Abstract
The hormone ghrelin promotes eating and is widely considered to be a hunger signal. Ghrelin receptors, growth hormone secretagogue receptors (GHSRs), are found in a number of specific regions throughout the brain, including the lateral septum (LS), an area not traditionally associated with the control of feeding. Here we investigated whether GHSRs in the LS play a role in the control of food intake. We examined the feeding effects of ghrelin and the GHSR antagonists ([d-Lys3]-growth hormone-releasing peptide-6 and JMV-2959) at doses subthreshold for effect when delivered to the lateral ventricle. Intra-LS ghrelin significantly increased chow intake during the midlight phase, suggesting that pharmacological activation of LS GHSRs promotes feeding. Conversely, GHSR antagonist delivered to the LS shortly before dark onset significantly reduced chow intake. These data support the hypothesis that exogenous and endogenous stimulation of GHSRs in the LS influence feeding. Ghrelin is known to affect motivation for food, and the dorsal subdivision of LS (dLS) has been shown to play a role in motivation. Thus, we investigated the role of dLS GHSRs in motivation for food reward by examining operant responding for sucrose on a progressive ratio (PR) schedule. Intra-dLS ghrelin increased PR responding for sucrose, whereas blockade of LS GHSRs did not affect responding in either a fed or fasted state. Together these findings for the first time substantiate the LS as a site of action for ghrelin signaling in the control of food intake.
Keywords: ghrelin, food intake, food reward, lateral septum
INTRODUCTION
Ghrelin, the only known circulating hormone that increases feeding, is a gastrointestinal peptide produced primarily in the stomach (3, 7, 16). Levels of circulating ghrelin increase during fasting and peak just before meal initiation (4, 8, 35). Ghrelin is an endogenous ligand for the growth hormone secretagogue receptor (GHSR), and GHSR stimulation increases food intake and gastric motility, whereas central blockade of GHSRs has been demonstrated to decrease food intake (4). GHSRs are expressed throughout the brain, and specific nuclei mediating the food intake effects of ghrelin initially focused on hypothalamic and caudal brain stem nuclei (9–11). More recent studies have demonstrated that, in the nucleus accumbens, ventral tegmental area, and hippocampus, ghrelin promotes food intake, and this effect appears to be driven by an increase in motivation (1, 14, 15, 19, 21). Furthermore, blockade of GHSR signaling has been shown to decrease motivation for food, suggesting a role for endogenous ghrelin in the control of reward (22, 30, 31, 37).
GHSRs are expressed in multiple regions throughout the central nervous system, including the lateral septum (LS) (12). Until recently, the LS has not been considered a major player in central control of food intake, but several recent studies have now identified this brain region as a critical contributor to the physiological control of feeding in rats and mice (32–34). For example, Sweeney and colleagues have shown that an excitatory pathway from the ventral hippocampus to the LS suppresses feeding, as does an inhibitory pathway from the LS to the lateral hypothalamus (LH) (32, 33). Our laboratory demonstrated that endogenous glucagon-like peptide-1 (GLP-1) in the LS plays a physiological role in limiting intake of chow and several highly palatable foods. We also showed that blockade of dorsal LS (dLS) GLP-1 receptors (GLP-1Rs) in particular increases operant responding for sucrose pellets on a progressive ratio (PR) schedule.
The LS contains other receptors for peptides known to influence feeding, including GHSRs, which have been detected by PCR, Western blot, and immunohistochemistry throughout the LS (12). Electrophysiological evidence presented in that study also supports the presence of GHSRs in LS neurons. Previous studies have demonstrated that LS GHSR stimulation promotes gastric motility (12, 13), and we hypothesized that these receptors may have a role in food intake and motivation for food, as well. To investigate the role of LS GHSRs in the control of feeding, we examined the effect of intra-LS ghrelin injection on chow intake. We hypothesized that pharmacological activation of LS GHSRs, at doses that were ineffective when delivered to the lateral ventricle (LV), would increase chow intake during the midlight phase. To address the question of whether endogenous GHSR stimulation in the LS affects feeding, we asked if blockade of LS GHSRs with a selective antagonist decreases dark-onset chow intake.
Because ghrelin is known to affect motivation for food, we examined whether LS GHSR activation or blockade impacts operant responding for sucrose reinforcement on a PR schedule. For these studies, we targeted the dLS more selectively than in the other experiments. This choice was made because of our previous investigation of LS GLP-1R effects on feeding and motivation. In that series of studies, we determined that, although effects on food intake could be obtained with injection of GLP-1R antagonist in the intermediate LS (iLS) or dLS, only dLS injections elicited an increase in motivation for sucrose reinforcement (34). Previous studies in the literature support the idea that dLS may be more involved in operant responding than other subregions because of its connectivity with other brain areas of relevance to learning and motivation (17, 34). Therefore, when we began the present experiments investigating GHSR effects on motivation, rather than include a wider range of LS injection sites, we focused on the subregion we hypothesized to be most likely to play this role, the dLS.
