Abstract
The present study, performed in Dahl salt-sensitive (SS) and SS-Rag1−/− rats lacking T and B lymphocytes, tested the hypothesis that immune cells amplify salt-sensitive hypertension and kidney damage in response to a high-protein diet. After being weaned, SS and SS-Rag1−/− rats were placed on an isocaloric, 0.4% NaCl diet containing normal (18%) or high (30%) protein. At 9 wk of age, rats were switched to a 4.0% NaCl diet containing the same amount of dietary protein and maintained on the high-salt diet for 3 wk. After being fed the high-salt diet, SS rats fed high protein had amplified hypertension and albumin excretion (158.7 ± 2.6 mmHg and 140.8 ± 16.0 mg/day, respectively, means ± SE) compared with SS rats fed normal protein (139.4 ± 3.6 mmHg and 69.4 ± 11.3 mg/day). When compared with the SS rats, SS-Rag1−/− rats fed high protein were protected from exacerbated hypertension and albuminuria (142.9 ± 5.8 mmHg and 66.2 ± 10.8 mg/day). After 3 wk of the high-salt diet, there was a corresponding increase in total leukocyte infiltration (CD45+) in the kidneys of both strains fed high-protein diet. The SS-Rag1−/− rats fed high-protein diet had 74–86% fewer CD3+ T lymphocytes and CD45R+ B lymphocytes infiltrating the kidney versus SS rats, but there was no difference in the infiltration of CD11b/c+ monocytes and macrophages, suggesting that the protective effects observed in the SS-Rag1−/− rats are specific to the reduction of lymphocytes. With the SS-Rag1−/− rats utilized as a novel tool to explore the effects of lymphocyte deficiency, these results provide evidence that adaptive immune mechanisms contribute to the exacerbation of salt-induced hypertension and renal injury mediated by increased dietary protein intake.
Keywords: dietary protein, kidney disease, salt-sensitive hypertension, T lymphocytes
INTRODUCTION
Environmental factors, including components of the diet, can influence phenotypes related to hypertension and end-organ damage in patients and experimental animals. In humans, consumption of diets high in carbohydrates, saturated fats, and cholesterol is associated with elevated blood pressure, whereas high-protein diets are linked to decreased blood pressure (7, 11, 12, 17, 37). In contrast, increased dietary protein intake in patients with preexisting renal insufficiency has been suggested to lead to a regression in renal function (9, 18–20, 31), where dietary protein restriction is recommended in these patients. The Dahl salt-sensitive (SS) rat, a genetic model of hypertension, is susceptible to renal damage in response to a high-salt challenge. Because the SS rat demonstrates many phenotypic characteristics observed in patients with salt-sensitive hypertension (21), an understanding of the mechanisms whereby changes in dietary protein intake lead to disease in the SS rat may provide useful insight into human disease.
In animal models of hypertension, the elevation of blood pressure has been shown to be a function of the source of fat (36, 40), carbohydrate (27, 32, 39, 40), and protein (28) of the diet. Previous studies from our laboratory have indicated that sodium-independent components of the diet, in particular the source and amount of protein, can alter the development of sodium-sensitive hypertension and associated renal damage in SS rats fed a high-salt diet (5, 23, 25). As seen in humans, and given the predisposition of the SS rat to renal damage, we observed that increased protein intake in SS rats is associated with an exacerbation of salt-dependent hypertension and renal damage accompanied by an increase in immune cell infiltration into the kidney (5). Food intake has been shown to induce an inflammatory response in healthy subjects and animals and offers a potential mechanism whereby changes in dietary intake can alter functional phenotypes (6, 15, 38). Additional experimental data have indicated that immune cells, particularly T lymphocytes, can amplify the sodium-sensitive disease phenotypes in the SS rat (3–5, 22, 24, 34), but it is unknown whether these immune mechanisms are related to the effects seen during a high-protein diet.
SS-Rag1−/− rats have a mutation in the Rag1 gene, which is important for the variable-(diversity)-joining [V(D)J] recombination required for the proper generation of diverse antibody and T cell receptors. SS-Rag1−/− rats lack these functional receptors and are unable to produce mature T and B lymphocytes (24). By utilizing the SS-Rag1−/− rats, which lack lymphocytes, the present study was designed to test whether these immune cells mediate the effects of a high-protein diet in the amplification of SS rat hypertension and kidney damage. The present experiments first determined whether a normal (18%) or a high (30%)-protein diet altered growth and renal damage-associated phenotypes in SS and SS-Rag1−/− rats when maintained on a low-sodium diet. Subsequently, sodium-dependent changes in arterial blood pressure and renal damage were examined in SS rats and lymphocyte-deficient SS rats (SS-Rag1−/− rats) maintained on either a normal or high-protein diet from weaning.
METHODS
Animals and diets.
