Abstract
Generation of secondary alveolar septa occurs primarily after birth in humans and is complete in mice postnatally, when mechanical stresses vary as air space pressure oscillates. Alveolar mesenchymal cells deposit elastic fibers, which limit cell strain; although when the elastic fiber network is incomplete, this function is also served by the intracellular cytoskeleton. Intermediate filament proteins support deformation during cell division and migration, which occur during septal elongation. Because platelet-derived growth factor receptor-α (PDGFRα) signaling is essential for alveolar septation, we hypothesized that neuropilin-1 (NRP1) may link PDGFRα to cytoskeletal deformation. During cell migration, NRP1 links receptor tyrosine kinase signaling to cytoskeletal and focal adhesion remodeling. Therefore, we examined the consequences of nrp1 gene deletion in alveolar mesenchymal cells (myofibroblasts and pericytes). NRP1 depletion reduced the proportion of mesenchymal cells that contain nestin and desmin within the subpopulation that lacked PDGFRα but contained PDGFRβ. Desmin was reduced at alveolar entry rings, air spaces were enlarged, and surface area was reduced after NRP1 depletion. PDGFRα and NRP1 colocalized to membrane lipid rafts, which are known to contain Src kinase. NRP1 depletion reduced alveolar mesenchymal cell migration and PDGF-A-mediated activation of Src kinase, which may limit accumulation of desmin at septal tips (alveolar entry rings). Cooperation between NRP1 and PDGF signaling is required for secondary septation, and manipulation of NRP1 could promote alveolar regeneration without producing fibrosis.
Keywords: desmin, myofibroblast, nestin, pericyte, Src kinase
INTRODUCTION
Mice lack respiratory bronchioles, so their pulmonary acinar openings are demarcated by alveolar ducts, which reach their full complement at completion of the saccular development stage on postnatal day 4 (P4) (50). During the subsequent alveolar stage, secondary alveolar septa lift off the preexisting saccules (alveolar ducts) until P21, but additional alveoli are generated through P36 (56). Both alveolar ducts and alveoli contribute to gas exchange, but they arise by distinct processes and at different times. Alveolar ducts are the termini of airways, which increase by iterative branching, whereas alveolar septa are generated by the outgrowth of multiple thin walls from the ductal circumference.
Because alveolar duct formation largely occurs before, whereas alveolar septation exclusively occurs after, birth, their mechanical contexts differ widely. Airway branching occurs under a relatively constant distension pressure, whereas postnatal pressure continuously oscillates with tidal breathing. Predictive models using empirical anatomic and physiological measurements indicate geometric hysteresis of alveolar ducts during tidal breathing (35). Surfactant and the contractile structures of the alveolar entry ring (AER) make partially offsetting contributions to hysteresis. Three-dimensional synchrotron radiation-based X-ray tomography showed that the alveolar duct unfolds like an accordion and incurs the largest increase in volume as inspiration begins (63). In adults, the viscoelastic properties of the alveolar ducts are determined by extracellular cables, which contain both elastin and collagen (65). However, fiber cables are sparser, less cross-linked, and unevenly distributed during secondary septation. Therefore, the ductal viscoelastic properties also depend on the cellular cytoskeleton, which includes microtubules, filamentous actin, and intermediate filaments (IFs) (65).
IFs are particularly important for the viscoelastic properties of fibroblasts and myofibroblasts (MFs) (7). Because they are less rigid than microtubules and actin filaments, IFs critically influence cell mechanics when cells are distorted during mitosis and migration. Contractile mesenchymal cells [MFs and pericytes (PCs)] are abundant in the AER, which sustains maximal volumetric distortion during tidal breathing. Despite their potential importance, little is known about how IFs participate in septal outgrowth and how their abundance is regulated. IF proteins form composite “ultrafilaments,” enabling an array of viscoelasticity (41). During cell migration, IFs dynamically regulate branched actin formation, focal adhesion turnover, and stress fiber stability to change cell shape and location (10). Whereas fibroblasts usually contain only vimentin, MFs may contain both vimentin and desmin (DES), and PCs may also contain nestin (NES) (9). Outside the nervous system, NES (nes) expression is usually limited to development or regeneration of tissues (52).
Neuropilins (NRPs) are widely expressed transmembrane proteins with receptor and signaling functions, although they lack intrinsic kinase activity (22). Their extracellular domain binds class 3 semaphorins (SEMAs), augmenting signaling through plexins, to guide migration of neurocytes and cells forming the neural crest, heart, and smooth muscle (57). A different portion of the extracellular domain binds peptide growth factors with a terminal arginine, most notably human VEGF-165 (mouse VEGF-164) and platelet-derived growth factor (PDGF)-D (71). This enhances VEGF receptor 2 (VEGFR2), PDGF receptor (PDGFR)-α, and PDGFRβ signaling by recruiting adapter proteins to the NRP1 COOH terminus (55). The COOH-terminal, PDZ domain-binding portion of NRP1 recruits kinase substrates or downstream intermediates to regulate endosomal trafficking.
Pulmonary epithelial, endothelial, and interstitial mesenchymal cells express nrp1, and various strategies have been used to disrupt nrp1 in the lung (30, 31). In pdgfra-expressing pulmonary mesenchymal cells, nrp1 gene expression is higher at P7 than during the late embryonic stages (17, 18). When NRP1 could not bind SEMA3, mice exhibited immature, fragile, misplaced pulmonary microvessels with reduced PC coverage (30). Inducible promoters (surfactant protein C or a universally expressed estrogen receptor Cre) have been used to disrupt nrp1, yielding reversible air space enlargement (31). α-Smooth muscle actin (α-SMA)- and neural/glial antigen-2 (NG2, CSPG4)-containing mesenchymal cells were displaced from the septal tips (30, 31). Although these studies showed that NRP1 is required for development of the gas-exchange surface, several important questions remain. Which mesenchymal populations depend on NRP1 for positioning? Are the MF and PC populations similarly affected, and does one compensate for the other? Which mesenchymal signaling pathways require NRP1, and how do they regulate expansion of the gas-exchange surface? To address these questions, we studied the consequences of nrp1 deletion in pulmonary parenchymal PCs and MFs during secondary septation.
MATERIALS AND METHODS
Materials
Antibodies.
For flow cytometry [fluorescence-activated cell sorting (FACS)], phycoerythrin (PE)-anti-mouse CD304 (NRP1; catalog no. 145203) and PE/Cy7-anti-mouse CD45 (catalog no. 103113) were obtained from BioLegend (San Diego, CA); BV421-rat anti-mouse CD140a (PDGFRα; catalog no. 562774), Alexa Fluor 647-rat anti-mouse CD71 (catalog no. 563504), and Alexa Fluor 647-rat anti-mouse CD9 (catalog no. 564233) from BD Biosciences (San Jose, CA); CD140a-FITC, mouse (clone REA637; catalog no. 130-109-735), CD140b-PE, mouse (clone REA634; catalog no. 130-109-867), anti-NES-allophycocyanin (APC), mouse and rat (clone REA575; catalog no. 130-109-058) from Miltenyi Biotech (Auburn, CA); and mouse monoclonal anti-α-SMA-A405 (clone IA4; catalog no. IC1420V) and goat polyclonal anti-DES (catalog no. AF3844) from R&D Systems (Minneapolis, MN). For laser scanning confocal microscopy immunofluorescence, goat polyclonal anti-PDGFRα (catalog no. AF1062) and goat polyclonal anti-DES (catalog no. AF3844) were obtained from R&D Systems; rat IgG2a,κ-anti-PDGFRβ (catalog no. 136002) from BioLegend; mouse monoclonal anti-α-SMA-Cy3 (catalog no. C6198) and rabbit polyclonal anti-NES (catalog no. SAB4200394) from Sigma-Aldrich (St. Louis, MO); isolectin B4-AF568 (catalog no. I21412) and YO-PRO-1 (catalog no. Y3603) from Thermo Fisher Scientific (Waltham, MA); β-agarase (catalog no. M0392S) from New England Biolabs (Ipswich, MA); donkey anti-rat IgG-A647 (catalog no. 712-605-150) from Jackson ImmunoResearch (West Grove, PA); and Vectashield antifade mounting medium (catalog no. H-1000) from Vector Laboratories (Burlingame, CA). For Western blotting, rabbit anti-phosphorylated (Y410) p130Cas (catalog no. ab55263) was obtained from Abcam (Cambridge, MA); rabbit monoclonal anti-NRP1 (catalog no. 3725), rabbit anti-calveolin-1 (catalog no. 3267), rabbit anti-phosphorylated (Y416) Src (catalog no. 6943), rabbit anti-Src (catalog no. 2109), and rabbit anti-p130Cas (catalog no. 13846) from Cell Signaling Technology (Danvers, MA); rabbit polyclonal anti-PDGFRα (catalog no. sc-431) from Santa Cruz Biotechnology (Dallas, TX); and mouse monoclonal β-tubulin (catalog no. T-7816) from Sigma. For proximity ligation assay, goat polyclonal anti-NRP1 (catalog no. AF566) was obtained from R&D Systems; rabbit polyclonal anti-PDGFRα (catalog no. sc-431) from Santa Cruz Biotechnology; rabbit polyclonal anti-phosphorylated (Y410) p130Cas (BCAR-1; catalog no. ab55263) from Abcam; and Duolink in situ orange starter kit, goat/rabbit (catalog no. DUO92106-1KT) from Sigma Aldrich. Another reagent, recombinant human PDGF-AA (catalog no. 221-AA), was obtained from R&D Systems.
Mice
Pdgfrαtm11(EGFP)Sor/J mice (obtained from Philippe Soriano) have been described elsewhere and are referred to as PDGFRα-GFP mice (34). Production and nuclear localization of eGFP are under the control of the endogenous pdgfrα promoter. GFP expression spatially and temporally recapitulates endogenous pdgfrα gene expression (25). We used heterozygous mice carrying one pdgfrα-GFP allele (which does not encode active PDGFRα) and one functional pdgfrα allele, which are phenotypically identical to wild-type (GFPneg) mice (25). To delete nrp1, we crossed B6.129(SJL)-Nrp1tm2Ddg/J mice (LoxP-flanked exon 2; stock no. 005247, Jackson Laboratories) with B6.129S6-Taglntm2(cre)Yec/J mice (stock no. 006878). The DNA coding Cre recombinase was inserted into exon 1 of transgelin (tagln) and mediates Cre recombination postnatally, but not in the embryo (73). Transgelin is expressed in pulmonary MFs, PCs, and smooth muscle cells (SMCs). Protocols for animal use were approved by the Iowa City Veterans Affairs Medical Center Animal Use Committee (43). Mice with tagln-Cre-mediated deletion of LoxP-flanked pdgfrα are described elsewhere (45). Male and female mice were used.
Isolation of Parenchymal Mesenchymal Cells
Lung fibroblasts were isolated at P8 from heterozygous PDGFRα-GFP and TGCre+/−;Nrp1F/F (the heterozygous tagln-Cre allele excises the LoxP-flanked segment of nrp1 DNA) and littermate TGCre+/−;Nrp1F/− (only 1 nrp1 LoxP-flanked allele) mice for flow cytometry (FACS). The pulmonary parenchymal mesenchymal cells were isolated using a previously reported method involving digestion with collagenase (43). The mesenchymal cells were selected from the dispersed cells on the basis of their adherence to tissue culture dishes for 1 h at 37°C. Nearly all endothelial and epithelial cells fail to adhere during this short period. Nonadherent cells were removed by serial washes with PBS before release of the adherent cells with TrypLE Express. Purity of the released mesenchymal population was assessed by immunostaining for cell markers specific for epithelial (anti-pan-cytokeratin antibody), macrophage (CD 206), and endothelial (CD31) cells (46). Epithelial and endothelial cells comprised ~2.5 and 1.6%, respectively, of the mesenchymal cell population (46).
