Abstract
The sympathetic nerve activity (SNA) to brown adipose tissue (BAT) regulates BAT thermogenesis to defend body temperature in cold environments or to produce fever during immune responses. The vagus nerve contains afferents that inhibit the BAT SNA and BAT thermogenesis evoked by skin cooling. We sought to determine whether activation of transient receptor potential vanilloid 1 (TRPV1) channels in the nucleus tractus solitarius (NTS), which are prominently expressed in unmyelinated vagal afferents, would affect cold-evoked BAT thermogenesis, cardiovascular parameters, or their vagal afferent-evoked responses. In urethane-chloralose-anesthetized rats, during skin cooling, nanoinjection of the TRPV1-agonist resiniferatoxin in NTS decreased BAT SNA (from 695 ± 195% of baseline during cooling to 103 ± 8% of baseline after resiniferatoxin), BAT temperature (−0.8 ± 0.1°C), expired CO2 (−0.3 ± 0.04%), mean arterial pressure (MAP; −20 ± 5 mmHg), and heart rate (−44 ± 11 beats/min). Pretreatment of NTS with the TRPV1 antagonist capsazepine prevented these resiniferatoxin-mediated effects. Intravenous injection of the TRPV1 agonist dihydrocapsaicin also decreased all the measured variables (except MAP). Bilateral cervical or subdiaphragmatic vagotomy attenuated the decreases in BAT SNA and thermogenesis evoked by nanoinjection of resiniferatoxin in NTS but did not prevent the decreases in BAT SNA and BAT thermogenesis evoked by intravenous dihydrocapsaicin. We conclude that activation of TRPV1 channels in the NTS of vagus nerve intact rats inhibits BAT SNA and decreases BAT metabolism, blood pressure, and heart rate. In contrast, the inhibition of BAT thermogenesis following systemic administration of dihydrocapsaicin does not require vagal afferent activity, consistent with a nonvagal pathway through which systemic TRPV1 agonists can inhibit BAT thermogenesis.
Keywords: dihydrocapsaicin, raphe pallidus, resiniferatoxin, sympathetic nerve activity, thermoregulation
INTRODUCTION
Brown adipose tissue (BAT) is a principal thermogenic thermoeffector for defending body temperature in cold ambient environments and for producing fever during immune responses (20–23). The activation of BAT sympathetic nerve activity (SNA) is regulated by central neural circuits that are sensitive to a variety of metabolic inputs, including the availability of the fuel substrates, oxygen (18), and glucose (16). The neural circuitry responsible for the thermoregulatory control of BAT sympathetic outflow includes a significant role for the visceral vagal afferent input to neurons in the nucleus tractus solitarius (NTS) (17, 19).
The NTS contains second-order visceral sensory neurons that receive glutamatergic afferents from the vagus and glossopharyngeal nerves (7). Primary vagal afferent axons entering the NTS are predominantly unmyelinated C fibers that contain transient receptor potential vanilloid 1 (TRPV1) receptors (13). TRPV1 channels are specialized capsaicin-sensitive, temperature-sensitive, and proton-activated calcium channels expressed chiefly in primary afferent sensory neurons (28). Activation of TRPV1 channels in the NTS facilitates the release of glutamate onto second-order neurons (7, 14, 25, 26, 30). Since activation of neurons in the NTS can inhibit BAT SNA and BAT thermogenesis (3, 34), we hypothesized that activation of TRPV1 channels in the NTS would also decrease BAT SNA and BAT thermogenesis. Systemic administration of the TRPV1 agonist dihydrocapsaicin (DHC) decreases body temperature (4, 10, 11, 39). Activation of TRPV1 channels in the gastrointestinal space can increase (24) or decrease (31) BAT thermogenesis, likely dependant on the route of administration and the population of afferents affected. Since the location of the relevant TRPV1 channels responsible for this effect is unknown, we also determined whether the hypothermia evoked by systemic DHC involved an inhibition of BAT thermogenesis and whether vagal afferent nerve activity is necessary for this effect.
MATERIALS AND METHODS
General procedures.
All procedures were performed in accordance with the regulations detailed in the Guide for the Care and Use of Laboratory Animal (8th ed., National Research Council, National Academies Press, 2010) and were approved by the Animal Care and Use Committee of the Oregon Health & Science University.
Experiments were performed in 49 male Sprague-Dawley rats (300–375 g), anesthetized with urethane (750 mg/kg iv) and α-chloralose (60 mg/kg iv). Initially, while rats were under isoflurane (3% in 100% O2), a tracheotomy was performed to insert an endotracheal tube for artificial ventilation, and the femoral artery and vein were cannulated for measurement of arterial pressure and for systemic drug injections, respectively. Subsequently, rats were mounted in a stereotaxic apparatus (David Kopf Instruments) with a spinal clamp on the T8-T9 vertebral processes and the incisor bar at −12.0 mm. Rats were artificially ventilated (100% O2) and paralyzed with d-tubocurarine. Expired CO2 was continuously monitored. A water-perfused thermal blanket containing silicone tubing was wrapped around the rat’s shaved trunk from the shoulders to the hips to produce changes in skin temperature (TSKIN) by perfusing the tubing within the blanket with cold or warm water. Thermocouples were fixed into the rectum for measurement of core body temperature (TCORE), into the left interscapular BAT pad for BAT temperature (TBAT), and into the shaved abdominal skin surface under the water-perfused, thermal blanket for TSKIN. A partial occipital craniotomy was performed to visualize the dorsal surface of the caudal brainstem.
In some experiments (n = 11), we sectioned the cervical vagi (including the aortic depressor nerves), at the time of the tracheotomy, after their dissection from the common carotid artery in the neck (17). In other experiments (n = 6), at the time of the tracheotomy, we sectioned the subdiaphragmatic vagus by making a ventral incision, retracting the liver, and sectioning the anterior and posterior branches of the subdiaphragmatic vagus at the level of the caudal third of the esophagus, thus cutting the gastric and the celiac branches of the vagi but sparing the hepatic branch of the vagus (27). For cervical vagal nerve stimulation (VNS; n = 6), the right vagus nerve was isolated from the common carotid artery in the neck and sectioned. A bipolar hook-stimulating electrode was implanted on the central end of the sectioned vagus nerve and embedded in Kwik-Sil (World Precision Instruments). VNS (1-ms pulses; 100–200 μA; 1, 2, 5, 10 Hz) was delivered in 30-s trains at supramaximal stimulus intensities yielding complete inhibition of BAT SNA at 10 Hz (19).
