparisons was used for nonparametric data. Error bars indicate standard deviation (SD) unless otherwise indicated. A P value < 0.05 was considered statistically significant.
RESULTS
Early response to MI shows loss of contractility and increased HIF1 activity.
To study the early response to ischemic injury, we used permanent ligation of the LAD artery to produce MI and examined mouse hearts at 1 and 3 days after injury for functional and transcriptional changes. A substantial decrease in cardiac function was noted at both time points compared with sham-operated animals (Fig. 1A). To focus on changes in gene expression directly affected by MI, we dissected “ischemic” (I) ventricular heart tissue containing the infarct and border regions from the “remainder” (R) or remote region of the heart and used these fractions for subsequent analysis (Fig. 1B). We then chose a small group of transcripts to initially characterize gene expression in these hearts. As reported by others (32), we found several genes involved in excitation-contraction coupling (RyR2, SERCA2, Pln) to be progressively downregulated in ischemic tissue after MI (Fig. 1C). Importantly, hearts from MI mice also showed increased abundance of HIF1α transcript in ischemic tissue compared with sham hearts 1 and 3 days after MI. Pfkp (PFKP, phosphofructokinase, platelet), a direct HIF1α target gene (38), was also upregulated (Fig. 1C), indicating HIF1 was transcriptionally active at these time points.
Western blotting also showed a hypoxic response in infarcted heart tissue. Substantial amounts of both HIF1α and VEGF protein, a downstream target of HIF1 and several other ischemia related pathways, were observed in the ischemic portion of the heart at 1 day post-MI, compared with the noninfarcted ventricular tissue or either portion of sham hearts (Fig. 1D). HIF1α was barely detectable 3 days after MI, likely due to compensatory upregulation of genes involved in HIF1α degradation (43) and cell death in the infarct region; VEGF, however, remained abundant and continued to increase (Fig. 1E), suggesting ongoing angiogenic and hypoxic signaling.
Differential gene expression analysis.
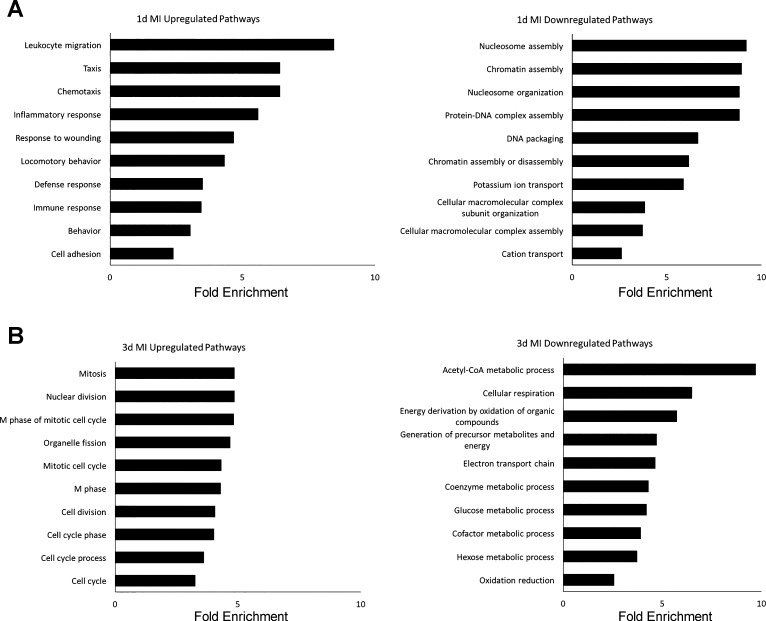
We next performed RNA-Seq on ischemic tissue from each time point to gain a comprehensive understanding of the transcriptional changes. The hearts of two mice from each cohort were selected, based on high HIF1α expression to maximize detection of hypoxia-driven transcriptional changes. We identified 719 upregulated and 183 downregulated genes 1 day after MI. DAVID analysis showed the most affected pathways included upregulation of the innate immune response and cell proliferation; by contrast, pathways involved in ion transport and DNA organization were downregulated (Fig. 2A). By 3 days after MI, 1,457 genes were upregulated and 1,645 genes were downregulated. Processes involved in cellular proliferation were still the predominantly upregulated pathways, while RNA abundance from genes involved in oxygen-dependent metabolic processes were decreased (Fig. 2B).
Fig. 2.
DAVID analysis of differentially expressed genes (DEGs) at 1 and 3 days post-MI. Both up- and downregulated DEGs by log2 (fold change) ≥2 or ≤−2 at 1 day (A) and 3 days after MI (B) were analyzed by DAVID to find most dysregulated pathways with gene ontology (GO) terms. Bar graphs show top 10 GO terms as ranked by P value (or EASE score, determined by modified exact Fisher’s test) as well as fold enrichment for each term. All P < 0.05.
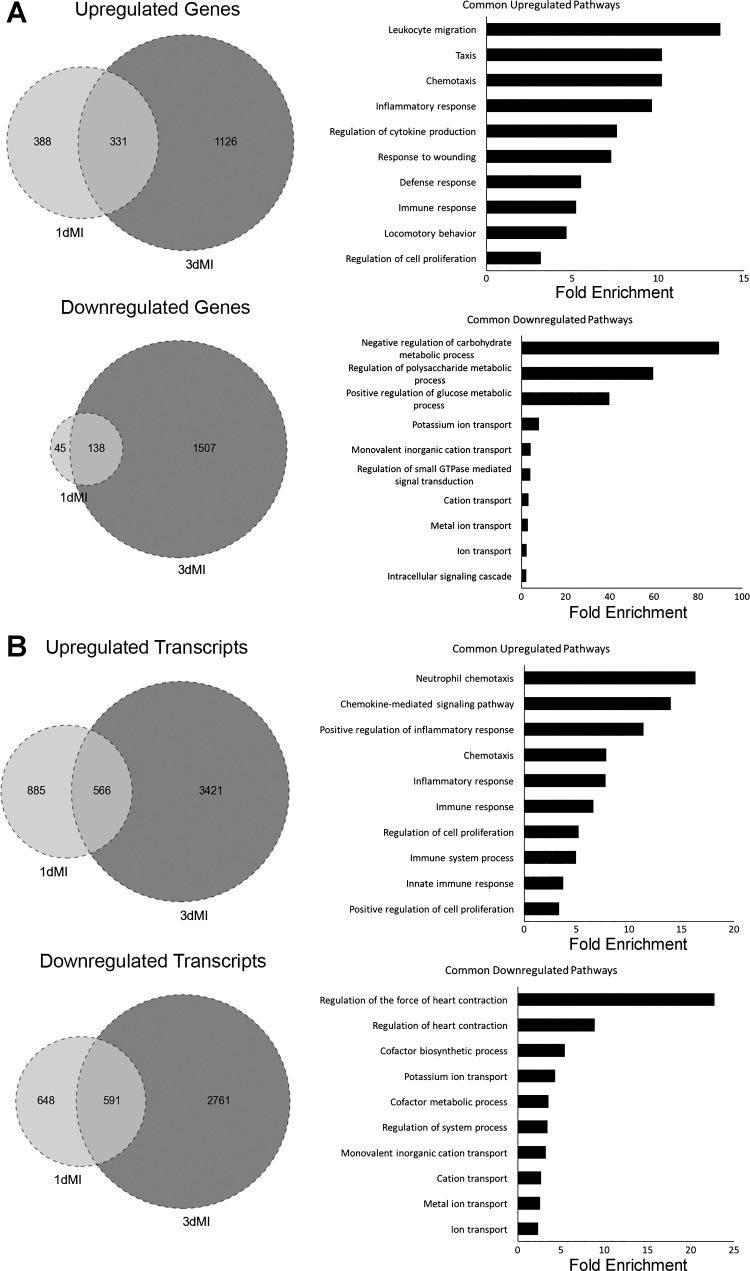
We then compared our data sets to determine which genes were commonly dysregulated at both time points and found a sizeable portion of dysregulated genes at 1 day post-MI were also affected at 3 days after injury. In total, 331 genes were substantially increased and 138 were decreased at both time points (Fig. 3A). Inflammatory and immune responses were most apparent along with decreased ion transport and oxidative metabolism (Fig. 3A). There was also overlap among the most dysregulated genes between 1 and 3 days post-MI (Supplemental Tables S1 and S2). (The online version of this article contains supplemental material.)