Analytical Flow Cytometry
Freshly isolated mesenchymal cells were fixed before staining, except when they were used to analyze membrane lipid rafts (MLRs). Fixed cells were permeabilized with 0.1% saponin and then stained for intracellular antigens. CD45pos cells (primarily macrophages, but possibly also fibrocytes) that had adhered to culture plastic were excluded from FACS analysis. Virtually all the PDGFRα-expressing fibroblasts were in the CD45neg fraction (46). Forward and side scatter were used to exclude small (presumably apoptotic) cells and aggregates, and compensation controls were always included to correct for spectral overlap. Events were captured from 2 × 104 gated cells using a flow cytometer (model LSR2, Becton Dickinson) and analyzed using Cell Quest software (BD Biosciences) (46).
Lung Inflation and Fixation
Lungs from TGCre+/−;Nrp1F/F and littermate TGCre+/−:Nrp1F/− control mice were uniformly inflated using fixative containing 0.8% low-melting-point agarose (50 μl/g body wt) and cooled to solidify, and the volume of both lungs was determined by displacement (48). The lungs were sectioned at 1-mm intervals and fixed for 6 h at 4°C in 0.1 M sodium phosphate (pH 7), 15% picric acid, and 1% paraformaldehyde. For immunostaining and imaging by laser scanning confocal microscopy, the 1-mm sections were embedded in OCT and sectioned at 100- or 7-μm intervals. Tissues used for stereological analysis of alveolar surface area and surface-weighted star volume were fixed for an additional 18 h in 4% paraformaldehyde and then dehydrated and embedded in paraffin before they were sectioned at 3.5-μm intervals. The sections were mounted on glass slides and stained with hematoxylin and eosin. Stereological analyses were performed using StereoInvestigator (MicroBrightField, Williston, VT) and the cycloids for surface density (Sv) probe to determine gas-exchange surface area. Distance between the intersections of a series of test lines with the opposing walls of alveolar ducts or alveoli was measured to ascertain the surface-weighted star volume of alveolar ducts and alveoli (58).
Colocalization of α-SMA and NES with PDGFRs
Lungs from TGCre+/−;Nrp1F/F and TGcre+/−;Nrp1F/− control mice were uniformly inflated and fixed, and 7-μm sections were cut using a Cryotome. Agarose that remained after the lung tissue was washed at 50°C and removed by incubation of the tissue with β-agarase before immunostaining. After the tissues were permeabilized with 0.1% Triton X-100 and blocked with 2% normal donkey serum, they were incubated overnight with 5 μg/ml anti-PDGFRα, 2.5 μg/ml anti-PDGFRβ, and either 1:800 dilution of anti-α-SMA-Cy3 or 4 μg/ml anti-NES; controls received goat, rat, and rabbit IgG, respectively, at equivalent concentrations. Secondary antibodies were donkey anti-goat Ig-A488 or anti-rabbit IgG-A568 at 1:1,000 dilution and anti-rat IgG-A647 at 1:800 dilution. Images were acquired at 1,024 × 1,024 pixel density from randomly selected fields using a Zeiss LSM710 confocal microscope equipped with a Zeiss Plan apochromat ×40/1.3 oil differential interference contrast M27 objective, uniform excitation laser power and detector gains, and appropriate excitation and emission filters and averaged from three scans. The uncompressed images were converted to TIF format, and colocalization was analyzed using uniform thresholding for both genotypes and the JACoP plugin Fiji ImageJ2 software (National Institutes of Health) (47).
Portions of uniformly inflated and fixed lungs from five TGCre+/−;Nrp1F/F and five littermate TGCre+/−;Nrp1F/− control mice were sectioned at 100-μm intervals (42) and incubated overnight with goat anti-DES and rabbit anti-NES, washed, and then incubated with donkey anti-rabbit IgG-A568, anti-goat IgG-A647, and anti-αSMA-FITC. After the tissues were washed and mounted, 2.5-μm-interval confocal z stacks were acquired using uniformly applied laser power and detector gains throughout each imaging session. Littermate nrp1-deleted and control mice were always included in the same staining and imaging cohort, enabling comparison between lungs from each sibling pair. AERs were identified by circumferential α-SMA around air space openings that ended with a discrete tissue plane at the base of the alveolus (42). Regions of interest were traced around the circumference of the AER, including the entire breadth of its comprising cells. Images were converted to 8-bit per color channel z stacks, and thresholding and segmentation were applied using Fiji ImageJ2, maintaining uniform criteria within a staining session. The aggregate area and integrated intensity of NES- or DES-containing pixels were determined for all alveoli or alveolar ducts within the imaged fields (4 for each tissue section for each mouse, with 5 mice used with each genotype).
Proximity Ligation Assay to Quantify Interactions Between NRP1 and PDGFRα or p130Cas
Mesenchymal cells were isolated and released from the tissue culture plastic with TrypLE Express, counted, and plated on fibronectin-coated glass (47). To identify protein interactions between NRP1 and PDGFRα or p130Cas, we performed proximity ligation assay, which identifies two proteins residing within a 40-nm radius. We used the Duolink (Olink Bioscience, Sigma Aldrich) kit according to the manufacturer’s instructions, as previously described, with the following modifications (47). Some cultures were incubated for 10 or 30 min with 50 ng/ml PDGF-A before fixation (49). To highlight the cytoplasm, F-actin was stained with Acti-stain 488-phalloidin and nuclei with TO-PRO-3. Granules were enumerated to calculate the mean number per cell.
Analysis of PDGFRα and NRP1 in MLRs
Distal pulmonary mesenchymal cells were isolated and cultured to confluence in 100-mm dishes, and some were stimulated with 20 ng/ml PDGF-A for 15 min. The washed cell layers were immediately placed on ice, and subsequent procedures were conducted on ice in an ambient cold (4°C) room. Cell layers from three dishes were combined, scraped, and recovered in 25 mM NaPhos (pH 7.4), 90 mM NaCl, 2 mM EDTA, 50 mM NaF, and 1 mM sodium orthovanadate containing 1% (by volume) protease inhibitor cocktail (catalog no. P8340, Sigma-Aldrich), 1 mM PMSF, and 1% Triton X-100. The cell layers were homogenized by extrusion through a 22-gauge needle, held on ice for 30 min, and then centrifuged at 120 g to remove nuclei and larger debris. The supernatants were layered on a discontinuous gradient of OptiPrep (catalog no. D1556, Sigma) and centrifuged at 200,000 g in a rotor (model SW55ti, Beckman) at 4°C for 4.25 h. Serial (numbered from the top to the bottom of the tube) 0.3-ml fractions were removed, equal volumes were subjected to SDS-PAGE in a 4–20% linear gradient denaturing polyacrylamide gel, and NRP1, PDGFRα, and caveolin were detected using immunoblotting.
The distribution of NRP1 and PDGFRα in MLRs was also analyzed in mesenchymal cells immediately after isolation from the lungs of PDGFRα-GFP mice at P8. Cells were kept on ice during all treatments and stained before fixation with 0.5 μg/ml BV421-anti-CD140a, 0.75 μg/ml anti-NRP1 (CD304), 0.5 μg/ml anti-CD9 (marker of MLRs), 0.5 μg/ml anti-CD71-A647 (transferrin receptor, not found in MLRs), and 0.4 μg/ml anti-CD45-PE-Cy7 for 1 h at 4°C in PBS with 3 mg/ml BSA and 2% goat serum. After the cells were washed, some were resuspended in 0.1% Triton X-100 for 5 min at 4°C and then immediately washed with PBS, and others were held on ice in the absence of Triton X-100; then all cells were fixed in 2% paraformaldehyde for 15 min and washed before FACS analysis.
Western Immunoblotting of Cell Lysates from Primary Cultures of Distal Mesenchymal Cells
Cells were isolated by differential adherence to tissue culture plastic and cultured to near confluence. Cells from wild-type mice were used, and the FBS concentration was reduced to 2% 16 h before 15 min of incubation with 50 ng/ml PDGF-AA. Cultured cells from TGCre+/−;Nrp1F/F mice were transduced with Ad5-CMV-empty or Ad5-CMV-Cre for 6 h terminating 54 h before stimulation with PDGF-AA. Replication-deficient adenovirus-5 (Ad5) constructs using the CMV promoter to transcribe Cre recombinase or an empty cassette were obtained from the University of Iowa Viral Vector Core. The cell layers were washed with PBS, cooled to 4°C, and lysed in situ in the presence of phosphatase and protease inhibitors (47). After protein quantification, the samples were subjected to SDS-PAGE, immunoblotting, and enhanced chemiluminescence detection (47). The fluorograms were digitally imaged, and the densities were quantified using ImageJ2.
Migration of Confluent Lung Fibroblasts into an Adjacent Empty Space
Vitronectin-coated glass sticky-Slides (Ibidi, Verona, WI) were fitted with silicone inserts that divide each well into two segments. After removal of the insert, a 500-µm gap remained, and viral stocks were diluted to a final multiplicity of infection of 25, incubated with cell monolayers for 6 h, and removed by washing; transduction was allowed to proceed for 36 h, as described in detail elsewhere (44). During the final 16 h before removal of the insert and initiation of time-lapse imaging, the FBS concentration was reduced to 2%, where it remained during imaging, when the HEPES concentration was increased to 30 mM and some wells were supplemented with PDGF-A to 20 ng/ml.
Statistical Methods
Box plots extend from the 25th to the 75th percentile, and whiskers were plotted based on Tukey’s interquartile range using GraphPad Prism; n is the number of different mice that were used or the number of different experiments that were performed using cell cultures (59). Analysis of variance (1- or 2-way) was performed using Systat software (Chicago, IL), and Student’s t-test (paired or unpaired) was performed using Microsoft Excel. P < 0.05 was considered significant.