The BAT SNA was recorded with bipolar hook electrodes under mineral oil from a small nerve bundle dissected from the ventral surface of the right interscapular BAT pad. BAT SNA was amplified (×10 K, bandpass: 1–300 Hz, CyberAmp 380; Axon Instruments) and digitized to a hard drive (Spike 2; Cambridge Electronic Design) along with all other variables. A continuous measurement (4-s bins) of BAT SNA amplitude was obtained from the autospectra of sequential 4-s segments of raw BAT SNA as the root mean square value (square root of the total power in the 0.1 to 20-Hz frequency band) of the BAT SNA.
TCORE was initially maintained above 36°C by perfusing the thermal blanket with warm water and with the aid of an infrared heat lamp. Skin cooling to reflexively increase BAT SNA was effected by turning off the heat lamp and pumping cold water through the thermal blanket while monitoring TSKIN. As skin cooling proceeded, the BAT SNA bursts increased in frequency and amplitude. Shortly, the level of BAT SNA and the other recorded variables settled to stable levels of activation during which the effects of drug nanoinjections in the NTS were tested.
Drug administration.
Drugs were nanoinjected into the medial NTS (mNTS) at the following coordinates relative to the calamus scriptorius: 0.5 mm rostral, 0.5 mm lateral, and 0.5 mm ventral. The following drugs were injected into mNTS: resiniferatoxin (RTX; Tocris Bioscience) dissolved in 1% ethanol and capsazepine (CPZ; Tocris Bioscience) dissolved in 1% ethanol. Fluorescent polystyrene microspheres (FluoSpheres, F8797, F8801, or F8803; Molecular Probes) were included in the injected drug solution (1:100 volume dilution of FluoSpheres in the injectate) to allow histological identification of the drug nanoinjection sites in the mNTS. DHC (Cayman Chemical) was dissolved in 1% DMSO and administered intravenously (0.3 ml in 30 s).
Histology.
After the experimental procedures were completed, rats were transcardially perfused with 5% formaldehyde. Brains were removed and placed in 30% sucrose solution overnight and sectioned at 60-μm thickness. Sites of drug nanoinjection in the mNTS were identified in coronal sections through the caudal brain stem and plotted on schematic drawings of representative sections through the mNTS.
Data analysis.
All signals were digitized using Spike2 software (Cambridge Electronic Design). Mean arterial pressure (MAP) and heart rate (HR) were obtained from the pulsatile arterial pressure signal. For each experiment, the baseline level of BAT SNA was determined as the mean BAT SNA amplitude during a 2-min period recorded when the rat was in a warm condition (TCORE and TSKIN > 36.5°C) and basal bursting of BAT SNA was absent (i.e., 100% of baseline BAT SNA is approximately equal to no ongoing BAT SNA).
Drug and VNS treatment effects consisted of reductions in BAT SNA and other BAT thermogenic parameters, as well as reductions in MAP and HR. To assess these treatment effects, statistical comparisons were made between the mean values of measured parameters during the 30-s control period just before the drug administration or VNS and the mean values during the 30-s period that captured the nadir of the response (i.e., the maximum reduction), within 15 min of the drug administration or VNS. For BAT SNA, we used the following formula to calculate the percent inhibition of cold-evoked BAT SNA that was produced by the treatment: {1-[(nadir BAT SNA – baseline BAT SNA)/(cold evoked BAT SNA – baseline BAT SNA)]} × 100.
Grouped data (means ± SE) were analyzed, and graphical representations were performed using Spike2 and Igor Pro software (Wavemetric). Statistical analysis was performed using StatView software (SAS Institute, Carey, NC). Grouped data were compared between drug-injected and vehicle-injected rats or between intact and vagotomized rats using an ANOVA or t-test, as appropriate (details of specific tests used for each experiment are included in the figure legends). For dose-dependent responses, simple regression analysis using log-scale values was performed using StatView software. An α-level of 0.05 was used to determine significance.
RESULTS
RTX nanoinjection into mNTS decreases BAT SNA and BAT thermogenesis.
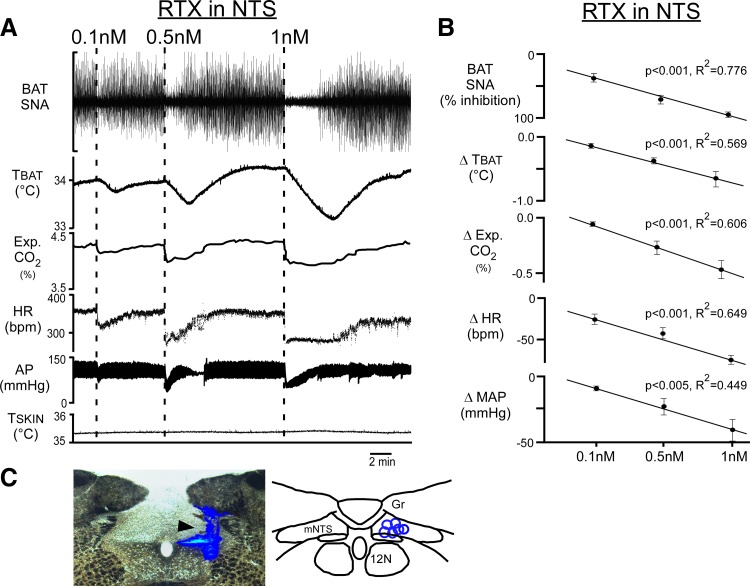
To determine if activation of TRPV1 in mNTS would affect BAT SNA and BAT thermogenesis, we nanoinjected (100 nl) the TRPV1 agonist RTX (0.1–1 nM) into the right mNTS of vagus-intact rats, in which TSKIN was cooled to 35.0 ± 0.5°C, which resulted in a TCORE of 35.0 ± 0.5°C and elevated levels of BAT SNA (Fig. 1A). In a dose-dependent manner (Fig. 1B; linear regression, P < 0.001), unilateral nanoinjection of RTX into the mNTS (Fig. 1C) abruptly inhibited cold-evoked BAT SNA, eliciting falls in TBAT and expired CO2 (Fig. 1, A and B). RTX nanoinjection in mNTS rapidly reduced MAP and HR (Fig. 1, A and B). Nanoinjection of 1 nM RTX in mNTS reduced (P < 0.001, n = 6) BAT SNA from a cooling-evoked level of 988 ± 232% of baseline to 216 ± 64% of baseline after the RTX injection, representing a 93 ± 2% inhibition of cooling-evoked BAT SNA (Fig. 1B). This nearly complete inhibition of the sympathetic outflow to BAT caused a marked decrease in BAT thermogenesis, reflected in the rapid fall in expired CO2 from 4.8 ± 0.3 to 4.3 ± 0.2% (P < 0.005, n = 6) and a more slowly developing decline in TBAT from 34.6 ± 0.5 to 33.9 ± 0.5°C (P < 0.001, n = 6). Nanoinjection of 1 nM RTX in mNTS also caused precipitous falls in MAP from 115 ± 9 to 80 ± 12 mmHg (P < 0.005, n = 6) and in HR from 391 ± 37 to 318 ± 38 beats/min (P < 0.001, n = 6). All variables returned to the initial cold-evoked level within 15–20 min (Fig. 1A). These responses to RTX nanoinjected into mNTS are consistent with strong cranial visceral afferent activation (1, 19).
