Abstract
Sickle cell disease (SCD) is a genetic disorder associated with hemolytic anemia, end-organ damage, reduced survival, and pain. One of the unique features of SCD is recurrent and unpredictable episodes of acute pain due to vasoocclusive crisis requiring hospitalization. Additionally, patients with SCD often develop chronic persistent pain. Currently, sickle cell pain is treated with opioids, an approach limited by adverse effects. Because pain can start at infancy and continue throughout life, preventing the genesis of pain may be relatively better than treating the pain once it has been evoked. Therefore, we provide insights into the cellular and molecular mechanisms of sickle cell pain that contribute to the activation of the somatosensory system in the peripheral and central nervous systems. These mechanisms include mast cell activation and neurogenic inflammation, peripheral nociceptor sensitization, maladaptation of spinal signals, central sensitization, and modulation of neural circuits in the brain. In this review, we describe potential preventive/therapeutic targets and their targeting with novel pharmacologic and/or integrative approaches to ameliorate sickle cell pain.
Keywords: analgesia, mast cell, neurogenic inflammation, opioid, pain, sickle cell disease, substance P, vasoocclusive crises
INTRODUCTION
Sickle cell disease (SCD) affects millions of people worldwide (65). Although the molecular basis of this genetic disease is a point mutation in the β-chain of hemoglobin, its consequences are manyfold (7, 86). One of the major comorbidities of SCD is pain. In SCD, pain can start in infancy and continue throughout life, often leading to hospitalization and poor quality of life (1, 77). Two types of pain have been characterized in SCD: acute pain and chronic pain. Acute pain is associated with vasoocclusion. Under low oxygen tension, sickle hemoglobin polymerizes into rigid fibers and makes red blood cells (RBCs) less deformable. Such stiff RBCs occlude microcirculation and cause vasoocclusive crisis (VOC) characterized by episodic, recurrent, and unpredictable acute pain (7, 8, 68). During VOC, there is impaired oxygenation of the distal tissue and ischemic-reperfusion injury. This injury leads to inflammation, oxidative stress, and endothelial dysfunction, resulting in acute pain (1, 8). In addition to the acute pain of VOC, patients with SCD may also experience chronic pain. Chronic pain may be the result of maladaptive changes in pain pathways. Current understanding of pain in SCD suggests that pain occurs by nociceptive, inflammatory, and neuropathic mechanisms (77). Emerging observations suggest several pain pathways that could be targeted to ameliorate or reduce pain.
In this review, we describe the mechanisms of acute and chronic pain in SCD, focusing on how sickle pathobiology evokes pain by activating the peripheral and central nervous systems (Fig. 1). We provide brief insight into the unique humanized transgenic mouse models of SCD, which have been critical in defining the mechanisms of sickle cell pain. We also elaborate on the targeting of these mechanisms with specific pharmacologic and/or integrative approaches with the potential to treat sickle cell pain more effectively.
Fig. 1.
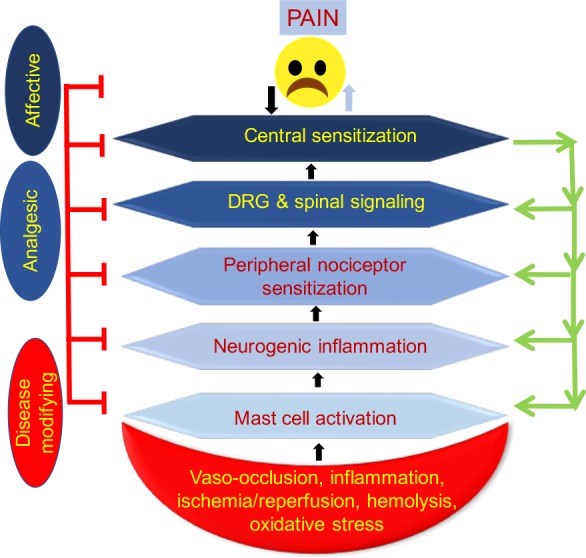
Pain pathways from the periphery to the brain and from the brain and central nervous system to the periphery. We propose that sickle pathobiology replete with vasoocclusion, ischemia-reperfusion injury, inflammation, and oxidative stress activates mast cells, which in turn leads to peripheral nociceptor sensitization. Signals from the periphery are transmitted to the dorsal horn of the spinal cord via primary afferents, which are modulated and further transmitted to the brain. However, sickle cell disease pathobiology may influence each of these components of pain independently. Moreover, sustained hyperexcitability of second-order neurons in the spinal cord may lead to an antidromic release of action potentials and neurotransmitters from the central nervous system to the periphery, leading to a feedback loop of peripheral and central sensitization. In addition to receiving signals from the spinal cord, the brain may modulate pain by releasing neurotransmitters through the descending inhibitory pathway into the dorsal horn, which may have an inhibitory or facilitatory effect on pain. DRG, dorsal root ganglion.
HUMANIZED MOUSE MODELS OF SCD
Transgenic mouse models of SCD expressing human sickle hemoglobin have played a key role in understanding the pathobiology of pain in SCD, reviewed by Tran et al. (77). There are two broad categories of such mice. The first type of mice expresses human β-sickle hemoglobin alongside mouse α- and β-globin; these include the NY1DD and S+SAntilles mice with a mild-to-moderate SCD phenotype (29, 30). The second type of mice expresses exclusively human α- and β-globin chains and no mouse α- and β-globin chains. These include the HbSS-BERK and HbSS-Townes sickle mice, which exclusively express >99% human sickle hemoglobin (HbS), and control HbAA-BERK or HbAA-Townes mice, which express normal human hemoglobin (HbA; 64, 69). HbSS-BERK and HbSS-Townes mice constitutively show mechanical, thermal, and deep tissue hyperalgesia, characteristic features of chronic pain in patients with SCD (1, 17, 37, 38, 45, 47, 56). Using hypoxia-reoxygenation treatments, we have been able to evoke acute pain in these sickle mice to simulate pain due to VOC (17, 81). BERK sickle mice exhibit a significantly greater degree of hyperalgesia for all behavioral measures compared with sex- and age-matched Townes sickle mice (39). These models have been highly instructive in examining the pathobiology of sickle cell pain and show translational potential. The genetically distinct “knock-in” strategy of human α- and β-transgene insertion in Townes mice compared with BERK mice may provide relative advantage for further genetic manipulations to examine specific mechanisms of pain (39).
PATHOBIOLOGY OF CHRONIC PAIN IN SCD
Chronic pain is defined as pain that continues for >3 mo (14). A significant majority of patients with SCD develop chronic pain with increasing age (54, 73). Development of new treatments is hampered by the long-term requirement of opioids, lack of objective pain measures, and relatively poor understanding of symptoms and mechanisms. A collaborative effort between the Analgesic, Anesthetic, and Addiction Clinical Trail Translations, Innovations, Opportunities, and Networks (ACTTION) and the American Pain Society has led to an evidence-based classification system to facilitate the diagnosis and management of chronic sickle cell pain (24), the ACTTION-American Pain Society Pain Taxonomy (AAPT). These criteria may improve the diagnosis of chronic pain, but new treatments are dependent on a better understanding of the sickle pathobiology that perpetuates pain.