RESULTS
PDGFRα-Expressing Mesenchymal Cells Also Contain PDGFRβ
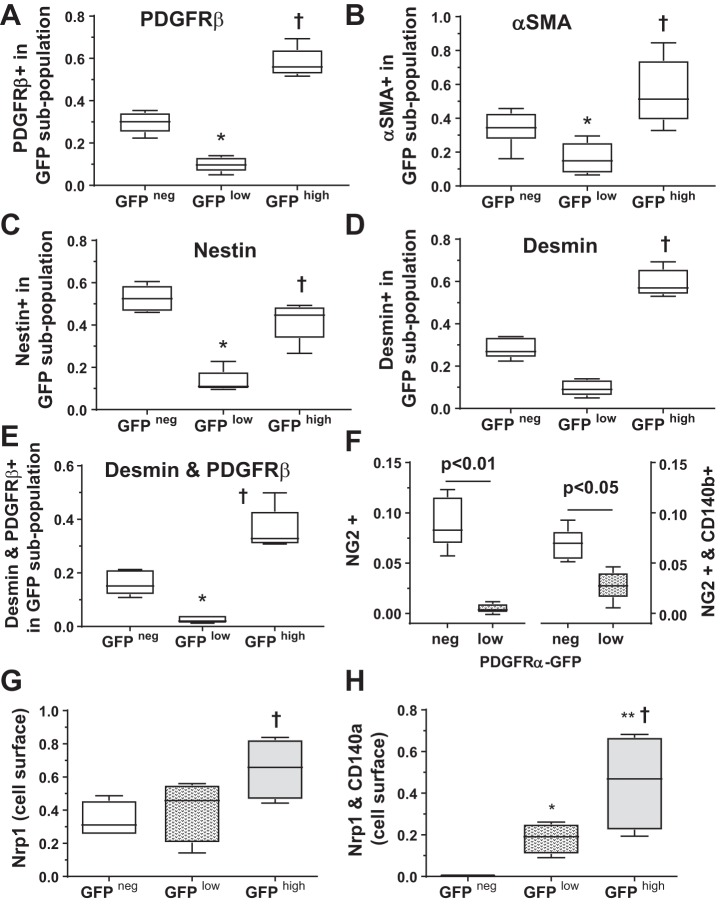
We previously classified pulmonary parenchymal cells from Pdgfrαtm11(EGFP)Sor/J (PDGFRα-GFP) and TGCre+/−;R26dTomato mice as follows: 1) those that bore both the GFP marker of pdgfra gene expression and the stop-Flox dTomato marker activated by transgelin (tagln) Cre and 2) those that bore only dTomato (46). The abundance of both populations decreased with age. This demonstrated that some, but not all, tagln-expressing cells also expressed pdgfra. We suspected that the dTomato+;GFPneg mesenchymal cells may be PCs, but they were not characterized. Because tagln is expressed in smooth muscle-like cells, we isolated parenchymal mesenchymal cells, which adhere to plastic within 1 h, whereas endothelial and epithelial cells do not. Approximately 15% of the adherent cells were macrophages, but these CD45+ cells were excluded from flow cytometric analyses and do not remain adherent in culture (46). Mesenchymal cells were isolated from heterozygous PDGFRα-GFP mice, which express the nuclear-targeted H2B-eGFP fusion protein when and where pdgfra is actively expressed. Using FACS, we distinguished three alveolar mesenchymal cell subpopulations based on the intensity of GFP fluorescence: GFPneg, GFPlow, and GFPhigh. If pdgfrb is exclusively expressed in PCs but not MFs, we reasoned that a minority of GFPhigh cells would stain for PDGFRβ. However, a majority of GFPhigh cells were also positive for PDGFRβ, indicating that MFs and PCs cannot be distinguished solely on the basis of their PDGFR profile (Fig. 1A). A significantly smaller proportion of GFPlow cells, which accumulate lipid droplets, contained PDGFRβ (46). A significantly larger proportion of GFPhigh than GFPneg cells contained ACTA2 (α-SMA), and a smaller proportion of GFPlow than either GFPneg or GFPhigh cells were α-SMApos (Fig. 1B). IF proteins are differentially expressed in PCs and MFs. Whereas both MFs and PCs contain vimentin, NES has been more frequently observed in PCs, and DES is more abundant in alveolar ducts, which accumulate GFPhigh cells (42, 69). A significantly larger proportion of GFPneg than either GFPlow or GFPhigh cells contained NES, whereas a larger proportion of GFPhigh cells contained DES (Fig. 1, C and D). As expected, the proportion of cells containing both DES and PDGFRβ was highest in the GFPhigh population (Fig. 1E). The PC marker NG2 (CSPG4) was most abundant in the GFPneg population (Fig. 1F) and was not detectable in the GFPhigh population, so only the GFPlow population is shown. A significantly larger proportion of GFPhigh mesenchymal cells externally displayed NRP, particularly among those that also had CD140a on their surface (Fig. 1, G and H). Because NRP1 regulates cardiovascular mesenchymal progenitors, we examined how NRP1 affects the phenotype of alveolar mesenchymal cells (66).
Fig. 1.
Flow cytometry [fluorescence-activated cell sorting (FACS)] demonstrated that platelet-derived growth factor (PDGF)-α receptor (PDGFRα)-expressing parenchymal cells contain neuropilin-1 (NRP1), PDGFRβ, desmin (DES), and nestin (NES). Parenchymal mesenchymal cells were isolated at postnatal day 8 from 5 mice, all from different litters, fixed, permeabilized, and stained for PDGFRβ, ACTA2 [α-smooth muscle actin (α-SMA)], NES, DES, and CD45 (a negative selection marker that excluded fibrocytes and macrophages). Cells were gated based on their level of GFP intensity, yielding 3 subpopulations: GFPneg (non-GFP-expressing) and GFPlow and GFPhigh, which had lower and higher levels of eGFP, respectively. A–E: proportion of each subpopulation that contains PDGFRβ [CD140b (A)], α-SMA [ACTA2 (B)], NES (C), DES (D), and DES and PDGFRβ (E). F: in a different cohort of 5 PDGFRα-GFP mice from 3 different litters, isolated mesenchymal cells were stained for neural/glial antigen-2 [NG2 (CSPG4)] and CD140b and analyzed by FACS. G and H: in a 3rd cohort of 4 mice from different litters, mesenchymal cells were isolated, fixed but not permeabilized, and analyzed by FACS to assess the abundance of cells with NRP1 and PDGFRα (CD140a) on the cell surface. Box-and-whisker (Tukey) plots show data from 5 (A–E) and 4 (F–H) separate cell isolations. *P < 0.05, GFPlow vs. GFPneg; †P < 0.05, GFP high vs. GFPneg; **P < 0.05, GFPhigh vs. GFPlow (by 2-way ANOVA).
Alveolar Structure of Mice with nrp1 Gene Deletion in Smooth Muscle-Like Parenchymal Cells
We queried whether tagln-Cre-mediated nrp1 gene deletion would differentially affect PC and PDGFRαpos MFs. We also reasoned that targeting smooth muscle-like mesenchymal cells might inform whether NRP1 influences secondary septal formation, which could not be addressed using germ-line deletion of the SEMA3-binding domain (31). Le and associates showed that deletion of nrp1 in alveolar epithelial cells of 6-wk-old mice rendered them more susceptible to cigarette smoke-induced emphysema, but in the absence of smoke exposure, epithelial-targeted nrp1 deletion did not alter alveolar structure (39). The body weights of TGCre+/−;Nrp1F/F mice were 6.7 ± 0.7 g (n = 7), 8.2 ± 0.7 g (n = 4), and 18.1 ± 1.7 g (n = 7) at P12, P21, and P42, respectively, whereas at the corresponding ages the weights of the TGcre+/−;Nrp1F/− controls were 7.4 ± 0.5 g (n = 7), 9.5 ± 1.3 g (n = 4), and 21.9 ± 3.0 g (n = 7), respectively [P = 0.049 at P12 and P = 0.03 at P42 and P21 (not significant), by unpaired t-test]. Other than the ~15% lower body weight, there were no other grossly obvious abnormalities. TGCre-mediated deletion significantly reduced, but did not eliminate, nrp1 gene expression in distal lung mesenchymal cells that had been isolated at P8 (Fig. 2B).
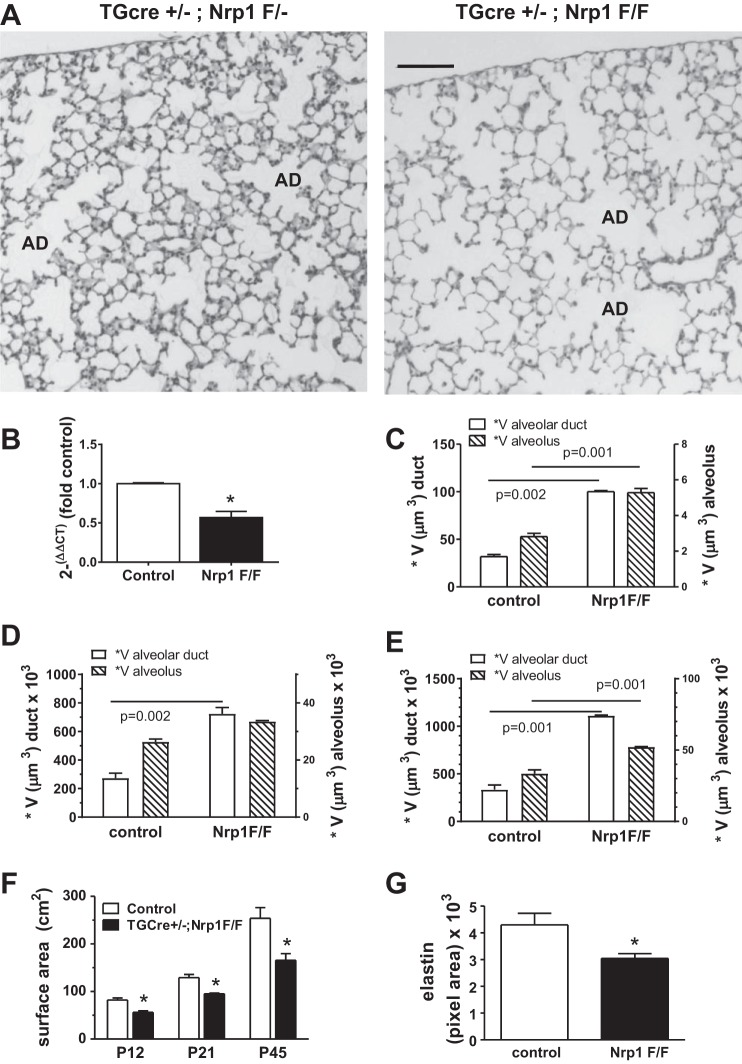
Fig. 2.
Transgelin (tagln)-targeted deletion of neuropilin-1 (nrp1) in myofibroblasts and pericytes reduces gas-exchange surface area. A: lungs from TGCre+/−;Nrp1F/F mice and littermate TGCre+/−;Nrp1F/− controls were fixed at postnatal day 21 (P21) at uniform inflation and stained with hematoxylin and eosin, and stereological analyses were performed. AD, alveolar duct. Scale bar = 100 μm. B: RNA was obtained from alveolar mesenchymal cells, which were isolated at P8 from TGCre+/−;Nrp1F/− (control) and littermate TGCre+/−;Nrp1F/F (Nrp1F/F) mice. Levels of NRP1 mRNA were determined by quantitative RT-PCR. Values are means ± SE; n = 5 mice for each genotype. *P < 0.05 (by paired t-test). C–E: surface-weighted star volume (*V) of alveolar ducts and alveoli was analyzed in paraffin-embedded sections from Nrp1F/F and littermate control lungs fixed at P12 (C), P21 (D), and P45 (E). Values are means ± SE; n = 4 mice for each genotype at each age (by paired t-test). F: the same lungs used in C–E were analyzed using the cycloids for surface density (Sv) stereological probe, and gas-exchange surface areas of control (TGCre+/−;Nrp1F/−) and Nrp1F/F mice were compared. Values are means ± SE; n = 4 pairs of mice at each age. *P < 0.01 (by 2-way ANOVA). G: uniformly inflated lungs from Nrp1F/F and littermate control mice at P45 were stained with Verhoeff-van Gieson, and pixel areas of elastic fibers in alveolar ducts were compared in 10 ducts for each mouse and 3 separate mice for each genotype. Values are means ± SE; n = 3 pairs from 3 separate litters. *P < 0.05 (by paired t-test).