METHODS
Subjects.
Naïve male Wistar rats (Harlan, Indianapolis, IN) were maintained individually in temperature-controlled vivariums on a 12:12-h light-dark cycle in plastic cages. Rats had ad libitum access to distilled water and rat chow (Purina 5001), except where otherwise noted. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee and conformed to the standard of the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Surgery.
Rats were implanted with unilateral 26-gauge guide cannulas under 2–4% isoflurane delivered at a rate of 1 l/min. Cannulas targeted the LV (1.5 mm lateral to midline, 0.9 mm posterior to bregma, and 2.7 mm ventral to skull surface), LS (0.6 mm lateral to midline, 1.0 mm anterior to bregma, and 4.0 mm ventral to skull surface), or the dLS (0.6 mm lateral to midline, 1.0 mm anterior to bregma, and 3.0 mm ventral to skull surface). Injectors (33 gauge) extending 2.0 mm below the end of the guide cannulas were used. Rats were given 1 wk to recover from surgery before the start of experimentation. LV cannula placements were verified before the start of experiments through observation of water intake (>5 ml ingested during a 45-min test) induced by 40 μg angiotensin II (Sigma-Aldrich, St. Louis, MO). LS and dLS placements were verified histologically after behavioral testing. Injection sites within the boundaries of the LS, as drawn in the atlas of Paxinos and Watson (20), were considered correct, and data from rats with accurate placements were included in analyses (86% hit rate) (Fig. 1).
Fig. 1.

Representative diagram of lateral septum (LS) injection placements; additional subjects’ injection sites were identified in similar locations at points between the anterior-posterior levels (mm relative to bregma) displayed here. Solid black circles, LS placements from studies 1 and 2. *Placements in the dorsal subdivision of LS (dLS) from studies 2, 3, and 4.
General methods for behavioral experiments.
Before the start of testing, rats were habituated to all experimental procedures. For habituation to intra-LV and intra-LS injection procedures, rats received a 2- or 0.5-μl injection of sterile 0.9% saline at a rate of 1.0 or 0.5 μl/min, respectively. For bilateral intra-dLS injection procedures, rats received a 0.25-μl injection of sterile 0.9% saline at a rate of 0.25 μl/min, delivered to each hemisphere, for a total volume of 0.5 μl distributed across the two dLS sites; injections in each hemisphere were given simultaneously. Injectors were left in place for an additional minute before removal. Body weights were recorded daily, and all drug treatments were separated by a minimum of 48 h.
Study 1: effects of LS GHSR stimulation on chow intake.
Initially, we conducted a within-subjects dose response for LV delivery of ghrelin to establish a subthreshold dose for use in studies targeting the LS directly. Food was removed 2 h before the start of the feeding test to ensure no subject consumed a significant meal before the onset of the experimental measurement period. In the midlight phase, rats (n = 8) received intra- LV injection of vehicle or 0.032, 0.1, 0.15, 0.32, or 1 nmol of ghrelin in 2 μl of saline. After intra-LV injections (45 min), chow was returned, and intake was measured 1, 2, 4, and 22 h later.
Using a within-subjects counterbalanced design, we determined the effect of LS GHSR stimulation on chow intake. Food was removed 4 h before the start of the feeding test. Rats (n = 15) implanted with unilateral cannulas targeting the LS were injected with vehicle or ghrelin (0.032 and 0.1 nmol) in 0.5 μl of saline during the midlight phase. Following intra-LS injections (45 min), chow was returned, and intake was measured 1, 2, 4, and 20 h later.
Study 2: effects of LS GHSR blockade on chow intake.
We determined doses of GHSR antagonist [d-Lys3]-growth hormone-releasing peptide (GHRP-6), which were subthreshold for an effect on chow intake when administered in the LV. Chow was removed for 2 h before the dark cycle. Before dark onset (45 min), rats (n = 7) received intra-LV vehicle or 2, 6, or 20 μg of [d-Lys3]-GHRP-6 in 2 μl of saline. Access to chow was returned immediately before the dark phase, and intake was measured at 30 min and 1, 2, 4, and 22 h following dark onset.
To examine the effect of LS GHSR blockade on chow intake, food was removed 4 h before the onset of the dark cycle, and, 45 min before dark onset, rats (n = 11) received unilateral intra-LS injection of saline vehicle or GHSR antagonist [d-Lys3]-GHRP-6 (1, 2, or 5 μg) in 0.5 μl of saline. Chow was returned immediately before dark onset, and intake was measured 1, 2, 4, and 20 h later.
