Abstract
Over the last decade, genome-wide association studies (GWAS) have propelled the discovery of thousands of loci associated with complex diseases. The focus is now turning toward the function of these association signals, determining the causal variant(s) among those in strong linkage disequilibrium, and identifying their underlying mechanisms, such as long-range gene regulation. Genome-editing techniques utilizing zinc-finger nucleases (ZFN), transcription activator-like effector nucleases (TALENs), and clustered regularly-interspaced short palindromic repeats with Cas9 nuclease (CRISPR-Cas9) are becoming the tools of choice to establish functionality for these variants, due to the ability to assess effects of single variants in vivo. This review will discuss examples of how these technologies have begun to aid functional analysis of GWAS loci for complex traits such as cardiovascular disease, Type 2 diabetes, cancer, obesity, and autoimmune disease. We focus on analysis of variants occurring within noncoding genomic regions, as these comprise the majority of GWAS variants, providing the greatest challenges to determining functionality, and compare editing strategies that provide different levels of evidence for variant functionality. The review describes molecular insights into some of these potentially causal variants and how these may relate to the pathology of the trait and look toward future directions for these technologies in post-GWAS analysis, such as base-editing.
Keywords: CRISPR-Cas9, functionality, GWAS, SNP, TALENs
INTRODUCTION
Genome-wide association studies (GWAS) have transformed our ability to detect genetic loci for many complex diseases and traits. Fine-mapping arrays (9a, 53), exome-sequencing, and whole-genome sequencing (24, 71a) have helped to define regions of genetic association and identify 99% credible sets of variants (75). Despite the success of these methodologies to detect associations in large, well-phenotyped cohorts, the identity of the causal variant(s) often remains hidden (61). The primary cause for this can be attributed to the strong linkage disequilibrium (LD) present in much of the genome, where the sentinel variant providing the strongest association signal at a locus e.g., a single nucleotide polymorphism (SNP), may be tagging (r2 > 0.8) hundreds of other co-inherited variants. An additional level of complexity for determining the functionality of trait-associated variants occurs since the majority of associated SNPs from GWAS occur within noncoding regions of the genome (61): thus an even bigger challenge compared with coding variants to predict function using in silico tools, as well as to verify experimentally. Occasionally signals may appear close to strong candidate genes for the trait, but often, there are no genes that have shown a prior link to the trait, or they are located within gene deserts without obvious mechanisms for functionality.
To steer GWAS variant discoveries toward functionality in order to identify new mechanisms of disease, several complementary public resources are available. Global efforts to annotate the coding and noncoding functional elements of the genome, such as the Encyclopedia of DNA Elements (ENCODE) Project (21) and Roadmap Epigenomics (3), have uncovered a wealth of data to enable researchers to speculate the potential role of variants, such as their location within transcription factor binding sites, histone modifications, or DNA methylation sites for a number of transformed and primary cell types. Summary data from these resources can be readily accessed from a number of databases, such as HaploReg v4.1 (74) and RegulomeDB (7). The predicted effects of variants on transcription factor binding sites can be examined through databases such as TRANSFAC (50) and JASPAR (49), although such predictions in transcription factor binding affinity do not accurately predict functional outcomes as with coding variants, and laboratory verification with the suitable cell type and conditions is required. Analysis of expression quantitative trait loci (eQTL) can provide valuable data regarding regulation of gene targets possibly affected by GWAS variants, and resources such as the Genotype-Tissue Expression (GTEx) project are facilitating these efforts by expanding the tissues and numbers of samples available for such analyses (30a). Tools such as Combined Annotation Dependent Depletion (CADD) attempt to predict pathogenicity by highly scoring variants that are not stabilized by evolutionary selection (40).
Studies into allele-specific effects on chromatin accessibility can aid the localization of potentially functional variants (17, 62), and examination of the three-dimensional organization of chromatin, based on chromosome conformation capture (3C) techniques can give clues toward the target genes for variants located within distal enhancers (60). Other emerging techniques include the massively parallel reporter assay (MPRA), which can screen thousands of potential functional variants in a single assay to determine effects on gene expression (51).
Despite these complementary tools, there are very few methods available to reliably test functionality of single variants in vivo, due to either the nature of LD in the examined genomic region or the necessity to perform the experiments outside of the context of the cell’s chromatin organization. New genome-editing techniques, however, are beginning to revolutionize the methods of studying functionality of variants from GWAS. This review will discuss the current progress and future directions for these technologies.
GENOME-EDITING TECHNOLOGIES
Until recently, efforts to modify genetic material of an organism or cell have been hindered by a lack of specificity and inefficiency, relying on site-directed mutagenesis or recombination-based methods (48, 65). Since the development of engineered nucleases (25), genome-editing has fast become an efficient and highly targeted approach to modify the genetic architecture of a cell and a promising tool to investigate functionality of GWAS loci. Three genome-editing techniques have been widely used to date: zinc-finger nucleases (ZFNs) (38), transcription activator-like effector nucleases (TALENs) (75a), and clustered regularly interspaced short palindromic repeats (CRISPR) with Cas9 nuclease (CRISPR/Cas9) (33, 47). These methods rely on the ability to create synthetic vectors to target specific oligonucleotide sequences and to induce a targeted double-strand break in the genome (Fig. 1). These breaks can then be repaired by the cell’s imperfect repair mechanisms: nonhomologous end joining (NHEJ) or by homology-directed repair (HDR), to create alterations in the genome at the site of the break. Several comprehensive articles are available detailing genome-editing technologies (12, 20, 33, 36, 57, 59, 72, 76): this review will only briefly outline the principles of the three major techniques, followed by an examination of where these have been, or have potential to be applied in the post-GWAS era to characterize causal variants or pathways.
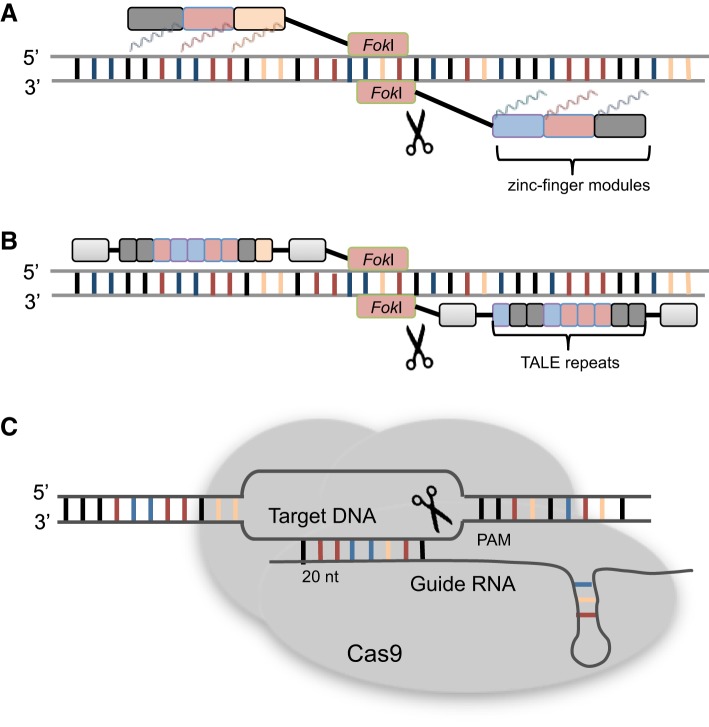
Fig. 1.
Genome-editing technologies employing different methods to create a targeted double-strand break. A: zinc-finger nucleases (ZFNs) target the genome with zinc-finger modules that each identify nucleotide triplets. This series of targeting modules is attached to a FokI nuclease, which when dimerized can form a double-strand break in the DNA. B: transcription activator-like effector nucleases (TALENs) target the genome with transcription activator-like effectors (TALEs), which target single nucleotides, as with ZFNs, these are attached to FokI to form the double-strand break. C: clustered regularly interspaced short palindromic repeats with Cas9 nuclease (CRISPR/Cas9) uses a guide RNA to base-pair with the target DNA, adjacent to the protospacer adjacent motif (PAM), and to recruit Cas9 nuclease to create the double-strand break.
ZFNs
The earliest targeted genome-editing tools to realistically allow for follow-up of GWAS targets were the ZFNs, pioneered by Kim et al. (39), who created a chimeric restriction endonuclease that demonstrated in vitro activity. The zinc-finger domain comprises ~30 amino acids in a ββα configuration, where the α-helix can contact 3 bp of DNA in a sequence-specific manner (56). Zinc-finger modules have now been designed that identify almost all 64 potential nucleotide triplets; by engineering synthetic zinc-finger domains between a conserved linker sequence, zinc-finger proteins can be created to distinguish DNA sequences up to 18 bp in length, allowing for sequence specificity within the human genome. Genome-editing relies on the creation of a double-strand break in the target DNA, and this can be achieved by engineering a FokI cleavage domain to the zinc-finger modules. The FokI cleavage domain must dimerize to cleave DNA (4), and this requirement of two targeted ZFNs for a single genomic locus results in strong sequence specificity and low likelihood for off-target effects (Fig. 1A).