Stereological examination of lungs from TGCre+/−;Nrp1F/− control and littermate TGCre+/−;Nrp1F/F mice was performed at P12, P21, and P42, and representative fields at P21 are shown in Fig. 2A. The surface-weighted star volume of alveolar ducts and alveoli was determined at all three ages (Fig. 2, C–E). In TGCre+/−;Nrp1F/F mice, the surface-weighted star volume, which reflects the orthogonal distance between opposing ductal or alveolar walls and, therefore, the size of the intervening air space, was significantly higher in nrp1-deleted lungs for both alveolar ducts and alveoli (Fig. 2, C–E). Correspondingly, the gas-exchange surface area was smaller in TGCre+/−;Nrp1F/F mice at all ages (Fig. 2F). The pixel area occupied by elastic fibers in the walls of alveolar ducts was lower in nrp1-deleted mice, commensurate with the diminished surface area occupied by these structures (Fig. 2G). Therefore, disruption of nrp1 gene expression in mesenchymal cells disturbed alveolar architecture, indicating that mesenchymal NRP1 is important for alveolarization.
Deletion of nrp1 Selectively Disrupts PDGFRβ and Cytoskeletal Proteins in Mesenchymal Cells Without PDGFRα (CD140a)
Parenchymal mesenchymal cells were isolated at P8 from TGCre+/−;Nrp1F/F and TGCre+/1;Nrp1F/− control littermates and analyzed using FACS. CD45neg cells were gated into CD140aneg and CD140apos populations, and the proportions of mesenchymal cells staining for CD140b (PDGFRβ), α-SMA, NES, or DES were ascertained for each population (Fig. 3). Deletion of nrp1 reduced the proportions of PDGFRβpos, α-SMApos, NESpos, and DESpos cells only in the CD140aneg population. Although a larger proportion of PDGFRα-GFPhigh than PDGFRα-GFPneg mesenchymal cells expressed PDGFRβ, α-SMA, and DES (Fig. 1), nrp1 deletion only reduced the proportions of cells bearing these antigens in the CD140aneg subpopulation. The abundance of NG2 was greater in the CD140aneg than CD140apos mesenchymal cell population in control lungs, but not in mesenchymal cells bearing the nrp1 deletion. Deletion of nrp1 led to a significant increase in the proportion of NG2pos cells in the CD140apos population. In their perivascular niche, PCs support the endothelial barrier function; however, they also may serve as oligopotent progenitors, giving rise to muscle, adipose, or neuronal tissues (6). PCs universally express PDGFRβ, but they may also express PDGFRα, particularly in profibrotic states (29).
Fig. 3.
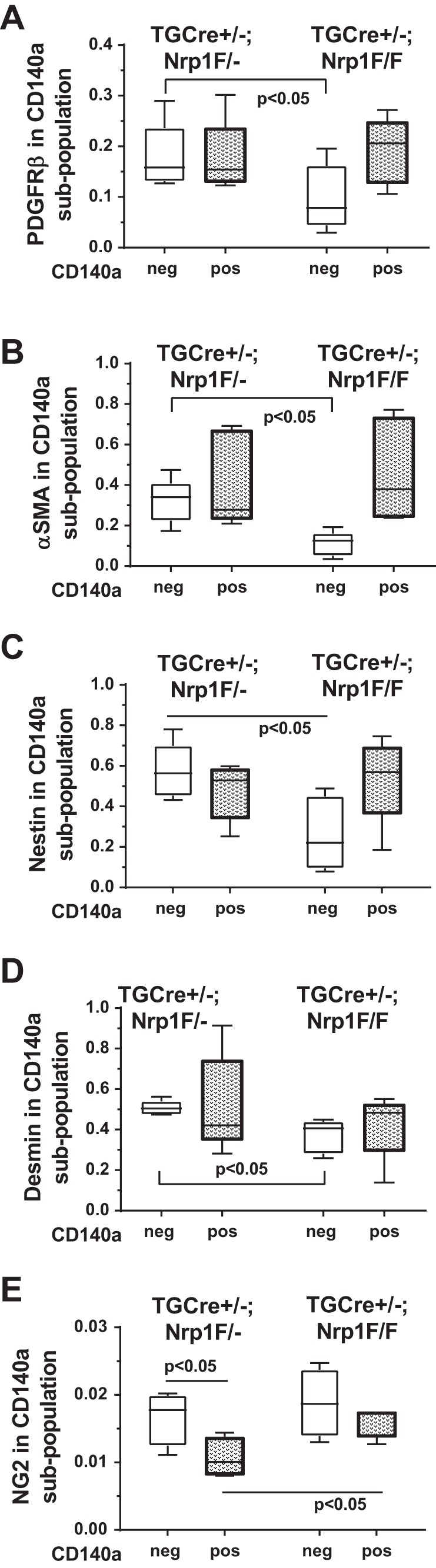
Targeted neuropilin-1 (nrp1) deletion preferentially alters transgelin (tagln)-expressing mesenchymal cells lacking platelet-derived growth factor (PDGF)-α receptor [PDGFRα (CD140a)]. A−D: fibroblasts were isolated at postnatal day 8 from 5 TGCre+/−;Nrp1F/F mice and 5 littermate TGCre+/−;Nrp1F/− controls (obtained from 4 separate litters) and analyzed as described in Fig. 1 legend, including negative selection for CD45; however, PDGFRα-containing cells were identified by staining with anti-CD140a (PDGFRα). Ordinate scale shows proportion of a population (designated along the abscissa) that stains for a particular marker: PDGFRβ (A), α-smooth muscle actin [α-SMA (B)], nestin [NES (C)], and desmin [DES (D)]. E: in a different cohort of 4 TGCre+/−;Nrp1F/F mice and 4 littermate controls, isolated mesenchymal cells were stained with neural/glial antigen-2 (NG2) and PDGFRα (CD140a) and analyzed by fluorescence-activated cell sorting. Box-and-whisker (Tukey) plots show data from 5 (A−D) or 4 (E) separate isolations. CD140aneg and CD140apos populations were compared across genotypes by 2-way ANOVA.
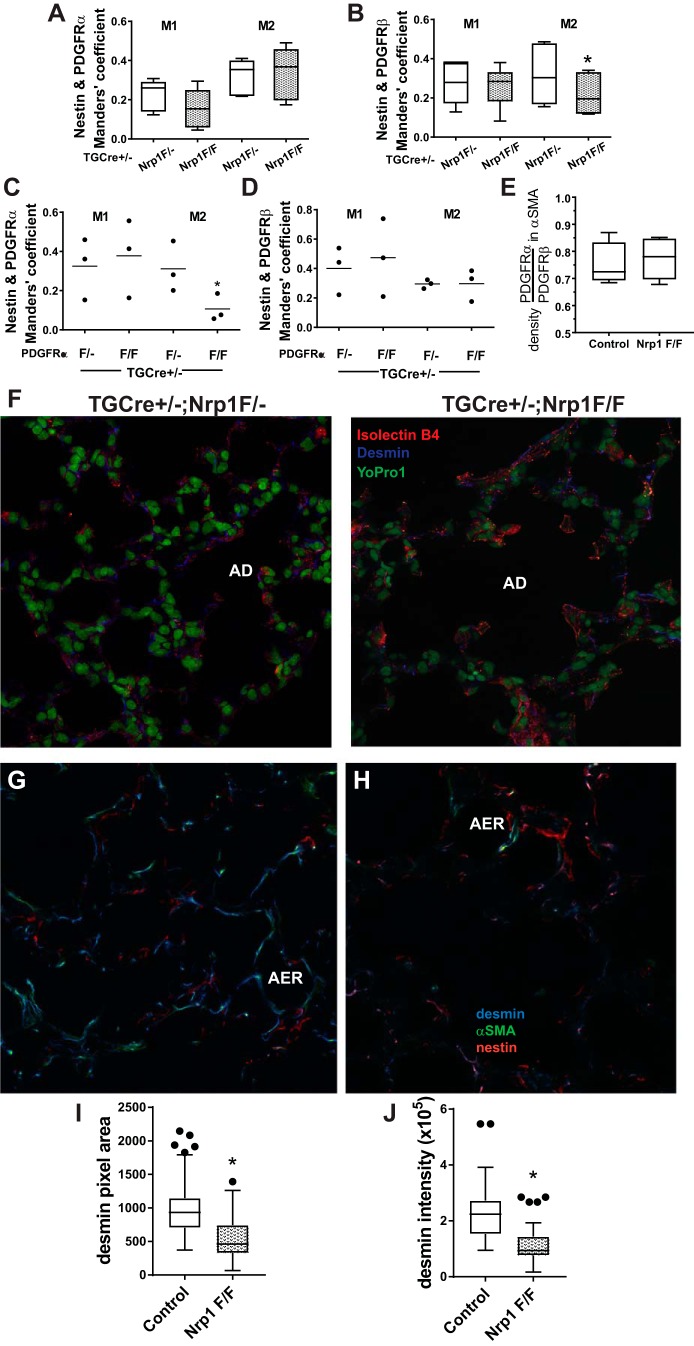
Colocalization of NES with PDGFRβ Is Reduced and DES Is Abnormally Distributed in Lungs of nrp1-Deleted Mice
To confirm our observations using isolated pulmonary mesenchymal cells, we analyzed the alveolar colocalization of NES with PDGFRα or PDGFRβ at P12. The conditions for imaging and thresholding were consistently applied to nrp1-deleted mice and their littermate controls, and colocalization was assessed using Mander’s coefficients (14). Colocalization of NES with PDGFRα was not altered (Fig. 4A), whereas fewer pixels occupied by PDGFRβ were also occupied by NES in mice bearing the targeted nrp1 deletion (Fig. 4B). Because NES is not observed in epithelial cells and endothelial cells do not contain both NES and PDGFRβ, NES resides within interstitial mesenchymal cells. We also analyzed colocalization of NES and PDGFRα in mice bearing a tagln-Cre-mediated deletion of PDGFRα. As expected, when PDGFRα was deleted, colocalization of NES with PDGFRα (M2) was reduced (Fig. 4C). In nrp1-deleted mesenchymal cells, the abundance of PDGFRα relative to PDGFRβ overlapping α-SMA did not differ between TGCre+/−;Nrp1F/F and TGCre+/−;Nrp1F/− control mice (Fig. 4E).
Fig. 4.