With the use of the same design as the experiment described above, a second cohort of rats (n = 8) with bilateral cannulas targeting the dLS received intra-dLS injection of saline vehicle or GHSR antagonist [d-Lys3]-GHRP-6 (5 μg) or JMV-2959 (2 or 10 μg) 45 min before dark onset. Each dose was evenly divided between the two hemispheres (i.e., 2.5 μg on each side for [d-Lys3]-GHRP-6). Dose selection for JMV-2959 was based on previous reports (2, 14, 30). Chow was returned immediately before dark onset, and intake was measured 1, 2, 4, and 20 h later.
Study 3: effects of dLS GHSR stimulation or blockade on operant responding in fed rats.
To evaluate the role of dLS GHSRs in motivation, we examined operant responding for sucrose using a PR schedule during the midlight phase. Initially, unilateral dLS cannulated rats (n = 12) were trained to lever press for 45-mg sucrose pellets (TestDiet, Richmond, IN) in operant chambers (Coulbourn Instruments, Allentown, PA) on fixed-ratio (FR1 and then FR3) schedules with a 5-s timeout after each reinforcement. Two levers were presented in each chamber, and only responses on the active lever were reinforced. Reponses on the inactive lever were not reinforced (rats seldom pressed the inactive lever). Food was removed 45 min before all operant training and testing sessions to ensure that no subject may have consumed a large meal right before entering the test chamber.
After 14 days of FR training, variation between training sessions was low (<10% day-to-day variation in responding), and rats moved to a PR schedule of reinforcement. This schedule followed the algorithm of Richardson and Roberts (23): 1, 2, 4, 6, 9, 12, 16, 20, 28, 36, 48, etc., lever presses for each successive reinforcement. PR sessions ended when rats failed to earn the next reinforcer within 10 min of earning the previous reinforcer with a maximum possible session duration of 1 h. After the operant session, rats were returned to their home cages with ad libitum access to chow and water. Experimentation began after 13 days of PR training when PR schedule responding was stable and consistent. On experiment days, rats received a unilateral intra-dLS injection of vehicle, 0.1 nmol ghrelin, or 1 μg [d-Lys3]-GHRP-6 in 0.5 μl of saline 30 min before the start of the PR session. Overnight chow intake was measured 20 h after the PR sessions. Rats received all treatment conditions in counterbalanced order, and rats were given 48 h between experiment days, during which PR training sessions continued.
We used the same basic design as described above in another group of rats (n = 8) with bilateral dLS cannulas. Rats were trained to lever press for sucrose pellets over 7 days on a FR1 schedule of reinforcement, followed by 8 days on a FR3 schedule of reinforcement. Rats were then placed on a PR schedule of reinforcement and trained for 14 days before the start of the experiment. On experimental days, rats received an intra-dLS injection of saline vehicle or GHSR antagonist [d-Lys3]-GHRP-6 (5 μg) or JMV-2959 (2 or 10 μg) 45 min before the start of the session. Each dose of the antagonists was evenly divided between the two hemispheres. Chow intake was measured 20 h after injections. Rats received both treatment conditions in counterbalanced order. Between treatment days, rats continued to receive PR training sessions.
Study 4: effects of dLS GHSR blockade on operant responding in overnight-fasted rats.
In the same cohorts of rats from study 3, we tested PR responding following an overnight food deprivation intended to increase endogenous ghrelin levels. For the unilateral dLS-cannulated group (n = 12), the experiment began 3 days after completion of study 3. Experimental days were as described above, with the addition of overnight food deprivation before the PR session. After an overnight food deprivation, rats received intra-dLS injections of either vehicle or 1 μg [d-Lys3]-GHRP-6 30 min before the start of the PR session. In the bilaterally dLS-cannulated cohort of rats (n = 8), this experiment began 3 wk after the completion of study 3. After overnight food deprivation, rats received intra-dLS injections of either saline vehicle or GHSR antagonist [d-Lys3]-GHRP-6 (5 μg) or JMV-2959 (2 or 10 μg), with each dose evenly divided between the two hemispheres. Again, chow intake was measured 20 h after the PR sessions, and rats were given 48 h between experiment days, during which daily PR training sessions continued.
Statistical analysis.
Data are reported as means ± SE. Effects were evaluated by paired Student’s t-test or repeated-measures one-way ANOVA, as appropriate. Post hoc comparisons were made with Holm-Bonferroni tests. P values of <0.05 were taken as significant.