Fig. 3.
Comparison of differentially expressed genes and transcripts at 1 day and 3 days post-MI. A: analysis of differentially expressed genes. All genes included in analysis had fold change values of log2 ≥2 (or ≤−2). Venn diagrams show number of genes commonly up- and downregulated at both 1 and 3 days post-MI. Genes common to both time points were then analyzed by DAVID to find commonly dysregulated pathways by gene ontology (GO) terms. Bar graphs show top 10 GO terms as ranked by P value (or EASE score, determined by modified exact Fisher’s test) as well as fold enrichment for each term. All P < 0.05. B: analysis of differentially expressed transcripts. All parameters for Venn diagrams and DAVID analysis are the same as described for A.
Analysis of transcript expression.
RNA-Seq provides fine resolution of transcript detail in which one can detect variation in isoform expression. We therefore wanted to determine if analysis of individual transcript expression would reveal additional information about the specific RNAs responding to injury. Several of the dysregulated transcripts were already detected by differential gene expression analysis, but a number of new transcripts were also revealed (Supplemental Tables S3, S4). As with the gene analysis, we found a number of transcripts to be commonly dysregulated at both time points (Fig. 3B). Of the 1,451 and 3,987 upregulated transcripts at 1 and 3 days post-MI, respectively, 566 of these were common to both data sets. For downregulated transcripts, 591 of the 1,239 transcripts at 1 day post-MI overlapped with the 3,352 transcripts identified at 3 days post-MI. Pathways associated with commonly upregulated transcripts were similar to those found in Fig. 3A, including immune signaling and cell proliferation pathways. By contrast, downregulated genes mapped to pathways of metabolism while transcripts were preferentially linked to muscle contraction pathways (Fig. 3B).
In addition to examining overall patterns of transcript expression, we were interested in identifying divergent isoform transcript expression from a single gene. We identified 17 genes at 1 day after MI to have splice-variants that were substantially distinct compared with sham hearts (Supplemental Table S5). At 3 days after MI, 72 genes were found to have transcripts with divergent expression patterns (Supplemental Table S6). From these candidates, we focused on the changes in PKM expression because of its importance in cellular metabolism and the relatively well-characterized functional differences between its two main isoforms.
Pkm2 expression upregulated in response to MI.
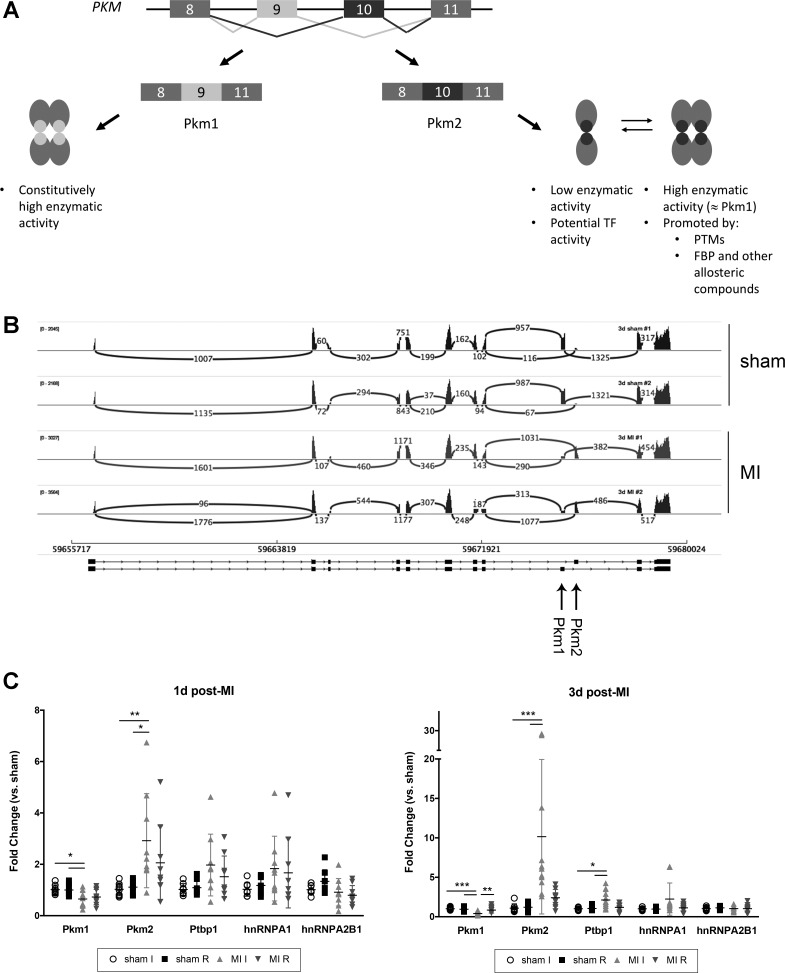
Pyruvate kinase is a key enzyme in glycolytic metabolism. PKM produces two isoforms. While the two sequences are otherwise identical, the inclusion of exon 9 in Pkm1 or exon 10 in Pkm2 transcripts confers important differences in the activity of each protein (Fig. 4A). Pkm1 acts as a tetramer that is highly efficient at converting pyruvate, while Pkm2 can act as a dimer or tetramer, with the balance between the two states affected by a number of posttranslational modifications including hydroxylation, phosphorylation, oxidation, and allosteric regulation by FBP and many other molecules (15). The dimer is much less efficient at converting pyruvate (30). Sashimi plot analysis of our RNA-Seq showed that while some Pkm2 transcript was made at baseline, Pkm1 was the predominant isoform in sham hearts (Fig. 4B). In contrast, by three days after MI Pkm1 message abundance was dramatically reduced (~4-fold), while Pkm2 message was increased 14-fold. We used a larger cohort of mice to confirm this data by semiquantitative PCR and found Pkm1 to be decreased by 70% and Pkm2 to be upregulated 10-fold in the ischemic tissue compared with both sham hearts and the noninfarcted remainder of heart tissue (Fig. 4C). Although this switch from Pkm1 to Pkm2 expression was not evident in our initial analysis 1 day after MI, further examination of our RNA-Seq data showed that Pkm1 was modestly decreased in MI hearts (data not shown). Semiquantitative PCR with a larger group of mice confirmed that Pkm1 transcript was consistently downregulated and Pkm2 increased in ischemic heart tissue 1 day after infarct, albeit to a lesser degree than after 3 days (Fig. 4C).