Nestin (NES) and desmin (DES) are differentially distributed in mesenchymal cells after neuropilin-1 (nrp1) gene deletion. A−H: at postnatal day 12 (P12), lungs from TGCre+/−;Nrp1F/F mice and littermate TGCre+/−;Nrp1F/− controls were uniformly inflated and fixed, and 7-μm frozen sections were prepared. Tissues were immunostained for platelet-derived growth factor (PDGF)-α receptor (PDGFRα), PDGFRβ, and NES and imaged using confocal microscopy with uniform parameters within staining and imaging sessions, which always included both paired littermates. Consistent thresholding criteria and the ImageJ2 JACoP plugin were used to calculate Mander’s coefficients for the proportion of pixels occupied by one of the PDGFRs (PDGFRα or PDGFRβ) that overlap pixels occupied by NES (M1) or the proportion of pixels occupied by NES that overlap pixels occupied by one of the PDGFRs (M2). Box-and-whisker (Tukey) plots show data from lungs of 5 separate mice for each genotype; Manders’ coefficients for TGCre+/−;Nrp1F/F mice are compared with those of their respective TGCre+/−;Nrp1F/− littermate mice. *P < 0.01 for comparison of M2 for NES within PDGFRβ (by 2-way ANOVA). C and D: lungs from 3 TGCre+/−;PDGFRαF/F and 3 TGCre+/−;PDGFRαF/− littermate control mice were prepared and analyzed as described in A. Manders’ coefficients for TGCre+/−;PDGFRα1F/F mice were compared with controls. *P < 0.05 for comparison of M2 for NES within PDGFRα. E: sections prepared from the same mice used for experiments described in A and B were stained for α-smooth muscle actin (α-SMA), PDGFRα, and PDGFRβ. Consistent criteria were used to threshold pixels containing α-SMA, and intensities of PDGFRα or PDGFRβ within α-SMA-containing pixels were ascertained. Data are presented as the ratio of PDGFRα intensity to PDGFRβ intensity within α-SMA-containing pixels. Box-and-whisker (Tukey) plots show data from 5 separate mice for each genotype. F: sections of lungs from TGCre+/−;Nrp1F/− and TGCre+/−;Np1F/F mice at P21 were stained for DES, the endothelial marker isolectin B4, and the nuclear stain YO-PRO-1 and imaged using confocal microscopy. Images are representative of those obtained from 4 fields using sections prepared from 3 mice of each genotype. AD, alveolar duct. G−J: images of confocal z stacks from 100-μm sections from 5 TGCre+/−;Nrp1F/F and 5 littermate TGCre+/−;Nrp1F/− control mice immunostained for DES, NES, and α-SMA. For image analysis, uniform thresholding and segmentation criteria were applied to compare nrp1-deleted and control lungs analyzed within the same staining and imaging session. G and H: representative images for lungs from control (G) and nrp1-deleted (H) mice at z levels that contain an alveolar entry ring (AER). Pixel areas and integrated intensities of pixels occupied by DES were ascertained for all 5 littermate pairs, obtained from 4 separate litters. I and J: areas and intensities were pooled for all AERs analyzed for each genotype (n = 73 TGCre+/−;Nrp1F/− and n = 56 TGCre+/−;Nrp1F/F). *P < 0.05 (by Student’s t-test for unpaired variables and Bonferroni’s correction for multiple comparisons). ●, Outliers.
Using mice bearing an inactivating germline mutation in the SEMA3-binding portion of nrp1, Joza and associates concluded that vascular defects accounted for pulmonary air sac disruption (30). To evaluate the vascular consequences of nrp1 deletion in tagln-expressing pulmonary mesenchymal cells (PCs and MFs), we stained lungs from mice at P21 for DES and the endothelial marker isolectin B4. The distribution and apparent abundance of endothelial cells in the gas-exchange region did not differ between control and TGCre+/−;Nrp1F/F mice. However, casual inspection suggested that DES was less abundant in the gas-exchange region of lungs from mice bearing the nrp1 deletion. This was systematically evaluated using confocal microscopy to image z stacks of 100-μm sections, which enabled identification of AERs, and the circumference of alveolar ducts (42). Representative slices containing AERs in lungs from control and nrp1-deleted mice are shown in Fig. 4, G and H. Application of uniform thresholding and segmentation criteria to analyze the pixel areas (Fig. 4I) and to integrate pixel intensities (Fig. 4J) showed that DES was less abundant in AERs of nrp1-deleted than control lungs. The area and integrated intensities of DES in the circumference of alveolar ducts did not differ between the two genotypes (not shown). Therefore, FACS and confocal microscopy demonstrated that nrp1 deletion alters the abundance of IFs in mesenchymal cells.
PDGF-A Signaling Coordinates Interactions Between PDGFRα and NRP1
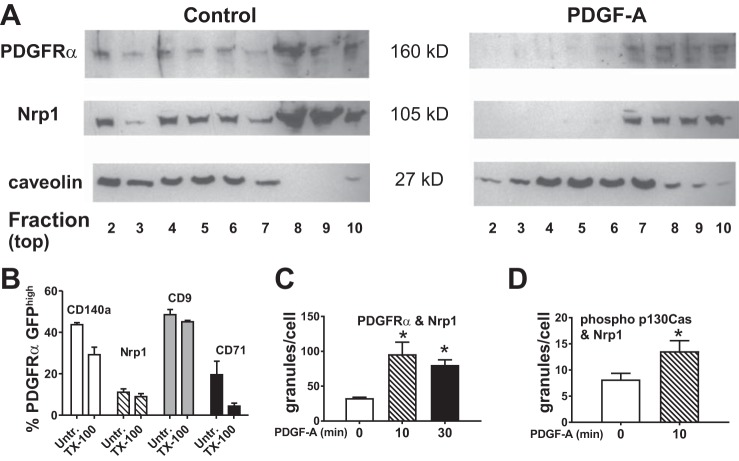
The NRP1 extracellular b1 domain contains a binding site that interacts with receptor tyrosine kinases, including PDGF-D and VEGF-(164) [VEGF-(165) in humans] to recruit the adapter protein p130Cas (72). Others have shown that NRP1 enhances PDGFRα-dependent phosphorylation of p130Cas, but the mechanism is unknown (3, 55). Lung fibroblasts from wild-type mice were isolated and cultured at P12 and stimulated with PDGF-A. The cell layers were used to prepare MLRs, which were fractionated by isopycnic ultracentrifugation and subjected to SDS-PAGE. Immunoblotting was performed to determine whether NRP1 or PDGFRα sedimented at the same density as caveolin, a marker of MLR (Fig. 5A). We observed that a portion of both PDGFRα and NRP1 partitioned with caveolin and that PDGFRα and NRP1 moved into higher-density fractions after exposure to PDGF-A. Using flow cytometry, we also evaluated MLRs in freshly isolated lung mesenchymal cells from Pdgfrαtm11(EGFP)Sor/J mice at P8. The percentages of PDGFRα-GFPhigh cells that retained NRP1 or PDGFRα in detergent-resistant membrane domains (identified by the MLR marker CD9) are shown in Fig. 5B. A majority of the cells that bore PDGFRα or NRP1 (untreated) also bore these antigens after treatment with Triton X-100, indicating that they resided in MLRs (61).
Fig. 5.
Platelet-derived growth factor (PDGF)-A promotes interactions between PDGF receptor (PDGFR)-α and neuropilin-1 (NRP1). Distal pulmonary mesenchymal cells from wild-type C57BLK/6J mice at postnatal day 12 were isolated by differential adherence to tissue culture plastic (which selects for fibroblasts and pericytes) and cultured. Confluent cells that had been exposed to PDGF-A or remained unexposed were used to isolate membrane lipid rafts (MLRs), and samples were fractionated in a discontinuous gradient of OptiPrep. Gradient fractions are numbered from the least-dense fraction 1 [containing buoyant Triton X-100-resistant membrane (cholesterol-rich fraction)] to the more-dense fraction 10 (cytosolic proteins). A: caveolin is retained in MLRs, whereas exposure to PDGFR-A shifts NRP1 and PDGFRα to more-dense fractions. Immunoblot is representative of cell isolations from 2 additional litters. B: freshly isolated distal pulmonary mesenchymal cells obtained from 4 mice from separate litters were stained for PDGFRα (CD140a), NRP1, CD9 (marker of caveolin-rich MLRs), or CD71 (marker of clathrin-coated vesicles), briefly exposed to Triton X-100 (TX-100) or remained unexposed (Untr), fixed, and then analyzed by fluorescence-activated cell sorting. A majority of CD140a and NRP1 antigens were resistant to Triton X-100, indicating that they reside in MLRs. C and D: pulmonary mesenchymal cells isolated from Pdgfrαtm11(EGFP)Sor/J mice adhered to fibronectin-coated glass. Duolink proximity ligation assay was used to quantify cellular granules in cultures treated with PDGF-A for 0−30 min. Values are means ± SE; n = 4 separate cell isolations of granule counts from 3 separate experiments for comparisons between 2 treatment conditions. *P < 0.05 [by 1-way ANOVA (C) and t-test for paired variables (D)].
We used the proximity ligation assay to assess the propinquity (residence within a 40-nm radius) between NRP1 and components of the PDGFRα signaling pathway. Stimulation of subconfluent cultured wild-type mesenchymal cells with PDGF-A increased the proximity between NRP1 and PDGFRα or between NRP1 and phosphorylated p130Cas, suggesting that NRP1 is recruited along with p130Cas during PDGFRα signaling (Fig. 5, C and D).
Deletion of nrp1 Reduces Migration of Cultured Pulmonary Mesenchymal Cells
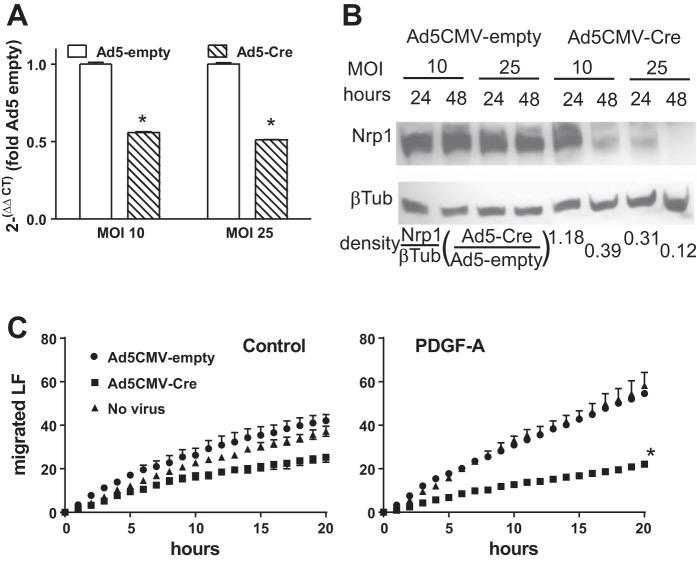
We previously showed that PDGF-AA and PDGFRα signal through Src kinase and β-PIX/paxillin to increase Rac1-GTP and cell migration (49). During SMC migration, PDGF-A initiates complex formation between PDGFRα and NRP1, which recruits Src and the adapter protein p130Cas to the NRP1 intracellular domain (3, 55). Using adenovirus with a CMV promoter to drive Cre, we depleted nrp1 in mesenchymal cells isolated from the lungs of Nrp1F/F mice and quantified cells migrating away from a confluent monolayer. By 48 h after introduction of Cre, nrp1 was reduced by ~85% (Fig. 6A). Depletion of nrp1 significantly reduced cell migration during the final 16 h of the 48-h period after transduction. Therefore, like vascular SMCs, NRP1 regulates PDGF-A-stimulated migration of isolated mesenchymal cells. NRP1 may augment PDGF-A-stimulated mesenchymal migration by coordinating PDGFRα signaling at MLRs, assembling cytoplasmic adapter proteins at the membrane, or regulating the composition of IF proteins, or a combination of these actions.
Fig. 6.
Disruption of neuropilin-1 (nrp1) gene expression in cultured mesenchymal cells impaired their migration. Parenchymal pulmonary mesenchymal cells were isolated from lungs of Nrp1F/F mice at postnatal day 10 (P10) or P12 and cultured. Cre recombinase was introduced using adenovirus 5 (Ad5) and the CMV promoter driving transcription of Cre recombinase (Ad5-CMV-Cre) or an empty cassette (Ad5-CMV-empty) at multiplicity of infection (MOI) of 10 or 25. A: Ad5-Cre significantly reduced NRP1 mRNA by 12 h and NRP1 protein by 24 and 48 h. Values are means ± SE; n = 3 separate cell isolations. *P < 0.01 (by unpaired t-test). B: density of NRP1 relative to density of β-tubulin (βTub) for that lane. For cultures that received Ad5-CMV-Cre, this ratio was expressed relative to that for the culture that was comparably transduced with Ad5-CMV-empty and is shown below the 4 lanes. Results are representative of 2 other similar experiments. C: fibroblasts were isolated from lungs of Nrp1F/F mice and adhered to Vitronectin-coated glass within 8-well sticky-Slide chambers. At 24 h after transduction with Ad5-CMV-Cre or Ad5-CMV-empty, lung fibroblasts (LF) were allowed to migrate to an intervening empty space in the presence or absence (control) of platelet-derived growth factor (PDGF)-A. Some wells received no virus. Cumulative number of cells that had entered the previously empty space is plotted at 1-h intervals. Values are means ± SE; n = 3 separate cell isolations from separate litters for each genotype. *P < 0.01 vs. Ad5-empty (medium containing PDGF-A) (by repeated-measures ANOVA).