RESULTS
Study 1: effects of LS GHSR stimulation on chow intake.
Significant main effects of intra-LV ghrelin injection were seen at 2 [F(5,35) = 2.863, P < 0.05], 4 [F(5,35) = 4.535, P < 0.01], and 22 [F(5,35) = 2.835, P < 0.05] h postinjection. At 2 and 4 h, 1 nmol ghrelin produced significant increase in feeding (P < 0.05), and, at 22 h, 0.15, 0.32, and 1 nmol ghrelin significantly increased chow intake (P < 0.01) (Fig. 2A). Doses of 0.032 and 0.1 nmol ghrelin were found to be subthreshold for an effect on chow intake when administered in the LV and were therefore selected for subsequent experiments.
Fig. 2.

Dose response for the chow intake effects of lateral ventricle (LV)-injected ghrelin (n = 8) (*P < 0.05 relative to saline vehicle) (A) and LV-injected growth hormone secretagogue receptor (GHSR) antagonist (GA) [d-Lys3]-growth hormone-releasing peptide (GHRP)-6 (B) (n = 7). Data are means ± SE. Data were analyzed using repeated-measures 1-way ANOVA (*P < 0.05 relative to saline vehicle).
Intra-LS injections of ghrelin, at doses subthreshold for effect when delivered to the ventricle, significantly increased chow intake at 1 [F(2,28) = 6.815, P < 0.01], 2 [F(2,28) = 9.695, P < 0.01], 4 [F(2,28) = 9.889, P < 0.01], and 20 [F(2,28) = 9.218, P < 0.01] h postinjection relative to saline. Post hoc analysis revealed that the lower dose of ghrelin, 0.032 nmol, did not significantly increase chow intake at any time points, whereas the higher dose, 0.1 nmol, significantly increased intake at 1, 2, 4, and 20 h postinjection (P < 0.01 for each time point) (Fig. 3A).
Fig. 3.

A: unilateral intra-lateral septum (LS) injection of 0.1 nmol ghrelin significantly increased chow intake at 1, 2, 4, and 20 h postinjection (n = 15) (*P < 0.01 relative to vehicle). B: unilateral LS growth hormone secretagogue receptor (GHSR) antagonist [d-Lys3]-growth hormone-releasing peptide (GHRP)-6 injection decreased chow intake at 1, 2, and 4 h postdark onset (n = 11). Data are means ± SE. Data were analyzed using repeated-measures 1-way ANOVA (*P < 0.05 relative to vehicle).
Study 2: effects of LS GHSR blockade on chow intake.
Significant effects of intra-LV [d-Lys3]-GHRP-6 ghrelin receptor antagonist were seen only at the 4-h time point [F(3,18) = 3.553, P < 0.05]. Post hoc analysis revealed that the 20-μg dose of antagonist significantly suppressed intake when administered to the LV (P < 0.05) (Fig. 2B). There was a trend for the 6-μg dose to suppress chow intake at 4 h; however, this failed to reach significance. Thus, experimental doses of 1, 2, and 5 μg [d-Lys3]-GHRP-6 were selected for subsequent testing targeted to the LS.
Unilateral intra-LS injection of [d-Lys3]-GHRP-6 significantly suppressed chow intake at 1 [F(3,30) = 6.206, P < 0.01], 2 [F(3,30) = 5.592, P < 0.01], and 4 [F(3,30) = 6.251, P < 0.01] h postdark onset. There were no effects on overnight chow intake. Post hoc analysis revealed that all doses (1, 2, or 5 μg) of [d-Lys3]-GHRP-6 significantly suppressed intake at 1, 2, and 4 h postdark onset (P < 0.05) (Fig. 3B).
Bilateral intra-dLS injection of ghrelin antagonists JMV-2959 and [d-Lys3]-GHRP-6 significantly suppressed chow intake at each time point measured: 1 [F(3,21) = 3.700, P < 0.05], 2 [F(3,21) = 3.675, P < 0.05], 4 [F(3,21) = 4.604, P < 0.05], and 20 [F(3,21) = 7.154, P < 0.01] h postdark onset. Post hoc analysis revealed that, at 1 and 20 h, 2 μg of JMV-2959 produced a significant decrease in chow intake (P < 0.05); at 20 h, 10 μg of JMV-2959 produced a significant decrease in feeding (P < 0.05); and at 1, 4, and 20 h, 5 μg [d-Lys3]-GHRP-6 suppressed chow intake (P < 0.01) (Fig. 4).
Fig. 4.