SICKLE PATHOBIOLOGY UNDERLYING THE ACTIVATION OF SOMATOSENSORY MECHANISMS LEADING TO PAIN
The unique sickle pathobiology of vasoocclusion, inflammation, and oxidative stress along with ischemia-reperfusion injury may create a noxious environment in the periphery, spinal cord, and/or brain, contributing to a complex pathology underlying pain in SCD (Fig. 1). Two key processes that may drive chronic as well as acute pain are neurogenic inflammation and mast cell activation.
Neurogenic Inflammation in SCD
Inflammation caused by nerve stimulation is called “neurogenic inflammation” (36). It leads to the release of cytokines and neuropeptides including substance P (SP) and calcitonin gene-related peptide (CGRP) from nerve fibers, which stimulate vascular dilatation and increased venular permeability. Sickle BERK mice show neurogenic inflammation as evidenced by an increase in vascular leakage compared with control BERK mice (84). This process may underlie enhanced peripheral nociceptor sensitization observed by us in these mice (79) described in central and peripheral sensitization of chronic pain pathways in scd. Significantly higher circulating SP has been described in patients with SCD at steady state compared with healthy controls (55). One of the sources of SP appears to be mast cells as described below.
Mast Cell Activation in SCD
Mast cells are tissue-resident leukocytes, unlike neutrophils, which are found in circulation (36). Mast cells display tremendous heterogeneity in their activation, which is specific to the surrounding microenvironment (36). An important feature of mast cell activation is their ability to release a variety of substances by degranulation and/or de novo synthesis, including proteases, adhesion molecules, neuropeptides, and cytokines, which act in a paracrine as well as autocrine manner. Notably, mast cells also release extracellular traps, vesicles, podia, and nanotubes, which physically interact directly with other cellular structures. Since mast cells are located in the vicinity of vasculature and nerve fibers, their activation has direct influence on circulatory and neural physiology. We found degranulating mast cells in the skin in close proximity to nerve fibers, particularly in the nerve plexus of BERK sickle mice (36, 84). We found that mast cells release tryptase, which activates protease-activated receptor 2 (PAR2) and transient receptor potential cation channel V1 (TRPV1) channels on nerve endings, leading to the release of neuropeptides SP and CGRP. These neuropeptides act on the vasculature leading to increased permeability and neurogenic inflammation and a vicious cycle of mast cell activation and pain. Treatment of sickle mice with imatinib, a c-kit inhibitor, or cromolyn sodium, a mast cell stabilizer, led to reduced inflammation, neuroinflammation, and hyperalgesia. Deletion of mast cells from sickle mice ameliorated chronic pain; this finding suggests that mast cell activation contributes to pain. As described below in mechanism-based strategies to target sickle cell pain, imatinib treatment completely ameliorated VOC in patients with SCD (61, 75). It is likely that mast cell activation in SCD underlies the stimulation of selectins on the endothelial surface and activation of neutrophils, as described below, which orchestrate sickle RBC adhesion and vasoocclusion, as well as contribute to coagulopathy by activating platelets.
PATHOBIOLOGY OF ACUTE PAIN IN SCD
A characteristic and unique feature of SCD is unpredictable and recurrent VOC due to occlusion of venules accompanied by ischemia-reperfusion injury, inflammation, hemolysis, and oxidative stress (8, 68, 73). Acute pain, considered worse than the pain of childbirth, requires hospitalization and is often challenging to treat (8). Under low oxygen tension, sickle erythrocyte HbS polymerizes into rigid fibers resulting in the typical sickle shape of erythrocytes (16). Rigid sickle RBCs aggregate and adhere to activated endothelium, along with circulating leukocytes and platelets (90, 92). Leukocytes, specifically neutrophils, are activated in sickle mice and patients with SCD (50). These interactions of sickle RBC and leukocytes with vascular endothelium are facilitated by adhesion molecules including E- and P-selectin (52, 66). Vasoocclusion is prevented in sickle mice lacking E- and P-selectin (78). Blockade of P-selectin with heparin improved microvascular blood flow in patients with SCD (46, 52). Crizanlizumab, an antibody to P-selectin, significantly reduced pain crises in patients with SCD in a phase II trial, described in detail in mechanism-based strategies to target sickle cell pain (6). However, it remains to be determined how vasoocclusion leads to acute pain.
Upregulation of selectins on the endothelial surface is critical for adhesion of sickle RBCs and leukocytes. Mast cell activation induces P-selectin-dependent leukocyte rolling and adhesion in postcapillary venules in vivo (32, 76). Activation of mast cells leads to endothelial leukocyte adhesion molecule (ELAM) expression on vasculature in the skin (44). In the Klein et al. (44) study, neonatal human foreskins, incubated with morphine, a known activator of mast cell degranulation, led to increased ELAM expression. We observed extremely large aggregates of activated mast cells releasing extracellular traps in close association with the intact vasculature in the skin of sickle mice (36). Thus, activation of mast cells in SCD may play a critical role in VOC and acute pain in addition to chronic pain.
CENTRAL AND PERIPHERAL SENSITIZATION OF CHRONIC PAIN PATHWAYS IN SCD
In chronic pain, constitutively hyperexcitable nociceptors on peripheral afferents and/or second-order neurons in the spinal cord continue to transmit action potentials on a sustained basis in the absence of noxious insult. Hypersensitivity to noxious and innocuous stimuli—called “hyperalgesia” and “allodynia,” respectively—has been observed in sickle mice and patients (12, 17, 18, 38, 42, 45, 47, 84). Electrophysiological recordings in the dorsal horn neurons of sickle BERK mice compared with control BERK mice demonstrate a higher rate of spontaneous discharge, enlarged receptive fields, reduced mechanical threshold, and prolonged after-discharges after mechanical stimulation (20). These electrophysiological changes are accompanied by increased phosphorylation of the MAPK family members p42/44 MAPK, ERK, JNK, and p38 MAPK in the spinal cord, which is consistent with chronic pain pathways (20).
In addition to central sensitization, we observed sensitization of cutaneous nociceptors in the periphery in sickle BERK mice (79). Electrophysiological recordings of the tibial nerve in vivo in live anesthetized mice demonstrated spontaneous activity in the Aδ and C fiber nociceptors. The C fibers showed increased response to mechanical and thermal stimuli in sickle BERK compared with control BERK mice. Complementary to these observations, TRPV1 channels on the primary afferent terminals and somata have been shown to be activated and contribute to mechanical hypersensitivity in sickle BERK mice (38). The molecular signatures of these electrophysiological outcomes are emerging. We found that oxidative stress, SP, glial activation, glial fibrillary acidic protein, and inflammation are increased significantly in the dorsal horn of sickle BERK mice compared with control BERK mice (84, 85). The spontaneous nociceptor activity in the periphery and spinal cord and hypersensitivity to mechanical and/or thermal stimuli are suggestive of central sensitization in sickle mice, which may underlie chronic pain and be recalcitrant to analgesic therapy. It is likely that this hyperexcitability of dorsal horn neurons is a continuum of peripherally evoked sensory activity and/or may be due to the glial activation by sickle pathobiology involving mast cells and ischemia-reperfusion injury in the spinal cord (Fig. 1).