Deletion of Nrp1 Reduces PDGFRa Signaling Through Src Kinase
To learn how nrp1 deletion modifies PDGF-A signaling pathways, we examined the phosphorylation of PDGFRα downstream targets. Stimulation of primary cultures of wild-type lung mesenchymal cells with PDGF-A increased phosphorylation of Src (Fig. 7A). Next, cultured cells from the lungs of TGCre+/−;Nrp1F/F mice were transduced with Ad5-CMV-Cre or a control virus not bearing Cre (Ad5-CMV-empty). After 60 h, the cultures were stimulated with PDGF-A, and cell lysates were subjected to phosphoprotein immunoblotting. PDGF-A increased phosphorylated Src in control cultures, but not after Cre-mediated nrp1 deletion. This suggests that NRP1 promotes Src phosphorylation, which, through its downstream targets focal adhesion kinase and Crk, may support cell migration.
Fig. 7.
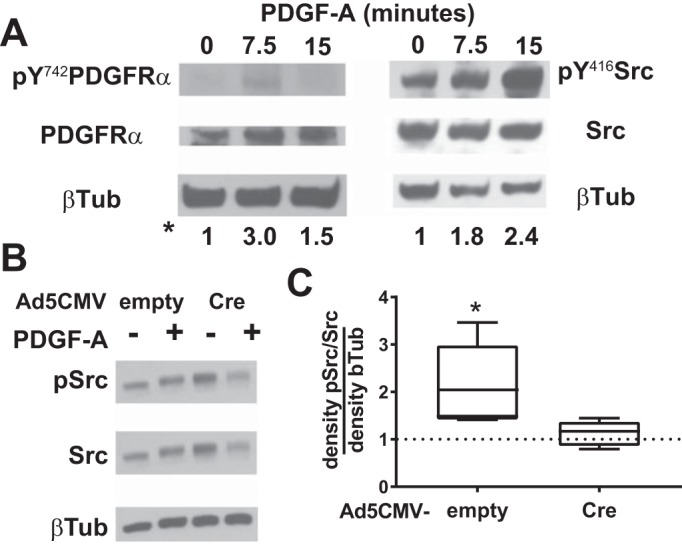
Neuropilin-1 (NRP1) regulates platelet-derived growth factor (PDGF) receptor (PDGFR)-α signaling through Src kinase. A: pulmonary mesenchymal cells were isolated at postnatal day 10 (P10) or P12 from wild-type mice. B: cells were isolated from Nrp1F/F mice, cultured, and transduced with Ad5-CMV-empty (control) or Ad5-CMV-Cre to delete nrp1. Transduced cells were incubated with and without PDGF-A for 15 min, and cell layers were harvested and subjected to Western immunoblotting. Membranes were probed for phosphorylated (Y742) PDGFRα and PDGFRα or phosphorylated (Y416) Src and Src and then for β-tubulin (βTub) to account for inadvertent differences in protein loading. Proteins were detected with enhanced chemiluminescence, and densities of the appropriate bands on fluorograms were quantified. Density of a particular phosphoprotein is expressed relative to the total abundance of that protein and then divided by the density of β-tubulin in that sample. Ratios shown below the lanes in A are representative of 2 other similar experiments. C: summary of densities of 4 other experiments, as well as that shown in B. *P < 0.05 vs. PDGF-A treatment without or without nrp1 deletion (by t-test for unpaired variables).
DISCUSSION
Neither NES nor how it is altered by mesenchymal nrp1 gene deletion has been studied in detail during alveolar septation. Using FACS to analyze freshly isolated mesenchymal cells, we observed NES in pdgfra-expressing and -nonexpressing populations (Fig. 1), which was confirmed by colocalization using confocal microscopy (Fig. 4). Deletion of nrp1 primarily altered the proportions of NES- and DES-containing cells within the PDGFRα (CD140a)neg and PDGFRβ (CD140b)pos populations, which more likely comprised PCs (Fig. 3). This is consistent with findings from studies of mural cells in the brain, heart, and skeletal muscle (21, 68). Less DES was observed in the AERs, and the gas-exchange surface area was lower in mice bearing the tagln-targeted nrp1 deletion. This indicates that NRP1 regulates DES in mesenchymal cells at the septal tips, although it does not reduce the proportion of DES-containing PDGFRαpos cells within the parenchyma as a whole (Fig. 3D). The finding that DES was not reduced at the circumference of the alveolar ducts (corresponding to the primary septum) argues that DES-containing cells fail to migrate away from the primary septa in TGCre+/−;Nrp1F/F mice. Our observation that nrp1 deletion reduces lung fibroblast migration in vitro (Fig. 6) also supports this contention.
Phenotypes Vary when Different Smooth Muscle Cre Drivers Are Used to Delete nrp1
Our tagln (Sm22α)-Cre driver differed from that used by Wang and coworkers to delete nrp1 in mesenchymal cells (66). Their tagln-Cre driver enabled deletion during embryonic life, yielding a predominantly cardiac phenotype, whereas the tagln-Cre we used is only active postnatally (73). Using myh11 (expressed in differentiated SMCs)-Cre to delete nrp1, Yamaji and associates observed a phenotype of colonic dysmotility in adult mice, although myh11-Cre was also active in the heart and aorta (38, 70). Analyses at P7 showed that SMCs failed to throttle proliferation and retained a synthetic, rather than contractile, phenotype. Therefore, the outcome of targeted nrp1 deletion in mesenchymal smooth muscle-like cells is dependent on when and where the deletion occurs (66, 70). Expression of tagln-Cre varies among different retinal PC precursor subpopulations (38). Lower tagln expression in some PC and MF precursor populations may explain why we observed incomplete deletion of nrp1 (Fig. 2B). However, our approach disrupted mesenchymal populations that are required for alveolar septation (Fig. 2).
Overlapping Characteristics of Alveolar Mesenchymal Cells
We observed that nrp1 depletion diminished NES and DES in CD140aneg, but not CD140apos, mesenchymal cells at P8 (Fig. 3). The tagln-expressing, CD140aneg-, CD140b-enriched population exhibits PC characteristics, but immunostaining cannot unequivocally distinguish MFs from PCs. Endale and associates compared gene expression profiles in CD140apos and CD140aneg mesenchymal cells, which were isolated after negative selection for CD45 (hematopoietic), CD326 (epithelial), and CD31 (endothelial) markers (17). They observed that the kinetics for the abundance of α-SMA, and surface-CD29 (β1-integrin), and CD34 were similar in CD140aneg and CD140apos cells, further illustrating the overlapping characteristics of these cells.
Recent lineage-tracing studies assessed the contributions of different α-SMA-containing alveolar populations to fibrosis (16). Using α-SMA-CreER2 for lineage tracing and varying the timing of tamoxifen induction, El Agha and associates showed that bleomycin induced MF characteristics in resident fibroblasts and that the same population adopted a lipofibroblast phenotype during the repair phase of pulmonary fibrosis (16). Using FoxD1-CreER2, Hung and coworkers demonstrated that PC progenitors increased, expressed collagen type I-α1 (col1A1) and acta2, and congregated in “fibroblast foci” following bleomycin-induced injury (28). They observed a second Col1A1-producing MF population, which expressed PDGFRα, but not PDGFRβ, during lung development and comprised 53% of the Col1A1-producing cells in fibroblast foci. Therefore, Gli1pos MF and FoxD1-labeled PC populations similarly contribute to both alveolar development and fibrosis. Overlapping profiles of tagln, pdgfra, pdgfrb, and acta2 gene expression in PCs and MFs prevent assignment of unique roles to various mesenchymal cell populations, which is a limitation of our study (4).
NES as a Marker of Mesenchymal Progenitor Cells
We and others have shown that PDGFRα marks alveolar mesenchymal cells, which can alternatively exhibit an adipogenic or MF (fibrogenic) phenotype (16, 40, 46, 53). Studies of mesenchymal progenitors in other organs showed that NESpos cells can assume two different phenotypes, which correlate with their resident location: 1) perivascular, which display CSPG4 (NG2) on their surface, participate in microvascular formation, and regulate capillary permeability (12, 33, 68), and 2) NESpos progenitors, which differentiate into Gli1pos, Col1A2pos, and PDGFRαpos MFs and migrate away from their perivascular niche to participate in organ fibrosis in the heart, kidney, and bone marrow (36, 37, 62). During secondary alveolar septation, NESpos cells are found in both PDGFRαpos and PDGFRαneg populations and are observed in the AER (alveolar ducts) and alveolar walls (Figs. 4 and 5). In adipose tissue, NESpos perivascular cells express pdgfra, which, if constitutively active, represses adipogenesis and stimulates fibrogenesis (29, 64). Similarly, we found a lower proportion of NESpos cells in the PDGFRα-GFPlow, lipid-accumulating population than PDGFRα-GFPhigh MF population (Fig. 1).
Potential Contributions of IFs to Alveolar Septation
Alveolar septation involves rapid and extensive changes in the numbers, positioning, and function of interstitial mesenchymal cells. At birth, elastic fibers subtend the distal saccules, and during septal outgrowth, a more extensive elastic fiber network forms, connecting the alveolar ducts with the septa. Because nascent elastic fibers are discontinuous and incompletely cross-linked, IFs and other cytoskeletal proteins may contribute to hysteresis. Sequestering hydrophobic IF α-helical residues from water enables IF “ultrafilaments” to stretch up to 3.5 times their length and relax, thereby storing and then releasing energy, the hallmark of hysteretic viscoelastic materials (7).
Using hyperpolarized 3H-dilution MRI, others showed that the radius of the alveolar duct increases and the subtending alveoli flatten during inspiration (23, 24). This “cup-to-saucer” shape transition increases tension at the AER. Before there are sufficient cross-linked elastic fibers, this strain must be accommodated by intracellular proteins such as DES and α-SMA. We posit that the presence of both NES and DES at the AER may stabilize the cytoplasm yet allow sufficient deformability for cells to migrate.
NRP1 Impacts PDGFRα Signaling in Alveolar Mesenchymal Cells
The impact of NRP1 on PDGFRα signaling is likely more complex than our data show. Signaling by PDGFRα through the PI3K/Akt pathway is the most vital signaling pathway during embryonic development, and our prior studies showed that it regulates MF proliferation, survival, and migration (45, 49). Cultured PDGFRα-expressing lung fibroblasts signal through PI3K/Akt, which determines whether the cells remain quiescent (G0) or transition to the G1/S phase. Mesenchymal cells in bone and heart, with high levels of PDGFRα, also signal through p53 or p16 to regulate cell survival (19, 51, 54). NRP intracellular domains regulate the endosomal trafficking of receptor tyrosine kinases (EGFR and VEGFR2) to enhance kinase activity, cell proliferation, and cytoskeletal function (15, 20). Additional studies are required to more completely elucidate shared signaling pathways.