Fig. 1.
Unilateral nanoinjection of resiniferatoxin (RTX) into the medial nucleus tractus solitarius (mNTS) dose-dependently decreases brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired (Exp.) CO2, heart rate (HR), and arterial pressure (AP). A: representative example of the effects of nanoinjection of RTX into the mNTS (0.1, 0.5, or 1 nM in 100 nl, dashed lines) on the recorded variables. B: group data (means ± SE; n = 6), showing dose-dependent effects of RTX nanoinjections into mNTS. MAP, mean arterial pressure. *Significant linear regression between different doses of RTX and BAT SNA inhibitions, reductions in TBAT, and reductions in expired CO2, in HR, and in MAP. C, left: photomicrograph of a partial coronal section illustrating a representative nanoinjection site in mNTS (arrowhead indicating blue bead deposit). C, right: locations of RTX nanoinjection sites (blue circles, n = 6) plotted on a schematic drawing of a partial coronal section at 13.5 to 14 mm caudal to bregma. TSKIN, skin temperature; Gr, gracile nucleus; 12N, hypoglossal nucleus.
Pretreatment of mNTS with CPZ blocks the RTX-evoked reductions in BAT SNA and TBAT.
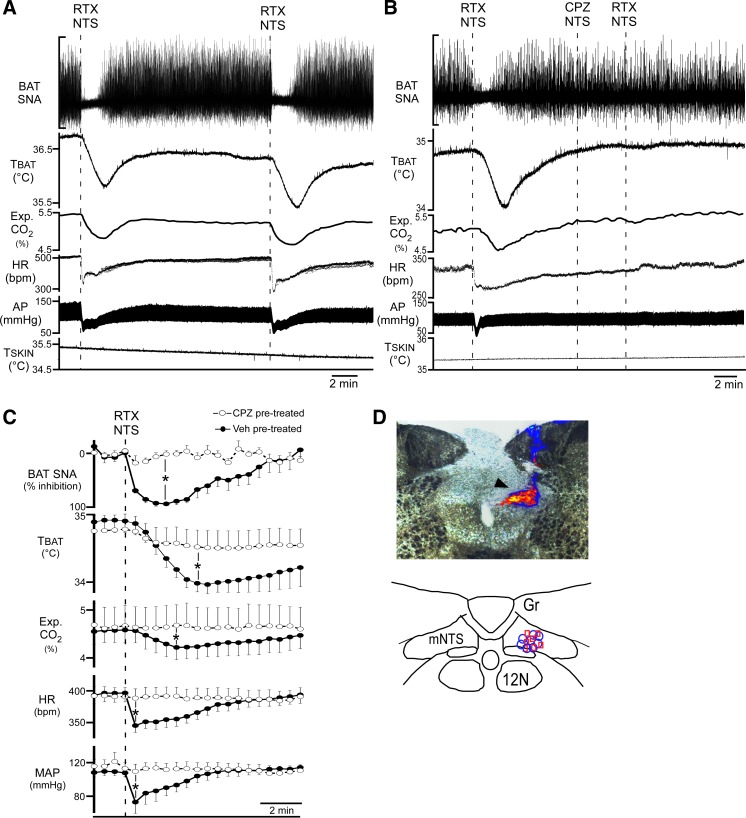
To confirm that the observed BAT sympathoinhibitory responses to RTX in mNTS were due to the activation of TRPV1 receptors in mNTS, we tested the responses to nanoinjection of RTX in mNTS (Fig. 2D) after antagonizing TRPV1 receptors with a nanoinjection of the TRPV1 antagonist CPZ into mNTS (Fig. 2D). We observed nearly identical (P > 0.05, n = 4) BAT and cardiovascular sympathoinhibitory responses to sequential nanoinjections of RTX (1 nM, 100 nl) into the mNTS that were separated by at least 10 min (Fig. 2A). The skin cooling-evoked amplitudes of the recorded variables were not affected by a unilateral nanoinjection of CPZ (100 μM, 100 nl; n = 7; Fig. 2B) or of vehicle (1% ethanol, n = 8) into the mNTS (Fig. 2, B and C). When compared with vehicle, pretreatment of the mNTS with CPZ significantly attenuated the RTX-induced reflex reductions in cooling-evoked BAT SNA, TBAT, CO2, HR, and MAP (Fig. 2, B and C). Thus the vanilloid competitive antagonist effectively prevented the actions of RTX confirming that TRPV1 located within mNTS mediates these responses.
Fig. 2.
Nanoinjection of the transient receptor potential vanilloid 1 (TRPV1) antagonist capsazepine (CPZ), into medial nucleus tractus solitarius (mNTS) blocks the sympathoinhibitory effects of resiniferatoxin (RTX) in the mNTS. A: representative example of the consistency of the effects on brown adipose tissue (BAT) and cardiovascular variables of sequential nanoinjections of RTX (1 nM in 100 nl) into the mNTS. SNA, sympathetic nerve activity; TBAT, BAT temperature; HR, heart rate heart rate; AP, arterial pressure; TSKIN, skin temperature; Gr, gracile nucleus; 12N, hypoglossal nucleus. B: representative example of the blockade of the sympathoinhibitory effects of the nanoinjection of RTX in the mNTS, following nanoinjection of CPZ (100 µM, 100 nl) in mNTS. C: group data (means ± SE; n = 8 in each group) for the time courses and amplitudes of the responses to RTX nanoinjections into mNTS after pretreatment of the mNTS with vehicle (Veh, filled circles, solid lines) or with CPZ (open circles, dashed lines). *P < 0.05, significant difference by t-test between the nadir of the RTX-evoked responses in vehicle-pretreated and CPZ-pretreated groups. MAP, mean arterial pressure. D, top: photomicrograph of representative RTX (blue beads) and CPZ (red beads) nanoinjection sites in the mNTS (arrowhead indicates overlapping blue and red bead deposits). D, bottom: locations of RTX (blue circles; n = 8) and CPZ (red squares; n = 7; 1 site not recovered due to absence of beads in injectate) nanoinjection sites plotted on a schematic drawing of a partial coronal section at 13.5–14 mm caudal to bregma.
Section of either the cervical vagi or the subdiaphragmatic vagi prevents BAT thermogenic responses evoked by RTX in the mNTS.