Experiments were performed on age-matched, inbred, male Dahl SS rats (SS/JrHSDMcwi), referred to as SS rats throughout the paper, and SS rats with a 13-base mutation in exon 1 of the Rag1 gene (SS-Rag1em1Mcwi), referred to as SS-Rag1−/− rats (24). Additional experiments were performed on SS rats lacking Cd247 (SS-Cd247−/− rats), which were generated via zinc finger nuclease technology, as previously described (34). Breeders from all strains were maintained on a 0.4% NaCl AIN-76A diet containing normal (18%) protein (Dyets). Immediately after being weaned, SS and SS-Rag1−/− rats were placed on isocaloric 0.4% NaCl diets containing normal (18%) or high (30%) protein (casein). At 9 wk of age, rats were switched to a 4.0% NaCl diet containing the same amount of dietary protein and maintained on this high-salt diet for 3 wk. The custom diets utilized in this study were based on the standard AIN-76A formulation (Dyets) with changes in protein or NaCl content matched by equal and opposite changes in carbohydrate (sucrose). All experimental animal procedures were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Surgical procedure and blood pressure monitoring.
At 10.5 wk of age, after ~1.5 wk of the 4.0% NaCl diet, the rats underwent surgery for catheter implantation in the femoral artery as previously described (5, 22, 24). The rats were deeply anesthetized with an intraperitoneal injection of ketamine (35 mg/kg), xylazine (10 mg/kg), and acepromazine (0.5 mg/kg) with supplemental anesthesia administered when needed. With the use of aseptic technique, polyvinyl catheters were implanted in the femoral artery, tunneled subcutaneously, and exteriorized at the back of the neck in a lightweight tethering spring. Both antibiotic (cefazolin, 25 mg/kg) and analgesic (carprofen, 5 mg/kg) agents were administered postsurgically, and the rats were allowed to fully awaken from anesthesia on a temperature-controlled pad. Rats were placed in individual stainless-steel cages that permit daily measurement of arterial blood pressure and overnight urine collection. After 5 days of recovery, blood pressure was measured via a pressure transducer connected to the femoral catheter while the rats were between 11 and 12 wk of age.
Experimental procedure.
Overnight urine was collected during the low-salt period and after 7, 14, and 21 days of 4.0% NaCl intake. Urine electrolytes were measured by flame photometry (model 410; Corning). Urine creatinine values were measured by an autoanalyzer with an assay based on the Jaffé reaction (ACE; Alfa Wasserman, Fairfield, NJ). Urine albumin was quantified with a fluorescent assay that utilized Albumin Blue 580 dye (Sigma-Aldrich, St. Louis, MO) and a fluorescent plate reader (FL-600; BioTek, Winooski, VT).
Immune cell isolation and flow cytometry.
At the conclusion of the experiment, rats were anesthetized, and the kidneys were flushed with heparinized PBS, minced, and incubated in RPMI 1640 media containing collagenase to isolate immune cells as we have previously described (14, 34, 35). Mononuclear cells were separated by Percoll density gradient centrifugation, counted on a hemocytometer, and incubated for 30 min with extracellular markers anti-CD3 (eBioscience) for T cells, anti-CD45R (BD Bioscience) for B cells, and anti-CD11b/c (eBioscience) for monocytes and macrophages. All cells were analyzed by flow cytometry (LSRII; Becton Dickinson) with FACSDIVA software (Becton Dickinson) and FlowJo software (TreeStar).
Histological analysis of renal damage.
Kidneys were collected for histological analysis as previously described (22–25). Tissues were fixed with 10% paraformaldehyde, paraffin embedded, cut into 4-µm sections, mounted, and stained with Masson’s trichrome. Images of slides were taken at different magnifications using a Nikon E400 microscope fitted with a Spot Insight camera. As described previously, individual glomeruli (at least 50 per rat) were semiquantitatively scored from 0 (best) to 4 (worst) on the basis of glomerulosclerosis and mesangial expansion (22, 23, 25). The outer medullary cast percentage was determined by color inclusion via MetaMorph software. Glomeruli were scored and medullary cast formation was assessed in a blinded manner.
Statistical analysis.
Data are expressed as means ± SE. Data were assessed for significance using a two-way analysis of variance (ANOVA) with a Holm-Sidak post hoc test or a two-way repeated-measures ANOVA with a Holm-Sidak post hoc test, as appropriate. A value of P < 0.05 was considered statistically significant.
RESULTS
No differences were detected in body weight, serum albumin, or sodium and potassium excretion rates between strains fed the normal and high-protein diets.
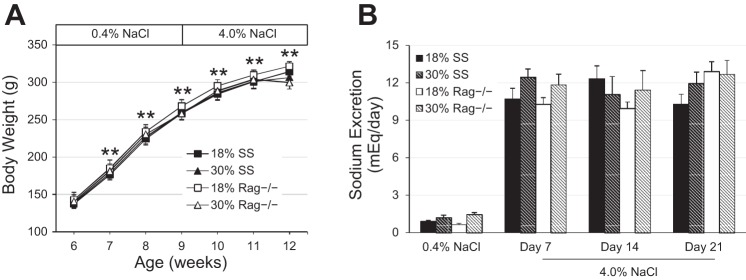
Although body weight progressively increased in both SS and SS-Rag1−/− rats (Fig. 1A) from 6 to 12 wk of age, no differences were detected in body weight between the SS and SS-Rag1−/− rats at any time point in the protocol. At 9 wk of age, the chow salt content was increased from 0.4 to 4.0% NaCl, and the steady-state sodium excretion rate significantly increased in all rats (Fig. 1B). However, there was no difference in sodium excretion between the SS and SS-Rag1−/− rats at any of the time points demonstrating that there were minimal, if any, differences in total food intake between groups. Although rats were not pair fed, the similarities in sodium excretion and body weight indicate that any potential difference in blood pressure or renal disease phenotypes is likely not due to differences in sodium or food intake between groups. Additionally, there were no differences in urinary potassium excretion or serum albumin concentration between the two strains at any time point.