NEUROMODULATORY PATHWAYS
Signals in the dorsal horn of the spinal cord are also modulated by neurotransmitters including dopamine, endogenous opioids, γ-aminobutyric acid, glycine, and others released from the nerve fiber projections from the brain stem. These top-down mechanisms originating in the brain may be influenced by perception and lead to affective modulation, which may have an inhibitory or facilitatory effect on pain transmission. Neuroimaging studies in sickle cell subjects with chronic pain have shown abnormal activity in two key neuronal networks of the brain, namely, the default mode network (DMN) and executive control network (ECN; 19, 25). The DMN is a network of highly correlated areas of the brain that is active when the brain is engaged in nonpurposeful actions, such as daydreaming, not conscious and purposeful action. The key zones of the DMN are the ventral medial prefrontal cortex, the inferior parietal lobule, the posterior cingulate cortex, the precuneus, areas of the medial frontal gyri, the hippocampal formation, and the posterior lateral temporal cortex (15). In patients with SCD, the neuronal circuits that enhance pain are sensitized, and the circuits that suppress pain are desensitized. In SCD subjects with increased pain, pronociceptive connections between the anterior cingulate cortex and areas of the DMN such as the precuneus and the inferior parietal lobule are increased (25). In contrast, SCD subjects with infrequent pain show increased connectivity between the opioid-rich antinociceptive perigenual and subgenual cingulate and the DMN. There is also increased connectivity between the perigenual anterior cingulate area and the salience network (SLN; 25). The SLN is the main area for pain processing and includes the bilateral primary and secondary somatosensory cortexes, the anterior insula, the primary motor cortex, and the sensory motor area (9). The ECN is the part of the brain that is active when the brain is engaged in conscious, purposeful action. The ECN is involved in external processing such as nociception and decision making. The ECN comprises the dorsolateral prefrontal cortex and the posterior parietal cortex (53). In patients with SCD, this part of the brain is hyperactive and may contribute to exaggerated pain perception by the patient. Sociopsychological factors leading to cognitive impairment have been described to influence pain and opioid requirement in patients with SCD (31). In addition, cerebral infarction in SCD subjects also results in a significant decline of neuropsychological functions, IQ, and cognitive score (5, 10, 13, 22, 26, 71, 87). However, molecular events underlying these neural processes in the brain remain unknown. It is likely that local ischemia-reperfusion injury, mast cell activation, and other inflammatory processes may directly influence the neural circuits and/or may be a result of somatosensory inputs from the periphery.
MECHANISM-BASED STRATEGIES TO TARGET SICKLE CELL PAIN
Opioids have been the mainstay of symptomatic management of sickle cell pain. Use of opioids is fraught with side effects including hyperalgesia, dependence, and tolerance, reviewed by Gupta et al. (37). Furthermore, opioids adversely influence RBC rheology by altering their membrane structure, increase mast cell degranulation-induced inflammation, and influence organ pathology via their coactivation of receptor tyrosine kinases leading to mitogenic signaling (35, 37). Importantly, opioids do not treat the underlying mechanisms evoking pain, and considerable concerns regarding opioid use have led to opioid phobia, often leading to undertreatment of sickle cell pain (8). Recent understanding of the mechanism-based targets of pain described above has the potential to lead to improved analgesic therapies in SCD, described below (Table 1).
Table 1.
Mechanism-based therapies to target sickle cell pain
| Intervention | Mechanism | Known Outcomes and Current Clinical Trials |
|---|---|---|
| Crizanlizumab | Anti-P-selectin antibody | Reduced the frequency of painful VOC by 45% and tripled the median time of first VOC in patients with SCD; current clinical trial (ClinicalTrials.gov identifier NCT03264989: “Pharmacokinetics and Pharmacodynamics Study of Crizanlizumab in Adult SCD Patients with VOC”) |
| Sivelestat | Leukocyte elastase inhibitor | Decreased neuronal injury to the DRG and reduced neuropathic pain in BERK sickle mice |
| Imatinib | cKIT/mast cell inhibitor | Reduced neurogenic inflammation and prevented hypoxia-reoxygenation-induced hyperalgesia in sickle mice; decreased frequency of VOC in patients with SCD |
| Cromolyn | Mast cell stabilizer | Increased the analgesic effect of a suboptimal dose of morphine in BERK sickle mice |
| Trifluoperazine | CaMKIIα inhibitor; reduces calpain-1 activity | In a phase I clinical trial, ameliorated neurogenic pain and caused 50% reduction in chronic pain in patients with SCD without severe sedation or supplemental opioid analgesics |
| Simvastatin | Decreases calpain-1 activation? | In patients with SCD, reduced frequency of pain, oral analgesic use, and markers of inflammation, acting synergistically with hydroxyurea; 4 completed clinical trials are cited on the ClinicalTrials.gov website |
| CP55,940 | Cannabinoid receptor 1 and 2 agonist | Ameliorated chronic and hypoxia-reoxygenation-induced hyperalgesia in sickle mice; mitigated mast cell activation, inflammation, and neurogenic inflammation in sickle mice; current clinical trial (ClinicalTrials.gov identifier NCT01771731: “Vaporized Cannabis for Chronic Pain Associated with SCD”) |
| Rapamycin everolimus | mTOR inhibitor: increases fetal hemoglobin levels | Ameliorated the nociception phenotype in sickle mice; in 1 renal transplant recipient, increased fetal hemoglobin levels from 4.8 to 15% and was well tolerated |
| Omega-3 fatty acids | Antioxidant | Reduced frequency of VOC and transfusion requirements in a randomized study of 140 patients in Sudan; current clinical trial (ClinicalTrials.gov identifier NCT02947100: “Omega–3 Fatty Acids in Sickle Cell Disease”) |
| Curcumin and CoQ10 | Anti-inflammatory and antioxidant | Attenuated glial activation, neuroinflammation, and oxidative stress in spinal cords of BERK sickle mice, resulting in attenuation of hyperalgesia; reduced inflammation, oxidative stress, and VOC-associated pain in patients with SCD |
| AT-200 | Nociceptin opioid receptor agonist and mast cell inhibitor | Ameliorated chronic and hypoxia-reoxygenation-induced mechanical, thermal, and deep tissue/musculoskeletal hyperalgesia in BERK sickle mice without causing tolerance |
| Acupuncture | Inhibits inflammation peripherally and in the spinal cord | In awake BERK sickle mice, reduced inflammatory cytokines, substance P, and neurogenic inflammation in the periphery and signaling pathways of nociception in the spinal cord and potentiated the effect of a suboptimal dose of morphine; acupuncture significantly reduced VOC-associated pain in patients with SCD |
| Hypnosis | Modulates vascular physiology | Decreased pain intensity and increased peripheral blood flow during anticipation and experience of pain in patients with SCD |
CoQ10, coenzyme Q10; DRG, dorsal root ganglion; mTOR, mammalian target of rapamycin; SCD, sickle cell disease; VOC, vasoocclusive crisis.