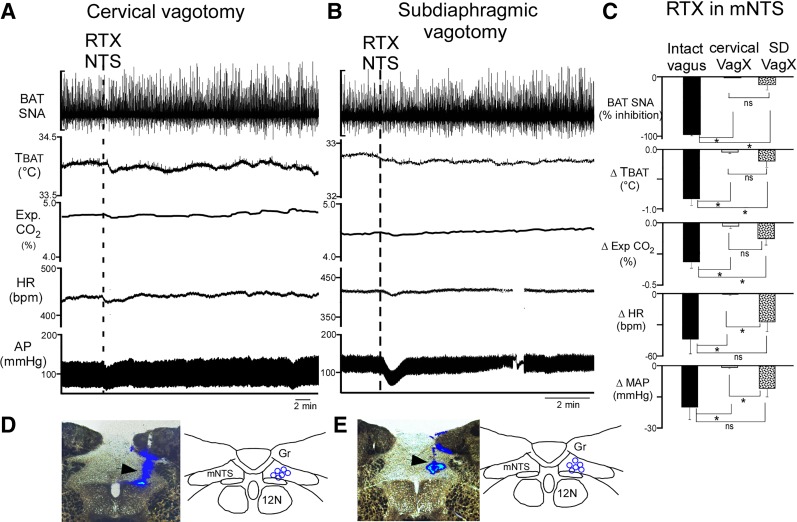
To determine whether the reduction in BAT thermogenic and in cardiovascular parameters evoked by activation of TRPV1 receptors in mNTS (Figs. 1, A and B, and 2, A–C) requires the activity of a population of afferent fibers in the vagus nerve, we nanoinjected RTX into mNTS following acute section of either the cervical vagus nerve bilaterally (n = 6) or the anterior and posterior branches of the subdiaphragmatic vagus nerve (27) (n = 6). The cooling-evoked levels of the BAT thermogenic variables and of MAP were not different between intact and vagotomized rats; however, the basal HR was higher after cervical vagotomy than in intact or subdiaphragmatic vagotomized rats. RTX nanoinjections into the mNTS consistently decreased cooling-evoked BAT SNA, TBAT, expired CO2, MAP, and HR in vagus-intact rats (Fig. 3C, n = 8 from Fig. 2C). Bilateral cervical vagotomy prevented all of the BAT and cardiovascular responses to nanoinjections of RTX in the mNTS (Fig. 3, A, C, and D). Interestingly, subdiaphragmatic vagotomy did not alter the cardiovascular responses to RTX administration into the mNTS, although the subdiaphragmatic vagotomy did significantly reduce the inhibitions of BAT SNA, TBAT, and expired CO2 produced by nanoinjection of RTX in mNTS (Fig. 3, B–D).
Fig. 3.
Section of the cervical or the subdiaphragmatic gastric vagi attenuates the sympathoinhibitory effects evoked by nanoinjection of resiniferatoxin (RTX) in the medial nucleus tractus solitarius (mNTS). A: representative example showing that following cervical vagotomy (VagX), nanoinjection of RTX (1 nM in 100 nl, dashed line) into the mNTS has minimal effects on all measured variables. AP, arterial pressure. B: with a subdiagphragmatic VagX of the anterior and posterior vagal branches, RTX injection into mNTS evokes changes in heart rate (HR) and mean arterial pressure (MAP) but not in brown adipose tissue (BAT) sympathetic nerve activity (SNA) or BAT temperature (TBAT). C: group data (means ± SE, n = 8 in the intact vagus group, n = 6 in each vagotomized group) show that cervical VagX and subdiaphragmatic (SD) VagX are effective in preventing the BAT thermogenic inhibition evoked by RTX in NTS but that cervical VagX is significantly more effective than SD VagX in attenuating cardiovascular responses to RTX injection in NTS. *P < 0.005, significant difference (least significant difference post hoc comparison between each group following significant ANOVA). For all variables, the RTX-evoked responses were significantly different between the intact vagus group and the cervical VagX group. The RTX-evoked responses in BAT SNA, TBAT, and expired CO2 differed significantly between the intact vagi and SD VagX groups; in contrast, the RTX-evoked changes in HR and MAP were not significantly different between the intact vagus and SD VagX groups, (ns, P > 0.05). The RTX-evoked responses in BAT SNA, TBAT, and expired CO2 were not significantly different (ns, P > 0.05) between the cervical and the SD VagX groups. The HR and MAP responses to RTX were significantly different between the cervical and the SD VagX groups. D and E: photomicrographs of representative RTX nanoinjection sites in mNTS in a rat with a cervical VagX (D, arrowhead indicates blue bead deposit) and one with a SD VagX (E, arrowhead indicates blue bead deposit) and schematic representations of the respective RTX nanoinjection sites (blue circles, n = 6 in each group) plotted on schematic drawings of a partial coronal section at 13.5 to–14 mm caudal to bregma. Gr, gracile nucleus; 12N, hypoglossal nucleus.
Pretreatment of mNTS with CPZ does not alter VNS-evoked responses.