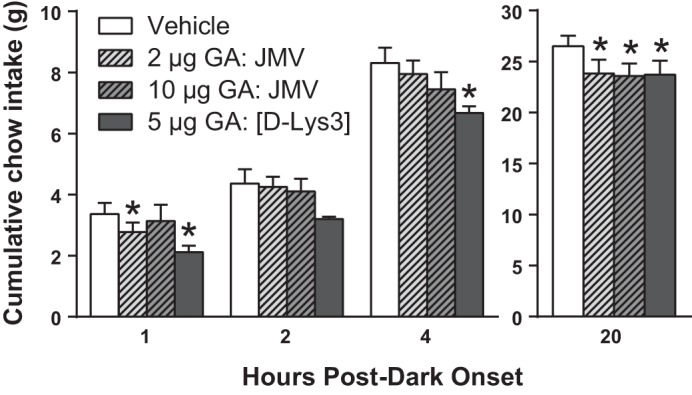
Bilateral dorsal subdivision of LS (dLS) growth hormone secretagogue receptor (GHSR) blockade with antagonists [d-Lys3]-growth hormone-releasing peptide (GHRP)-6 or JMV-2959 decreased chow intake at 1, 4, and 20 h postdark onset (n = 8). Data are means ± SE. Data were analyzed using repeated-measures 1-way ANOVA (*P < 0.05 relative to vehicle).
Study 3:effects of dLS GHSR stimulation or blockade on operant responding in fed rats.
In unilateral dLS-cannulated rats, there was a significant main effect of drug treatment on active lever presses [F(2,22) = 25.429, P < 0.01], reinforcers earned [F(2,22) = 20.664, P < 0.01], breakpoint (the last completed ratio of the session) [F(2,22) = 20.140, P < 0.01], and overnight food intake (20 h) following the PR session [F(2,22) = 6.421, P < 0.01] (Fig. 4A). Post hoc analysis revealed that only ghrelin administered in the dLS significantly increased all measures (P values <0.01) (Fig. 5). Unilateral intra-dLS injection of 1 µg of [d-Lys3]-GHRP-6 had no effect on any PR parameters measured here (Fig. 5). There was no effect on session duration after dLS stimulation or blockade (data not shown).
Fig. 5.
In ad libitum-fed unilaterally cannulated rats (n = 12) responding on a progressive ratio (PR) schedule of reinforcement, dorsal lateral septum (dLS) ghrelin (0.1 nmol) significantly increased active lever presses (A), number of reinforcers earned (B), breakpoint (C), and overnight chow intake (D). Growth hormone secretagogue receptor (GHSR) antagonist [d-Lys3]-growth hormone-releasing peptide (GHRP)-6 (1 μg) had no effect on PR parameters or overnight chow intake. Data are means ± SE. Data were analyzed using repeated-measures 1-way ANOVA (*P < 0.01 relative to saline vehicle).
In bilateral dLS-cannulated rats, there was no effect of dLS GHSR blockade on active lever presses, reinforcers earned, breakpoint, or overnight chow intake (Fig. 6).
Fig. 6.
In ad libitum-fed bilaterally cannulated rats (n = 8) responding on a progressive ratio (PR) schedule of reinforcement, dorsal lateral septum (dLS) growth hormone secretagogue receptor (GHSR) antagonists JMV-2959 (2 and 10 µg) and [d-Lys3]-growth hormone-releasing peptide (GHRP)-6 (5 μg) had no effect on active lever presses (A), number of reinforcers earned (B), breakpoint (C), or overnight chow intake (D). Data are means ± SE. Data were analyzed using repeated-measures 1-way ANOVA.
Study 4: effects of dLS GHSR blockade on operant responding in overnight-fasted rats.
After overnight food deprivation, neither unilateral (Fig. 7) nor bilateral (Fig. 8) blockade of dLS GHSRs had an effect on active lever presses, reinforcers earned, breakpoint, session duration, or overnight chow intake following the PR session.
Fig. 7.
In overnight-fasted rats (n = 12), unilateral blockade of the dorsal subdivision of LS (dLS) growth hormone secretagogue receptors (GHSRs) had no effect on active lever presses (A), reinforcers earned (B), breakpoint (C), or overnight chow intake (D) following the progressive ratio (PR) session. Data are means ± SE. Data were analyzed using paired t-test.
Fig. 8.
In overnight-fasted rats (n = 8) with bilateral dorsal subdivision of LS (dLS) cannulas, blockade of growth hormone secretagogue receptors (GHSRs) had no effect on active lever presses (A), reinforcers earned (B), breakpoint (C), or overnight chow intake (D) following the progressive ratio (PR) session. Data are means ± SE. Data were analyzed using repeated-measures 1-way ANOVA.