Targeting VOC in SCD
During the initial phases of VOC, P-selectin initiates the adhesion of sickle erythrocytes and leukocytes to the endothelium and mediates the adhesion of activated platelets to neutrophils, resulting in vasoocclusion and inflammation (68). The anti-P-selectin antibody, crizanlizumab, inhibits P-selectin-mediated cell-cell adhesion and has been shown to reduce the frequency of painful VOC by 45% and triple the median time of first VOC in a phase II clinical trial (6). Crizanlizumab may be the first drug to be approved by the US Food and Drug Administration (FDA) to prevent VOC and subsequent pain at its source.
Inhibiting Inflammation
Elastase released from activated leukocytes mediates inflammation in the peripheral tissues and the dorsal root ganglion (DRG), contributing to pain. Vicuña et al. showed that upon peripheral nerve injury in rodents, elastase is released from infiltrating T cells in the DRG, contributing to neuropathic pain [82a; see Weyer and Stucky (88)]. SerpinA3N, an endogenous inhibitor of serine proteases, and the drug sivelestat, a leukocyte elastase inhibitor, alleviated pain in this mouse model of neuropathic pain as measured by the paw withdrawal threshold and responses to von Frey filaments (P < 0.05). We found that leukocyte elastase activity is significantly increased in the DRG and lungs of sickle mice (2). Sivelestat treatment decreased neuropathic pain in BERK sickle mice (2). The safety of sivelestat is already established in humans, and it has been approved for treatment of acute lung injury in Japan (3). Trials of this drug in the United States were previously halted because of concerns that it increased long-term mortality rate in mechanically ventilated patients with acute lung injury (93) Sivelestat and other similar drugs in development require preclinical evaluation for sickle-specific pain and associated adverse effects.
Inhibiting Mast Cell Activation
Activation of mast cell plays a critical role in neurogenic inflammation and nociceptor activation via the release of SP in the skin and DRG of sickle mice. We found that imatinib, a mast cell inhibitor, significantly reduces neurogenic hyperalgesia and prevents hypoxia-reoxygenation-induced hyperalgesia in BERK sickle mice (P < 0.05; 84). In separate case reports of two patients with chronic myeloid leukemia and SCD, imatinib significantly reduced painful episodes, hospitalizations, and daily pain (21, 74). Imatinib is FDA approved for several indications. At this time, we are not aware of any clinical trials of imatinib in SCD, but the rationale for such a trial would be compelling. We also found that pretreatment with the mast cell stabilizer cromolyn sodium potentiated the analgesic effect of otherwise ineffective low doses of morphine in BERK sickle mice (84). Therefore, it is likely that morphine activates mast cells, thereby contributing to pain, but concomitantly acts as an analgesic via its activity in the nervous system. Mast cell targeting thus offers an opioid-reducing strategy in the management of sickle cell pain.
Calpain-1 contributes to IgE-mediated mast cell activation and plays an important role in neurotransmission and neurogenic pain (89). We have demonstrated that genetic ablation of calpain-1 in Townes sickle mice ameliorates tonic hyperalgesia in sickle mice, but not that evoked by hypoxia-reoxygenation (62). Trifluoperazine, an FDA-approved antipsychotic agent, acts as a potent CaMKIIα inhibitor and reduces calpain-1 activity (49). It ameliorated neurogenic pain and caused 50% reduction in chronic pain in patients with SCD without severe sedation or supplemental opioid analgesics in a phase I clinical trial (57). Statins also decrease calpain-1 activation (91). In a single-center pilot study in adolescents and adults with SCD, simvastatin treatment for up to 3 mo led to an 85% reduction in the rate of sickle cell-related pain and oral analgesic use, improved soluble biomarkers of inflammation, and acted synergistically with hydroxyurea (39). Despite the marked decline in the rate of occurrence of pain, the intensity of pain did not change with simvastatin (39). To explain this disparate effect of simvastatin on frequency and intensity of pain, the authors of this study hypothesized that the visual analog scale, used as the sole measure of intensity of pain in this study, may not have fully captured the multidimensional nature of intensity of pain in SCD (39).
Stimulating Cannabinoid Receptors
Cannabinoid receptor type 1 (CB1R) and cannabinoid receptor type 2 (CB2R) activation on mast cells has been shown to inhibit degranulation and inflammation, respectively (72). CB1R and CB2R are expressed in both the central nervous system and non-central nervous system tissues, including inflammatory cells (59, 82). Activation of CB2R peripherally generates an antinociceptive response in inflammatory and neuropathic pain (41). Targeting CB1R has psychotropic effects, but targeting CB2R does not have psychotropic effects (27). CB2R agonists and/or knockout mice provide compelling evidence that CB2R activation mitigates neuropathic and inflammatory pain and is protective against ischemia-reperfusion injury by decreasing the endothelial expression of adhesion molecules and secretion of chemokines (58, 60, 67) and by attenuating leukocyte adhesion to the endothelium, transendothelial migration, and interrelated oxidative nitrosative damage (63), all of which are consistent with the pathobiology of SCD. We showed that cannabinoids mitigate mast cell activation, inflammation, and neurogenic inflammation in BERK sickle mice via both CB1R and CB2R, but activation of CB1R is required to ameliorate hyperalgesia (83). In 86 human patients with HbSS, HbSC, and HbS-β thalassemia, a structured self-administered anonymous questionnaire found that that 31 (36%) had used cannabis in the previous 12 mo to relieve symptoms associated with SCD (40). The main route in all but two patients was by smoking. The most common reasons for use were to reduce pain (in 52%) and to induce relaxation or relieve anxiety and depression (in 39%). We are currently performing a double-blind, placebo-controlled, FDA-approved clinical trial to test the effect of vaporized cannabis on chronic pain in SCD (ClinicalTrials.gov identifier NCT01771731).
Elevating Fetal Hemoglobin
Chronic pain has been found to be inversely associated with circulating fetal hemoglobin (HbF) in patients with SCD (25). Notably, higher HbF in patients with SCD has been shown to be associated with reduced frequency of painful crises (51). HbF-increasing strategies have been examined in preclinical studies. The mammalian target of rapamycin (mTOR) plays a central role in regulating many fundamental cell processes, from protein synthesis to autophagy, and deregulated mTOR signaling is implicated in a variety of pathological conditions (70). The mTOR inhibitor, rapamycin, increases HbF levels and ameliorates the nociception phenotype in sickle cell mice (43). Gaudre et al. reported a case of a kidney transplant recipient with SCD who was treated with everolimus, an mTOR inhibitor (33). At 10 mo after initiating therapy, the patient’s HbF level increased dramatically from 4.8 to 15%, and there was excellent tolerance to treatment. Reinduction of HbF by depleting DNA methyltransferase 1 with a combination of decitabine and tetrahydrouridine led to a significant increase in HbF and improved RBC quality as well as hemolysis and inflammation in patients with SCD in a phase I clinical trial (NCT01685515). Together, these preclinical and clinical observations suggest the potential of HbF-enhancing strategies in reducing the activation of pain pathways.
Anti-inflammatory and Antioxidant Strategies
Omega-3 fatty acids have antiaggregatory, antiadhesive, anti-inflammatory, and vasodilatory properties. Omega–3 fatty acid was shown to reduce frequency of VOC and transfusion requirements in a randomized study of 140 patients in Sudan (23). In the United States, a phase I/II clinical trial to determine the safety of a new formulation of the omega–3 fatty acids docosahexaenoic acid and eicosapentaenoic acid and to assess whether it decreases inflammation and inflammatory pain in children and young adults with SCD has been undertaken (NCT02947100).