Despite the remarkable advances made by ZNFs to genome-editing, the technique does have drawbacks, primarily the difficulty in constructing the zinc-finger domain.
TALENs
Following from the success of ZFNs to target and cleave specific genomic regions, TALEN technology was developed to facilitate construction of targeting nucleases. This technique uses transcription activator-like effectors (TALEs): proteins originating from Xanthomonas spp. proteobacteria that can alter gene transcription in host cells (5). An individual TALE repeat recognizes and binds to a single nucleotide, where the hypervariable residues located at positions 12 and 13 of the TALE are key to specificity, and bind in the major groove of the DNA. These hypervariable residues can either be NN or NK (recognizing guanine), NI (adenine), HD (cytosine), or NG (thymine), and the construction of TALE repeats can be engineered to recognize almost any genomic sequence (19). The only restriction for TALE repeats is the necessity for a 5′-thymine preceding the first base of the TALE sequence. As with ZFNs, engineering a nuclease such as FokI results in a synthetic protein capable of targeted genomic cleavage (Fig. 1B).
Although TALENs provide a similar efficiency to ZNFs and also have a low chance of off-target effects due to their requirement for dimerization of FokI constructs to enable cleavage, they are significantly easier to assemble, making this a much more viable option for targeted genome-editing. One drawback is their sensitivity to cytosine methylation, requiring the methylation state of the target site to be known before design.
CRISPR-CaS9
More recently, Cas9, a nuclease from Streptococcus pyogenes, has been exploited to facilitate the targeting of genomic sequences: a protein that relies on association with RNA to target the nuclease toward the DNA target (35). The CRISPR system has evolved as a protection mechanism for bacteria against foreign nucleic acids such as plasmids and viruses, where these sequences can be incorporated between CRISPR repeat sequences encoded within the host genome. When these sequences are processed into CRISPR RNA (crRNA), hybridization with a transactivating CRISPR RNA (tracrRNA) is facilitated, forming a complex with Cas9 nuclease. Cas9 is directed toward the DNA sequence to be cleaved by the crRNA, where it requires a protospacer adjacent motif (PAM) to be present (5′-NGG). This CRISPR system has been adapted for use in genome-editing, whereby a guide RNA (gRNA), formed from a fusion between tracrRNA and crRNA, and Cas9 are introduced in a cell or organism. Cas9 is guided toward a specific site by RNA-DNA base pairing from the sequence of 20 nucleotides at the 5′-end of the gRNA. It has been shown that double-strand breaks created by Cas9 are able to result in indels from NHEJ and HDR from either single-stranded oligonucleotides or double-stranded plasmid DNA (Fig. 1C).
The use of CRISPR-Cas9 for genome-editing has many advantages over ZNFs and TALENs for the majority of situations, primarily the ease of creating targeting plasmids based directly on DNA sequence and the ability to multiplex. Unlike TALENs, CRISPR-Cas9 is not methylation sensitive. The key disadvantage with this system, however, occurs with the increased likelihood for off-target effects. There are several ways to reduce this possibility, such as dimeric CRISPR RNA-guided FokI nuclease (RFN) technology (70). RFN relies on the expression of two gRNAs targeting opposing strands flanking the target site and FokI-Cas9 fusion proteins, resulting in similar specificity to ZFNs and TALENs. Use of paired single-strand “nickase” mutants has a similar effect (46). The CRISPR system also relies on Cas9 to recognize the PAM: in situations where simply knocking out a gene is required, this is not normally a concern. When attempting to alter a particular nucleotide with HDR or using RFNs or nicking Cas9, this can provide difficulties, particularly with the spacing constraints required for RFNs and nickases. Different PAM sequences are available, such as an engineered Cas9 from Francisella novicida recognizing 5′-YG-3′ (32), or the Cpf1 nuclease from this bacteria that recognizes 5′-TTN-3′ (81) or 5′-YTN-3′ (23).
CHARACTERIZATION OF LOCI IDENTIFIED BY GWAS
There are multiple ways genome-editing is set to transform the functional analysis of GWAS loci, and we will discuss some early examples of the two main approaches in relation to noncoding variants: that of examining the effects of the variant on downstream pathways and that of identifying the causal variant, and these are summarized in Table 1. There is opportunity to use genome-editing to study the majority of traits examined by GWAS, and here we will focus on a subset of these.
Table 1.
Use of genome-editing techniques to examine functionality of noncoding variants
| Genome-editing Tool | Trait | Locus | Lead SNPs | Investigated SNP | Functionality Demonstrated through Genome-editing | Reference |
|---|---|---|---|---|---|---|
| Zinc-finger nuclease | breast cancer | FGFR2 | rs2981578 rs1219648 rs2981582 rs2981579 | rs2981578 | effects on cell proliferation, cell cycle progression, and transcription factor binding examined, showing no allele-specific effects | (43) |
| TALEN | prostate cancer | RFX6 | rs339331 | rs339331 | prostate cancer cell lines homozygous for risk alleles demonstrated increased RFX6 gene expression and increased HOXB13 transcription factor occupancy | (44) |
| CRISPR-Cas9 | Type 2 diabetes | TCF7L2 | rs7903146 | rs7903146 | allele-specific effects demonstrated in a human colorectal carcinoma cell line on ACSL5 gene expression and long-range chromatin contacts | (31) |
| Type 2 diabetes | PPARG2 | n/a | rs4684847 | preadipocytes with nonrisk allele introduced showed increased PPARG2 transcript levels, mediated by PRRX1 transcription factor | (32) | |
| lipids | CPNE1 | rs2277862 | rs2277862 | allele-specific effects on gene CPNE1 gene expression observed upon differentiation of hPSCs into adipocytes | (39) | |
| coronary artery disease | PHACTR1 | rs9349379 | rs9349379 | deletion surrounding rs9349379 resulted in decrease of PHCTR1 gene expression | (40) | |
| obesity | IRX3/IRX5 | rs1558902 | rs1421085 | allele-specific gene expression observed upon adipocyte differentiation in presence of the repressor, ARID5B | (41) | |
| renal cancer | MYC/PVT1 | rs35252396 | rs35252396 | mutations introduced to an HIF-binding site surrounding rs35252396 led to reduction in MYC and PVT1 gene expression | (46) | |
| Parkinson's disease | SNCA | rs356220 rs356219 rs356182 | rs356168 | iPSCs differentiated into neuronal precursors showed higher levels of SNCA gene expression for carriers of the risk allele | (47) | |
| ankylosing spondylitis | PTGER4 | n/a | rs9283753 | using lymphoblast cell lines, allele-specific effects on PTGER4 gene expression were observed | (48) |
CRISPR-Cas9, clustered regularly interspaced short palindromic repeats with Cas9 nuclease; iPSC, induced pluripotent stem cell; SNP, single-nucleotide polymorphism; TALEN, transcription activator-like effector nucleases.
TYPE 2 DIABETES
A study by Claussnizter et al. (10) sought to examine the functionality of cis-regulatory variants causing predispositions to Type 2 diabetes (T2D) with integrative computational analysis of phylogenetic conservation. They examined enrichment for transcription factor binding within conserved transcription factor binding site modules at GWAS loci, and their analysis identified clustering of distinct homeobox transcription factor binding sites, specifically, identifying the paired-related homeobox 1 (PRRX1) factor as a repressor of peroxisome proliferator activated receptor gamma gene (PPARG2) expression in adipose cells. The authors demonstrated that PRRX1 showed adverse effect on lipid metabolism and systemic insulin sensitivity, according to rs4684847 genotype, where the risk allele triggers PRRX1 binding. The authors employed CRISPR-Cas9 homology-directed repair genome editing to introduce the rs4684847 nonrisk allele into human Simpson-Golabi-Behmel syndrome preadipocytes. Introduction of this allele led to a 5.4-fold increase in PPARG2 transcript levels, which was in accordance with experiments performing PRRX1 knockdown in human adipose stromal cells, where the risk allele-driven suppression of PPARG2 expression was reversed by silencing PRRX1. PRRX1 silencing did not, however, affect expression of PPARG2 in cells harboring the nonrisk allele.