Fig. 4.
Pyruvate kinase, muscle (Pkm) transcript expression at 1 and 3 days after MI. A: schematic highlighting the differences between Pkm1 and Pkm2 isoforms. TF, transcription factor; PTMs, posttranslational modifications; FBP, fructose-1,6-bisphosphonate. B: sashimi plot illustrating sequencing reads across exon-exon junctions for PKM gene for each RNA sample (total reads per sample >35 million). Numbers indicate quantification of reads at each junction. Exons specific to Pkm1 and Pkm2 are denoted with arrows. C: semiquantitative PCR analysis of Pkm transcripts and associated splicing factors at 1 and 3 days after MI. Data shown with means ± SD n = 8–11 mice for each time point. For C, statistical significance was determined by one-way ANOVA with Tukey’s multiple comparisons or Kruskal-Wallis with Dunn’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001.
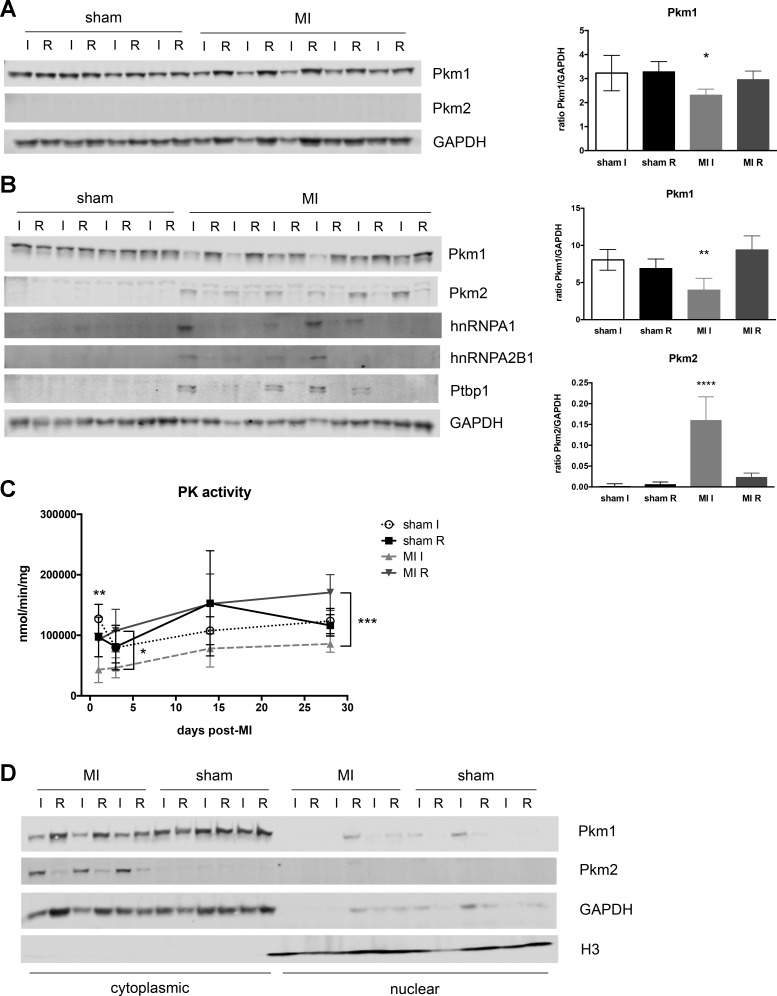
These data were recapitulated at the protein level as well. Pkm1 was decreased by 1 day after MI in ischemic tissue, but Pkm2 was not detectable at this time point (Fig. 5A), possibly due to insufficient time for the protein to accumulate to detectable levels. By 3 days after MI, Western blots showed that ischemic heart tissue had a substantial loss of Pkm1 expression, while Pkm2 protein was increased compared with sham and remote heart tissue (Fig. 5B).
Fig. 5.
Pkm protein expression and function after myocardial infarction (MI). Western blotting for Pkm isoforms and splicing factors at 1 day (A) and 3 days post-MI (B). Right: quantification of blots for Pkm1 and Pkm2 expression. GAPDH used as loading control and normalization. Quantification shown as means ± SD. C: overall pyruvate kinase activity was measured in ischemic free wall fractions at 1, 3, 14, and 28 days post-MI. For A–C, one-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. When specific comparisons are not indicated, statistical significance is for MI I compared with all other groups. D: Western blots of Pkm1 and Pkm2 expression in cytoplasmic and nuclear fractions of heart tissue. GAPDH was used to identify cytoplasmic portion and histone H3 used to denote nuclear localization.
The preference for Pkm1 vs. Pkm2 expression is largely mediated by three splicing factors (Ptbp1, hnRNPA1, hnRNPA2B1), which prevent inclusion of exon 9 and promote inclusion of exon 10 into the final mRNA product (6). We therefore examined expression of these splicing factors in MI hearts. Analysis at 1 day after infarction indicated a trend toward increased Ptbp1 and hnRNPA1 transcripts (Fig. 4C), but these did not reach statistical significance, and none of these factors were detectable at the protein level (data not shown). However, both Ptbp1 and hnRNPA1 transcripts were more abundant by 3 days after MI, although only Ptbp1 reached statistical significance (Fig. 4C). Western blotting also showed that all three factors were present in the ischemic tissue and absent in all other fractions (Fig. 5B).
Pyruvate kinase activity reduced after MI and Pkm2 retained in cytoplasm.
We next wanted to determine the effect of this isoform switch on pyruvate kinase function. Unfortunately, since the main enzymatic difference between Pkm1 and Pkm2 is its turnover rate, we could not distinguish the contributions of the individual isoforms. We therefore measured total pyruvate kinase activity in post-MI hearts and found a substantial decrease in pyruvate production as early as 1 day post-MI (Fig. 5C). This drop in activity, compared with sham hearts, persisted throughout the 28-day time course, although activity in the ischemic fraction increased modestly over time. These data suggest that despite the significant increase in Pkm2 expression, and the potential for modulation to increase kinase activity, these changes were not enough to overcome the sizeable loss of Pkm1.