Central nociceptive mechanisms contribute to chronic pain and hyperalgesia in SCD, releasing reactive oxidative species, inflammatory cytokines, neurotrophic factors, and prostaglandins that excite nociceptive neurons by activated microglia contributing to the persistence of chronic pain in SCD (34). We found that glial activation, neuroinflammation, and oxidative stress in spinal cords of sickle mice are ameliorated with curcumin and/or coenzyme Q10 (CoQ10), resulting in attenuation of hyperalgesia in BERK sickle mice (80). Curcumin and CoQ10 are likely to be relatively nontoxic adjuvants. In a preliminary study in a subpopulation of patients with SCD, CoQ10 treatment reduced inflammation and oxidative stress as measured by circulating C-reactive protein and thiobarbituric acid reactive substances, respectively, and reduced pain during VOC (75).
Nociceptin Opioid Receptor Agonists
Once pain has been evoked, analgesic strategies are required. Opioids exert most of their antinociceptive effect via the µ-opioid receptor (MOP/R). In addition to MOP/R, the opioid receptor family includes the nociceptin opioid receptor (NOP/R), which is involved in nociceptive signaling via its endogenous peptide ligand nociceptin/orphanin FQ. NOP/Rs are found in the DRG, spinal cord, and supraspinal regions of the brain involved in nociception. We have shown that AT-200, a high-affinity NOP/R agonist with low efficacy at the MOP/R, ameliorated chronic pain and hypoxia-reoxygenation-induced mechanical, thermal, and deep tissue/musculoskeletal hyperalgesia in HbSS-BERK sickle mice (81). Daily treatment for 7 days with AT-200 did not cause tolerance and showed a sustained antinociceptive effect, which improved over time and led to reduced plasma serum amyloid protein, neuropeptides, inflammatory cytokines, and mast cell activation in the periphery. These data suggest that AT-200 ameliorates pain in sickle mice via NOP/R by reducing inflammation and mast cell activation without causing tolerance. Thus, NOP/R agonists are promising drugs for treating pain in SCD because of their effect on sickle pathobiology as well as nociceptive mechanisms.
Electroacupuncture
We found that electroacupuncture in awake BERK sickle mice influenced the sickle microenvironment as well as nociception centrally. We observed varied antinociceptive response among identical electroacupuncture-treated mice. To analyze individualized responses, we categorized all electroacupuncture-treated mice into three groups on the basis of their increase in pain threshold after electroacupuncture treatment: high responders (>200%), moderate responders (~100–200%), and nonresponders (≤100%). Electroacupuncture led to reduction in inflammatory cytokines, SP, and neurogenic inflammation in the periphery and signaling pathways of nociception in the spinal cord and potentiated the effect of a suboptimal dose of morphine in moderate responders, resulting in analgesia equivalent to that in high responders (85). In a retrospective review and descriptive analysis of 24 patients with SCD with pain treated at the National Institutes of Health, 9 patients underwent only inpatient acupuncture for VOC, 11 patients received only outpatient acupuncture treatment for chronic pain, and 4 patients received both inpatient and outpatient treatments (48). For the patients who received inpatient acupuncture treatment for VOC, there was a significant reduction of reported pain score immediately after acupuncture treatment with an average pain reduction of 2.1 points on the 0–10 numeric pain scale (P < 0.0001). Five of the 15 outpatients had only 1 session per course of acupuncture and thus did not contribute to outpatient data. Of the 10 outpatients who accepted >1 session per course of acupuncture, 2 patients accounted for 79.2% of the 231 sessions. The investigators therefore analyzed the results from these two patients separately from the other eight patients. Six out of the remaining eight outpatients described their pain as improved. The two high-usage patients, who had pain mostly related to chronic ulcers, reported improved pain compared with the prior session in 36.1% of sessions, stable pain in 28.5%, and worse pain in 11.5%. Data were not available for 23% of the sessions (48). This analysis raises the possibility of acupuncture as an adjuvant for pain management in SCD (48).
Perception-Based Therapies
Nonpharmacological techniques such as guided imagery, mindfulness, relaxation, hypnosis, and cognitive behavioral therapy are effective in alleviating chronic pain in SCD (4, 11, 28). Activation of the descending neuromodulatory pathway by nonpharmacologic interventions (e.g., acupuncture, meditation, and yoga) has been shown to reduce peripheral and central sensitization. In a pilot study of patients with SCD and healthy controls, a single 30-min hypnosis session decreased pain intensity by a moderate amount in patients with SCD (11). Pain threshold and tolerance increased after hypnosis in the control group, but not in patients with SCD. Patients with SCD exhibited lower baseline peripheral blood flow and a greater increase in blood flow after hypnosis than controls, suggesting that hypnosis may increase peripheral vasodilation during both the anticipation and experience of pain in patients with SCD.
FUTURE PERSPECTIVES
Emerging pain therapies are in development that target SCD pathobiology, peripheral and central sensory mechanisms, and descending neuromodulatory pathways. Notably, many therapeutic strategies have the potential to reduce opioid requirement and improve opioid analgesia. Targeting pain at its source is perhaps the most effective method of preventing the pain of SCD compared with treating pain once it has been evoked. Targeting mast cells with imatinib, an FDA-approved drug, to ameliorate the evoking of somatosensory mechanisms by sickle pathobiology appears to be a highly promising pharmacotherapeutic strategy to prevent/treat sickle cell pain. Increased attention is required to apply perception-based affective modulation to minimize the use of analgesics. A major gap still exists in diagnostic criteria for quantifying and phenotyping pain on the basis of circulating biomarkers, physiological measures, and neuroimaging to guide the use of specific therapies and/or combination therapies. Finally, well-designed clinical trials are required to test these pharmacological and integrative approaches.
GRANTS
This effort was supported by National Heart, Lung, and Blood Institute Grant U01-HL-117664 to Kalpna Gupta.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
Kalpna Gupta is a consultant for TauTona Group.
AUTHOR CONTRIBUTIONS
K.G. and K.G. prepared figures; K.G., O.J., and K.G. drafted manuscript; K.G., O.J., and K.G. edited and revised manuscript; K.G., O.J., and K.G. approved final version of manuscript; Kalpna G. conceived and designed research.
ACKNOWLEDGMENTS
We thank Michael Franklin for editorial suggestions and Barb Benson for assistance with manuscript preparation. We apologize for our inability to include many studies due to space limitations, but we deeply appreciate their contribution to the study of pain/SCD.