The TCF7L2 locus has been associated with T2D with a relative-risk of 1.36 per allele: the strongest effect size observed for T2D for a common variant (30). An intronic variant in TCF7L2, rs7903146 t > C, has long been considered to be functional not only due to its consistent appearance as the lead SNP at the locus (53), but also from allele-specific effects on reporter gene expression and the presence of open chromatin at this locus in pancreatic islets (27). Other tissues implicated in the role of TCF7L2 in T2D include the liver (6, 37), adipose tissue (37), and intestinal cells (79). To ascertain a potential effect of this variant on gene regulation in intestinal cells, Xia et al. (77) used CRISPR-Cas9 tools, which generated a 1.4 kb deletion surrounding the variant in HCT116 cells, a human colorectal carcinoma cell line, followed by an examination of global gene expression and chromosome conformation capture-based techniques to assess chromatin structure in the mutant vs. wild-type cell line. The authors identified 99 genes that showed differential expression in the mutant line carrying the deletion, compared with the wild type, but only one gene whose promoter formed intrachromosomal contacts in proximity to the variant location in the wild-type cell line: Acyl-CoA synthetase long-chain family member 5 (ACSL5). To further define the regulatory effects surrounding rs7903146, further deletions of 66 and 104 bp surrounding the variant were performed, leading to similar reductions in ACSL5 protein levels. The authors speculated that this revealed a role for rs7903146 in creating a colon-specific enhancer associated with expression of ACSL5, producing a protein with known roles in fatty acid metabolism and involvement in T2D. The authors also note, however, that studies in different tissues need to be performed to demonstrate tissue-specific effects, particularly as chromatin accessibility surrounding this variant has only been detected in pancreatic cells (27). The authors did not examine the effects of rs7903146 alleles on ACSL5 expression or protein levels outside the context of large deletions, and so these initial findings do not characterize SNP functionality at this locus specifically for T2D.
Genome-editing not only provides means to explore functionality of genes implicated in disease through GWAS but also offers the potential to screen potential therapeutics. Using isogenic human embryonic stem cells (hESCs), Zeng et al. (80) created insertion or deletion mutations with CRISPR/Cas9 to create early frame shifts in three genes identified from GWAS for T2D: CDKAL1 (CDK5 regulatory subunit associated protein 1 like 1), KCNQ1 (potassium voltage-gated channel subfamily Q member 1), and KCNJ11 (potassium voltage-gated channel subfamily J member 11). These mutant hESCs were differentiated into functional islet cells that did not affect insulin production, although they showed effects on impaired glucose secretion and glucose homeostasis. Using a high-content chemical screen on these cells, the authors identified a candidate drug, T5224, which was able to rescue the CDKAL1-specific defects by inhibition of the FOS/JUN pathway.
CARDIOVASCULAR TRAITS
A number of continuous traits such as blood pressure and circulating lipid levels are well-established risk factors for cardiovascular disease. The MTHFR-NPPB locus has been associated with blood pressure in a number of GWASs (34a, 73). Since the locus contains many candidate genes for hypertension, a study by Flister et al. (22) employed genome-editing to establish which genes at this locus could be implicated with the trait. Using ZFN tools, the authors introduced deleterious mutations into rodent models of genetic hypertension, resulting in frame shifts or disruption to functional protein domains into each of the six genes spanning this locus: Agtrap (angiotensin II receptor associated protein), Mthfr (methylenetetrahydrofolate reductase), Clcn6 (chloride voltage-gated channel 6), Nppa/Nppb (natriuretic peptides A and B), and Plod1 (procollagen-lysine, 2-oxoglutarate 5-dioxygenase 1). The study revealed that five of the six genes could affect hypertension by modifying blood pressure or renal phenotypes, with mutations in Nppa, Plod1, and Mthfr increasing hypertension risk and mutations in Agtrap and Clcn6 decreasing disease susceptibility.
The transcription factor nuclear receptor 2 family 2 gene (NR2F2) has been implicated in essential hypertension through GWAS (8). To demonstrate this, Kumarasamy et al. (44) created a ZFN-based 15 bp deletion in the Nr2f2 gene, leading to a deletion within the hinge region of the protein. The authors demonstrated that the mutant rats had significantly lower blood pressure than control rats (systolic BP 179 ± 3 vs. 197 ± 5 mmHg), due to the interaction of the hinge region of Nr2f2 and zinc finger protein, FOG family member 2 (Fog2), which was increased following the mutation, and that the interaction of these two transcription factors was an important regulator of blood pressure. Such studies where specific deletions are created within proteins cannot only give answers to which pathways may be involved from GWAS, but also specific molecular mechanisms leading to the trait.
The use of large-scale GWAS has confirmed and revealed new loci for lipid traits (67, 68, 75a). A study by Pashos et al. (55) employed multiple approaches involving induced pluripotent stem cells (iPSCs) and hepatocyte-like cells to isolate functional variants, including genome-wide mapping of eQTLs, allele-specific expression, and an MPRA. Following characterization of likely functional variants, the authors focused on several loci with genome-editing. First, they used CRISPR/Cas9 in a human pluripotent stem cell (hPSC) line to knock in one minor allele at the CPNE1 locus into the rs2277862 CC homozygous cells, using single-strand DNA oligonucleotide as a template. This, however, led to low efficiency, resulting in only a single clone with the heterozygous allele, but did demonstrate decreased CPNE1 expression in both knock-in undifferentiated hPSCs and differentiated hepatocyte-like cells. Second, the authors deleted ~38 bp surrounding the SNP, allowing for higher efficiency. This demonstrated 31% decreased expression of CPNE1 and 20% decrease in ERIGC3 expression. The authors also used CRISPR/Cas9 to generate a knock-in mouse with a minor allele in the homozygous C57BL/6J CC homozygous strain. Again, a 37% decrease of Cpne1 was observed in the liver of TT mice compared with CC wild-type mice. In the same study, the authors also examined the ANGPTL3/DOCK7 locus, associated with a 4.9 mg/dl change in TG levels (68). Using single-strand template was not successful for HDR using CRISPR/Cas9, and the authors used a targeting vector with 500 bp homology arms on a transposon that was able to undergo scarless removal from a TTA site with piggyBac. Puromycin selection allowed for knock-in of rs10889356 minor alleles to both chromosomes resulting in decreased DOCK7 expression (36%) and increased ANGPTL3 expression (60%). Similar findings were seen when the authors multiplexed CRISPR/Cas9 to create a 36–39 bp deletion surrounding the variant.
A consistent finding in GWASs for coronary artery disease (CAD) is seen with variants at the phosphatase and actin regulator 1 gene locus (PHACTR1) (9a). To examine functionality of this locus, Beaudoin et al. (2) performed fine-mapping and DNA resequencing to prioritize rs9349379 as a causal variant, also determining the variant as an eQTL for PHACTR1 expression in the coronary artery. Using an endothelial cell extract, the authors demonstrated that rs9349379 differentially bound the transcription factor myocyte enhancer factor-2 (MEF2). Using siRNA to knockdown MEF2 in human umbilical vein endothelial cells was unsuccessful, and so the authors used CRISPR/Cas9 to introduce a heterozygous deletion surrounding rs9349379 and the MEF2-binding site in hESCs. Following differentiation into endothelial cells, a decrease of 35% PHACTR1 expression was observed validating the potentially causal role of rs9349379 in CAD.
OBESITY
The fat mass and obesity associated gene (FTO) locus has shown the strongest association with obesity in GWAS, although it was established that the variants in the FTO locus might be acting through long-range interactions with the IRX3 (iroquois homeobox 3) locus (60). A recent study performed phylogenetic module complexity analysis at the locus to determine the most likely functional variant, with rs1421085 achieving the highest score, and in perfect LD with the lead GWAS SNP, rs1558902 (11). Reporter assays and electrophoretic mobility shift assays indicated the loss of a repressor protein, AT-rich interaction domain 5B (ARID5B), binding to the risk allele in adipocytes, in line with effects that the authors observed on IRX3 and IRX5 gene expression. As with similar examples outlined previously, CRISPR/Cas9 genome-editing was performed in primary preadipocytes to create cells that differed only by rs1421085 genotype. It was found that conversion from the risk allele to the nonrisk allele restored low expression levels of IRX3 and IRX5 in the presence of ARID5B. Further examination of the differences between edited and nonedited preadipocytes during differentiation into white and beige adipocytes showed that peak expression occurred in days 0–2 of differentiation in the unedited risk alleles compared with those edited to wild type, which maintained low levels of expression throughout differentiation, indicating that rs1421085 plays a causal role in developmental gene expression.