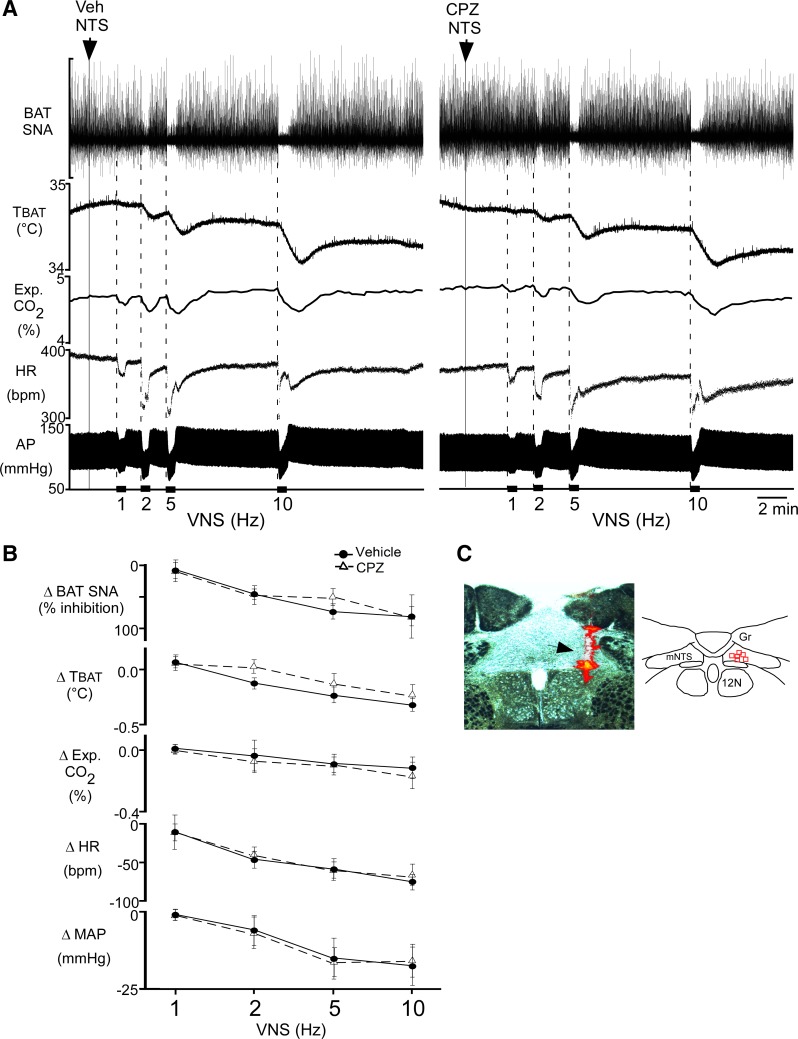
To test whether TRPV1 receptors in mNTS might contribute to regulation of the vagal afferent-activated, BAT sympathoinhibition (19), we electrically stimulated afferent fibers in the cervical vagus nerve (VNS) following injection of CPZ into mNTS. In control, following vehicle (1% ethanol) nanoinjection in mNTS (n = 6), VNS decreased BAT SNA, TBAT, expired CO2, HR, and MAP in a frequency-dependent manner (Fig. 4, A, left, and B). Injection of CPZ into the mNTS (Fig. 4C) ipsilateral to the VNS did not alter (P > 0.05, n = 6) these VNS-evoked responses (Fig. 4, A, right, and B) compared with those evoked after vehicle control injections. These observations are consistent with a lack of tonic or VNS-induced TRPV1 activation in mNTS during these responses.
Fig. 4.
Nanoinjection of capsazepine (CPZ) in the medial nucleus tractus solitarius (mNTS) does not attenuate the effects of electrical vagal nerve stimulation (VNS). A: representative example illustrating the decreases in brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), expired CO2, heart rate (HR), and arterial pressure (AP) evoked by unilateral VNS (30-s stimulation periods are indicated by black bars along the time axis) after nanoinjection of vehicle (left, arrowhead and dotted line) or CPZ (right, arrowhead and dotted line) into the mNTS ipsilateral to the VNS. B: group data (means ± SE; n = 6 in each group) of VNS-evoked responses following vehicle pretreatment (filled circles, solid line) or CPZ pretreatment (open triangles, dashed line) into mNTS. There were no significant differences between vehicle and CPZ trials at any frequency (P > 0.05, paired t-test at each frequency). MAP, mean arterial pressure. C, left: photomicrograph of a representative CPZ nanoinjection site in mNTS (arrowhead indicates red bead deposit). C, right: locations of CPZ nanoinjection sites (red squares) plotted on a schematic drawing of a coronal section at 13.5–14 mm caudal to bregma.
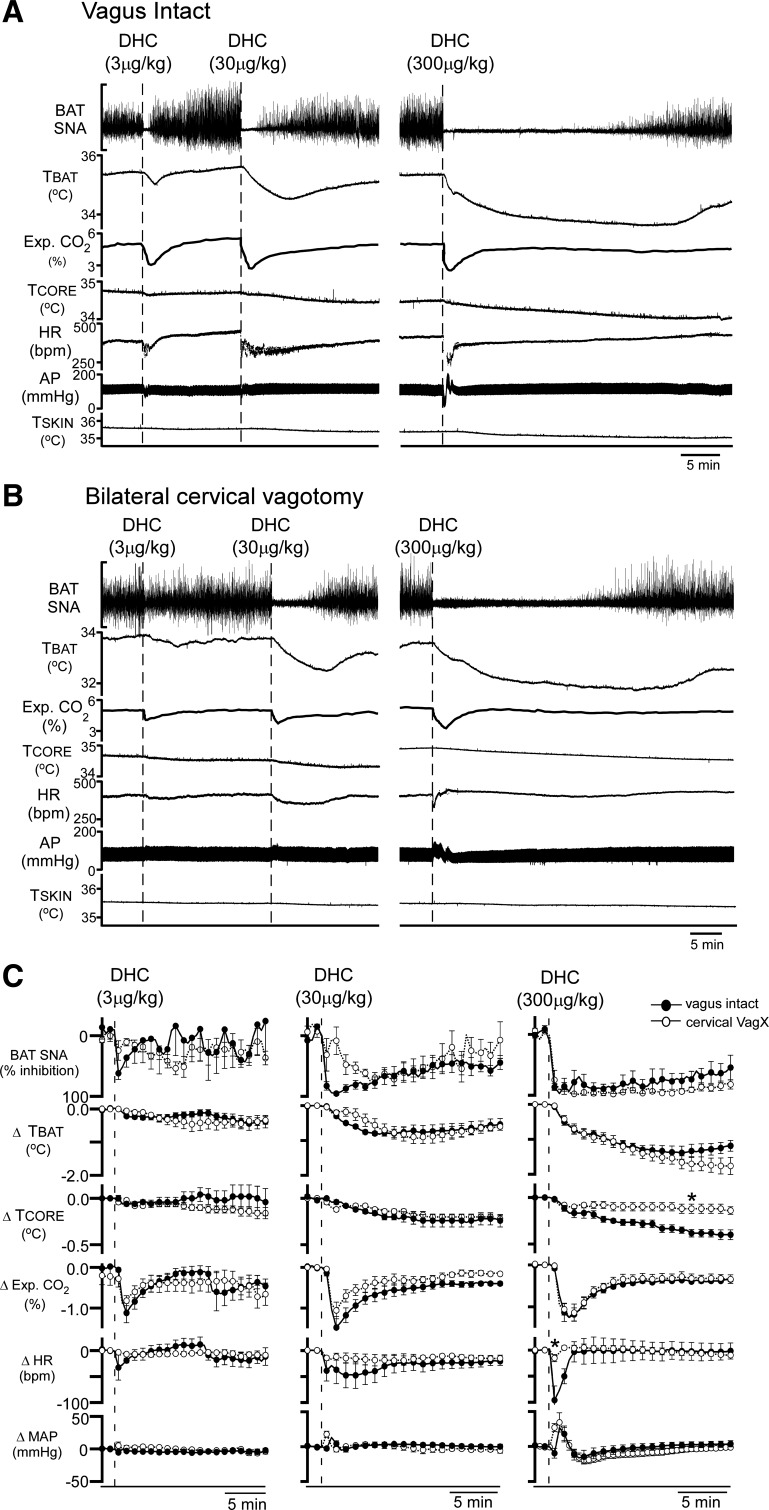
Systemic DHC decreases BAT SNA and BAT thermogenesis after bilateral cervical vagotomy.