REFERNCES
- 1.Aich A, Beitz AJ, Gupta K. Mechanisms of pain in sickle cell disease. In: Sickle Cell Disease: Pain and Common Chronic Complications, edited by Inusa BP London: InTech, 2016, chapt. 5, p. 69–91. doi: 10.5772/64647 https://www.intechopen.com/books/sickle-cell-disease-pain-and-common-chronic-complications/mechanisms-of-pain-in-sickle-cell-disease. [DOI] [Google Scholar]
- 2.Aich A, Paul J, Lei J, Wang Y, Bagchi A, Gupta K. Regulation of elastase by SerpinA3N contributes to pain in sickle cell disease. Blood 128: 858, 2016. [Google Scholar]
- 3.Aikawa N, Kawasaki Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther Clin Risk Manag 10: 621–629, 2014. doi: 10.2147/TCRM.S65066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9: 463–484, 2005. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong FD, Thompson RJ Jr, Wang W, Zimmerman R, Pegelow CH, Miller S, Moser F, Bello J, Hurtig A, Vass K; Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease . Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Pediatrics 97: 864–870, 1996. [PubMed] [Google Scholar]
- 6.Ataga KI, Kutlar A, Kanter J, Liles D, Cancado R, Friedrisch J, Guthrie TH, Knight-Madden J, Alvarez OA, Gordeuk VR, Gualandro S, Colella MP, Smith WR, Rollins SA, Stocker JW, Rother RP. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med 376: 429–439, 2017. doi: 10.1056/NEJMoa1611770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballas SK. Sickle Cell Pain. Philadelphia, PA: Lippincott Williams & Wilkins, 2015. [Google Scholar]
- 8.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood 120: 3647–3656, 2012. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 9.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013, 2005. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernaudin F, Verlhac S, Fréard F, Roudot-Thoraval F, Benkerrou M, Thuret I, Mardini R, Vannier JP, Ploix E, Romero M, Cassé-Perrot C, Helly M, Gillard E, Sebag G, Kchouk H, Pracros JP, Finck B, Dacher JN, Ickowicz V, Raybaud C, Poncet M, Lesprit E, Reinert PH, Brugières P. Multicenter prospective study of children with sickle cell disease: radiographic and psychometric correlation. J Child Neurol 15: 333–343, 2000. doi: 10.1177/088307380001500510. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt RR, Martin SR, Evans S, Lung K, Coates TD, Zeltzer LK, Tsao JC. The effect of hypnosis on pain and peripheral blood flow in sickle-cell disease: a pilot study. J Pain Res 10: 1635–1644, 2017. doi: 10.2147/JPR.S131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol 88: 37–43, 2013. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown RT, Davis PC, Lambert R, Hsu L, Hopkins K, Eckman J. Neurocognitive functioning and magnetic resonance imaging in children with sickle cell disease. J Pediatr Psychol 25: 503–513, 2000. doi: 10.1093/jpepsy/25.7.503. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan G, Yawn B, Afenyi-Annan A, Ballas S, Hassell K, James A, Jordan L, Lanzkron S, Lottenberg R, Savage W, Tanabe P, Ware RE. Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014. Bethesda, MD: National Heart, Lung, and Blood Institute, 2014. https://www.nhlbi.nih.gov/health-topics/evidence-based-management-sickle-cell-disease. [DOI] [PubMed] [Google Scholar]
- 15.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38, 2008. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 16.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 337: 762–769, 1997. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 17.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol 156: 535–544, 2012. doi: 10.1111/j.1365-2141.2011.08977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell CM, Moscou-Jackson G, Carroll CP, Kiley K, Haywood C Jr, Lanzkron S, Hand M, Edwards RR, Haythornthwaite JA. An evaluation of central sensitization in patients with sickle cell disease. J Pain 17: 617–627, 2016. doi: 10.1016/j.jpain.2016.01.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Case M, Zhang H, Mundahl J, Datta Y, Nelson S, Gupta K, He B. Characterization of functional brain activity and connectivity using EEG and fMRI in patients with sickle cell disease. Neuroimage Clin 14: 1–17, 2017. doi: 10.1016/j.nicl.2016.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cataldo G, Rajput S, Gupta K, Simone DA. Sensitization of nociceptive spinal neurons contributes to pain in a transgenic model of sickle cell disease. Pain 156: 722–730, 2015. doi: 10.1097/j.pain.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Close JL, Lottenberg R. Effectiveness of imatinib therapy for a patient with sickle cell anemia and chronic myelocytic leukemia. Blood 114: 2559, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Cohen MJ, Branch WB, McKie VC, Adams RJ. Neuropsychological impairment in children with sickle cell anemia and cerebrovascular accidents. Clin Pediatr (Phila) 33: 517–524, 1994. doi: 10.1177/000992289403300902. [DOI] [PubMed] [Google Scholar]
- 23.Daak AA, Ghebremeskel K, Hassan Z, Attallah B, Azan HH, Elbashir MI, Crawford M. Effect of omega–3 (n-3) fatty acid supplementation in patients with sickle cell anemia: randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 97: 37–44, 2013. doi: 10.3945/ajcn.112.036319. [DOI] [PubMed] [Google Scholar]
- 24.Dampier C, Palermo TM, Darbari DS, Hassell K, Smith W, Zempsky W. AAPT diagnostic criteria for chronic sickle cell disease pain. J Pain 18: 490–498, 2017. doi: 10.1016/j.jpain.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Darbari DS, Hampson JP, Ichesco E, Kadom N, Vezina G, Evangelou I, Clauw DJ, Taylor Vi JG, Harris RE. Frequency of hospitalizations for pain and association with altered brain network connectivity in sickle cell disease. J Pain 16: 1077–1086, 2015. doi: 10.1016/j.jpain.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBaun MR, Schatz J, Siegel MJ, Koby M, Craft S, Resar L, Chu JY, Launius G, Dadash-Zadeh M, Lee RB, Noetzel M. Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology 50: 1678–1682, 1998. doi: 10.1212/WNL.50.6.1678. [DOI] [PubMed] [Google Scholar]
- 27.Desroches J, Bouchard JF, Gendron L, Beaulieu P. Involvement of cannabinoid receptors in peripheral and spinal morphine analgesia. Neuroscience 261: 23–42, 2014. doi: 10.1016/j.neuroscience.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 28.Evans S, Seidman L, Sternlieb B, Casillas J, Zeltzer L, Tsao J. Clinical case report: yoga for fatigue in five young adult survivors of childhood cancer. J Adolesc Young Adult Oncol 6: 96–101, 2017. doi: 10.1089/jayao.2016.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fabry ME, Nagel RL, Pachnis A, Suzuka SM, Costantini F. High expression of human beta S- and alpha-globins in transgenic mice: hemoglobin composition and hematological consequences. Proc Natl Acad Sci USA 89: 12150–12154, 1992. doi: 10.1073/pnas.89.24.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fabry ME, Sengupta A, Suzuka SM, Costantini F, Rubin EM, Hofrichter J, Christoph G, Manci E, Culberson D, Factor SM, Nagel RL. A second generation transgenic mouse model expressing both hemoglobin S (HbS) and HbS-Antilles results in increased phenotypic severity. Blood 86: 2419–2428, 1995. [PubMed] [Google Scholar]