CANCERS
Variants in the fibroblast growth factor receptor 2 (FGFR2) have shown a strong association with development of breast cancer (34). To examine one of the putative functional variants at the locus, an estrogen receptor alpha-positive breast cancer cell line, MCF7, was edited with ZFN technology to modify the lead GWAS variant: rs2981578, from wild type to heterozygous status (58). The resulting mutant cell line showed no effects on cell proliferation, cell cycle progression, or indeed, binding of the transcription factor runt-related transcription factor 2 (RUNX2), as previously indicated. In this case genome-editing was used to refute the role of a putative functional SNP as a single causal variant at the locus.
Spisak et al. (64) examined a GWAS locus on 6q22.1 for pancreatic cancer, which also acts as an eQTL for regulatory factor X6 (RFX6) gene expression. Following approaches to reduce the number of candidate causal variants that included fine-mapping and analyses of chromatin annotations in the prostate cancer cell line LNCaP, the authors confirmed a previous finding that the risk allele of rs339331 was able to create a binding site for the prostate lineage-specific homeobox B13 (HOXB13) transcription factor. In an effort to characterize the locus further, genome-editing using TALE-based tools were performed. Initially, the authors used a fusion protein, where the TALE element was fused to LSD1, a histone lysine-specific demethylase, previously shown to remove H34K methylation marks at the site of DNA-targeting (52), thereby decreasing their regulatory activity. By targeting the HOXB13 site at rs339331, the authors observed a threefold decrease in RFX6 expression levels. A similar experiment was performed replacing LSD1 with VP64, a transcriptional activator, where a twofold increase in RFX6 expression was observed, confirming the locus as a key regulator of RFX6 expression. To determine whether rs339331 was responsible for this effect, TALEN-mediated HDR was employed to create isogenic 22Rv1 prostate cancer cell lines containing each of the three rs339331 genotypes. The cells homozygous for the protective alleles showed decreased RFX6 expression and those homozygous for the risk alleles showed increased expression compared with the heterozygous cell lines. In concordance with these findings, the authors demonstrated increased HOXB13 occupancy in the risk allele carriers to account for the effects on gene expression. When performing a global analysis of gene expression with RNA-Seq in the edited cell lines, the authors observed enrichment in genes affected by androgenic compounds and androgen receptor.
Renal cancer susceptibility has been examined with GWAS, where a study identified a single intergenic variant, rs35252396, in a region located between the oncogenes v-myc avian myelocytomatosis viral oncogene homolog (MYC) and pvt1 oncogene (PVT1) (31). A study by Grampp et al. (29) examined ENCODE annotations for chromatin accessibility and chromatin immunoprecipitation (ChIP)-seq data for hypoxia inducible factor (HIF), a transcription factor implicated in MYC and PVT1 regulation. The authors identified a HIF-binding signal that coincided with the renal cancer susceptibility SNP rs35252396 and showed by chromosome conformation capture techniques that this formed physical connections to both the MYC and PVT1 promoters. To confirm whether the HIF binding site surrounding rs35252396 or another HIF site was responsible for the effects on regulation, the authors targeted the HIF-response element in 786-O renal cancer cells with CRISPR/Cas9 technology. Using seven clones of cells with mutations that affected the HIF-binding site, the authors confirmed reduced binding of HIF to the locus and observed a reduction in MYC and PVT1 RNA expression by 40 and 32%, respectively. The results indicated that the enhancer site affected by rs35252396 interacts with MYC and PVT1 promoters and is necessary for HIF-mediated transactivation of both genes to influence downstream effects of MYC in disease.
NEURODEGENERATIVE DISORDERS
While patient-specific iPSCs can be useful for examining effects of pathogenic variants in vitro, biological heterogeneity hinders the identification of causal variants, particularly for more complex traits. Genome-editing using iPSCs is becoming an increasingly attractive option for studies into GWAS variants as the technology to create lineage-specific cell lines continues to improve. A study by Soldner et al. (63) employed CRISPR/Cas9 genome editing to explore two risk variants, rs356168 and rs3756054, for Parkinson’s disease in a noncoding distal enhancer element that regulates synuclein alpha (SNCA), an important gene in the pathogenesis of the disease, by inserting all genotype combinations into the iPSCs and measuring alteration in gene expression. The cells were differentiated into neuronal precursors or mixed neuronal cultures and expression examined by qRT-PCR. The authors observed that cells carrying the rs356168 G allele showed significantly higher expression, independent of other variants, in line with the GWAS findings.
AUTOIMMUNE DISORDERS
Tewhey et al. (69) used MPRA to identify a number of potentially functional variants at GWAS locations in lymphoblast cell lines. Looking at 32,373 variants, the study identified 842 whose alleles showed differential gene expression. The authors focused on one variant, rs9283753, associated with both risk for ankylosing spondylitis and allele-specific reporter expression. Using a CRISPR/Cas9 system to switch only this allele in their cellular system, the study demonstrated that rs9283753 affected enhancer activity on Prostaglandin E Receptor 4 (PTGER4) gene expression, providing strong indications of causality at this GWAS locus.
CONCLUSIONS AND FUTURE DIRECTIONS
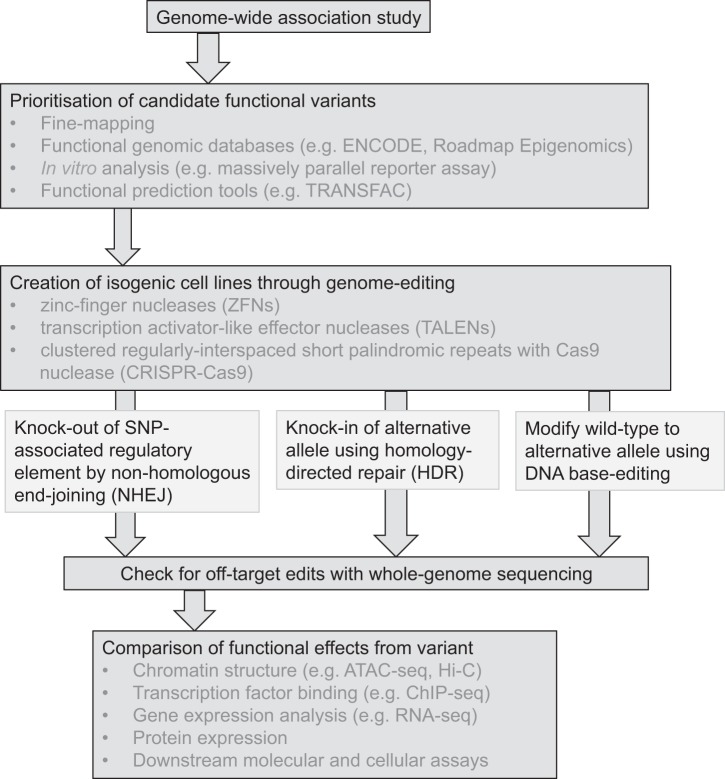
The examples described here provide insight into the multiple ways genome-editing promises to transform studies into the functionality of GWAS variants, primarily within regulatory genomic regions, and these processes are summarized in Fig. 2. The approaches used to examine these variants also highlight some of the advantages and disadvantages of study design when using genome-editing technology, where focus should be directed toward examination of an individual variant rather than deletion of large regions and use of appropriate tissues. Tools such as TALENs or CRISPR/Cas9 are still relatively recent, and there is substantial scope to expand and further develop genome-editing technology to facilitate these goals. Recent advances have been made with base-editing, a form of genetic engineering that facilitates irreversible conversion of base pairs without requiring double-strand DNA breaks or donor DNA templates (26, 41, 42, 54). These new tools are increasing their capabilities to allow for different PAM compatibilities, enhanced DNA specificity, improved editing efficiency, and narrowed editing windows and have the potential to overtake CRISPR/Cas9 in future genome-editing strategies.
Fig. 2.
Experimental methodologies to build functional evidence for genome-wide association study (GWAS) variants using genome-editing technologies.
Due to the lengthy process required to isolate edited cell lines with the desired variant, studies have so far focused on loci where there are only a few candidate functional SNPs either due to low LD in the region or through fine-mapping and analysis of chromatin marks before commencing genome-editing. The latter requires that “reduction” methodologies do not overlook potentially functional variants, which will always be a concern, particularly if there may be more than one causal variant at a locus. Future directions for improving genome-editing to streamline GWAS variant analysis will focus on increasing the efficiency and targeting of genome-editing, facilitating the investigation of multiple variants, and perform unbiased functional analysis in the same way that GWAS performs unbiased association analyses.