Fig. 1.
A: body weight in Dahl salt-sensitive (SS) and SS-Rag1−/− rats fed either the normal (18%) or high (30%)-protein chow. B: steady-state sodium excretion between SS and SS-Rag1−/− rats fed either the normal (18%) or high (30%)-protein chow during low salt (0.4% NaCl) and throughout 3 wk of high salt (4.0% NaCl). Two-way repeated-measures ANOVA with Holm-Sidak post hoc test, **P < 0.001 vs. week 6. Here, 18% SS, n = 8; 30% SS, n = 7; 18% SS-Rag1−/−, n = 8; 30% SS-Rag1−/−, n = 5–7.
Salt-induced hypertension and renal damage in high protein-fed SS-Rag1−/− rats lacking T and B lymphocytes.
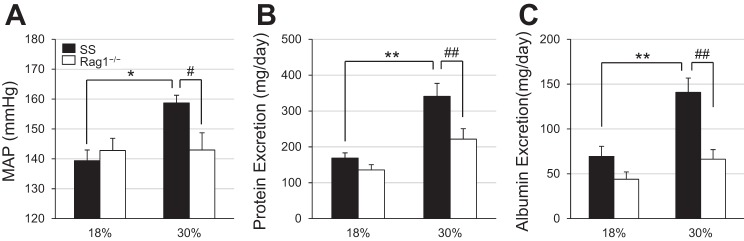
At baseline, when the rats were fed the low-salt diet, no differences were observed in the albumin excretion rate between high protein-fed SS and SS-Rag1−/− rats (data not shown, 37.3 ± 3.9 vs. 27.1 ± 8.3 mg/day). After 3 wk of the 4.0% NaCl challenge, mean arterial pressure (MAP) was significantly higher in SS rats maintained on the high-protein diet compared with SS rats on the normal protein diet (158.7 ± 2.6 vs. 139.4 ± 3.6 mmHg; Fig. 2A). This rise in blood pressure was accompanied with a corresponding increase in urinary protein (341.0 ± 36.2 vs. 168.8 ± 14.5 mg/day) and albumin excretion (140.8 ± 16.0 vs. 69.4 ± 11.3 mg/day) compared with SS rats fed the normal protein diet (Fig. 2, B and C), indicating that consumption of the high-protein diet exacerbated salt-induced hypertension and renal damage in SS rats. Interestingly, there was no difference in MAP or albumin or protein excretion rate between SS-Rag1−/− rats fed the normal protein diet and those fed the high-protein diet at the end of the high-salt period. However, after 3 wk of high salt intake, MAP (142.9 ± 5.8 vs. 158.7 ± 2.6 mmHg), protein excretion (221.5 ± 29.1 vs. 341.0 ± 36.2 mg/day), and albumin excretion (66.2 ± 10.8 vs. 140.8 ± 16.0 mg/day) in the SS-Rag1−/− rats were all reduced compared with the SS rats fed the 30% high-protein diet, importantly demonstrating protection from the high protein-induced exacerbation of hypertension and renal damage in the SS-Rag1−/− rats lacking lymphocytes. At the conclusion of the experiment, left kidney weight was significantly greater in SS rats fed the high-protein diet versus those fed the normal protein diet (2.08 ± 0.10 vs. 1.71 ± 0.09 g). Left kidney weight was not different between the SS-Rag1−/− rats fed the normal protein diet and those fed the high-protein diet (1.82 ± 0.07 g) at the same time point, suggesting that the differences in kidney weight in the SS rats are likely due to hypertrophy related to kidney damage.
Fig. 2.
Mean arterial blood pressure (MAP, A), urinary protein excretion (B), and urinary albumin excretion (C) after 3 wk of 4.0% high-salt challenge in Dahl salt-sensitive (SS) and SS-Rag1−/− rats fed either the normal (18%) or high (30%)-protein chow. Two-way ANOVA with Holm-Sidak post hoc test, *P < 0.01 and **P < 0.001 vs. 18% SS, #P < 0.01 and ##P < 0.001 vs. 30% SS. Here, 18% SS, n = 8; 30% SS, n = 7; 18% SS-Rag1−/−, n = 8; 30% SS-Rag1−/−, n = 5–7.
Effect of high protein consumption on circulating and renal immune cell infiltration profile.