- 31.Finan PH, Carroll CP, Moscou-Jackson G, Martel MO, Campbell CM, Pressman A, Smyth JM, Tremblay JM, Lanzkron SM, Haythornthwaite JA. Daily opioid use fluctuates as a function of pain, catastrophizing, and affect in patients with sickle cell disease: an electronic daily diary analysis. J Pain 19: 46–56, 2018. doi: 10.1016/j.jpain.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaboury JP, Johnston B, Niu XF, Kubes P. Mechanisms underlying acute mast cell-induced leukocyte rolling and adhesion in vivo. J Immunol 154: 804–813, 1995. [PubMed] [Google Scholar]
- 33.Gaudre N, Cougoul P, Bartolucci P, Dörr G, Bura-Riviere A, Kamar N, Del Bello A. Improved fetal hemoglobin with mTOR inhibitor-based immunosuppression in a kidney transplant recipient with sickle cell disease. Am J Transplant 17: 2212–2214, 2017. doi: 10.1111/ajt.14263. [DOI] [PubMed] [Google Scholar]
- 34.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol 14: 217–231, 2014. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta K. Iatrogenic angiogenesis. In: Morphine and Metastasis, edited by Parat MO Dordrecht, The Netherlands: Springer, 2013, p. 63–78. doi: 10.1007/978-94-007-5678-6_5. [DOI] [Google Scholar]
- 36.Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev 282: 168–187, 2018. doi: 10.1111/imr.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta M, Msambichaka L, Ballas SK, Gupta K. Morphine for the treatment of pain in sickle cell disease. Sci World J 2015: 540154, 2015. doi: 10.1155/2015/540154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood 118: 3376–3383, 2011. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppe C, Jacob E, Styles L, Kuypers F, Larkin S, Vichinsky E. Simvastatin reduces vaso-occlusive pain in sickle cell anaemia: a pilot efficacy trial. Br J Haematol 177: 620–629, 2017. doi: 10.1111/bjh.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howard J, Anie KA, Holdcroft A, Korn S, Davies SC. Cannabis use in sickle cell disease: a questionnaire study. Br J Haematol 131: 123–128, 2005. doi: 10.1111/j.1365-2141.2005.05723.x. [DOI] [PubMed] [Google Scholar]
- 41.Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP Jr. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA 100: 10529–10533, 2003. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacob E, Chan VW, Hodge C, Zeltzer L, Zurakowski D, Sethna NF. Sensory and thermal quantitative testing in children with sickle cell disease. J Pediatr Hematol Oncol 37: 185–189, 2015. doi: 10.1097/MPH.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khaibullina A, Almeida LE, Wang L, Kamimura S, Wong EC, Nouraie M, Maric I, Albani S, Finkel J, Quezado ZM. Rapamycin increases fetal hemoglobin and ameliorates the nociception phenotype in sickle cell mice. Blood Cells Mol Dis 55: 363–372, 2015. doi: 10.1016/j.bcmd.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Klein LM, Lavker RM, Matis WL, Murphy GF. Degranulation of human mast cells induces an endothelial antigen central to leukocyte adhesion. Proc Natl Acad Sci USA 86: 8972–8976, 1989. doi: 10.1073/pnas.86.22.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood 116: 456–465, 2010. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kutlar A, Ataga KI, McMahon L, Howard J, Galacteros F, Hagar W, Vichinsky E, Cheung AT, Matsui N, Embury SH. A potent oral P-selectin blocking agent improves microcirculatory blood flow and a marker of endothelial cell injury in patients with sickle cell disease. Am J Hematol 87: 536–539, 2012. doi: 10.1002/ajh.23147. [DOI] [PubMed] [Google Scholar]
- 47.Lei J, Benson B, Tran H, Ofori-Acquah SF, Gupta K. Comparative analysis of pain behaviours in humanized mouse models of sickle cell anemia. PLoS One 11: e0160608, 2016. doi: 10.1371/journal.pone.0160608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu K, Cheng MC, Ge X, Berger A, Xu D, Kato GJ, Minniti CP. A retrospective review of acupuncture use for the treatment of pain in sickle cell disease patients: descriptive analysis from a single institution. Clin J Pain 30: 825–830, 2014. doi: 10.1097/AJP.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther 325: 267–275, 2008. doi: 10.1124/jpet.107.132167. [DOI] [PubMed] [Google Scholar]
- 50.Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood 122: 3892–3898, 2013. doi: 10.1182/blood-2013-05-498311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashon RS, Dash PM, Khalkho J, Dash L, Mohanty PK, Patel S, Mohanty RC, Das BS, Das UK, Das PK, Patel DK. Higher fetal hemoglobin concentration in patients with sickle cell disease in eastern India reduces frequency of painful crisis. Eur J Haematol 83: 383–384, 2009. doi: 10.1111/j.1600-0609.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 52.Matsui NM, Borsig L, Rosen SD, Yaghmai M, Varki A, Embury SH. P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood 98: 1955–1962, 2001. doi: 10.1182/blood.V98.6.1955. [DOI] [PubMed] [Google Scholar]
- 53.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 12: 592–605, 2015. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McClish DK, Smith WR, Levenson JL, Aisiku IP, Roberts JD, Roseff SD, Bovbjerg VE. Comorbidity, pain, utilization, and psychosocial outcomes in older versus younger sickle cell adults: the PiSCES project. BioMed Res Int 2017: 4070547, 2017. doi: 10.1155/2017/4070547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood 92: 3148–3151, 1998. [PubMed] [Google Scholar]
- 56.Mittal A, Gupta M, Lamarre Y, Jahagirdar B, Gupta K. Quantification of pain in sickle mice using facial expressions and body measurements. Blood Cells Mol Dis 57: 58–66, 2016. doi: 10.1016/j.bcmd.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molokie RE, Wilkie DJ, Wittert H, Suarez ML, Yao Y, Zhao Z, He Y, Wang ZJ. Mechanism-driven phase I translational study of trifluoperazine in adults with sickle cell disease. Eur J Pharmacol 723: 419–424, 2014. doi: 10.1016/j.ejphar.2013.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB2 cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J Mol Cell Cardiol 46: 612–620, 2009. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 59.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365: 61–65, 1993. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 60.Murikinati S, Jüttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A, Kalinke U, Schwaninger M. Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J 24: 788–798, 2010. doi: 10.1096/fj.09-141275. [DOI] [PubMed] [Google Scholar]