Genome-editing applied to model pathogenic variants is becoming increasingly reliant on hPSCs and their differentiation to cell types that closely resemble those relevant to the traits being examined. Such cells are readily edited, and differentiation to multiple cell types allows for potential effects in multiple tissues to be examined, an important issue when a GWAS variant shows no clear target tissue. Continued development of hPSC differentiation protocols will increase the number of cell types available for meaningful genome-editing research. In parallel to hPSCs, the use of genome-editing to create specific animal models of disease by targeting protein-coding regions is becoming well established (20, 71). One of the next challenges for examination of GWAS variants will involve expanding gene-editing techniques to study noncoding variations in the same manner in animal models to gain a fuller understanding of the physiological role of risk variants. Such studies have begun, for example, the deletion of the mouse Myc-355, a putative regulatory element in mouse, which in humans, contains rs6983267, a variant that accounts for more cancer-related morbidity than any other variant (66). These mice showed reduced expression of Myc and increased resistance to tumorigenesis induced by APC mutations. The use of technologies such as CRISPR/Cas9 is likely to facilitate such studies. However, since noncoding elements are less conserved between species, and these enhancers often differ in critical components (9), it will be a greater challenge, likely to involve insertion of human regulatory sequences at the orthologous location in the animal model to examine allele-specific phenotypic differences.
In addition to single nucleotide variants, future studies may also examine structural variations in more detail, where differences in genomic DNA can range from kilobase to chromosomal magnitude. Such variations are often associated with disease and can contribute large portions of genomic variability. Structural variants have been created using ZFN (45) and TALENs (1) and CRISPR-based systems (43, 78). Many of these structural variants encompass noncoding regions of the genome, and studies in the relevant human cellular models are needed to evaluate their impact.
The last 10 yr of genetic association studies was transformed by the development of low-cost, high-throughput genotyping arrays. The next 10 yr and beyond is likely to be focused on analyzing of this wealth of association signals, with genome-editing one of the major tools to facilitate this endeavor. As with genotyping and sequencing technologies, the challenge for genome editing will be to develop cheaper, highly parallel methodologies that will allow us to test every variant in the genome for function.
GRANTS
This work is supported by the National Institute for Health Research Biomedical Research Centre at Barts. A. J. P. Smith is a British Heart Foundation (BHF) Intermediate Fellow (FS/13/6/29977). P. Deloukas is supported by BHF Grant RG/14/5/30893.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.P.S. prepared figures; A.J.P.S., P.D., and P.B.M. drafted manuscript; A.J.P.S., P.D., and P.B.M. edited and revised manuscript; A.J.P.S., P.D., and P.B.M. approved final version of manuscript.
APPENDIX: USEFUL URLs:
GWAS Catalog, https://www.ebi.ac.uk/gwas/
Encyclopedia of DNA Elements - ENCODE, http://genome.ucsc.edu/ENCODE/
Roadmap Epigenomics Project, http://www.roadmapepigenomics.org
HaploReg, https://www.broadinstitute.org/mammals/haploreg
RegulomeDB, http://www.regulomedb.org/
Addgene guide to CRISPR/Cas9, https://www.addgene.org/crispr/guide/
Addgene guide to TALENs, https://www.addgene.org/talen/guide/
REFERENCES
- 1.Bauer DE, Kamran SC, Lessard S, Xu J, Fujiwara Y, Lin C, Shao Z, Canver MC, Smith EC, Pinello L, Sabo PJ, Vierstra J, Voit RA, Yuan GC, Porteus MH, Stamatoyannopoulos JA, Lettre G, Orkin SH. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 342: 253–257, 2013. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaudoin M, Gupta RM, Won HH, Lo KS, Do R, Henderson CA, Lavoie-St-Amour C, Langlois S, Rivas D, Lehoux S, Kathiresan S, Tardif JC, Musunuru K, Lettre G. Myocardial Infarction-Associated SNP at 6p24 Interferes With MEF2 Binding and Associates With PHACTR1 Expression Levels in Human Coronary Arteries. Arterioscler Thromb Vasc Biol 35: 1472–1479, 2015. doi: 10.1161/ATVBAHA.115.305534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernstein BE, Stamatoyannopoulos JA, Costello JF, Ren B, Milosavljevic A, Meissner A, Kellis M, Marra MA, Beaudet AL, Ecker JR, Farnham PJ, Hirst M, Lander ES, Mikkelsen TS, Thomson JA. The NIH Roadmap Epigenomics Mapping Consortium . The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol 28: 1045–1048, 2010. doi: 10.1038/nbt1010-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitinaite J, Wah DA, Aggarwal AK, Schildkraut I. FokI dimerization is required for DNA cleavage. Proc Natl Acad Sci USA 95: 10570–10575, 1998. doi: 10.1073/pnas.95.18.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boch J, Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol 48: 419–436, 2010. doi: 10.1146/annurev-phyto-080508-081936. [DOI] [PubMed] [Google Scholar]
- 6.Boj SF, van Es JH, Huch M, Li VSW, José A, Hatzis P, Mokry M, Haegebarth A, van den Born M, Chambon P, Voshol P, Dor Y, Cuppen E, Fillat C, Clevers H. Diabetes risk gene and Wnt effector Tcf7l2/TCF4 controls hepatic response to perinatal and adult metabolic demand. Cell 151: 1595–1607, 2012. doi: 10.1016/j.cell.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 7.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, Karczewski KJ, Park J, Hitz BC, Weng S, Cherry JM, Snyder M. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res 22: 1790–1797, 2012. doi: 10.1101/gr.137323.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browning BL, Browning SR. Haplotypic analysis of Wellcome Trust Case Control Consortium data. Hum Genet 123: 273–280, 2008. doi: 10.1007/s00439-008-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP, Luc S, Kurita R, Nakamura Y, Fujiwara Y, Maeda T, Yuan GC, Zhang F, Orkin SH, Bauer DE. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature 527: 192–197, 2015. doi: 10.1038/nature15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.CARDIoGRAMplusC4D Consortium; Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, König IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikäinen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do R, DIAGRAM Consortium, CARDIOGENICS Consortium, Doney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Müller-Nurasyid M, MuTHER Consortium; Nikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schäfer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJ, Wellcome Trust Case Control Consortium, Wells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrières J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kähönen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Trégouët DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvänen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimäki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O’Donnell C, Reilly MP, März W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 45: 25–33, 2013. doi: 10.1038/ng.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claussnitzer M, Dankel SN, Klocke B, Grallert H, Glunk V, Berulava T, Lee H, Oskolkov N, Fadista J, Ehlers K, Wahl S, Hoffmann C, Qian K, Rönn T, Riess H, Müller-Nurasyid M, Bretschneider N, Schroeder T, Skurk T, Horsthemke B, Spieler D, Klingenspor M, Seifert M, Kern MJ, Mejhert N, Dahlman I, Hansson O, Hauck SM, Blüher M, Arner P, Groop L, Illig T, Suhre K, Hsu YH, Mellgren G, Hauner H, Laumen H, Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Boström KB, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney ASF, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PRV, Jørgensen T, Kao WHL, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JRB, Petersen A-K, Platou C, Proença C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparsø T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Mohlke KL, Morris AD, Palmer CNA, Pramstaller PP, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Wareham NJ, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Hu FB, Meigs JB, Pankow JS, Pedersen O, Wichmann H-E, Barroso I, Florez JC, Frayling TM, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI; DIAGRAM+Consortium . Leveraging cross-species transcription factor binding site patterns: from diabetes risk loci to disease mechanisms. Cell 156: 343–358, 2014. doi: 10.1016/j.cell.2013.