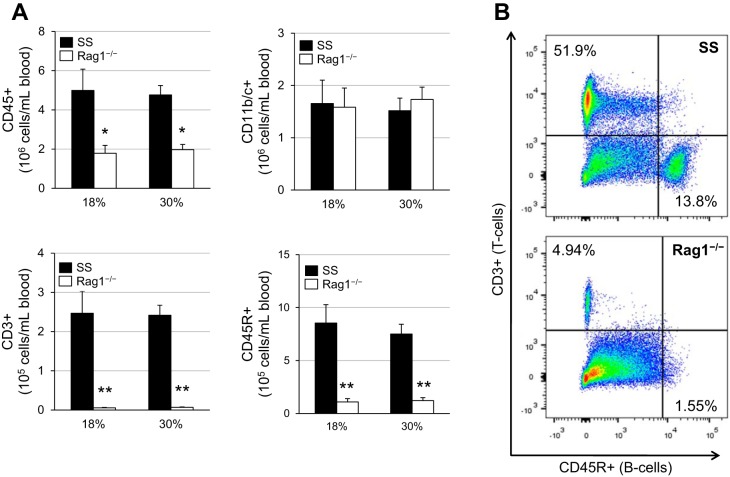
At the end of the study, the blood was analyzed for the following peripheral immune cell populations: CD45+ leukocytes, CD11b/c+ monocytes and macrophages, CD3+ T cells, and CD45R+ B cells. When comparing SS rats on the normal and high-protein diets, no differences were observed in any of the immune cell populations (Fig. 3A). As expected, SS-Rag1−/− rats had significantly fewer CD45+ leukocytes compared with the SS rats, which was due to the depletion of CD3+ T cells and CD45R+ B cells. There was no difference in the number of circulating CD11b/c+ monocytes and macrophages between SS and SS-Rag1−/− rats. A representative flow cytometric plot is shown in Fig. 3B, which depicts the depletion of the T and B cell populations in the circulation of SS-Rag1−/− rats.
Fig. 3.
A: circulating immune cell profile after 3 wk of 4.0% high-salt challenge in Dahl salt-sensitive (SS) and SS-Rag1−/− rats fed either the normal (18%) or high (30%)-protein chow. CD45, leukocytes; CD11b/c, monocytes and macrophages; CD3, T lymphocytes; CD45R, B lymphocytes. Two-way ANOVA with Holm-Sidak post hoc test, *P < 0.01 and **P < 0.001 vs. SS. Here, 18% SS, n = 8; 30% SS, n = 7; 18% SS-Rag1−/−, n = 8; 30% SS-Rag1−/−, n = 7. B: representative flow cytometric plots demonstrating T and B lymphocyte depletion in SS-Rag1−/− rats.
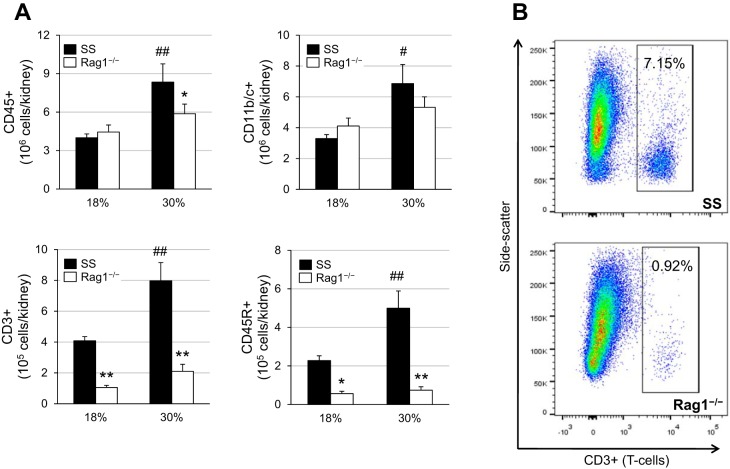
As illustrated in Fig. 4A, SS rats maintained on the high-protein diet exhibited a significant increase in total CD45+ leukocyte infiltration into the kidneys compared with SS rats on the normal protein diet after 3 wk of 4.0% high salt (8.3 ± 1.4 × 106 vs. 4.0 ± 0.3 × 106 CD45+ cells). This increase in high-protein, high-salt-fed SS rats was evident across all other populations of immune cells evaluated in the kidney, including CD11b/c+ monocytes and macrophages, CD3+ T cells, and CD45R+ B cells. As expected in the SS-Rag1−/− rats, there was a drastic reduction in the number of T and B cells in the kidneys compared with SS rats. Most interestingly, there was no difference in the infiltration of CD11b/c+ monocytes and macrophages between the SS and SS-Rag1−/− rats fed the high-protein diet (6.9 ± 1.2 × 106 vs. 5.3 ± 0.7 × 106 CD11b/c+ cells), importantly suggesting that the protection from high protein-exacerbated hypertension and renal damage observed in the SS-Rag1−/− rats is not due to an accompanying reduction in renal infiltrating monocytes and macrophages but due to the specific absence of lymphocytes. A representative flow cytometric plot is shown in Fig. 4B, demonstrating the reduction of T lymphocytes in the kidneys of SS-Rag1−/− rats.
Fig. 4.
A: renal infiltrating immune cell profile after 3 wk of 4.0% high-salt challenge in Dahl salt-sensitive (SS) and SS-Rag1−/− rats fed either the normal (18%) or high (30%)-protein chow. CD45, leukocytes; CD11b/c, monocytes and macrophages; CD3, T lymphocytes; CD45R, B lymphocytes. Two-way ANOVA with Holm-Sidak post hoc test, *P < 0.01 and **P < 0.001 vs. SS, #P < 0.01 and ##P < 0.001 vs. 18% SS. Here, 18% SS, n = 8; 30% SS, n = 7; 18% SS-Rag1−/−, n = 8; 30% SS-Rag1−/−, n = 7. B: representative flow cytometric plots demonstrating T lymphocyte depletion in SS-Rag1−/− rats.