Additionally, while pyruvate kinase is thought to function principally as a metabolic enzyme, several studies have suggested that Pkm2 can also act as a transcription factor (11, 45). We therefore performed subcellular fractionation to determine the localization of the Pkm isoforms in our ischemic hearts. As expected, Pkm1 remained in the cytoplasmic fraction for all samples (Fig. 5D). Interestingly, Pkm2 expression was also restricted to the cytoplasmic fraction (Fig. 5D). Neither protein was detected in the nuclear fraction of sham or MI hearts. These data suggest that Pkm2 function in the response to MI is largely limited to its role in cellular metabolism.
Pkm isoforms in long-term remodeling and heart failure.
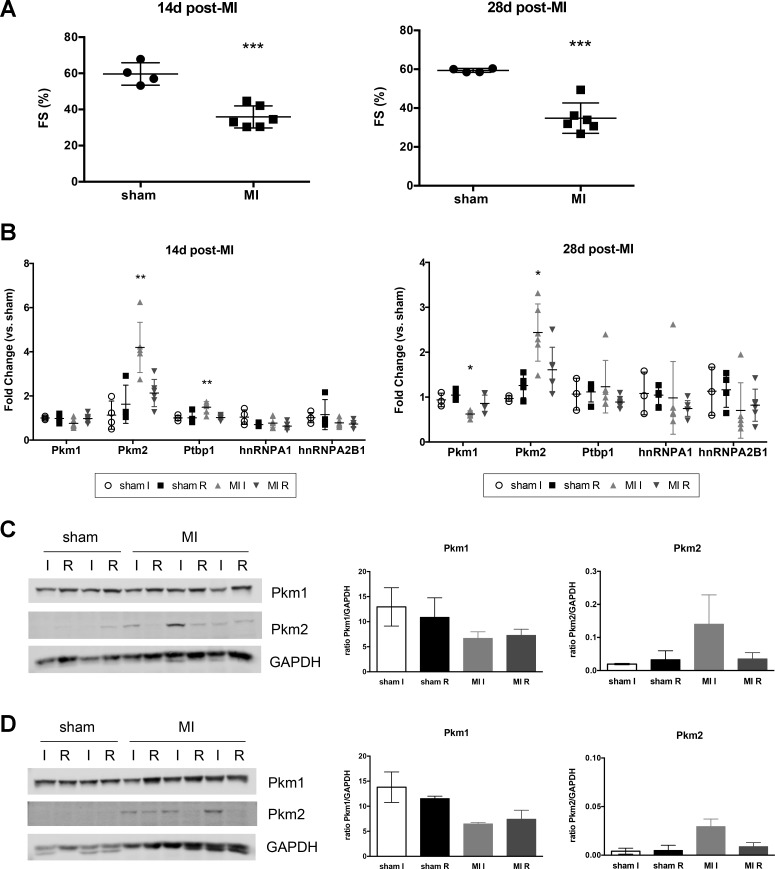
Given the potential effects that a switch in Pkm isoform expression could have on glycolytic metabolism in the heart, we wanted to determine if these changes persisted in the remodeling heart after ischemic injury. We therefore examined Pkm1 and Pkm2 expression in mouse hearts 14 and 28 days after MI. As expected, echocardiography showed MI hearts had persistently decreased function compared with sham hearts (Fig. 6A). RNA and protein analysis revealed that both time points had similar expression patterns of decreased Pkm1 and increased Pkm2, as seen at earlier time points (Fig. 6, B–D). These data suggest that the observed alteration in Pkm expression is not simply a short-term response to injury, but likely a sustained change during recovery from ischemic damage.
Fig. 6.
Functional analysis and Pkm expression at 14 and 28 days post-MI. A: fractional shortening (FS) in sham and MI mice at baseline at 14 and 28 days post-MI. Student’s t-test vs. sham mice. ***P = 0.0003 for both 14 and 28 days after MI. B: semiquantitative PCR analysis of Pkm transcripts and associated splicing factors. Data shown with means ± SD. One-way ANOVA with Tukey’s multiple comparisons. *P < 0.05, **P < 0.01. Statistical significance for MI I compared with all other groups. Western blotting for Pkm1 and Pkm2 at 14 days (C) and 28 days (D) after MI. Right: quantification of expression. GAPDH used as loading control and normalization.
Pkm1 and Pkm2 expression in cardiomyocytes.
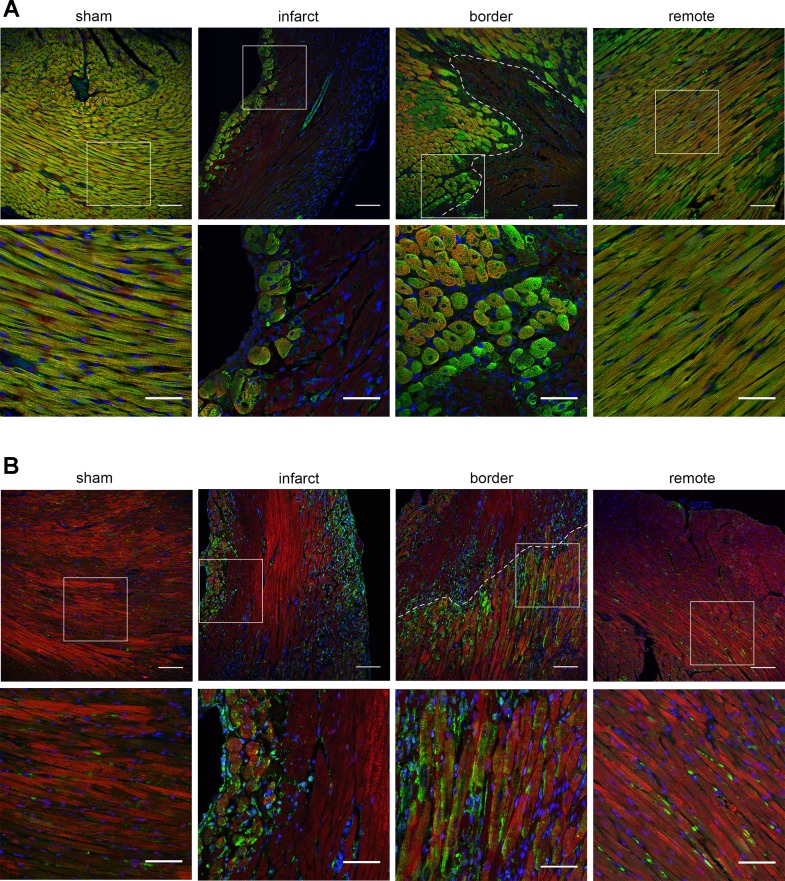
Since cardiomyocytes require large amounts of ATP and are sensitive to changes in metabolism, we used immunohistochemistry to identify whether both Pkm isoforms were present in cardiomyocytes. Figure 7 shows that in sham hearts Pkm1 expression was prominent but restricted to cardiomyocytes, while Pkm2 was detected in a few nonmyocytes but not seen in cardiomyocytes. The remote areas of MI hearts had similar patterns to those observed in sham mice. After injury, Pkm1 staining is almost entirely absent in the infarct region, where many cardiomyocytes are presumed to be apoptotic or necrotic (Fig. 7A, α-actinin staining). Within the infarct Pkm1 expression was maintained in only a few cells close to blood vessels and the outer edges of the left ventricle. By contrast, numerous cardiomyocytes within the infarct and border regions had substantial Pkm2 expression (Fig. 7B). Pkm2 was seen along the outer edges of the infarct region and in cardiomyocytes within the border zones near healthy tissue (Fig. 7B). Similar, though less dramatic patterns for Pkm1 and Pkm2 expression were also observed in heart tissue at 1 day after MI (data not shown). Interestingly, several areas within the infarct and border regions with Pkm2-positive cardiomyocytes also appeared to express Pkm1, which suggests that surviving cardiomyocytes within the infarct and peri-infarct region may express Pkm1 and Pkm2 simultaneously. Several other cell types in the infarcted hearts also appear to express Pkm2. These are likely fibroblasts and macrophages responding to the acute injury. Others have reported that macrophages can express Pkm2 upon activation (39) and histological staining from our laboratory indicates fibroblasts in the infarcted tissue can also express Pkm2 (data not shown).