DISCUSSION
Our data show that ghrelin and GHSRs in the LS play a role in the control of food intake. Exogenous application of ghrelin to the LS, at doses that were ineffective when delivered to the LV, potently stimulated feeding during the light phase, when baseline food intake is typically low. Stimulation of LS GHSRs significantly increased intake up to 20 h after intra-LS administration. The effect was greatest in magnitude during the first few hours after treatment. Conversely, blockade of LS GHSRs with ventricle-subthreshold doses of the GHSR antagonist significantly suppressed chow intake for up to 20 h into the dark phase. These data suggest that endogenous ghrelin activity at these receptors contributes to ad libitum feeding throughout the dark cycle. Previous studies had demonstrated the presence of GHSRs in the LS and showed that LS GHSR stimulation promotes gastric motility (12), but this is the first demonstration of a physiological role for LS GHSRs in the central control of food intake.
Ghrelin is known to affect motivation for food (21, 29, 30), so we hypothesized that the ability of LS GHSR activation or blockade to affect chow intake is mediated through an effect on motivation. As discussed in the introduction, we narrowed our LS target to the dorsal subregion based in part on our recent work examining effects of GLP-1 receptors in the LS, in which we found that the dLS was the location from which effects on motivation could be elicited (34). We assessed motivation for food using a PR operant responding task, and, importantly, we used doses of ghrelin or one of two different GHSR antagonists that had significant effects on chow intake. We found that intra-dLS ghrelin significantly increased active lever presses for sucrose pellets, total reinforcers earned, and breakpoint, confirming that pharmacological dLS GHSR stimulation can promote motivation for food. In this experiment, we also saw a significant increase in overnight chow intake after intra-dLS ghrelin, consistent with our hypothesis that the intake-promoting effects of LS ghrelin are mediated through increased motivation for food. However, these data do not speak to a role of endogenous GHSR stimulation within the LS, so we applied an antagonist strategy to address this. We blocked dLS GHSRs using doses of [d-Lys3]-GHRP-6 that had reliably suppressed dark-onset chow intake, as well as 2 and 10 µg JMV-2959, another specific GHSR antagonist. Bilateral dLS GHSR antagonist treatment also had no effect on any PR performance variable. These PR test sessions occurred during the midlight phase when endogenous ghrelin levels were likely not very high, so we hypothesized that this could be the reason the antagonist failed to impact motivation in this study. To increase endogenous ghrelin and thus perhaps increase the likelihood of finding an effect of GHSR blockade, we examined the effect of dLS antagonist administration after an overnight fast, a manipulation well known to elevate plasma ghrelin levels. Overnight food deprivation before the PR test session elevated PR operant responding compared with the nonfasted condition, as expected. However, unilateral and bilateral blockade of dLS GHSRs still had no effect on operant performance, despite the fact that these doses of each antagonist suppressed ad libitum chow intake. These data suggest that, while pharmacological stimulation of dLS GHSRs can drive motivation for sucrose rewards, under our testing conditions, endogenous GHSR activation in the dLS is not necessary for the maintenance of motivation for food in ad libitum-fed rats, nor is it necessary for fasting to increase motivation for food. The lack of antagonist effect on PR performance also suggests that the ability of these antagonists to suppress chow intake does not rely on a change in motivation for food. This result is surprising in light of the well-established ability of brain GHSRs to increase motivation for food reinforcers, but it may be that ghrelin action in other locations is sufficient for this effect without a contribution from dLS GHSRs (22, 30, 31, 37).
Our studies suggest that endogenous GHSR stimulation in the LS promotes food intake but does not identify the source of the endogenous ligand for these receptors. There have been a number of reports suggesting that ghrelin is synthesized in neurons in the brain (12, 16), including one study hypothesizing that ghrelin-producing neurons in the arcuate nucleus of the hypothalamus project to the LS and provide the ghrelin stimulation of LS GHSRs (12). However, there are a variety of methodological concerns about all of the evidence for neuronal ghrelin production to date, reviewed in Cabral et al. (6), and so this remains controversial. An alternative possible source of ligand for these receptors is circulating ghrelin released by the gastrointestinal tract. Transport of active acylated ghrelin across the blood-brain barrier is limited; however, des-acyl ghrelin readily enters the brain by a nonsaturable mechanism (5). This would seem to suggest that acyl-ghrelin would not access brain GHSRs outside of circumventricular organs. However, the enzyme ghrelin-O-acyltransferase, which acylates ghrelin, is found in the brain (18, 38), supporting the idea that inactive ghrelin from the periphery can be converted within the brain to acyl-ghrelin. Additional studies will be required to determine whether ghrelin-O-acyltransferase is present in the LS, making this a potential mechanism for peripherally derived ghrelin to activate LS GHSRs.