Renal histological and morphological damage in SS and SS-Rag1−/− rats on the normal and high-protein diets.
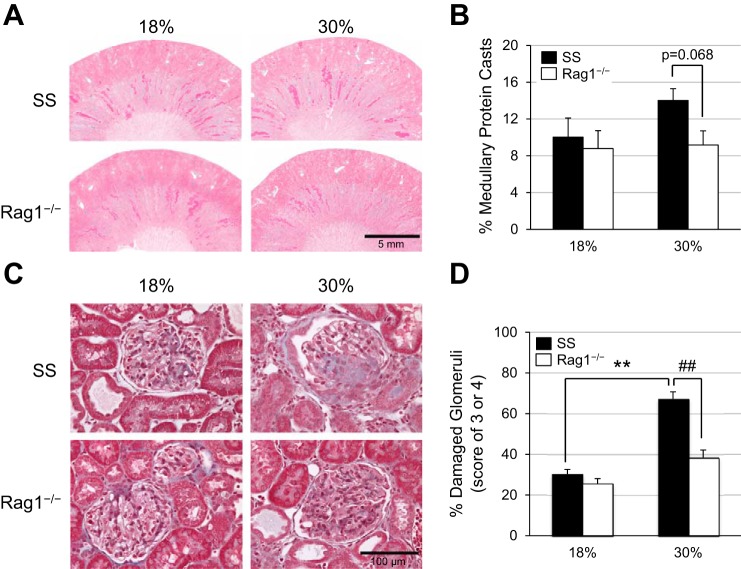
Corresponding with the increase in salt-induced proteinuria and albuminuria in the SS rats fed the high- versus normal protein diets, an increase in histological renal damage, marked by the presence of outer medullary protein casts and damaged glomeruli, was also observed in the SS rats fed the high-protein diet compared with those fed the normal protein diet (Fig. 5, A and C). However, there was a reduction in outer medullary protein cast formation (14.0 ± 1.3 vs. 9.2 ± 1.5%) and in the percentage of severely damaged glomeruli with glomerular injury scores of 3 or 4 (67.2 ± 3.5 vs. 38.3 ± 3.8%) in the high protein-fed SS-Rag1−/− rats compared with the parental SS rats, indicating the deleterious role of T and B lymphocytes in the exacerbation of salt-induced renal damage by a high-protein diet. The percentages of outer medullary protein casts and damaged glomeruli are quantified in Fig. 5, B and D.
Fig. 5.
A: representative light microscopy images of trichrome-stained kidneys after 3 wk of high-salt challenge in Dahl salt-sensitive (SS) and SS-Rag1−/− rats fed either the normal (18%) or high (30%)-protein chow (×4), with the quantification of the percentage of outer medullary protein casts summarized in B. C: light microscopy images of representative glomeruli (×40) from kidneys obtained from SS and SS-Rag1−/− rats after 4.0% NaCl normal protein or high-protein diet, with the percentage of damaged glomeruli summarized in D. Two-way ANOVA with Holm-Sidak post hoc test, **P < 0.001 vs. 18% SS, ##P < 0.001 vs. 30% SS. Here, 18% SS, n = 8; 30% SS, n = 7; 18% SS-Rag1−/−, n = 8; 30% SS-Rag1−/−, n = 6.
Effect of high-protein diet on renal damage and immune cell infiltration in T lymphocyte-deficient SS-Cd247−/− rats.
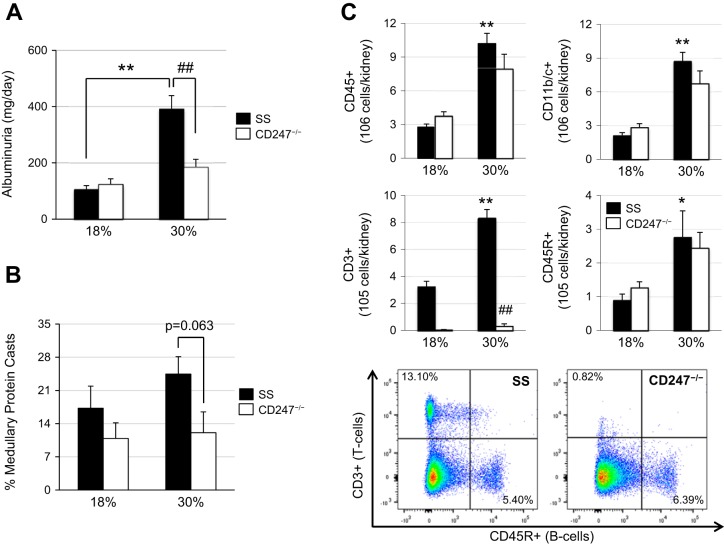
Experiments were also performed in SS-Cd247−/− rats to determine the specific contribution of T lymphocytes to high protein-exacerbated hypertension and renal damage in SS rats. At baseline, when the rats were fed the 0.4% NaCl chow, no differences were observed in the albumin excretion rate between high protein-fed SS and SS-Cd247−/− rats (data not shown, 41.0 ± 11.4 vs. 23.2 ± 6.0 mg/day). As seen previously, after 3 wk of the 4.0% high-salt challenge, SS rats on the high-protein diet had an exacerbation of renal damage and albuminuria compared with SS rats on the normal protein chow (392.7 ± 46.4 vs. 106.0 ± 13.0 mg/day; Fig. 6A), which was significantly attenuated in the high protein-fed SS-Cd247−/− rats (186.1 ± 26.8 mg/day). As with the SS-Rag1−/− rats, there was no difference in albuminuria and the extent of renal damage between SS and SS-Cd247−/− rats fed the normal protein chow (106.0 ± 13.0 vs. 124.6 ± 18.8 mg/day). Summarized in Fig. 6B, the reduction in albuminuria observed in the high protein-fed SS-Cd247−/− rats was also accompanied with a reduction in outer medullary protein casts after 3 wk of high-salt challenge. At the end of the study, the immune cell profile in the blood was analyzed, and as expected, the only immune cell population different between the high protein-fed SS and SS-Cd247−/− rats was the CD3+ T cells, which were depleted in the SS-Cd247−/− rats (data not shown). As shown in Fig. 6C, there was a significant increase in infiltrating CD45+ total leukocytes, CD11b/c+ monocytes and macrophages, CD3+ T cells, and CD45R+ B cells into the kidneys of SS rats on high protein compared with SS rats on normal protein, whereas in both the normal and high protein-fed SS-Cd247−/− rats, there was a specific depletion of CD3+ T cells. This protection from high protein-induced renal damage observed in the SS-Cd247−/− rats highlights the detrimental role of these infiltrating T lymphocytes in this exacerbated disease process.