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claussnitzer M, Hui CC, Kellis M. FTO Obesity Variant and Adipocyte Browning in Humans. N Engl J Med 374: 192–193, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science 339: 819–823, 2013. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degner JF, Pai AA, Pique-Regi R, Veyrieras JB, Gaffney DJ, Pickrell JK, De Leon S, Michelini K, Lewellen N, Crawford GE, Stephens M, Gilad Y, Pritchard JK. DNase I sensitivity QTLs are a major determinant of human expression variation. Nature 482: 390–394, 2012. doi: 10.1038/nature10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng D, Yan C, Pan X, Mahfouz M, Wang J, Zhu JK, Shi Y, Yan N. Structural basis for sequence-specific recognition of DNA by TAL effectors. Science 335: 720–723, 2012. doi: 10.1126/science.1215670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding Q, Lee YK, Schaefer EA, Peters DT, Veres A, Kim K, Kuperwasser N, Motola DL, Meissner TB, Hendriks WT, Trevisan M, Gupta RM, Moisan A, Banks E, Friesen M, Schinzel RT, Xia F, Tang A, Xia Y, Figueroa E, Wann A, Ahfeldt T, Daheron L, Zhang F, Rubin LL, Peng LF, Chung RT, Musunuru K, Cowan CA. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 12: 238–251, 2013. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature 489: 57–74, 2012. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flister MJ, Tsaih SW, O’Meara CC, Endres B, Hoffman MJ, Geurts AM, Dwinell MR, Lazar J, Jacob HJ, Moreno C. Identifying multiple causative genes at a single GWAS locus. Genome Res 23: 1996–2002, 2013. doi: 10.1101/gr.160283.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fonfara I, Richter H, Bratovič M, Le Rhun A, Charpentier E. The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA. Nature 532: 517–521, 2016. doi: 10.1038/nature17945. [DOI] [PubMed] [Google Scholar]
- 24.Futema M, Plagnol V, Li K, Whittall RA, Neil HA, Seed M, Simon Broome Consortium; Bertolini S, Calandra S, Descamps OS, Graham CA, Hegele RA, Karpe F, Durst R, Leitersdorf E, Lench N, Nair DR, Soran H, Van Bockxmeer FM; UK10K Consortium, Humphries SE. Whole exome sequencing of familial hypercholesterolaemia patients negative for LDLR/APOB/PCSK9 mutations. J Med Genet 51: 537–544, 2014. doi: 10.1136/jmedgenet-2014-102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaj T, Gersbach CA, Barbas CF III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31: 397–405, 2013. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A·T to G·C in genomic DNA without DNA cleavage. Nature 551: 464–471, 2017. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaulton KJ, Nammo T, Pasquali L, Simon JM, Giresi PG, Fogarty MP, Panhuis TM, Mieczkowski P, Secchi A, Bosco D, Berney T, Montanya E, Mohlke KL, Lieb JD, Ferrer J. A map of open chromatin in human pancreatic islets. Nat Genet 42: 255–259, 2010. doi: 10.1038/ng.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grampp S, Platt JL, Lauer V, Salama R, Kranz F, Neumann VK, Wach S, Stöhr C, Hartmann A, Eckardt KU, Ratcliffe PJ, Mole DR, Schödel J. Genetic variation at the 8q24.21 renal cancer susceptibility locus affects HIF binding to a MYC enhancer. Nat Commun 7: 13183, 2016. doi: 10.1038/ncomms13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groves CJ, Zeggini E, Minton J, Frayling TM, Weedon MN, Rayner NW, Hitman GA, Walker M, Wiltshire S, Hattersley AT, McCarthy MI. Association analysis of 6,736 U.K. subjects provides replication and confirms TCF7L2 as a type 2 diabetes susceptibility gene with a substantial effect on individual risk. Diabetes 55: 2640–2644, 2006. doi: 10.2337/db06-0355. [DOI] [PubMed] [Google Scholar]
- 30a.GTEx Consortium Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348: 648–660, 2015. doi: 10.1126/science.1262110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudmundsson J, Sulem P, Gudbjartsson DF, Masson G, Petursdottir V, Hardarson S, Gudjonsson SA, Johannsdottir H, Helgadottir HT, Stacey SN, Magnusson OT, Helgason H, Panadero A, van der Zanden LF, Aben KK, Vermeulen SH, Oosterwijk E, Kong A, Mayordomo JI, Sverrisdottir A, Jonsson E, Gudbjartsson T, Einarsson GV, Kiemeney LA, Thorsteinsdottir U, Rafnar T, Stefansson K. A common variant at 8q24.21 is associated with renal cell cancer. Nat Commun 4: 2776, 2013. doi: 10.1038/ncomms3776. [DOI] [PubMed] [Google Scholar]
- 32.Hirano H, Gootenberg JS, Horii T, Abudayyeh OO, Kimura M, Hsu PD, Nakane T, Ishitani R, Hatada I, Zhang F, Nishimasu H, Nureki O. Structure and Engineering of Francisella novicida Cas9. Cell 164: 950–961, 2016. doi: 10.1016/j.cell.2016.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157: 1262–1278, 2014. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover RN, Thomas G, Chanock SJ. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39: 870–874, 2007. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.International Consortium for Blood Pressure Genome-Wide Association Studies, Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WHL, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND, CARDIoGRAM Consortium, CKDGen Consortium, KidneyGen Consortium, EchoGen Consortium; CHARGE-HF Consortium, Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478: 103–109, 2011. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821, 2012. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joung JK, Sander JD. TALENs: a widely applicable technology for targeted genome editing. Nat Rev Mol Cell Biol 14: 49–55, 2013. doi: 10.1038/nrm3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaminska D, Kuulasmaa T, Venesmaa S, Käkelä P, Vaittinen M, Pulkkinen L, Pääkkönen M, Gylling H, Laakso M, Pihlajamäki J. Adipose tissue TCF7L2 splicing is regulated by weight loss and associates with glucose and fatty acid metabolism. Diabetes 61: 2807–2813, 2012. doi: 10.2337/db12-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S, Kim JS. Targeted genome engineering via zinc finger nucleases. Plant Biotechnol Rep 5: 9–17, 2011. doi: 10.1007/s11816-010-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA 93: 1156–1160, 1996. doi: 10.1073/pnas.93.3.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46: 310–315, 2014. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533: 420–424, 2016. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, Koblan LW, Kim YB, Badran AH, Liu DR. Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci Adv 3: eaao4774, 2017. doi: 10.1126/sciadv.aao4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kraft K, Geuer S, Will AJ, Chan WL, Paliou C, Borschiwer M, Harabula I, Wittler L, Franke M, Ibrahim DM, Kragesteen BK, Spielmann M, Mundlos S, Lupiáñez DG, Andrey G. Deletions, inversions, duplications: engineering of structural variants using CRISPR/Cas in mice. Cell Rep 10: 833–839, 2015. doi: 10.1016/j.celrep.2015.01.01. [DOI] [PubMed] [Google Scholar]