Intravenous delivery of the TRPV1 agonist DHC triggers hypothermic responses (10, 11). We reasoned that if systemic DHC inhibited BAT thermogenesis through activation of TRPV1 receptors in the mNTS, then cervical vagotomy should eliminate the BAT inhibitory response to systemic DHC, as it did for the similar responses to activation of TRPV1 receptors in mNTS with RTX.
In vagus-intact rats (n = 6), intravenous injection of DHC abruptly inhibited cooling-evoked BAT SNA in a dose-dependent manner and reduced TBAT and expired CO2. These prolonged inhibitions of BAT thermogenesis were accompanied by early, transient falls in HR and MAP at the lowest dose of DHC, with the addition of transient increases in MAP at higher doses of DHC (Fig. 5, A and C). In vagus-intact rats, DHC (300 μg/kg iv) reduced (P < 0.005, n = 6) BAT SNA from a cooling-evoked level of 885 ± 154% of baseline to 132 ± 19% of baseline after the DHC injection, representing an inhibition of 95.0 ± 4% of the cooling-evoked BAT SNA (Fig. 5C, right). Acute bilateral cervical vagotomy did not alter the overall response patterns to intravenous DHC (Fig. 5, A–C). In particular, DHC (300 μg/kg iv) produced decreases in cooling-evoked BAT SNA (−95.0 ± 4 vs. −97 ± 4%, P = 0.5), TBAT (−1.4 ± 0.1 vs. −1.7 ± 0.2°C, P = 0.2), and expired CO2 (−1.4 ± 0.2 vs. −1.5 ± 0.2%, P = 0.3) that were not different between vagus-intact and vagotomized rats. In contrast, in vagus-intact rats, DHC (300 μg/kg iv) evoked a larger, late-developing decrease in TCORE (−0.3 ± 0.0 vs. −0.1 ± 0.0°C, P < 0.001) and a larger rapid transient fall in HR (−111 ± 20 vs. −18 ± 6 beats/min, P < 0.01) than in the vagotomized rats. MAP responses were similarly multiphasic between intact and vagotomized rats (Fig. 5, A–C), although it should be noted that the initial depressor response to DHC (300 μg/kg iv) in intact rats (−10 ± 7 mmHg) was absent in the vagotomized rats. The later developing pressor response (27 ± 8 mmHg) in intact rats did not differ from that in vagotomized rats (34 ± 14 mmHg, P = 0.13). The later, long-lasting depressor response was unaffected by vagotomy (−16 ± 10 vs. −20 ± 5 mmHg, P = 0.15).
Fig. 5.
Vagal afferent activity is not necessary for the intravenous dihydrocapsaicin (DHC)-evoked inhibition of BAT thermogenesis. A and B: representative examples of the decreases in brown adipose tissue (BAT) sympathetic nerve activity (SNA), BAT temperature (TBAT), arterial pressure (AP), expired CO2, core body temperature (TCORE), heart rate (HR), and skin temperature (TSKIN) produced by intravenous DHC (3, 30, and 300 µg/kg, injection indicated by the vertical dashed lines) in a vagus intact rat (A) and in a rat with a bilateral cervical vagotomy (B). C: group data (means ± SE) of the BAT thermogenic and cardiovascular effects of DHC injections in vagus intact (filled circles, n = 6) and cervical vagotomized (open circles, n = 5) rats. MAP, mean arterial pressure. *P < 0.05, significant difference between the intact and vagotomized groups by t-test at the nadir of the DHC-evoked responses.
DISCUSSION
TRPV1 receptors on vagal afferents are involved in the NTS regulation of a variety of functions including gastrointestinal, respiratory and cardiovascular regulation (2, 12, 29, 35, 36). Here, we demonstrate the potential for a significant modulation of BAT thermogenesis, as well as HR and MAP, by TRPV1 receptors in the mNTS. Activation of TRPV1 receptors in the NTS in vitro augments glutamate release from vagal afferent terminals, leading to increased miniature excitatory postsynaptic current frequency that can generate action potentials in postsynaptic, second-order sensory neurons in NTS (7, 9, 14). Activation of glutamate receptors in NTS, including via cervical VNS, can inhibit cooling-evoked BAT SNA and reduce BAT thermogenesis (19). The major finding of the present study is that RTX activation of TRPV1 receptors in the mNTS, located in the axon terminals of C-fiber primary afferent neurons (6, 33) inhibits BAT SNA and reduces TBAT, expired CO2, HR, and MAP. Thus activation of TRPV1 receptors in the mNTS on the axon terminals of unmyelinated vagal and glossopharyngeal afferents can exert a potent inhibitory influence on cooling-evoked BAT SNA and BAT thermogenesis, as well as on cardiovascular function. The reductions in BAT metabolism and thermogenesis and in HR and MAP evoked by TRPV1 activation in the mNTS are expected to arise from augmented glutamate release onto second-order neurons in the NTS whose activity drives a sympathoinhibition of BAT, HR, and MAP.
The potent inhibitions of BAT SNA and BAT thermogenesis by both RTX in the mNTS and by cervical VNS (19) are consistent with a VNS-evoked glutamate release from C-fiber terminals onto second-order, BAT sympathoinhibitory neurons in the mNTS. Our finding that the reductions in BAT SNA, TBAT, expired CO2, MAP, and HR evoked by cervical VNS were not affected by blockade of TRPV1 receptors in the mNTS indicates that TRPV1 receptors in mNTS are not required for the cervical VNS-evoked sympathoinhibitory responses. These results are consistent with the observation that blockade of TRPV1 does not affect the solitary tract-evoked, synchronous glutamatergic postsynaptic response in NTS neurons in vitro (8).
A puzzling finding of our study is the consistent observation that bilateral section of the cervical vagus substantially impaired the RTX-evoked BAT sympathoinhibitory responses. This finding would suggest that the sympathoinhibitory effects on BAT thermogenesis, HR, and MAP mediated by TRPV1 activation in the mNTS require ongoing activity in a population of vagal afferents. However, the action of RTX in mNTS to activate second-order neurons in NTS is preserved in vitro (14), a situation in which there is presumed to be no ongoing activity in vagal afferents synapsing in NTS. The resolution of this dichotomy must await further study. It is of interest to note, however, that there is a similar dependency in vivo on an intact cervical vagus nerve and glutamatergic neurotransmission in the mNTS for the reduced cold-evoked activation of BAT thermogenesis and metabolism in rats on a high-fat diet (17). A role for TRPV1 receptors in NTS in this BAT inhibitory effect of a high-fat diet remains to be determined.