The current data along with findings in the literature show that the LS plays a physiological role in the control of food intake, with involvement of both LS GHSR and GLP-1R (28, 32–34). Previous data suggested that LS ghrelin-sensitive neurons receive sensory information about gastric distention, and the activity of these cells can affect gastric motility (12, 13). Distention and other gastrointestinal sensory information also potently influence food intake, so it is likely that LS GHSR neurons are integrating multiple types of gastrointestinal feedback to affect feeding. Because we know that GLP-1-producing neurons in the hindbrain are activated by gastric distention (36), and that they project to the LS (24), these neurons may be the source for that gut sensory information. It is not known whether GHSRs and GLP-1Rs are coexpressed by the same LS neurons, but, even if not, a GLP-1 signal to LS GHSR-bearing neurons could be indirect, transmitted via LS interneurons, for example. Further research on how the LS receives viscerosensory information will be informative. The majority of LS cells are GABAergic medium spiny neurons (25, 39). These LS neurons have a number of reciprocal connections with other brain regions that are also known to play an important role in the control of feeding, including the LH, hippocampus, and ventral tegmental area (17, 26, 27). Therefore, LS GHSR- and GLP-1R-bearing neurons may be integrating afferent information from a number of different locations, and there are several potential efferent pathways from the LS that could mediate the effect of LS GHSR and GLP-1R stimulation on feeding. Thus, future studies investigating these pathways are warranted.
Perspectives and Significance
Previous work has already established that ghrelin plays an important role in the control of feeding behavior, and several CNS GHSR populations have been identified as contributors. The data reported here add to our understanding of how ghrelin influences food intake and motivation by demonstrating a role for the LS GHSR population. It is notable that many of the other brain nuclei in which ghrelin can act to influence behavior, such as the LH, hippocampus, ventral tegmental area, and nucleus of the solitary tract, connect directly with the LS. This raises the possibility of coordinated ghrelin action across a network of feeding and reward-relevant brain sites. Further research will be necessary to unravel the anatomical organization and functional connections among these pathways.
GRANTS
This work was funded by grants from the National Institute of Diabetes and Digestive and Kidney Diseases to D. L. Williams (R01-DK-095757) and to S. J. Terrill (F31-DK-115102).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.J.T. and D.L.W. conceived and designed research; S.J.T., K.D.W., N.D.M., and C.B.M. performed experiments; S.J.T., K.D.W., and D.L.W. analyzed data; S.J.T., K.D.W., and D.L.W. interpreted results of experiments; S.J.T. and K.D.W. prepared figures; S.J.T., K.D.W., and D.L.W. drafted manuscript; S.J.T., K.D.W., C.B.M., and D.L.W. edited and revised manuscript; S.J.T., K.D.W., N.D.M., C.B.M., and D.L.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Charles Badland for assistance with figure production.
Present address of N. D. Medina: Department of Psychology, University of Chicago, Chicago, IL 60637.
REFERENCES
- 1.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest 116: 3229–3239, 2006. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez-Crespo M, Skibicka KP, Farkas I, Molnár CS, Egecioglu E, Hrabovszky E, Liposits Z, Dickson SL. The amygdala as a neurobiological target for ghrelin in rats: neuroanatomical, electrophysiological and behavioral evidence (Abstract). PLoS One 7: e46321, 2012. doi: 10.1371/journal.pone.0046321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 52: 947–952, 2003. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther 302: 822–827, 2002. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 6.Cabral A, López Soto EJ, Epelbaum J, Perelló M. Is ghrelin synthesized in the central nervous system? (Abstract). Int J Mol Sci 18: 638, 2017. doi: 10.3390/ijms18030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37: 649–661, 2003. doi: 10.1016/S0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 8.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 50: 1714–1719, 2001. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 9.Currie PJ, Mirza A, Fuld R, Park D, Vasselli JR. Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. Am J Physiol Regul Integr Comp Physiol 289: R353–R358, 2005. doi: 10.1152/ajpregu.00756.2004. [DOI] [PubMed] [Google Scholar]
- 10.Dickson SL, Leng G, Robinson ICAF. Systemic administration of growth hormone-releasing peptide activates hypothalamic arcuate neurons. Neuroscience 53: 303–306, 1993. doi: 10.1016/0306-4522(93)90197-N. [DOI] [PubMed] [Google Scholar]
- 11.Faulconbridge LF, Cummings DE, Kaplan JM, Grill HJ. Hyperphagic effects of brainstem ghrelin administration. Diabetes 52: 2260–2265, 2003. doi: 10.2337/diabetes.52.9.2260. [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Xu L, Guo F, Pang M, Shi Z, Gao S, Sun X. Effects of ghrelin on gastric distension sensitive neurons and gastric motility in the lateral septum and arcuate nucleus regulation. J Gastroenterol 49: 219–230, 2014. doi: 10.1007/s00535-013-0789-y. [DOI] [PubMed] [Google Scholar]