- 44.Kumarasamy S, Waghulde H, Gopalakrishnan K, Mell B, Morgan E, Joe B. Mutation within the hinge region of the transcription factor Nr2f2 attenuates salt-sensitive hypertension. Nat Commun 6: 6252, 2015. doi: 10.1038/ncomms7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee HJ, Kweon J, Kim E, Kim S, Kim JS. Targeted chromosomal duplications and inversions in the human genome using zinc finger nucleases. Genome Res 22: 539–548, 2012. doi: 10.1101/gr.129635.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol 31: 833–838, 2013. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science 339: 823–826, 2013. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature 336: 348–352, 1988. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 49.Mathelier A, Fornes O, Arenillas DJ, Chen CY, Denay G, Lee J, Shi W, Shyr C, Tan G, Worsley-Hunt R, Zhang AW, Parcy F, Lenhard B, Sandelin A, Wasserman WW. JASPAR 2016: a major expansion and update of the open-access database of transcription factor binding profiles. Nucleic Acids Res 44, D1: D110–D115, 2016. doi: 10.1093/nar/gkv1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, Kloos DU, Land S, Lewicki-Potapov B, Michael H, Münch R, Reuter I, Rotert S, Saxel H, Scheer M, Thiele S, Wingender E. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res 31: 374–378, 2003. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melnikov A, Murugan A, Zhang X, Tesileanu T, Wang L, Rogov P, Feizi S, Gnirke A, Callan CG Jr, Kinney JB, Kellis M, Lander ES, Mikkelsen TS. Systematic dissection and optimization of inducible enhancers in human cells using a massively parallel reporter assay. Nat Biotechnol 30: 271–277, 2012. doi: 10.1038/nbt.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendenhall EM, Williamson KE, Reyon D, Zou JY, Ram O, Joung JK, Bernstein BE. Locus-specific editing of histone modifications at endogenous enhancers. Nat Biotechnol 31: 1133–1136, 2013. doi: 10.1038/nbt.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, Prokopenko I, Kang HM, Dina C, Esko T, Fraser RM, Kanoni S, Kumar A, Lagou V, Langenberg C, Luan J, Lindgren CM, Müller-Nurasyid M, Pechlivanis S, Rayner NW, Scott LJ, Wiltshire S, Yengo L, Kinnunen L, Rossin EJ, Raychaudhuri S, Johnson AD, Dimas AS, Loos RJ, Vedantam S, Chen H, Florez JC, Fox C, Liu CT, Rybin D, Couper DJ, Kao WH, Li M, Cornelis MC, Kraft P, Sun Q, van Dam RM, Stringham HM, Chines PS, Fischer K, Fontanillas P, Holmen OL, Hunt SE, Jackson AU, Kong A, Lawrence R, Meyer J, Perry JR, Platou CG, Potter S, Rehnberg E, Robertson N, Sivapalaratnam S, Stančáková A, Stirrups K, Thorleifsson G, Tikkanen E, Wood AR, Almgren P, Atalay M, Benediktsson R, Bonnycastle LL, Burtt N, Carey J, Charpentier G, Crenshaw AT, Doney AS, Dorkhan M, Edkins S, Emilsson V, Eury E, Forsen T, Gertow K, Gigante B, Grant GB, Groves CJ, Guiducci C, Herder C, Hreidarsson AB, Hui J, James A, Jonsson A, Rathmann W, Klopp N, Kravic J, Krjutškov K, Langford C, Leander K, Lindholm E, Lobbens S, Männistö S, Mirza G, Mühleisen TW, Musk B, Parkin M, Rallidis L, Saramies J, Sennblad B, Shah S, Sigurðsson G, Silveira A, Steinbach G, Thorand B, Trakalo J, Veglia F, Wennauer R, Winckler W, Zabaneh D, Campbell H, van Duijn C, Uitterlinden AG, Hofman A, Sijbrands E, Abecasis GR, Owen KR, Zeggini E, Trip MD, Forouhi NG, Syvänen AC, Eriksson JG, Peltonen L, Nöthen MM, Balkau B, Palmer CN, Lyssenko V, Tuomi T, Isomaa B, Hunter DJ, Qi L, Wellcome Trust Case Control Consortium, Meta-Analyses of Glucose and Insulin-related traits Consortium (MAGIC) Investigators, Genetic Investigation of ANthropometric Traits (GIANT) Consortium, Asian Genetic Epidemiology Network–Type 2 Diabetes (AGEN-T2D) Consortium, South Asian Type 2 Diabetes (SAT2D) Consortium, Shuldiner AR, Roden M, Barroso I, Wilsgaard T, Beilby J, Hovingh K, Price JF, Wilson JF, Rauramaa R, Lakka TA, Lind L, Dedoussis G, Njølstad I, Pedersen NL, Khaw KT, Wareham NJ, Keinanen-Kiukaanniemi SM, Saaristo TE, Korpi-Hyövälti E, Saltevo J, Laakso M, Kuusisto J, Metspalu A, Collins FS, Mohlke KL, Bergman RN, Tuomilehto J, Boehm BO, Gieger C, Hveem K, Cauchi S, Froguel P, Baldassarre D, Tremoli E, Humphries SE, Saleheen D, Danesh J, Ingelsson E, Ripatti S, Salomaa V, Erbel R, Jöckel KH, Moebus S, Peters A, Illig T, de Faire U, Hamsten A, Morris AD, Donnelly PJ, Frayling TM, Hattersley AT, Boerwinkle E, Melander O, Kathiresan S, Nilsson PM, Deloukas P, Thorsteinsdottir U, Groop LC, Stefansson K, Hu F, Pankow JS, Dupuis J, Meigs JB, Altshuler D, Boehnke M, McCarthy MI; DIAbetes Genetics Replication And Meta-analysis (DIAGRAM) Consortium . Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet 44: 981–990, 2012. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, Shimatani Z, Kondo A. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353: aaf8729, 2016. doi: 10.1126/science.aaf8729. [DOI] [PubMed] [Google Scholar]
- 55.Pashos EE, Park Y, Wang X, Raghavan A, Yang W, Abbey D, Peters DT, Arbelaez J, Hernandez M, Kuperwasser N, Li W, Lian Z, Liu Y, Lv W, Lytle-Gabbin SL, Marchadier DH, Rogov P, Shi J, Slovik KJ, Stylianou IM, Wang L, Yan R, Zhang X, Kathiresan S, Duncan SA, Mikkelsen TS, Morrisey EE, Rader DJ, Brown CD, Musunuru K. Large, diverse population cohorts of hiPSCs and derived hepatocyte-like cells reveal functional genetic variation at blood lipid-associated loci. Cell Stem Cell 20: 558–570, 2017. doi: 10.1016/j.stem.2017.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pavletich NP, Pabo CO. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science 252: 809–817, 1991. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- 57.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8: 2281–2308, 2013. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robbez-Masson LJ, Bödör C, Jones JL, Hurst HC, Fitzgibbon J, Hart IR, Grose RP. Functional analysis of a breast cancer-associated FGFR2 single nucleotide polymorphism using zinc finger mediated genome editing. PLoS One 8: e78839, 2013. doi: 10.1371/journal.pone.0078839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 32: 347–355, 2014. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gómez-Marín C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, Lee JH, Puviindran V, Tam D, Shen M, Son JE, Vakili NA, Sung HK, Naranjo S, Acemel RD, Manzanares M, Nagy A, Cox NJ, Hui CC, Gomez-Skarmeta JL, Nóbrega MA. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507: 371–375, 2014. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith AJ, Humphries SE, Talmud PJ. Identifying functional noncoding variants from genome-wide association studies for cardiovascular disease and related traits. Curr Opin Lipidol 26: 120–126, 2015. doi: 10.1097/MOL.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 62.Smith AJP, Howard P, Shah S, Eriksson P, Stender S, Giambartolomei C, Folkersen L, Tybjærg-Hansen A, Kumari M, Palmen J, Hingorani AD, Talmud PJ, Humphries SE. Use of allele-specific FAIRE to determine functional regulatory polymorphism using large-scale genotyping arrays. PLoS Genet 8: e1002908, 2012. doi: 10.1371/journal.pgen.1002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soldner F, Stelzer Y, Shivalila CS, Abraham BJ, Latourelle JC, Barrasa MI, Goldmann J, Myers RH, Young RA, Jaenisch R. Parkinson-associated risk variant in distal enhancer of α-synuclein modulates target gene expression. Nature 533: 95–99, 2016. doi: 10.1038/nature17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spisák S, Lawrenson K, Fu Y, Csabai I, Cottman RT, Seo JH, Haiman C, Han Y, Lenci R, Li Q, Tisza V, Szállási Z, Herbert ZT, Chabot M, Pomerantz M, Solymosi N, Gayther SA, Joung JK, Freedman ML; GAME-ON/ELLIPSE Consortium . CAUSEL: an epigenome- and genome-editing pipeline for establishing function of noncoding GWAS variants. Nat Med 21: 1357–1363, 2015. doi: 10.1038/nm.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Storici F, Resnick MA. The delitto perfetto approach to in vivo site-directed mutagenesis and chromosome rearrangements with synthetic oligonucleotides in yeast. Methods Enzymol 409: 329–345, 2006. doi: 10.1016/S0076-6879(05)09019-1. [DOI] [PubMed] [Google Scholar]
- 66.Sur IK, Hallikas O, Vähärautio A, Yan J, Turunen M, Enge M, Taipale M, Karhu A, Aaltonen LA, Taipale J. Mice lacking a Myc enhancer that includes human SNP rs6983267 are resistant to intestinal tumors. Science 338: 1360–1363, 2012. doi: 10.1126/science.1228606. [DOI] [PubMed] [Google Scholar]