Our finding that section of subdiaphragmatic vagal branches markedly reduced the BAT, but not the cardiovascular, sympathoinhibitory effects of RTX nanoinjection in mNTS suggests that the vagal afferent discharge relevant to the BAT inhibitory effects of RTX arises from a subdiaphragmatic source, while that for the cardiovascular effects of RTX in mNTS arises from afferents entering the vagus nerve above the diaphragm. In this regard, it should be noted that our cervical vagotomies may have included section of the aortic depressor nerve. In addition, since our subdiaphragmatic vagotomies were at the level of the caudal third of the esophagus, we likely transected the gastric and celiac branches of the subdiaphragmatic vagus during the surgeries; therefore, these data suggest that the TRPV1 activation potentiates sensory influences arising from organs innervated by these branches. However, our surgeries preserved the vagal afferent input conveyed by the common hepatic branch; thus whether elimination of a tonic vagal afferent input from the liver or other areas innervated by the common hepatic branch of the vagus would be sufficient to prevent the inhibitions of BAT SNA and BAT thermogenesis evoked by RTX in NTS remains to be determined.
Long-term desensitization of the TRPV1 receptor can occur following administration of high doses of RTX (28, 31, 32). However, in the present study, we employed low, nondesensitizing doses of RTX, and the response to RTX in the mNTS was repeatable and was selectively blocked by the TRPV1 antagonist CPZ. Together, these results are consistent with the BAT sympathoinhibitory effects of RTX in mNTS reflecting an activation, rather than a desensitization, of the TRPV1 channel in mNTS. In addition, administration of RTX in mNTS inhibited BAT SNA, a response that is consistent with the known mechanism of TRPV1 receptor activation in NTS (i.e., glutamate release) (30) and the effect of glutamate receptor activation in the NTS to inhibit BAT SNA (19, 34).
In dorsal root ganglion neurons, in vitro, the conductance of the TRPV1 receptor was negligible below 40°C and was maximal between 42°C and 45°C (5). This range of thermal sensitivity has led to the suggestion that TRPV1 channels mediate the sensation of noxious temperatures and that they do not have a role in thermosensation within the normal physiological temperature range. However, in NTS slices, TRPV1-mediated effects are enhanced as bath temperature is increased from 32 to 36°C (14). Thus it seems likely that the cooperativity among modes of TRPV1 activation could modulate the temperature range of TRPV1 receptor opening in vivo and thereby allow the thermal sensitivity of TRPV1 receptors in the mNTS to influence BAT thermogenesis and contribute in some way to thermoregulation and metabolic homeostasis.
Our finding that intravenous injection of the TRPV1 agonist DHC inhibited BAT SNA and reduced BAT thermogenesis and TCORE confirmed and extended previous observations (4, 11, 28) that systemic administration of TRPV1 agonists induces a mild hypothermia by demonstrating that inhibition of BAT SNA and BAT thermogenesis contributes to this hypothermia. Since cervical vagotomy prevented the BAT sympathoinhibitory effect of a TRPV1 agonist in mNTS but failed to block similar responses to systemic DHC, we conclude that the decrease in BAT SNA and BAT thermogenesis in response to systemic DHC must be mediated by its action at a site other than the mNTS. The hyperthermia following either the systemic administration of TRPV1 antagonists or the elimination of TRPV1 receptor-mediated effects with large, desensitizing doses of TRPV1 agonists (15, 31, 38) reveals the existence of at least one population of TRPV1 channels that is tonically activated by endogenous “ligands” (i.e., vanilloids, heat, or H+) and whose activation promotes a reduction body temperature. The data of Steiner et al. (28, 31) are consistent with the localization of the relevant TRPV1 channels responsible for the tonic inhibition of BAT thermogenesis and the suppression of body temperature on spinal afferents accessible from the peritoneum. Indeed, many of the TRPV1 channels in the gastrointestinal tract are located on spinal afferents (37). Further studies will be required to determine the site of action for systemic DHC to inhibit BAT SNA and BAT thermogenesis. The reductions in HR and in TCORE following DHC administration were attenuated in vagotomized rats, consistent with a role for cardiac vagal efferents and for vagally mediated effects of DHC on other, non-BAT thermoeffectors.
Perspectives and Significance
TRPV1 receptors in NTS act presynaptically to augment glutamate release from C-fiber afferents. Activation of TRPV1 receptors in NTS can contribute to the inhibitory regulation of BAT SNA by increasing activity of neurons in NTS that drive an inhibition of BAT SNA (i.e., BAT sympathoinhibitory neurons). TRPV1-evoked inhibition of BAT SNA via NTS was eliminated by cervical or subdiaphragmatic vagotomy, consistent with the possibility that at least one population of C-fiber vagal afferents that originates in the gut express TRPV1 is tonically active in our preparation and exerts an inhibitory influence on the cooling-evoked activation of BAT SNA and thermogenesis. The inhibition of BAT thermogenesis by the systemic administration of the TRPV1-agonist DHC is mediated principally via a nonvagal neural pathway. The present study provides a framework for identifying the neural pathways that mediate the BAT and cardiovascular sympathoinhibitory effects of TRPV1 activation in the NTS and for understanding the physiological or pathological (e.g., high-fat diet) conditions that activate these pathways. The synaptic neurotransmission and sensory information processing within the NTS represents a critical point in metabolic, respiratory, and cardiovascular homeostatic regulation, where alterations might impact multiple regulatory pathways and thereby contribute to pathological conditions such as metabolic syndrome, heart failure, or sudden infant death syndrome.
GRANTS
This work was supported by National Institutes of Health Grants R01-NS-091066 (to S. F. Morrison), R01-DK-112198 (to C. J. Madden), and R01-HL-133505 (to M. C. Andresen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C. J. M. and S.F.M. conceived and designed research; M.M. and C.J.M. performed experiments; M.M., C.J.M., and S.F.M. analyzed data; M.M., C.J.M., M.C.A., and S.F.M. interpreted results of experiments; M.M., C.J.M., and S.F.M. prepared figures; M.M. drafted manuscript; M.M., C.J.M., M.C.A., and S.F.M. edited and revised manuscript; M.M., C.J.M., M.C.A., and S.F.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rubing Xing for excellent histological support.