Perivascular Cells Expressing nrp1 Regulate Angiogenesis
In the lymphatic endothelium, nrp1 is abundantly expressed in PCs and binds SEMA3 produced by the underlying endothelium (32). Without NRP1, PCs migrate away from lymphatic walls, leaving dilated channels and dysfunctional valves. PCs also regulate microvascular formation in the kidney. PC-like mesangial cells migrate into the S-shaped body and regulate formation of the endothelial-epithelial interface. Deletion of nrp1 in mesangial cells disrupts their migration into the glomerular tuft, leaving dilated capillaries and a more porous filter (5, 67). Others showed that pulmonary alveolar interstitial mesenchymal cells lie subjacent to and display α-SMA-rich protrusions that interdigitate with the membrane of endothelial cells (69). We observed that mesenchymal cells that display NG2 (CSPG4) were more abundant in the CD140aneg, CD140apos population (Figs. 1F), but nrp1 deletion negated this distinction (Fig. 3E). Therefore, NRP1 may maintain the alveolar perivascular PC phenotype, and after deletion, PCs may adopt more characteristics of CD140apos MFs.
NRP1 and Expansion of the Alveolar Capillary Surface
The alveolar microvasculature forms through both sprouting and nonsprouting angiogenesis [intussusceptive microvascular growth (IMG)]. Others showed that nrp1 deletion during the saccular stage results in fewer and dilated alveolar capillaries (30). NRP1 is essential for sprouting angiogenesis in the retina, where it limits TGFβ and bone morphogenetic protein-9 SMAD-dependent signaling (2). During postpneumonectomy compensatory alveolarization, IMG is driven by increases in mechanical stretch and blood flow, both of which are greatest in the cardiac lobe (mice) or left caudal lobe (dogs) (1, 11). Both sprouting angiogenesis and IMG are controlled by components of the TGFβ/SMAD signaling pathway, endoglin, bone morphogenetic protein-9, and activin receptor-like kinase-1 or -5, which are regulated by NRP1 (8, 13, 26, 27).
Potential Clinical Implications
Although it is required for alveolar septal formation, constitutively active PDGFRα produces fibrosis. Therefore, if PDGFRα signaling is manipulated to promote alveolar regeneration, it must be carefully tuned. NRP1 is a potential rheostat, because it impacts PDGFR signaling and can be used to regulate mesenchymal cell function through cell-penetrating CendR (C-end rule) peptides, which require NRPs for cell entry (60). Carefully regulated PDGFR signaling could help establish a structurally sound septal core with sufficient capillaries to restore gas transfer after alveolar destruction.
GRANTS
This study was funded by a Merit Review Award from the Department of Veterans Affairs Research Service. Flow cytometry was performed at the Flow Cytometry Facility, a Carver College of Medicine/Holden Comprehensive Cancer Center core research facility at the University of Iowa, which is funded through user fees and the generous financial support of the Carver College of Medicine, Holden Comprehensive Cancer Center, and Iowa City Veterans Administration Medical Center. The Aria flow cytometer was funded by National Center for Research Resources Grant 1S10 RR-027219.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E. conceived and designed research; S.E. and D.M.M. performed experiments; S.E. and D.M.M. analyzed data; S.E. interpreted results of experiments; S.E. prepared figures; S.E. drafted manuscript; S.E. and D.M.M. edited and revised manuscript; S.E. and D.M.M. approved final version of manuscript.
REFERENCES
- 1.Ackermann M, Houdek JP, Gibney BC, Ysasi A, Wagner W, Belle J, Schittny JC, Enzmann F, Tsuda A, Mentzer SJ, Konerding MA. Sprouting and intussusceptive angiogenesis in postpneumonectomy lung growth: mechanisms of alveolar neovascularization. Angiogenesis 17: 541–551, 2014. doi: 10.1007/s10456-013-9399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aspalter IM, Gordon E, Dubrac A, Ragab A, Narloch J, Vizán P, Geudens I, Collins RT, Franco CA, Abrahams CL, Thurston G, Fruttiger M, Rosewell I, Eichmann A, Gerhardt H. Alk1 and Alk5 inhibition by Nrp1 controls vascular sprouting downstream of Notch. Nat Commun 6: 7264, 2015. doi: 10.1038/ncomms8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball SG, Bayley C, Shuttleworth CA, Kielty CM. Neuropilin-1 regulates platelet-derived growth factor receptor signalling in mesenchymal stem cells. Biochem J 427: 29–40, 2010. doi: 10.1042/BJ20091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barron L, Gharib SA, Duffield JS. Lung pericytes and resident fibroblasts: busy multitaskers. Am J Pathol 186: 2519–2531, 2016. doi: 10.1016/j.ajpath.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett CS, Scott RP, Carota IA, Wnuk ML, Kanwar YS, Miner JH, Quaggin SE. Glomerular mesangial cell recruitment and function require the co-receptor neuropilin-1. Am J Physiol Renal Physiol 313: F1232–F1242, 2017. doi: 10.1152/ajprenal.00311.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 128: 81–93, 2015. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block J, Schroeder V, Pawelzyk P, Willenbacher N, Köster S. Physical properties of cytoplasmic intermediate filaments. Biochim Biophys Acta 1853: 11 Pt B: 3053–3064, 2015. doi: 10.1016/j.bbamcr.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Cao S, Yaqoob U, Das A, Shergill U, Jagavelu K, Huebert RC, Routray C, Abdelmoneim S, Vasdev M, Leof E, Charlton M, Watts RJ, Mukhopadhyay D, Shah VH. Neuropilin-1 promotes cirrhosis of the rodent and human liver by enhancing PDGF/TGF-β signaling in hepatic stellate cells. J Clin Invest 120: 2379–2394, 2010. doi: 10.1172/JCI41203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabot A, Meus MA, Naud P, Hertig V, Dupuis J, Villeneuve L, El Khoury N, Fiset C, Nattel S, Jasmin JF, Calderone A. Nestin is a marker of lung remodeling secondary to myocardial infarction and type I diabetes in the rat. J Cell Physiol 230: 170–179, 2015. doi: 10.1002/jcp.24696. [DOI] [PubMed] [Google Scholar]
- 10.Cheng F, Eriksson JE. Intermediate filaments and the regulation of cell motility during regeneration and wound healing. Cold Spring Harb Perspect Biol 9: a022046, 2017. doi: 10.1101/cshperspect.a022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dane DM, Yilmaz C, Gyawali D, Iyer R, Ravikumar P, Estrera AS, Hsia CC. Perfusion-related stimuli for compensatory lung growth following pneumonectomy. J Appl Physiol (1985) 121: 312–323, 2016. doi: 10.1152/japplphysiol.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Toro R, Chèvre R, Rodríguez C, Ordóñez A, Martínez-González J, Andrés V, Méndez-Ferrer S. Nestin+ cells direct inflammatory cell migration in atherosclerosis. Nat Commun 7: 12706, 2016. doi: 10.1038/ncomms12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delcourt N, Quevedo C, Nonne C, Fons P, O’Brien D, Loyaux D, Diez M, Autelitano F, Guillemot JC, Ferrara P, Muriana A, Callol C, Hérault JP, Herbert JM, Favre G, Bono F. Targeted identification of sialoglycoproteins in hypoxic endothelial cells and validation in zebrafish reveal roles for proteins in angiogenesis. J Biol Chem 290: 3405–3417, 2015. doi: 10.1074/jbc.M114.618611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol 300: C723–C742, 2011. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta S, Roy S, Polavaram NS, Stanton MJ, Zhang H, Bhola T, Hönscheid P, Donohue TM Jr, Band H, Batra SK, Muders MH, Datta K. Neuropilin-2 regulates endosome maturation and EGFR trafficking to support cancer cell pathobiology. Cancer Res 76: 418–428, 2016. doi: 10.1158/0008-5472.CAN-15-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Agha E, Moiseenko A, Kheirollahi V, De LS, Crnkovic S, Kwapiszewska G, Kosanovic D, Schwind F, Schermuly RT, Henneke I, Mackenzie B, Quantius J, Herold S, Ntokou A, Ahlbrecht K, Morty RE, Gunther A, Seeger W, Bellusci S. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20: 1–13, 2017. doi: 10.1016/j.stem.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch M, Xu Y, Perl AK. Dataset on transcriptional profiles and the developmental characteristics of PDGFRα expressing lung fibroblasts. Data Brief 13: 415–431, 2017. doi: 10.1016/j.dib.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endale M, Ahlfeld S, Bao E, Chen X, Green J, Bess Z, Weirauch MT, Xu Y, Perl AK. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev Biol 425: 161–175, 2017. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantauzzo KA, Soriano P. PI3K-mediated PDGFRα signaling regulates survival and proliferation in skeletal development through p53-dependent intracellular pathways. Genes Dev 28: 1005–1017, 2014. doi: 10.1101/gad.238709.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fantin A, Lampropoulou A, Senatore V, Brash JT, Prahst C, Lange CA, Liyanage SE, Raimondi C, Bainbridge JW, Augustin HG, Ruhrberg C. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J Exp Med 214: 1049–1064, 2017. doi: 10.1084/jem.20160311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhardt H, Ruhrberg C, Abramsson A, Fujisawa H, Shima D, Betsholtz C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn 231: 503–509, 2004. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 22.Guo H-F, Vander Kooi CW. Neuropilin functions as an essential cell surface receptor. J Biol Chem 290: 29120–29126, 2015. doi: 10.1074/jbc.R115.687327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajari AJ, Yablonskiy DA, Quirk JD, Sukstanskii AL, Pierce RA, Deslée G, Conradi MS, Woods JC. Imaging alveolar-duct geometry during expiration via 3He lung morphometry. J Appl Physiol (1985) 110: 1448–1454, 2011. doi: 10.1152/japplphysiol.01352.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajari AJ, Yablonskiy DA, Sukstanskii AL, Quirk JD, Conradi MS, Woods JC. Morphometric changes in the human pulmonary acinus during inflation. J Appl Physiol (1985) 112: 937–943, 2012. doi: 10.1152/japplphysiol.00768.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor-α receptor signaling mechanisms. Mol Cell Biol 23: 4013–4025, 2003. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota S, Clements TP, Tang LK, Morales JE, Lee HS, Oh SP, Rivera GM, Wagner DS, McCarty JH. Neuropilin 1 balances β8-integrin-activated TGFβ signaling to control sprouting angiogenesis in the brain. Development 142: 4363–4373, 2015. doi: 10.1242/dev.113746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hlushchuk R, Styp-Rekowska B, Dzambazi J, Wnuk M, Huynh-Do U, Makanya A, Djonov V. Endoglin inhibition leads to intussusceptive angiogenesis via activation of factors related to COUP-TFII signaling pathway. PLoS One 12: e0182813, 2017. doi: 10.1371/journal.pone.0182813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, Gharib SA, Schnapp LM, Duffield JS. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med 188: 820–830, 2013. doi: 10.1164/rccm.201212-2297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwayama T, Steele C, Yao L, Dozmorov MG, Karamichos D, Wren JD, Olson LE. PDGFRα signaling drives adipose tissue fibrosis by targeting progenitor cell plasticity. Genes Dev 29: 1106–1119, 2015. doi: 10.1101/gad.260554.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joza S, Wang J, Fox E, Hillman V, Ackerley C, Post M. Loss of semaphorin-neuropilin-1 signaling causes dysmorphic vascularization reminiscent of alveolar capillary dysplasia. Am J Pathol 181: 2003–2017, 2012. doi: 10.1016/j.ajpath.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 31.Joza S, Wang J, Tseu I, Ackerley C, Post M. Fetal, but not postnatal, deletion of semaphorin-neuropilin-1 signaling affects murine alveolar development. Am J Respir Cell Mol Biol 49: 627–636, 2013. doi: 10.1165/rcmb.2012-0407OC. [DOI] [PubMed] [Google Scholar]