Fig. 7.
Immunohistochemical analysis of Pkm1 and Pkm2 expression in cardiomyocytes in sham and infarcted heart tissue 3 days post-MI. A: Pkm1 (green) costaining with sarcomeric α-actinin (red, cardiomyocytes). B: Pkm2 (green) costaining with troponin T (red, cardiomyocytes). DAPI staining (blue) denotes nuclei in all images. Representative views of sham mice as well as infarct, border, and remote regions from MI mice included. Top: lower magnification view with dotted line indicating approximate edge of border region. Bottom: zoomed-in view of tissue portion denoted by white box. Scale bars = 100 μm for top panels and 50 μm for bottom panels.
HIF1 regulation of PKM isoform expression.
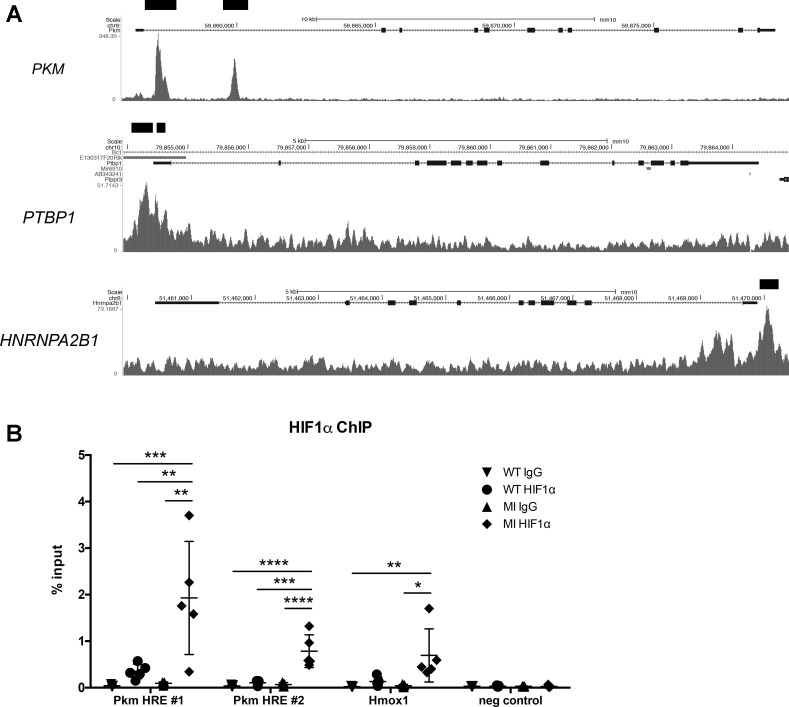
We hypothesized that HIF1 could have a direct effect on both the abundance of transcripts derived from the PKM gene and the switch in Pkm isoform expression, by binding to promoters associated with the PKM gene and its related splicing factors. While HIF1 is stabilized in the heart during ischemic injury, it is also normally expressed in cardiac tissue during development. We therefore examined ChIP-seq data for HIF1α from embryonic day 12.5 embryonic hearts that were previously generated by Guimaraes-Camboa et al. (12) of the Zambon laboratory to see if HIF1 was bound to any of our target genes. Strikingly, three of the four genes (PKM, PTBP1, and HNRNPA2B1) had sites that were significantly enriched over the input (Fig. 8A) and contained the core HIF1 binding sequence 5′-RCGTG-3′, further supporting the possibility that HIF1 can directly mediate the observed change in PKM expression.
Fig. 8.
Chromatin immunoprecipitation sequencing (ChIP) analysis of HIF1 binding to PKM (and related splicing factors). A: analysis of HIF1 binding sites in embryonic mouse hearts by ChIP-seq from Guimarães-Camboa et al. (12). Genome browser plots of PKM, PTBP1, and HNRNPA2B1 show signals from HIF1α-bound sequences. Black bars denote statistically significant peaks. B: semiquantitative PCR analysis of ChIP DNA using an HIF1α antibody on naïve wild-type (WT) and ischemic heart tissue 1 day after MI (MI). HIF1α showed increased binding at hypoxia response element (HRE) sites compared with both WT and IgG controls for Pkm and Hmox1, a known HIF1 target, while no binding was detected in the negative control, a site with no transcriptional activity. n = 5 for each group. Data shown as % of total input DNA and displayed with means ± SD. Statistical significance determined by one-way ANOVA with Tukey’s multiple comparisons or Kruskal-Wallis with Dunn’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We next confirmed direct HIF1 binding in our infarction model by ChIP and found both HIF1α sites observed in embryonic hearts for PKM (12) were substantially enriched in ischemic heart tissue compared with naïve mice (Fig. 8B). We compared these results to another known HIF1 target, Hmox1, and found HIF1 binding to the Pkm site was enriched to an even greater degree than the site by which HIF1 is known to regulate Hmox1 expression (24) (Fig. 8B). However, the two HIF1α sites for PTBP1 and single site in HNRNPA2B1 were not significantly enriched in MI samples over WT hearts (0.46% ± 0.3 vs. 0.25% ± 0.1 and 0.29% ± 0.16 vs. 0.14% ± 0.05 for PTBP1 sites, 0.26% ± 0.13 vs. 0.19% ± 0.05 for HNRNPA2B1). These data suggest that while HIF1 may directly increase Pkm transcript expression after MI, it does not appear to substantially alter transcription of pertinent splicing factors.