REFERENCES
- 1.Andresen MC, Kunze DL. Nucleus tractus solitarius–gateway to neural circulatory control. Annu Rev Physiol 56: 93–116, 1994. doi: 10.1146/annurev.ph.56.030194.000521. [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Peters JH. TRPV1, hypertension, and cardiovascular regulation. Cell Metab 12: 421, 2010. doi: 10.1016/j.cmet.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126: 229–240, 2004. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Cao Z, Balasubramanian A, Marrelli SP. Pharmacologically induced hypothermia via TRPV1 channel agonism provides neuroprotection following ischemic stroke when initiated 90 min after reperfusion. Am J Physiol Regul Integr Comp Physiol 306: R149–R156, 2014. doi: 10.1152/ajpregu.00329.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 6.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31: 5067–5077, 2011. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci 22: 8222–8229, 2002. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawley JA, Hofmann ME, Andresen MC. Cannabinoid 1 and transient receptor potential vanilloid 1 receptors discretely modulate evoked glutamate separately from spontaneous glutamate transmission. J Neurosci 34: 8324–8332, 2014. doi: 10.1523/JNEUROSCI.0315-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawley JA, Hofmann ME, Andresen MC. Distinct calcium sources support multiple modes of synaptic release from cranial sensory afferents. J Neurosci 36: 8957–8966, 2016. doi: 10.1523/JNEUROSCI.1028-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feketa VV, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Shivering and tachycardic responses to external cooling in mice are substantially suppressed by TRPV1 activation but not by TRPM8 inhibition. Am J Physiol Regul Integr Comp Physiol 305: R1040–R1050, 2013. doi: 10.1152/ajpregu.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fosgerau K, Weber UJ, Gotfredsen JW, Jayatissa M, Buus C, Kristensen NB, Vestergaard M, Teschendorf P, Schneider A, Hansen P, Raunsø J, Køber L, Torp-Pedersen C, Videbaek C. Drug-induced mild therapeutic hypothermia obtained by administration of a transient receptor potential vanilloid type 1 agonist. BMC Cardiovasc Disord 10: 51, 2010. doi: 10.1186/1471-2261-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeusler G, Osterwalder R. Is substance P the transmitter at the first synapse of the baroreceptor reflex in rats and cats? Clin Sci (Lond) 59, Suppl 6: 295s–297s, 1980. doi: 10.1042/cs059295s. [DOI] [PubMed] [Google Scholar]
- 13.Hermes SM, Andresen MC, Aicher SA. Localization of TRPV1 and P2X3 in unmyelinated and myelinated vagal afferents in the rat. J Chem Neuroanat 72: 1–7, 2016. doi: 10.1016/j.jchemneu.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann ME, Andresen MC. Vanilloids selectively sensitize thermal glutamate release from TRPV1 expressing solitary tract afferents. Neuropharmacology 101: 401–411, 2016. doi: 10.1016/j.neuropharm.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jancsó-Gábor A, Szolcsányi J, Jancsó N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J Physiol 208: 449–459, 1970. doi: 10.1113/jphysiol.1970.sp009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol 302: R224–R232, 2012. doi: 10.1152/ajpregu.00449.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden CJ, Morrison SF. A high-fat diet impairs cooling-evoked brown adipose tissue activation via a vagal afferent mechanism. Am J Physiol Endocrinol Metab 311: E287–E292, 2016. doi: 10.1152/ajpendo.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol 566: 559–573, 2005. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madden CJ, Santos da Conceicao EP, Morrison SF. Vagal afferent activation decreases brown adipose tissue (BAT) sympathetic nerve activity and BAT thermogenesis. Temperature (Austin) 4: 89–96, 2017. doi: 10.1080/23328940.2016.1257407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohammed M, Yanagisawa M, Blessing W, Ootsuka Y. Attenuated cold defense responses in orexin neuron-ablated rats. Temperature (Austin) 3: 465–475, 2016. doi: 10.1080/23328940.2016.1184366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison SF. Central control of body temperature. F1000 Res 5: 800, 2016. doi: 10.12688/f1000research.7958.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301: R1207–R1228, 2011. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci 11: 62–71, 2008. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol (1985) 110: 789–798, 2011. doi: 10.1152/japplphysiol.00128.2010. [DOI] [PubMed] [Google Scholar]
- 25.Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS One 6: e25015, 2011. doi: 10.1371/journal.pone.0025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prechtl JC, Powley TL. Organization and distribution of the rat subdiaphragmatic vagus and associated paraganglia. J Comp Neurol 235: 182–195, 1985. doi: 10.1002/cne.902350204. [DOI] [PubMed] [Google Scholar]
- 28.Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61: 228–261, 2009. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sessa WC. A new way to lower blood pressure: pass the chili peppers please! Cell Metab 12: 109–110, 2010. doi: 10.1016/j.cmet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci 30: 14470–14475, 2010. doi: 10.1523/JNEUROSCI.2557-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steiner AA, Turek VF, Almeida MC, Burmeister JJ, Oliveira DL, Roberts JL, Bannon AW, Norman MH, Louis JC, Treanor JJ, Gavva NR, Romanovsky AA. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci 27: 7459–7468, 2007. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szallasi A, Blumberg PM. Vanilloid receptor loss in rat sensory ganglia associated with long term desensitization to resiniferatoxin. Neurosci Lett 140: 51–54, 1992. doi: 10.1016/0304-3940(92)90679-2. [DOI] [PubMed] [Google Scholar]
- 33.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543, 1998. doi: 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 34.Tupone D, Madden CJ, Morrison SF. Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat. J Neurosci 33: 14512–14525, 2013. doi: 10.1523/JNEUROSCI.1980-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Kaminski NE, Wang DH. VR1-mediated depressor effects during high-salt intake: role of anandamide. Hypertension 46: 986–991, 2005. doi: 10.1161/01.HYP.0000174596.95607.fd. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang DH. Neural control of blood pressure: focusing on capsaicin-sensitive sensory nerves. Cardiovasc Hematol Disord Drug Targets 7: 37–46, 2007. doi: 10.2174/187152907780059100. [DOI] [PubMed] [Google Scholar]
- 37.Ward SM, Bayguinov J, Won KJ, Grundy D, Berthoud HR. Distribution of the vanilloid receptor (VR1) in the gastrointestinal tract. J Comp Neurol 465: 121–135, 2003. doi: 10.1002/cne.10801. [DOI] [PubMed] [Google Scholar]
- 38.Woods AJ, Stock MJ, Gupta AN, Wong TT, Andrews PL. Thermoregulatory effects of resiniferatoxin in the rat. Eur J Pharmacol 264: 125–133, 1994. doi: 10.1016/0014-2999(94)00445-5. [DOI] [PubMed] [Google Scholar]
- 39.Wu D, Shi J, Elmadhoun O, Duan Y, An H, Zhang J, He X, Meng R, Liu X, Ji X, Ding Y. Dihydrocapsaicin (DHC) enhances the hypothermia-induced neuroprotection following ischemic stroke via PI3K/Akt regulation in rat. Brain Res 1671: 18–25, 2017. doi: 10.1016/j.brainres.2017.06.029. [DOI] [PubMed] [Google Scholar]