Fig. 6.
A: urinary albumin excretion after 3 wk of 4.0% high-salt challenge in Dahl salt-sensitive (SS) and SS-Cd247−/− rats fed either the normal (18%) or high (30%)-protein chow. B: quantification of the percentage of outer medullary protein casts in SS and SS-Cd247−/− rats after 3 wk of high salt. C: renal infiltrating immune cell profile after 3 wk of high-salt challenge in SS and SS-Cd247−/− rats with representative flow cytometric plots demonstrating T lymphocyte depletion in SS-Cd247−/− rats. CD45, leukocytes; CD11b/c, monocytes and macrophages; CD3, T lymphocytes; CD45R, B lymphocytes. Two-way ANOVA with Holm-Sidak post hoc test, *P < 0.01 and **P < 0.001 vs. 18% SS, ##P < 0.001 vs. 30% SS. Here, 18% SS, n = 5–6; 30% SS, n = 5; 18% SS-Cd247−/−, n = 6; 30% SS-Cd247−/−, n = 5.
DISCUSSION
The present study demonstrated that hypertension and renal damage associated with increased dietary NaCl intake are exaggerated in SS rats fed a high-protein diet from weaning. Moreover, the amplified disease phenotype was associated with an increase in renal immune cell infiltration (macrophages and lymphocytes). These effects of lifelong exposure to the high-protein diet in our SS rats, who are genetically predisposed to renal injury, are consistent with our previous studies where we have demonstrated that administration of the immunosuppressive drug mycophenolate mofetil attenuated the protein-dependent increase in disease severity in SS rats fed high salt (5). Because mycophenolate mofetil decreases the number of both lymphocytes and macrophages in the kidney (3, 22, 30) and because of its potential side effects, the present study was performed in SS and SS-Rag1−/− rats to specifically examine the importance of lymphocytes in this response. Whether these effects of a high-protein diet persist during a shorter exposure remains to be investigated, but lymphocyte-deficient SS-Rag1−/− rats (24) fed a high-protein diet throughout their entire life demonstrated that the effects of dietary protein to enhance salt-sensitive hypertension were eliminated in the absence of T and B cell infiltration into the kidney despite no significant change in the presence of CD11b/c+ cells (macrophages and monocytes).
The role of immunity in hypertension has been well described and is implicated in both clinical and experimental hypertension (13, 16, 21, 26, 33). Lymphocytes (2, 3) and macrophages (29) are localized to the kidneys of hypertensive animals and patients (16), and immune suppression has been shown to blunt hypertension and accompanying renal damage (1, 22, 24, 34). Revealing studies from Harrison and colleagues highlighted the importance of adaptive immunity in hypertension, where an adoptive cell transfer approach demonstrated the specific importance of T cells in the development of ANG II hypertension (10). To delineate the effect of T versus B cells in response to a high-protein diet, we performed additional experiments in SS-Cd247−/− rats. Cd247 encodes the CD3 ζ-chain of the T cell receptor complex, and SS rats lacking Cd247 have a reduction in circulating T cells (34). These SS rats that are specifically deficient in T cells appear to be protected from renal damage to the same extent as the SS-Rag1−/− rats, suggesting that T cells, and not B cells, may be the primary culprit in the exacerbation of dietary protein-induced kidney injury. Although this study has specifically shown that T lymphocytes somehow contribute to the rise in pressure and disease exacerbation, we are unable to discriminate between the protective effects of lymphocyte depletion, lower blood pressure, and improved renal function. In light of recent studies utilizing a novel servo-control technique to demonstrate that increased renal perfusion pressure contributes to the initiation of renal T cell infiltration in SS rats (8), our study perhaps altogether indicates a positive feedback relationship between high protein diet-induced hypertension, renal damage, and immune cell infiltration.