- 67.Surakka I, Horikoshi M, Mägi R, Sarin AP, Mahajan A, Lagou V, Marullo L, Ferreira T, Miraglio B, Timonen S, Kettunen J, Pirinen M, Karjalainen J, Thorleifsson G, Hägg S, Hottenga JJ, Isaacs A, Ladenvall C, Beekman M, Esko T, Ried JS, Nelson CP, Willenborg C, Gustafsson S, Westra HJ, Blades M, de Craen AJ, de Geus EJ, Deelen J, Grallert H, Hamsten A, Havulinna AS, Hengstenberg C, Houwing-Duistermaat JJ, Hyppönen E, Karssen LC, Lehtimäki T, Lyssenko V, Magnusson PK, Mihailov E, Müller-Nurasyid M, Mpindi JP, Pedersen NL, Penninx BW, Perola M, Pers TH, Peters A, Rung J, Smit JH, Steinthorsdottir V, Tobin MD, Tsernikova N, van Leeuwen EM, Viikari JS, Willems SM, Willemsen G, Schunkert H, Erdmann J, Samani NJ, Kaprio J, Lind L, Gieger C, Metspalu A, Slagboom PE, Groop L, van Duijn CM, Eriksson JG, Jula A, Salomaa V, Boomsma DI, Power C, Raitakari OT, Ingelsson E, Järvelin MR, Thorsteinsdottir U, Franke L, Ikonen E, Kallioniemi O, Pietiäinen V, Lindgren CM, Stefansson K, Palotie A, McCarthy MI, Morris AP, Prokopenko I, Ripatti S; ENGAGE Consortium . The impact of low-frequency and rare variants on lipid levels. Nat Genet 47: 589–597, 2015. doi: 10.1038/ng.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466: 707–713, 2010. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tewhey R, Kotliar D, Park DS, Liu B, Winnicki S, Reilly SK, Andersen KG, Mikkelsen TS, Lander ES, Schaffner SF, Sabeti PC. Direct identification of hundreds of expression-modulating variants using a multiplexed reporter assay. Cell 165: 1519–1529, 2016. [Erratum in Cell 172: 1132–1134, 2018] doi: 10.1016/j.cell.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai SQ, Wyvekens N, Khayter C, Foden JA, Thapar V, Reyon D, Goodwin MJ, Aryee MJ, Joung JK. Dimeric CRISPR RNA-guided FokI nucleases for highly specific genome editing. Nat Biotechnol 32: 569–576, 2014. doi: 10.1038/nbt.2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tschaharganeh DF, Lowe SW, Garippa RJ, Livshits G. Using CRISPR/Cas to study gene function and model disease in vivo. FEBS J 283: 3194–3203, 2016. doi: 10.1111/febs.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71a.UK10K Consortium; Walter K, Min JL, Huang J, Crooks L, Memari Y, McCarthy S, Perry JR, Xu C, Futema M, Lawson D, Iotchkova V, Schiffels S, Hendricks AE, Danecek P, Li R, Floyd J, Wain LV, Barroso I, Humphries SE, Hurles ME, Zeggini E, Barrett JC, Plagnol V, Richards JB, Greenwood CM, Timpson NJ, Durbin R, Soranzo N. The UK10K project identifies rare variants in health and disease. Nature 526: 82–90, 2015. doi: 10.1038/nature14962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11: 636–646, 2010. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 73.Wain LV, Verwoert GC, O’Reilly PF, Shi G, Johnson T, Johnson AD, Bochud M, Rice KM, Henneman P, Smith AV, Ehret GB, Amin N, Larson MG, Mooser V, Hadley D, Dörr M, Bis JC, Aspelund T, Esko T, Janssens ACJW, Zhao JH, Heath S, Laan M, Fu J, Pistis G, Luan J, Arora P, Lucas G, Pirastu N, Pichler I, Jackson AU, Webster RJ, Zhang F, Peden JF, Schmidt H, Tanaka T, Campbell H, Igl W, Milaneschi Y, Hottenga JJ, Vitart V, Chasman DI, Trompet S, Bragg-Gresham JL, Alizadeh BZ, Chambers JC, Guo X, Lehtimäki T, Kühnel B, Lopez LM, Polašek O, Boban M, Nelson CP, Morrison AC, Pihur V, Ganesh SK, Hofman A, Kundu S, Mattace-Raso FUS, Rivadeneira F, Sijbrands EJG, Uitterlinden AG, Hwang SJ, Vasan RS, Wang TJ, Bergmann S, Vollenweider P, Waeber G, Laitinen J, Pouta A, Zitting P, McArdle WL, Kroemer HK, Völker U, Völzke H, Glazer NL, Taylor KD, Harris TB, Alavere H, Haller T, Keis A, Tammesoo ML, Aulchenko Y, Barroso I, Khaw KT, Galan P, Hercberg S, Lathrop M, Eyheramendy S, Org E, Sõber S, Lu X, Nolte IM, Penninx BW, Corre T, Masciullo C, Sala C, Groop L, Voight BF, Melander O, O’Donnell CJ, Salomaa V, d’Adamo AP, Fabretto A, Faletra F, Ulivi S, Del Greco F, Facheris M, Collins FS, Bergman RN, Beilby JP, Hung J, Musk AW, Mangino M, Shin SY, Soranzo N, Watkins H, Goel A, Hamsten A, Gider P, Loitfelder M, Zeginigg M, Hernandez D, Najjar SS, Navarro P, Wild SH, Corsi AM, Singleton A, de Geus EJC, Willemsen G, Parker AN, Rose LM, Buckley B, Stott D, Orru M, Uda M, LifeLines Cohort Study, van der Klauw MM, Zhang W, Li X, Scott J, Chen YDI, Burke GL, Kähönen M, Viikari J, Döring A, Meitinger T, Davies G, Starr JM, Emilsson V, Plump A, Lindeman JH, Hoen PA, König IR, EchoGen consortium, Felix JF, Clarke R, Hopewell JC, Ongen H, Breteler M, Debette S, Destefano AL, Fornage M, AortaGen Consortium, Mitchell GF, CHARGE Consortium Heart Failure Working Group, Smith NL, KidneyGen consortium, Holm H, Stefansson K, Thorleifsson G, Thorsteinsdottir U, CKDGen consortium, Cardiogenics consortium; CardioGram, Samani NJ, Preuss M, Rudan I, Hayward C, Deary IJ, Wichmann HE, Raitakari OT, Palmas W, Kooner JS, Stolk RP, Jukema JW, Wright AF, Boomsma DI, Bandinelli S, Gyllensten UB, Wilson JF, Ferrucci L, Schmidt R, Farrall M, Spector TD, Palmer LJ, Tuomilehto J, Pfeufer A, Gasparini P, Siscovick D, Altshuler D, Loos RJ, Toniolo D, Snieder H, Gieger C, Meneton P, Wareham NJ, Oostra BA, Metspalu A, Launer L, Rettig R, Strachan DP, Beckmann JS, Witteman JC, Erdmann J, van Dijk KW, Boerwinkle E, Boehnke M, Ridker PM, Jarvelin MR, Chakravarti A, Abecasis GR, Gudnason V, Newton-Cheh C, Levy D, Munroe PB, Psaty BM, Caulfield MJ, Rao DC, Tobin MD, Elliott P, van Duijn CM. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet 43: 1005–1011, 2011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res 44, D1: D877–D881, 2016. doi: 10.1093/nar/gkv1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wellcome Trust Case Control Consortium, Maller JB, McVean G, Byrnes J, Vukcevic D, Palin K, Su Z, Howson JM, Auton A, Myers S, Morris A, Pirinen M, Brown MA, Burton PR, Caulfield MJ, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Mathew CG, Pembrey M, Satsangi J, Stratton MR, Worthington J, Craddock N, Hurles M, Ouwehand W, Parkes M, Rahman N, Duncanson A, Todd JA, Kwiatkowski DP, Samani NJ, Gough SC, McCarthy MI, Deloukas P, Donnelly P. Bayesian refinement of association signals for 14 loci in 3 common diseases. Nat Genet 44: 1294–1301, 2012. doi: 10.1038/ng.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75a.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson Å, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PKE, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney ASF, Döring A, Elliott P, Epstein SE, Ingi Eyjolfsson G, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJP, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJF, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TVM, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YI, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PEH, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BHR, Ordovas JM, Boerwinkle E, Palmer CNA, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR; Global Lipids Genetics Consortium . Discovery and refinement of loci associated with lipid levels. Nat Genet 45: 1274–1283, 2013. doi: 10.1038/ng.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wood AJ, Lo TW, Zeitler B, Pickle CS, Ralston EJ, Lee AH, Amora R, Miller JC, Leung E, Meng X, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Meyer BJ. Targeted genome editing across species using ZFNs and TALENs. Science 333: 307, 2011. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Q, Chesi A, Manduchi E, Johnston BT, Lu S, Leonard ME, Parlin UW, Rappaport EF, Huang P, Wells AD, Blobel GA, Johnson ME, Grant SFA. The type 2 diabetes presumed causal variant within TCF7L2 resides in an element that controls the expression of ACSL5. Diabetologia 59: 2360–2368, 2016. doi: 10.1007/s00125-016-4077-2. [DOI] [PubMed] [Google Scholar]
- 78.Xiao A, Wang Z, Hu Y, Wu Y, Luo Z, Yang Z, Zu Y, Li W, Huang P, Tong X, Zhu Z, Lin S, Zhang B. Chromosomal deletions and inversions mediated by TALENs and CRISPR/Cas in zebrafish. Nucleic Acids Res 41: e141, 2013. doi: 10.1093/nar/gkt464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by beta-catenin and glycogen synthase kinase-3beta. J Biol Chem 280: 1457–1464, 2005. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 80.Zeng H, Guo M, Zhou T, Tan L, Chong CN, Zhang T, Dong X, Xiang JZ, Yu AS, Yue L, Qi Q, Evans T, Graumann J, Chen S. An Isogenic Human ESC Platform for Functional Evaluation of Genome-wide-Association-Study-Identified Diabetes Genes and Drug Discovery. Cell Stem Cell 19: 326–340, 2016. doi: 10.1016/j.stem.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zetsche B, Gootenberg JS, Abudayyeh OO, Slaymaker IM, Makarova KS, Essletzbichler P, Volz SE, Joung J, van der Oost J, Regev A, Koonin EV, Zhang F. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163: 759–771, 2015. doi: 10.1016/j.cell.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]