DISCUSSION
Understanding the transcriptional response to infarction should direct us to the biochemical pathways that are most important in the cardiac response to this critical stressor. While prior gene expression studies have tended to characterize long-term responses to MI in myocardial remodeling, we focused on early changes in transcription that could direct the immediate response to loss of contractility, as well as potentially contribute to long-term effects. Although our small sequencing sample size and the heterogeneous nature of our ischemic tissue (infarct and border) limit the conclusions we can draw about infarction-related transcriptional changes, our analysis still serves as a useful tool to identify novel genes for follow up study. We identified several genes that had not previously been associated with the early response to MI. One example was Cxcl3, which was the most differentially expressed gene for both time points; its change in abundance is likely due to the substantial influx of leukocytes after injury. Additionally, Kcnj3, a cardiac inward rectifying potassium channel, was found to be dramatically less abundant at both time points. Its loss could affect heart function as Kcnj3 is a subunit of the acetylcholine-activated potassium current that controls heart rate and cardiac conduction (28). These genes, along with others in our data sets, may provide new insight into pathways involved in MI injury, including the immune response and the electrical instability of the ischemic myocardium.
In this study we used RNA-Seq to enrich the evaluation of transcriptional response through an assessment of alternative splicing. We hypothesized that genes undergoing a switch in isoform expression would be likely to have functional significance, particularly if the transcripts encode functionally distinct proteins. We found a number of genes with differentially expressed splice variants and focused on PKM because of its importance in metabolism. Our results revealed upregulation of Pkm2 in cardiomyocytes, which is of particular interest due to the lower enzymatic activity of Pkm2 that disfavors oxidative phosphorylation and may provide a useful adaptation to hypoxia. Several studies have also demonstrated that Pkm2 can translocate to the nucleus to modulate transcription (15). While we did not observe any nuclear Pkm2 in infarcted heart tissue, we cannot exclude the possibility that nuclear Pkm2 exists in the ischemic heart below detectable levels. These data suggest that the role of Pkm2 after MI is mainly to modulate cellular metabolism. It will be important to determine if Pkm2 expression is part of the proliferative process or a response to the challenging metabolic milieu in the hypoxic myocardium. Interestingly though, we also noted an increase in the kinase activity of the remote fraction (Fig. 5C). It is known that the remaining viable heart tissue will try to compensate for loss of function after infarction (33), and we detect low levels of Pkm2 expression in the remote region (Figs. 4C and 5B). It is therefore possible that increased Pkm2 expression in remaining healthy heart can boost pyruvate kinase activity and thereby augment ATP production.
Pkm2 is widely expressed during development, and it has been shown that muscle cells express Pkm2 in their undifferentiated state but gradually switch to Pkm1 expression as they differentiate (7, 8). This expression pattern is similar to that of HIF1α, with high expression during development that tapers off as tissues differentiate and become oxygenated. Previous work in cancer cells has shown that HIF1 can upregulate Pkm1 and Pkm2 expression, and chromatin immunoprecipitation analysis of these cells demonstrated direct HIF1 binding to the human Pkm gene (PKM2) (38). While we present evidence that HIF1 can directly regulate Pkm isoform expression (Fig. 8), we cannot exclude that other factors may be involved. Insulin, EGFR, and mTOR-dependent signaling pathways have all been shown to affect Pkm2 levels under various conditions (49). Additionally, gain of SP1 and loss of SP3 binding to the PKM promoter have been shown to increase transcription (10, 36). Expression of Pkm splicing factors has also been shown to be dependent on another transcription factor, c-myc (7). Transcription of MYC is upregulated in our infarcted hearts, so it is possible that multiple activation pathways are contributing to the upregulation of Pkm2 expression. Interestingly, mass spectroscopy analysis of prolyl hydroxylation sites regulated by PHD2 in human induced-pluripotent cardiomyocytes found both Pkm2 and hnRNPA1 to be modified (44), suggesting the expression of Pkm2 may also be modulated by posttranslational modifications in response to oxygen levels.
Given its role in development, Pkm2 has been hypothesized to be required for cell proliferation. When Pkm2 was deleted in primary mouse embryonic fibroblasts, subsequent upregulation of Pkm1 caused cells to lose their proliferative capacity because of limited nucleotide synthesis (27). However, recent work has questioned the supposed essential role of Pkm2 by demonstrating that germline deletion of Pkm2 produced viable mice through increased expression of Pkm1 (8). Interestingly, Pkm2−/− mice showed altered systemic metabolism, particularly in glycolytic metabolites, and over time develop hepatic steatosis and hepatocellular carcinoma (8). These data suggest Pkm1 and Pkm2 have distinct roles that cannot be easily interchanged and that Pkm2 plays an important role in energy homeostasis. Unlike Pkm1, Pkm2 can also act as a dimer, which reduces the overall rate of glycolysis and allows upstream metabolites to be utilized in other pathways that are important for cell proliferation (e.g., pentose phosphate pathway and nucleotide biosynthesis) (15, 48). It will be useful to further elucidate how Pkm2 modifies metabolism in physiological and pathological settings, and how mice with loss of Pkm2 respond to infarction.
Although much of the infarct area becomes apoptotic or necrotic within a few days after MI, the border zone has been shown to be a highly hypoxic region with active HIF1 signaling (19, 22). Our data support these findings, as seen by the upregulation of both HIF1 and Pkm2 in the infarct and border regions after injury. These results suggest that upon ischemic injury the heart activates hypoxic signaling and metabolic pathways that favor proliferation in an attempt to restore damaged tissue. These processes are also prominent during development. Meta-analysis of microarray data from heart tissue in a number of developmental and pathological settings revealed the HIF1α motif to be the most highly enriched transcription factor binding site across all samples, highlighting its importance in both of these settings (12). Data from other studies also support this hypothesis. For example, stem cells are typically maintained in a hypoxic environment, with HIF1 signaling and glycolytic metabolism helping them remain in an undifferentiated state (16). Others have demonstrated the importance of a hypoxic environment to support cardiomyocyte regeneration and protection from ischemic injury (31). While resident cardiomyocytes can only divide at a low rate in adult tissue, this proliferation is increased in response to MI (41). Additionally, rare cardiomyocytes within the normal heart have been shown to be hypoxic and retain the ability to proliferate (21); these cells may contribute to the reparative process after injury. While we did not observe any Pkm2-positive cardiomyocytes in uninjured hearts, these cells may be exceedingly rare or the hypoxic cardiomyocytes normally present at baseline may not express Pkm2 at any appreciable level.