- 61.Murphy M, Close J, Lottenberg R, Rajasekhar A. Effectiveness of imatinib therapy for sickle cell anemia and chronic myeloid leukemia. Am J Med Sci 347: 254–255, 2014. doi: 10.1097/MAJ.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 62.Nwankwo JO, Lei J, Xu J, Rivera A, Gupta K, Chishti AH. Genetic inactivation of calpain-1 attenuates pain sensitivity in a humanized mouse model of sickle cell disease. Haematologica 101: e397–e400, 2016. doi: 10.3324/haematol.2016.148106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pacher P, Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol 153: 252–262, 2008. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pászty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science 278: 876–878, 1997. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 65.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med 376: 1561–1573, 2017. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- 66.Polanowska-Grabowska R, Wallace K, Field JJ, Chen L, Marshall MA, Figler R, Gear AR, Linden J. P-selectin-mediated platelet-neutrophil aggregate formation activates neutrophils in mouse and human sickle cell disease. Arterioscler Thromb Vasc Biol 30: 2392–2399, 2010. doi: 10.1161/ATVBAHA.110.211615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajesh M, Pan H, Mukhopadhyay P, Bátkai S, Osei-Hyiaman D, Haskó G, Liaudet L, Gao B, Pacher P. Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis. J Leukoc Biol 82: 1382–1389, 2007. doi: 10.1189/jlb.0307180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 376: 2018–2031, 2010. doi: 10.1016/S0140-6736(10)61029-X. [DOI] [PubMed] [Google Scholar]
- 69.Ryan TM, Ciavatta DJ, Townes TM. Knockout-transgenic mouse model of sickle cell disease. Science 278: 873–876, 1997. doi: 10.1126/science.278.5339.873. [DOI] [PubMed] [Google Scholar]
- 70.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 168: 960–976, 2017. [Erratum in Cell 169: 361–371, 2017. 10.1016/j.cell.2017.03.035.] doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schatz J, Buzan R. Decreased corpus callosum size in sickle cell disease: relationship with cerebral infarcts and cognitive functioning. J Int Neuropsychol Soc 12: 24–33, 2006. doi: 10.1017/S1355617706060085. [DOI] [PubMed] [Google Scholar]
- 72.Small-Howard AL, Shimoda LM, Adra CN, Turner H. Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells. Biochem J 388: 465–473, 2005. doi: 10.1042/BJ20041682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith WR, Bauserman RL, Ballas SK, McCarthy WF, Steinberg MH, Swerdlow PS, Waclawiw MA, Barton BA; Multicenter Study of Hydroxyurea in Sickle Cell Anemia . Climatic and geographic temporal patterns of pain in the Multicenter Study of Hydroxyurea. Pain 146: 91–98, 2009. doi: 10.1016/j.pain.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Stankovic Stojanovic K, Thiolière B, Garandeau E, Lecomte I, Bachmeyer C, Lionnet F. Chronic myeloid leukaemia and sickle cell disease: could imatinib prevent vaso-occlusive crisis? Br J Haematol 155: 271–272, 2011. doi: 10.1111/j.1365-2141.2011.08670.x. [DOI] [PubMed] [Google Scholar]
- 75.Thakur AS, Littaru GP, Moesgaard S, Dan Sindberg C, Khan Y, Singh CM. Hematological parameters and RBC TBARS level of Q 10 supplemented tribal sickle cell patients: a hospital based study. Indian J Clin Biochem 28: 185–188, 2013. doi: 10.1007/s12291-012-0277-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thorlacius H, Raud J, Rosengren-Beezley S, Forrest MJ, Hedqvist P, Lindbom L. Mast cell activation induces P-selectin-dependent leukocyte rolling and adhesion in postcapillary venules in vivo. Biochem Biophys Res Commun 203: 1043–1049, 1994. doi: 10.1006/bbrc.1994.2287. [DOI] [PubMed] [Google Scholar]
- 77.Tran H, Gupta M, Gupta K. Targeting novel mechanisms of pain in sickle cell disease. Blood 130: 2377–2385, 2017. doi: 10.1182/blood-2017-05-782003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA 99: 3047–3051, 2002. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uhelski ML, Gupta K, Simone DA. Sensitization of C-fiber nociceptors in mice with sickle cell disease is decreased by local inhibition of anandamide hydrolysis. Pain 158: 1711–1722, 2017. doi: 10.1097/j.pain.0000000000000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valverde Y, Benson B, Gupta M, Gupta K. Spinal glial activation and oxidative stress are alleviated by treatment with curcumin or coenzyme Q in sickle mice. Haematologica 101: e44–e47, 2016. doi: 10.3324/haematol.2015.137489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vang D, Paul JA, Nguyen J, Tran H, Vincent L, Yasuda D, Zaveri NT, Gupta K. Small-molecule nociceptin receptor agonist ameliorates mast cell activation and pain in sickle mice. Haematologica 100: 1517–1525, 2015. doi: 10.3324/haematol.2015.128736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310: 329–332, 2005. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 82a.Vicuña L, Strochlic DE, Latremoliere A, Bali KK, Simonetti M, Husainie D, Prokosch S, Riva P, Griffin RS, Njoo C, Gehrig S, Mall MA, Arnold B, Devor M, Woolf CJ, Liberles SD, Costigan M, Kuner R, The serine protease inhibitor SerpinA3N attenuates neuropathic pain by inhibiting T cell-derived leukocyte elastase. Nat Med 21: 518–523, 2015. doi: 10.1038/nm.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vincent L, Vang D, Nguyen J, Benson B, Lei J, Gupta K. Cannabinoid receptor-specific mechanisms to alleviate pain in sickle cell anemia via inhibition of mast cell activation and neurogenic inflammation. Haematologica 101: 566–577, 2016. doi: 10.3324/haematol.2015.136523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood 122: 1853–1862, 2013. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Lei J, Gupta M, Peng F, Lam S, Jha R, Raduenz E, Beitz AJ, Gupta K. Electroacupuncture in conscious free-moving mice reduces pain by ameliorating peripheral and central nociceptive mechanisms. Sci Rep 6: 34493, 2016. doi: 10.1038/srep34493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet 390: 311–323, 2017. doi: 10.1016/S0140-6736(17)30193-9. [DOI] [PubMed] [Google Scholar]
- 87.Watkins KE, Hewes DK, Connelly A, Kendall BE, Kingsley DP, Evans JE, Gadian DG, Vargha-Khadem F, Kirkham FJ. Cognitive deficits associated with frontal-lobe infarction in children with sickle cell disease. Dev Med Child Neurol 40: 536–543, 1998. doi: 10.1111/j.1469-8749.1998.tb15412.x. [DOI] [PubMed] [Google Scholar]
- 88.Weyer AD, Stucky CL. Repurposing a leukocyte elastase inhibitor for neuropathic pain. Nat Med 21: 429–430, 2015. doi: 10.1038/nm.3861. [DOI] [PubMed] [Google Scholar]
- 89.Wu Z, Chen X, Liu F, Chen W, Wu P, Wieschhaus AJ, Chishti AH, Roche PA, Chen WM, Lin TJ. Calpain-1 contributes to IgE-mediated mast cell activation. J Immunol 192: 5130–5139, 2014. doi: 10.4049/jimmunol.1301677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wun T, Paglieroni T, Tablin F, Welborn J, Nelson K, Cheung A. Platelet activation and platelet-erythrocyte aggregates in patients with sickle cell anemia. J Lab Clin Med 129: 507–516, 1997. doi: 10.1016/S0022-2143(97)90005-6. [DOI] [PubMed] [Google Scholar]
- 91.Yin M, Liu Q, Yu L, Yang Y, Lu M, Wang H, Luo D, Rong X, Tang F, Guo J. Downregulations of CD36 and calpain-1, inflammation, and atherosclerosis by simvastatin in apolipoprotein E knockout mice. J Vasc Res 54: 123–130, 2017. doi: 10.1159/000464288. [DOI] [PubMed] [Google Scholar]
- 92.Zarbock A, Polanowska-Grabowska RK, Ley K. Platelet-neutrophil-interactions: linking hemostasis and inflammation. Blood Rev 21: 99–111, 2007. doi: 10.1016/j.blre.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, Bernard G; STRIVE Study Group . Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med 32: 1695–1702, 2004. doi: 10.1097/01.CCM.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]