- 32.Jurisic G, Maby-El Hajjami H, Karaman S, Ochsenbein AM, Alitalo A, Siddiqui SS, Ochoa Pereira C, Petrova TV, Detmar M. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ Res 111: 426–436, 2012. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estapé A, Pinho S, Ciero P, Nakahara F, Ma’ayan A, Bergman A, Merad M, Frenette PS. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science 351: 176–180, 2016. doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kimani PW, Holmes AJ, Grossmann RE, McGowan SE. PDGF-Rα gene expression predicts proliferation, but PDGF-A suppresses transdifferentiation of neonatal mouse lung myofibroblasts. Respir Res 10: 119, 2009. doi: 10.1186/1465-9921-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kojic M, Butler JP, Vlastelica I, Stojanovic B, Rankovic V, Tsuda A. Geometric hysteresis of alveolated ductal architecture. J Biomech Eng 133: 111005, 2011. doi: 10.1115/1.4005380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 16: 51–66, 2015. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramann R, Wongboonsin J, Chang-Panesso M, Machado FG, Humphreys BD. Gli1+ pericyte loss induces capillary rarefaction and proximal tubular injury. J Am Soc Nephrol 28: 776–784, 2017. doi: 10.1681/ASN.2016030297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, D’Souza SS, Moskvin OV, Toh H, Wang B, Zhang J, Swanson S, Guo LW, Thomson JA, Slukvin II. Specification and diversification of pericytes and smooth muscle cells from mesenchymoangioblasts. Cell Reports 19: 1902–1916, 2017. doi: 10.1016/j.celrep.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le A, Zielinski R, He C, Crow MT, Biswal S, Tuder RM, Becker PM. Pulmonary epithelial neuropilin-1 deletion enhances development of cigarette smoke-induced emphysema. Am J Respir Crit Care Med 180: 396–406, 2009. doi: 10.1164/rccm.200809-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C, Li M, Li S, Xing Y, Yang CY, Li A, Borok Z, De Langhe S, Minoo P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells 33: 999–1012, 2015. doi: 10.1002/stem.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lowery J, Kuczmarski ER, Herrmann H, Goldman RD. Intermediate filaments play a pivotal role in regulating cell architecture and function. J Biol Chem 290: 17145–17153, 2015. doi: 10.1074/jbc.R115.640359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-α-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 291: 1649–1661, 2008. doi: 10.1002/ar.20764. [DOI] [PubMed] [Google Scholar]
- 43.McGowan SE, McCoy DM. Fibroblasts expressing PDGF-receptor-α diminish during alveolar septal thinning in mice. Pediatr Res 70: 44–49, 2011. doi: 10.1203/PDR.0b013e31821cfb5a. [DOI] [PubMed] [Google Scholar]
- 44.McGowan SE, McCoy DM. Platelet-derived growth factor-A and sonic hedgehog signaling direct lung fibroblast precursors during alveolar septal formation. Am J Physiol Lung Cell Mol Physiol 305: L229–L239, 2013. doi: 10.1152/ajplung.00011.2013. [DOI] [PubMed] [Google Scholar]
- 45.McGowan SE, McCoy DM. Platelet-derived growth factor-A regulates lung fibroblast S-phase entry through p27kip1 and FoxO3a. Respir Res 14: 68, 2013. doi: 10.1186/1465-9921-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGowan SE, McCoy DM. Regulation of fibroblast lipid storage and myofibroblast phenotypes during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol 307: L618–L631, 2014. doi: 10.1152/ajplung.00144.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGowan SE, McCoy DM. Fibroblast growth factor signaling in myofibroblasts differs from lipofibroblasts during alveolar septation in mice. Am J Physiol Lung Cell Mol Physiol 309: L463–L474, 2015. doi: 10.1152/ajplung.00013.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGowan SE, McCoy DM. Glucocorticoids retain bipotent fibroblast progenitors during alveolar septation in mice. Am J Respir Cell Mol Biol 57: 111–120, 2017. doi: 10.1165/rcmb.2016-0376OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowan SE, McCoy DM. Platelet-derived growth factor receptor-α and Ras-related C3 botulinum toxin substrate-1 regulate mechano-responsiveness of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 313: L1174–L1187, 2017. doi: 10.1152/ajplung.00185.2017. [DOI] [PubMed] [Google Scholar]
- 50.Mercer RR, Crapo JD. Architecture of the gas exchange region of the lungs. In: Comparative Biology of the Normal Lung, edited by Parent RA. New York: Elsevier, 2015, p. 93–103. [Google Scholar]
- 51.Meyer K, Hodwin B, Ramanujam D, Engelhardt S, Sarikas A. Essential role for premature senescence of myofibroblasts in myocardial fibrosis. J Am Coll Cardiol 67: 2018–2028, 2016. doi: 10.1016/j.jacc.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 52.Michalczyk K, Ziman M. Nestin structure and predicted function in cellular cytoskeletal organisation. Histol Histopathol 20: 665–671, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD, Szibor M, Braun T, Morty RE, Seeger W, Voswinckel R, Ahlbrecht K. Characterization of the platelet-derived growth factor receptor-α-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol 309: L942–L958, 2015. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 54.Papke CL, Cao J, Kwartler CS, Villamizar C, Byanova KL, Lim SM, Sreenivasappa H, Fischer G, Pham J, Rees M, Wang M, Chaponnier C, Gabbiani G, Khakoo AY, Chandra J, Trache A, Zimmer W, Milewicz DM. Smooth muscle hyperplasia due to loss of smooth muscle α-actin is driven by activation of focal adhesion kinase, altered p53 localization and increased levels of platelet-derived growth factor receptor-β. Hum Mol Genet 22: 3123–3137, 2013. doi: 10.1093/hmg/ddt167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pellet-Many C, Frankel P, Evans IM, Herzog B, Jünemann-Ramírez M, Zachary IC. Neuropilin-1 mediates PDGF stimulation of vascular smooth muscle cell migration and signalling via p130Cas. Biochem J 435: 609–618, 2011. doi: 10.1042/BJ20100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pozarska A, Rodríguez-Castillo JA, Surate Solaligue DE, Ntokou A, Rath P, Mižíková I, Madurga A, Mayer K, Vadász I, Herold S, Ahlbrecht K, Seeger W, Morty RE. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am J Physiol Lung Cell Mol Physiol 312: L882–L895, 2017. doi: 10.1152/ajplung.00492.2016. [DOI] [PubMed] [Google Scholar]
- 57.Raimondi C, Brash JT, Fantin A, Ruhrberg C. NRP1 function and targeting in neurovascular development and eye disease. Prog Retin Eye Res 52: 64–83, 2016. doi: 10.1016/j.preteyeres.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reed MG, Howard CV. Surface-weighted star volume: concept and estimation. J Microsc 190: 350–356, 1998. doi: 10.1046/j.1365-2818.1998.00331.x. [DOI] [PubMed] [Google Scholar]
- 59.Rosner B. Fundamentals of Biostatistics. Boston: PWS-Kent, 1990. [Google Scholar]
- 60.Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev 110–111: 3–12, 2017. doi: 10.1016/j.addr.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salikhova A, Wang L, Lanahan AA, Liu M, Simons M, Leenders WP, Mukhopadhyay D, Horowitz A. Vascular endothelial growth factor and semaphorin induce neuropilin-1 endocytosis via separate pathways. Circ Res 103: e71–e79, 2008. doi: 10.1161/CIRCRESAHA.108.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schneider RK, Mullally A, Dugourd A, Peisker F, Hoogenboezem R, Van Strien PMH, Bindels EM, Heckl D, Büsche G, Fleck D, Müller-Newen G, Wongboonsin J, Ventura Ferreira M, Puelles VG, Saez-Rodriguez J, Ebert BL, Humphreys BD, Kramann R. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell 20: 785–800.e8, 2017. doi: 10.1016/j.stem.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sera T, Yokota H, Tanaka G, Uesugi K, Yagi N, Schroter RC. Murine pulmonary acinar mechanics during quasi-static inflation using synchrotron refraction-enhanced computed tomography. J Appl Physiol (1985) 115: 219–228, 2013. doi: 10.1152/japplphysiol.01105.2012. [DOI] [PubMed] [Google Scholar]
- 64.Sun C, Berry WL, Olson LE. PDGFRα controls the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development 144: 83–94, 2017. doi: 10.1242/dev.135962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner W, Bennett RD, Ackermann M, Ysasi AB, Belle J, Valenzuela CD, Pabst A, Tsuda A, Konerding MA, Mentzer SJ. Elastin cables define the axial connective tissue system in the murine lung. Anat Rec (Hoboken) 298: 1960–1968, 2015. doi: 10.1002/ar.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Cao Y, Yamada S, Thirunavukkarasu M, Nin V, Joshi M, Rishi MT, Bhattacharya S, Camacho-Pereira J, Sharma AK, Shameer K, Kocher JP, Sanchez JA, Wang E, Hoeppner LH, Dutta SK, Leof EB, Shah V, Claffey KP, Chini EN, Simons M, Terzic A, Maulik N, Mukhopadhyay D. Cardiomyopathy and worsened ischemic heart failure in sm22-α cre-mediated neuropilin-1 null mice: dysregulation of pgc1α and mitochondrial homeostasis. Arterioscler Thromb Vasc Biol 35: 1401–1412, 2015. doi: 10.1161/ATVBAHA.115.305566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wnuk M, Anderegg MA, Graber WA, Buergy R, Fuster DG, Djonov V. Neuropilin1 regulates glomerular function and basement membrane composition through pericytes in the mouse kidney. Kidney Int 91: 868–879, 2017. doi: 10.1016/j.kint.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 68.Wong SP, Rowley JE, Redpath AN, Tilman JD, Fellous TG, Johnson JR. Pericytes, mesenchymal stem cells and their contributions to tissue repair. Pharmacol Ther 151: 107–120, 2015. doi: 10.1016/j.pharmthera.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 69.Yamada M, Kurihara H, Kinoshita K, Sakai T. Temporal expression of α-smooth muscle actin and drebrin in septal interstitial cells during alveolar maturation. J Histochem Cytochem 53: 735–744, 2005. doi: 10.1369/jhc.4A6483.2005. [DOI] [PubMed] [Google Scholar]
- 70.Yamaji M, Mahmoud M, Evans IM, Zachary IC. Neuropilin 1 is essential for gastrointestinal smooth muscle contractility and motility in aged mice. PLoS One 10: e0115563, 2015. doi: 10.1371/journal.pone.0115563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zachary I. Neuropilins: role in signalling, angiogenesis and disease. Chem Immunol Allergy 99: 37–70, 2014. doi: 10.1159/000354169. [DOI] [PubMed] [Google Scholar]
- 72.Zachary IC. How neuropilin-1 regulates receptor tyrosine kinase signalling: the knowns and known unknowns. Biochem Soc Trans 39: 1583–1591, 2011. doi: 10.1042/BST20110697. [DOI] [PubMed] [Google Scholar]
- 73.Zhang J, Zhong W, Cui T, Yang M, Hu X, Xu K, Xie C, Xue C, Gibbons GH, Liu C, Li L, Chen YE. Generation of an adult smooth muscle cell-targeted Cre recombinase mouse model. Arterioscler Thromb Vasc Biol 26: e23–e24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]