Pkm2 expression has also been observed in other models of heart disease. In a transverse aortic constriction model, Pkm2 was found to be upregulated in rat hearts (25). Long-term administration of sunitinib, a tyrosine kinase inhibitor used in cancer treatment, also increased expression of Pkm2 and other glycolytic genes, which may indicate a shift toward fetal-like cardiac metabolism (35). That study also found patients with heart failure to have increased Pkm2 in the heart and this increase was partially reduced with mechanical unloading from left ventricular assist device therapy. Other work has suggested that Pkm2 expression may regulate cardiomyocyte survival after transplantation (42). These data suggest there may be a more general response to injury in which gene expression is altered to favor metabolic pathways that promote proliferation and tissue repair. Further study of the role of Pkm2 in cardiac metabolism after injury will be necessary to assess its role in the reparative response and any potential window for therapeutic manipulation.
In summary, our investigation of early transcriptional response to MI revealed a number of changes in alternative splicing. Increased HIF1 expression after ischemic injury allows sustained Pkm2 expression to be favored over the normally expressed Pkm1. Pkm2 can then be modified by signaling proteins and posttranslational modifications to fine-tune its enzymatic activity to favor higher proliferation or energy production as needed. This may contribute to the potential for cell proliferation and effective metabolism in surviving cardiomyocytes after infarction.
GRANTS
This work was supported by National Institutes of Health Grants R01 HL-080532 and P30 GM-103341 to R. V. Shohet, T32 HL-115505 to A. L. Williams, P30 CA-071789 and 5G12 MD-007601 to the Microscopy and Imaging Core, and P20 GM-103466, U54 MD-007584, and 5P30 GM-114737 to the Bioinformatics Core. This work was also supported by the American Heart Association: 16POST29960013 to A. L. Williams.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.L.W. and R.V.S. conceived and designed research; A.L.W., M.T., and A.A. performed experiments; A.L.W., V.K., M.T., A.A., K.J.S., and M.M. analyzed data; A.L.W., V.K., K.J.S., M.M., and R.V.S. interpreted results of experiments; A.L.W. prepared figures; A.L.W. drafted manuscript; A.L.W., V.K., K.J.S., M.M., and R.V.S. edited and revised manuscript; A.L.W., V.K., M.T., A.A., K.J.S., M.M., and R.V.S. approved final version of manuscript.
Supplemental Data
Supplementary Table 1: Top 100 upregulated genes at 1 day and 3 days post-MI; Supplementary Table 2: Top 100 downregulated genes at 1 day and 3 days post-MI; Supplementary Table 3: Top 100 upregulated transcripts at 1 day and 3 days post-MI; Supplementary Table 4: Top 100 downregulated transcripts at 1 day and 3 days post-MI; Supplementary Table 5: Genes with transcripts that are both up- and downregulated at 1 day post-MI; Supplementary Table 6: Genes with transcripts that are both up- and downregulated at 3 days post-MI. - .pdf (88 KB)
ACKNOWLEDGMENTS
We thank Michelle Tallquist for use of her Col1a1-GFP transgenic mice to identify cardiac fibroblasts after injury. We also thank Youping Deng at the John A. Burns School of Medicine (JABSOM) Bioinformatics Core for advice on sequencing analysis. We also thank Karolina Peplowska from the University of Hawaii Cancer Center Genomics Core and Aaron Tuia from the JABSOM Mouse Phenotyping Core for technical assistance. We thank the following facilities for use of equipment and processing samples: JABSOM Genomics Core Facility, JABSOM Histology Core Facility, JABSOM Bioinformatics Core, and University of Hawaii Health Sciences Microscopy and Imaging Core.
REFERENCES
- 1.Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169, 2015. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baralle FE, Giudice J. Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18: 437–451, 2017. doi: 10.1038/nrm.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekeredjian R, Walton CB, MacCannell KA, Ecker J, Kruse F, Outten JT, Sutcliffe D, Gerard RD, Bruick RK, Shohet RV. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS One 5: e11693, 2010. doi: 10.1371/journal.pone.0011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brampton C, Aherrahrou Z, Chen LH, Martin L, Bergen AA, Gorgels TG, Erdmann J, Schunkert H, Szabó Z, Váradi A, Le Saux O. The level of hepatic ABCC6 expression determines the severity of calcification after cardiac injury. Am J Pathol 184: 159–170, 2014. doi: 10.1016/j.ajpath.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452: 230–233, 2008. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 6.Clower CV, Chatterjee D, Wang Z, Cantley LC, Vander Heiden MG, Krainer AR. The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism. Proc Natl Acad Sci USA 107: 1894–1899, 2010. doi: 10.1073/pnas.0914845107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463: 364–368, 2010. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayton TL, Gocheva V, Miller KM, Israelsen WJ, Bhutkar A, Clish CB, Davidson SM, Luengo A, Bronson RT, Jacks T, Vander Heiden MG. Germline loss of PKM2 promotes metabolic distress and hepatocellular carcinoma. Genes Dev 30: 1020–1033, 2016. doi: 10.1101/gad.278549.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimauro I, Pearson T, Caporossi D, Jackson MJ. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes 5: 513, 2012. doi: 10.1186/1756-0500-5-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Discher DJ, Bishopric NH, Wu X, Peterson CA, Webster KA. Hypoxia regulates beta-enolase and pyruvate kinase-M promoters by modulating Sp1/Sp3 binding to a conserved GC element. J Biol Chem 273: 26087–26093, 1998. doi: 10.1074/jbc.273.40.26087. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell 45: 598–609, 2012. doi: 10.1016/j.molcel.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guimarães-Camboa N, Stowe J, Aneas I, Sakabe N, Cattaneo P, Henderson L, Kilberg MS, Johnson RS, Chen J, McCulloch AD, Nobrega MA, Evans SM, Zambon AC. HIF1α represses cell stress pathways to allow proliferation of hypoxic fetal cardiomyocytes. Dev Cell 33: 507–521, 2015. doi: 10.1016/j.devcel.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harpster MH, Bandyopadhyay S, Thomas DP, Ivanov PS, Keele JA, Pineguina N, Gao B, Amarendran V, Gomelsky M, McCormick RJ, Stayton MM. Earliest changes in the left ventricular transcriptome postmyocardial infarction. Mamm Genome 17: 701–715, 2006. doi: 10.1007/s00335-005-0120-1. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Ge H, Newman M, Liu K. OSA: a fast and accurate alignment tool for RNA-Seq. Bioinformatics 28: 1933–1934, 2012. doi: 10.1093/bioinformatics/bts294. [DOI] [PubMed] [Google Scholar]