The precise mechanisms linking changes in dietary protein to the amplification of salt-sensitive hypertension and end-organ damage are not clear. One potential mechanism whereby changes in dietary intake can alter functional phenotypes is by directly stimulating inflammation (6, 15, 38), which would be consistent with our present observations. A recent study demonstrated that refeeding of fasted mice induced IL-1β release from peritoneal macrophages (6); the authors subsequently demonstrated elevated glucose as a potential mediator of these inflammatory changes. Although the direct influence of alterations in protein intake have yet to be examined, the direct effects of food intake to induce an inflammatory response should not be discounted. Regardless of the mechanisms of immune cell activation, it is apparent from the present study that the enhanced disease phenotype in SS rats fed the high-salt, high-protein diet is associated with increased immune cell infiltration in the kidney. Moreover, SS-Rag1−/− rats were protected from the exacerbation of hypertension and renal damage when fed the high-salt, high-protein diet, demonstrating the crucial and deleterious role for T and B lymphocytes in this diet-enhanced disease response.
In summary, the present study demonstrates the detrimental effect of a high-protein diet on the severity of salt-sensitive hypertension in the SS rat, an animal model of disease that exhibits phenotypic similarities to human salt-sensitive hypertension. Accompanying the enhanced hypertension and end-organ damage in the SS rats fed the high-protein diet, we observed an increase in the number of macrophages, T cells, and B cells in the kidneys of these rats. Parallel studies demonstrated protection in SS-Rag1−/− rats lacking T and B cells from the start, providing evidence that adaptive immune mechanisms, specifically lymphocytes, mediate the exaggerated salt-sensitive hypertension and renal injury that occurs in SS rats fed a high-protein diet throughout their entire life span.
Perspectives and Significance
The present study utilized SS-Rag1−/− rats to reveal important adaptive immune mechanisms regarding the exacerbation of salt-induced hypertension and renal injury in response to a high-protein diet. Future studies could test more specific therapeutic immune interventions or perhaps explore the effect of removal of the high-protein diet. Because the SS rat demonstrates many phenotypic characteristics observed in patients with salt-sensitive hypertension, utilizing this model to understand the mechanisms whereby these environmental changes lead to or aggravate disease may provide insight into how we clinically prevent and treat hypertension and renal injury.
GRANTS
This study was supported by National Institutes of Health Grants DK-96859, HL-116264, and 1F32-HL-136161 and by American Heart Association Grants 15SFRN2391002 and 16POST29900004.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.A.-B., H.L., and D.L.M. conceived and designed research; J.M.A.-B., H.L., D.J.F., and J.H.D. performed experiments; J.M.A.-B., H.L., and D.J.F. analyzed data; J.M.A.-B., J.H.D., and D.L.M. interpreted results of experiments; J.M.A.-B. prepared figures; J.M.A.-B. and D.L.M. drafted manuscript; J.M.A.-B., H.L., D.J.F., J.H.D., and D.L.M. edited and revised manuscript; J.M.A.-B., H.L., D.J.F., J.H.D., and D.L.M. approved final version of manuscript.
REFERENCES
- 1.Bataillard A, Vincent M, Sassard J, Touraine JL. Antihypertensive effect of an immunosuppressive agent, cyclophosphamide, in genetically hypertensive rats of the Lyon strain. Int J Immunopharmacol 11: 377–384, 1989. doi: 10.1016/0192-0561(89)90084-2. [DOI] [PubMed] [Google Scholar]
- 2. Crowley SD, Frey CW, Gould SK, Griffiths R, Ruiz P, Burchette JL, Howell DN, Makhanova N, Yan M, Kim HS, Tharaux PL, Coffman TM. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am J Physiol Renal Physiol 295: F515–F524, 2008. [Erratum in Am J Physiol Renal Physiol 298: F1286, 2010. 10.1152/ajprenal.zh2-5901-corr.2010.] 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Miguel C, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol 300: F734–F742, 2011. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57: 269–274, 2011. doi: 10.1161/HYPERTENSIONAHA.110.154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dror E, Dalmas E, Meier DT, Wueest S, Thévenet J, Thienel C, Timper K, Nordmann TM, Traub S, Schulze F, Item F, Vallois D, Pattou F, Kerr-Conte J, Lavallard V, Berney T, Thorens B, Konrad D, Böni-Schnetzler M, Donath MY. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol 18: 283–292, 2017. doi: 10.1038/ni.3659. [DOI] [PubMed] [Google Scholar]
- 7.Elliott P, Stamler J, Dyer AR, Appel L, Dennis B, Kesteloot H, Ueshima H, Okayama A, Chan Q, Garside DB, Zhou B. Association between protein intake and blood pressure: the INTERMAP Study. Arch Intern Med 166: 79–87, 2006. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70: 543–551, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev 3: CD001892, 2009. doi: 10.1002/14651858.CD001892.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hajjar I, Kotchen T. Regional variations of blood pressure in the United States are associated with regional variations in dietary intakes: the NHANES-III data. J Nutr 133: 211–214, 2003. doi: 10.1093/jn/133.1.211. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar IM, Grim CE, George V, Kotchen TA. Impact of diet on blood pressure and age-related changes in blood pressure in the US population: analysis of NHANES III. Arch Intern Med 161: 589–593, 2001. doi: 10.1001/archinte.161.4.589. [DOI] [PubMed] [Google Scholar]