- 13.Gong Y, Xu L, Wang H, Guo F, Sun X, Gao S. Involvements of the lateral hypothalamic area in gastric motility and its regulation by the lateral septum. Gen Comp Endocrinol 194: 275–285, 2013. doi: 10.1016/j.ygcen.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, Nakamoto EM, Kanoski SE. Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife 4: 1–20, 2015. doi: 10.7554/eLife.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanoski SE, Fortin SM, Ricks KM, Grill HJ. Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol Psychiatry 73: 915–923, 2013. doi: 10.1016/j.biopsych.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656–660, 1999. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 17.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science 333: 353–357, 2011. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murtuza MI, Isokawa M. Endogenous ghrelin-O-acyltransferase (GOAT) acylates local ghrelin in the hippocampus. J Neurochem 144: 58–67, 2018. doi: 10.1111/jnc.14244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naleid AM, Grace MK, Cummings DE, Levine AS. Ghrelin induces feeding in the mesolimbic reward pathway between the ventral tegmental area and the nucleus accumbens. Peptides 26: 2274–2279, 2005. doi: 10.1016/j.peptides.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Perello M, Dickson SL. Ghrelin signalling on food reward: a salient link between the gut and the mesolimbic system. J Neuroendocrinol 27: 424–434, 2015. doi: 10.1111/jne.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perello M, Sakata I, Birnbaum S, Chuang JC, Osborne-Lawrence S, Rovinsky SA, Woloszyn J, Yanagisawa M, Lutter M, Zigman JM. Ghrelin increases the rewarding value of high-fat diet in an orexin-dependent manner. Biol Psychiatry 67: 880–886, 2010. doi: 10.1016/j.biopsych.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11, 1996. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 24.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34, 2010. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Risold PY, Swanson LW. Chemoarchitecture of the rat lateral septal nucleus. Brain Res Brain Res Rev 24: 91–113, 1997. doi: 10.1016/S0165-0173(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 26.Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev 24: 115–195, 1997. doi: 10.1016/S0165-0173(97)00009-X. [DOI] [PubMed] [Google Scholar]
- 27.Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J Neurosci 32: 4623–4631, 2012. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scopinho AA, Resstel LB, Corrêa FM. alpha(1)-Adrenoceptors in the lateral septal area modulate food intake behaviour in rats. Br J Pharmacol 155: 752–756, 2008. doi: 10.1038/bjp.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skibicka KP, Dickson SL. Ghrelin and food reward: the story of potential underlying substrates. Peptides 32: 2265–2273, 2011. doi: 10.1016/j.peptides.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience 180: 129–137, 2011. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Skibicka KP, Hansson C, Egecioglu E, Dickson SL. Role of ghrelin in food reward: impact of ghrelin on sucrose self-administration and mesolimbic dopamine and acetylcholine receptor gene expression. Addict Biol 17: 95–107, 2012. doi: 10.1111/j.1369-1600.2010.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweeney P, Yang Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat Commun 6: 10188, 2015. doi: 10.1038/ncomms10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sweeney P, Yang Y. An inhibitory septum to lateral hypothalamus circuit that suppresses feeding. J Neurosci 36: 11185–11195, 2016. doi: 10.1523/JNEUROSCI.2042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terrill SJ, Jackson CM, Greene HE, Lilly N, Maske CB, Vallejo S, Williams DL. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am J Physiol Regul Integr Comp Physiol 311: R124–R132, 2016. doi: 10.1152/ajpregu.00460.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 36.Vrang N, Phifer CB, Corkern MM, Berthoud H-R. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 285: R470–R478, 2003. doi: 10.1152/ajpregu.00732.2002. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg ZY, Nicholson ML, Currie PJ. 6-Hydroxydopamine lesions of the ventral tegmental area suppress ghrelin’s ability to elicit food-reinforced behavior. Neurosci Lett 499: 70–73, 2011. doi: 10.1016/j.neulet.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Wellman M, Abizaid A. Knockdown of central ghrelin O-acyltransferase by vivo-morpholino reduces body mass of rats fed a high-fat diet. Peptides 70: 17–22, 2015. doi: 10.1016/j.peptides.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Eisinger B, Gammie SC. Characterization of GABAergic neurons in the mouse lateral septum: a double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS One 8: e73750, 2013. doi: 10.1371/journal.pone.0073750. [DOI] [PMC free article] [PubMed] [Google Scholar]