- 15.Israelsen WJ, Vander Heiden MG. Pyruvate kinase: Function, regulation and role in cancer. Semin Cell Dev Biol 43: 43–51, 2015. doi: 10.1016/j.semcdb.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol 15: 243–256, 2014. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katz Y, Wang ET, Airoldi EM, Burge CB. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat Methods 7: 1009–1015, 2010. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res 12: 996–1006, 2002. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol 46: 2116–2124, 2005. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 20.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14: R36, 2013. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura W, Xiao F, Canseco DC, Muralidhar S, Thet S, Zhang HM, Abderrahman Y, Chen R, Garcia JA, Shelton JM, Richardson JA, Ashour AM, Asaithamby A, Liang H, Xing C, Lu Z, Zhang CC, Sadek HA. Hypoxia fate mapping identifies cycling cardiomyocytes in the adult heart. Nature 523: 226–230, 2015. doi: 10.1038/nature14582. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Maeda K, Takefuji M, Kikuchi R, Morishita Y, Hirashima M, Murohara T. Dynamics of angiogenesis in ischemic areas of the infarcted heart. Sci Rep 7: 7156, 2017. doi: 10.1038/s41598-017-07524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359, 2012. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381, 1997. doi: 10.1074/jbc.272.9.5375. [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Kenney JW, Manousopoulou A, Johnston HE, Kamei M, Woelk CH, Xie J, Schwarzer M, Garbis SD, Proud CG. Quantitative non-canonical amino acid tagging (QuaNCAT) proteomics identifies distinct patterns of protein synthesis rapidly induced by hypertrophic agents in cardiomyocytes, revealing new aspects of metabolic remodeling. Mol Cell Proteomics 15: 3170–3189, 2016. doi: 10.1074/mcp.M115.054312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550, 2014. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN, Hollern DP, Bellinger G, Dayton TL, Christen S, Elia I, Dinh AT, Stephanopoulos G, Manalis SR, Yaffe MB, Andrechek ER, Fendt SM, Vander Heiden MG. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell 57: 95–107, 2015. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mark MD, Herlitze S. G-protein mediated gating of inward-rectifier K+ channels. Eur J Biochem 267: 5830–5836, 2000. doi: 10.1046/j.1432-1327.2000.01670.x. [DOI] [PubMed] [Google Scholar]
- 29.Mirtschink P, Krishnan J, Grimm F, Sarre A, Hörl M, Kayikci M, Fankhauser N, Christinat Y, Cortijo C, Feehan O, Vukolic A, Sossalla S, Stehr SN, Ule J, Zamboni N, Pedrazzini T, Krek W. HIF-driven SF3B1 induces KHK-C to enforce fructolysis and heart disease. Nature 522: 444–449, 2015. doi: 10.1038/nature14508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan HP, O’Reilly FJ, Wear MA, O’Neill JR, Fothergill-Gilmore LA, Hupp T, Walkinshaw MD. M2 pyruvate kinase provides a mechanism for nutrient sensing and regulation of cell proliferation. Proc Natl Acad Sci USA 110: 5881–5886, 2013. doi: 10.1073/pnas.1217157110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature 541: 222–227, 2017. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- 32.Netticadan T, Temsah RM, Kawabata K, Dhalla NS. Sarcoplasmic reticulum Ca(2+)/Calmodulin-dependent protein kinase is altered in heart failure. Circ Res 86: 596–605, 2000. doi: 10.1161/01.RES.86.5.596. [DOI] [PubMed] [Google Scholar]
- 33.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 81: 1161–1172, 1990. doi: 10.1161/01.CIR.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 34.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26: 841–842, 2010. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rees ML, Subramaniam J, Li Y, Hamilton DJ, Frazier OH, Taegtmeyer H. A PKM2 signature in the failing heart. Biochem Biophys Res Commun 459: 430–436, 2015. doi: 10.1016/j.bbrc.2015.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schäfer D, Hamm-Künzelmann B, Brand K. Glucose regulates the promoter activity of aldolase A and pyruvate kinase M2 via dephosphorylation of Sp1. FEBS Lett 417: 325–328, 1997. doi: 10.1016/S0014-5793(97)01314-8. [DOI] [PubMed] [Google Scholar]
- 37.Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 27: 863–864, 2011. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117: e207–e217, 2011. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, Miyazawa H, Yamaguchi Y, Miura M, Jenkins DM, Choi H, Kim JW, Asagiri M, Cowburn AS, Abe H, Soma K, Koyama K, Katoh M, Sayama K, Goda N, Johnson RS, Manabe I, Nagai R, Komuro I. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun 7: 11635, 2016. doi: 10.1038/ncomms11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9: 47–71, 2014. doi: 10.1146/annurev-pathol-012513-104720. [DOI] [PubMed] [Google Scholar]
- 41.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493: 433–436, 2013. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi J, Yang X, Yang D, Li Y, Liu Y. Pyruvate kinase isoenzyme M2 expression correlates with survival of cardiomyocytes after allogeneic rat heterotopic heart transplantation. Pathol Res Pract 211: 12–19, 2015. doi: 10.1016/j.prp.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Stiehl DP, Wirthner R, Köditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem 281: 23482–23491, 2006. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- 44.Stoehr A, Yang Y, Patel S, Evangelista AM, Aponte A, Wang G, Liu P, Boylston J, Kloner PH, Lin Y, Gucek M, Zhu J, Murphy E. Prolyl hydroxylation regulates protein degradation, synthesis, and splicing in human induced pluripotent stem cell-derived cardiomyocytes. Cardiovasc Res 110: 346–358, 2016. doi: 10.1093/cvr/cvw081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamada M, Suematsu M, Saya H. Pyruvate kinase M2: multiple faces for conferring benefits on cancer cells. Clin Cancer Res 18: 5554–5561, 2012. doi: 10.1158/1078-0432.CCR-12-0859. [DOI] [PubMed] [Google Scholar]
- 46.Tarnavski O, McMullen JR, Schinke M, Nie Q, Kong S, Izumo S. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics 16: 349–360, 2004. doi: 10.1152/physiolgenomics.00041.2003. [DOI] [PubMed] [Google Scholar]
- 47.van den Boogaard M, Wong LY, Christoffels VM, Barnett P. Acquisition of high quality DNA for massive parallel sequencing by in vivo chromatin immunoprecipitation. Methods Mol Biol 977: 53–64, 2013. doi: 10.1007/978-1-62703-284-1_5. [DOI] [PubMed] [Google Scholar]
- 48.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324: 1029–1033, 2009. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang W, Lu Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett 339: 153–158, 2013. doi: 10.1016/j.canlet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Top 100 upregulated genes at 1 day and 3 days post-MI; Supplementary Table 2: Top 100 downregulated genes at 1 day and 3 days post-MI; Supplementary Table 3: Top 100 upregulated transcripts at 1 day and 3 days post-MI; Supplementary Table 4: Top 100 downregulated transcripts at 1 day and 3 days post-MI; Supplementary Table 5: Genes with transcripts that are both up- and downregulated at 1 day post-MI; Supplementary Table 6: Genes with transcripts that are both up- and downregulated at 3 days post-MI. - .pdf (88 KB)