- 13.Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc 125: 130–138, 2014. [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 311: F555–F561, 2016. doi: 10.1152/ajprenal.00594.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herieka M, Erridge C. High-fat meal induced postprandial inflammation. Mol Nutr Food Res 58: 136–146, 2014. doi: 10.1002/mnfr.201300104. [DOI] [PubMed] [Google Scholar]
- 16.Hughson MD, Gobe GC, Hoy WE, Manning RD Jr, Douglas-Denton R, Bertram JF. Associations of glomerular number and birth weight with clinicopathological features of African Americans and whites. Am J Kidney Dis 52: 18–28, 2008. doi: 10.1053/j.ajkd.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Kesteloot H, Joossens JV. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniversity Research on Nutrition and Health. Hypertension 12: 589–593, 1988. doi: 10.1161/01.HYP.12.6.589. [DOI] [PubMed] [Google Scholar]
- 18.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med 138: 460–467, 2003. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ko GJ, Obi Y, Tortorici AR, Kalantar-Zadeh K. Dietary protein intake and chronic kidney disease. Curr Opin Clin Nutr Metab Care 20: 77–85, 2017. doi: 10.1097/MCO.0000000000000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin WF, Armstrong LE, Rodriguez NR. Dietary protein intake and renal function. Nutr Metab (Lond) 2: 25, 2005. doi: 10.1186/1743-7075-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattson DL. Infiltrating immune cells in the kidney in salt-sensitive hypertension and renal injury. Am J Physiol Renal Physiol 307: F499–F508, 2014. doi: 10.1152/ajprenal.00258.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 23.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW Jr. Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiol Genomics 16: 194–203, 2004. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 24.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 45: 736–741, 2005. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 26.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori Y, Murakawa Y, Yokoyama J, Tajima N, Ikeda Y, Nobukata H, Ishikawa T, Shibutani Y. Effect of highly purified eicosapentaenoic acid ethyl ester on insulin resistance and hypertension in Dahl salt-sensitive rats. Metabolism 48: 1089–1095, 1999. doi: 10.1016/S0026-0495(99)90120-8. [DOI] [PubMed] [Google Scholar]
- 28.Nevala R, Vaskonen T, Vehniäinen J, Korpela R, Vapaatalo H. Soy based diet attenuates the development of hypertension when compared to casein based diet in spontaneously hypertensive rat. Life Sci 66: 115–124, 1999. doi: 10.1016/S0024-3205(99)00569-X. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa Y, Kobori H, Suzaki Y, Navar LG. Sustained renal interstitial macrophage infiltration following chronic angiotensin II infusions. Am J Physiol Renal Physiol 292: F330–F339, 2007. doi: 10.1152/ajprenal.00059.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 294: R1234–R1239, 2008. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 31.Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. The effect of dietary protein restriction on the progression of diabetic and nondiabetic renal diseases: a meta-analysis. Ann Intern Med 124: 627–632, 1996. doi: 10.7326/0003-4819-124-7-199604010-00002. [DOI] [PubMed] [Google Scholar]
- 32.Preuss HG, Knapka JJ, MacArthy P, Yousufi AK, Sabnis SG, Antonovych TT. High sucrose diets increase blood pressure of both salt-sensitive and salt-resistant rats. Am J Hypertens 5: 585–591, 1992. doi: 10.1093/ajh/5.9.585. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Iturbe B. Autoimmunity in the pathogenesis of hypertension. Hypertension 67: 477–483, 2016. doi: 10.1161/HYPERTENSIONAHA.115.06418. [DOI] [PubMed] [Google Scholar]
- 34.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65: 1111–1117, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimokawa T, Moriuchi A, Hori T, Saito M, Naito Y, Kabasawa H, Nagae Y, Matsubara M, Okuyama H. Effect of dietary alpha-linolenate/linoleate balance on mean survival time, incidence of stroke and blood pressure of spontaneously hypertensive rats. Life Sci 43: 2067–2075, 1988. doi: 10.1016/0024-3205(88)90356-6. [DOI] [PubMed] [Google Scholar]
- 37.Stamler J, Caggiula A, Grandits GA, Kjelsberg M, Cutler JA. Relationship to blood pressure of combinations of dietary macronutrients. Findings of the Multiple Risk Factor Intervention Trial (MRFIT). Circulation 94: 2417–2423, 1996. doi: 10.1161/01.CIR.94.10.2417. [DOI] [PubMed] [Google Scholar]
- 38.van Oostrom AJ, Sijmonsma TP, Rabelink TJ, Van Asbeck BS, Cabezas MC. Postprandial leukocyte increase in healthy subjects. Metabolism 52: 199–202, 2003. doi: 10.1053/meta.2003.50037. [DOI] [PubMed] [Google Scholar]
- 39.Young JB, Landsberg L. Effect of oral sucrose on blood pressure in the spontaneously hypertensive rat. Metabolism 30: 421–424, 1981. doi: 10.1016/0026-0495(81)90173-6. [DOI] [PubMed] [Google Scholar]
- 40.Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the Dahl salt sensitive rat. Am J Hypertens 12: 183–187, 1999. doi: 10.1016/S0895-7061(98)00238-6. [DOI] [PubMed] [Google Scholar]