Abstract
The common gain-of-function MUC5B promoter variant (rs35705950) is the strongest risk factor for the development of idiopathic pulmonary fibrosis (IPF). While the role of complement in IPF is controversial, both MUC5B and the complement system play a role in lung host defense. The aim of this study was to evaluate the relationship between complement component 3 (C3) and MUC5B in patients with IPF and in bleomycin-induced lung injury in mice. To do this, we evaluated C3 gene expression in whole lung tissue from 300 subjects with IPF and 175 healthy controls. Expression of C3 was higher in IPF than healthy controls {1.40-fold increase [95% confidence interval (CI) 1.31–1.50]; P < 0.0001} and even greater among IPF subjects with the highest-risk IPF MUC5B promoter genotype [TT vs. GG = 1.59-fold (95% CI 1.15–2.20); P < 0.05; TT vs. GT = 1.66-fold (95% CI 1.20–2.30); P < 0.05]. Among subjects with IPF, C3 expression was significantly higher in the lung tissue without microscopic honeycombing than in the lung tissue with microscopic honeycombing [1.40-fold increase (95% CI 1.23– 1.59); P < 0.01]. In mice, while bleomycin exposure increased Muc5b protein expression, C3-deficient mice were protected from bleomycin-induced lung injury. In aggregate, our findings indicate that the MUC5B promoter variant is associated with higher C3 expression and suggest that the complement system may contribute to the pathogenesis of IPF.
Keywords: complement component 3, host defense, idiopathic pulmonary fibrosis, MUC5B
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a fibroproliferative disorder, characterized by progressive scarring of the pulmonary parenchyma that leads to progressive loss of function with dyspnea and hypoxemia and ultimately respiratory failure and death. The prevalence of IPF is currently estimated at ~60 cases per 100,000 individuals in the United States (16), the median survival is 3–5 yr, and there are only noncurative treatment options available (14, 24).
Although a gain-of-function MUC5B promoter variant is the strongest risk factor for the development of IPF (28), the mechanism(s) by which the MUC5B polymorphism predisposes individuals to pulmonary fibrosis is unknown. MUC5B appears to be critical to host defense. Muc5b-deficient mice have impaired innate immunity (26), and IPF subjects with the gain-of-function MUC5B promoter variant may have improved host defense (20). Based on these findings, we hypothesized that the increased MUC5B concentration is associated with better lung host defense and can affect the activation of complement and other components of innate immunity in the lung.
Complement activation, a key component of the innate immune system, is triggered in response to multiple types of tissue injury. The complement system is involved in the pathogenesis of various inflammatory lung conditions (21), including autoimmune diseases (15), chronic lung transplant rejection (31), experimental allergic asthma (32, 37), infectious disease (13), and chronic obstructive pulmonary disease (17). The anaphylatoxins complement components 3a and 5a (C3a and C5a) have been implicated in renal fibrosis through epithelial mesenchymal transition (33). Previous studies have demonstrated evidence of circulating immune complexes and complement activation in the lungs of subjects with IPF (4, 11). In experimental mouse models of pulmonary fibrosis, the deletion of complement component 5 (C5) significantly reduced the fibroproliferative response (1, 22). Similarly, C3a and C5a are profibrotic in the IPF lung (8). However, the current understanding of complement activation in the pathogenesis of IPF remains controversial.
We conducted a cross-sectional study in subjects with IPF to attempt to clarify the role of complement component 3 (C3), a central component of the complement activation cascade, in the pathogenesis of IPF and the relationship between C3 and the presence of the MUC5B promoter variant. We further investigated the role of C3 in lung fibrosis using a bleomycin-induced lung injury model in mice. Our findings indicate that the MUC5B promoter variant is associated with increased expression of C3 and suggest that the complement system may be involved in the pathogenesis of IPF.
MATERIALS AND METHODS
Human samples.
Standard criteria from the American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association were used to establish an IPF diagnosis (23). Cases with alternative explanations for fibrotic interstitial pneumonias were excluded. Diagnoses were made based on a multidisciplinary review that integrated clinical, radiologic, and pathologic data. Human lung tissue samples were obtained from the Lung Tissue Research Consortium (LTRC) and from National Jewish Health (NJH). A total of 175 control lung tissue samples (88 from the LTRC; 87 from NJH) and 300 IPF lung tissue samples from biopsy samples (167 from the LTRC; 133 from NJH) were included in this study. LTRC control samples came from histologically normal areas of lung obtained during tumor resection or biopsies for malignancy. Control samples from NJH were obtained from lungs of deceased donors deemed unsuitable for transplantation. Tissue, nucleic acid, and clinical data were obtained under a protocol approved by NJH and the Colorado Multiple Institutional Review Board (Protocol No. NJH HS-1441a).
Genotyping for MUC5B promoter polymorphism rs35705950.
Taqman genotyping assays (C_1582254_20; Thermo Fisher Scientific, Waltham, MA) and a Viaa7 real-time PCR system (Applied Biosystems, Foster City, CA) were used to genotype all cases and controls at rs35705950 as described previously (28).
Gene expression in lung tissue.
Gene expression was performed on RNA extracted from human lung tissue (MirVana Kit; Thermo Fisher Scientific) and RNA extracted from mouse lung (RNeasy Mini Kit; Qiagen, Germantown, MD) as described previously (18). Samples with an RNA integrity number less than 5 were excluded. cDNA was synthesized from RNA (SuperScript III reverse transcriptase; Thermo Fisher Scientific). cDNA was used with real-time PCR gene expression assay for C3 (Hs00163811_m1, Mm01232779_m1; Thermo Fisher Scientific) and MUC5B (Hs00861595_m1, Mm00466391_m1; Thermo Fisher Scientific) normalized to GAPDH (Hs02758991_g1, Mm99999915_g1; Thermo Fisher Scientific) using standardized TaqMan protocol (TaqMan Fast Advanced Master Mix; Thermo Fisher Scientific) and Viaa7 real-time PCR system. Delta cycle threshold (ΔCT) values were calculated by subtracting the CT values of GAPDH from the CT values of target genes for a sample. Relative gene expression was determined using the ΔΔCT method (27).
Histological evaluation.
Histological correlates of differential expression were examined using hematoxylin and eosin-stained tissue of 251 IPF/usual interstitial pneumonia cases as described previously (36). Each slide was examined independently by two experienced pulmonary pathologists. In the case of disagreement, the pathologists conferred and determined a final score. Each sample was given a score of 0, 1, or 2 depending on the presence and extent of microscopic honeycombing and fibroblastic foci in the entire sample. If insufficient tissue was available for examination, the specimen was not scored. The sections of lung tissue evaluated by pathologists were adjacent sections of lung tissue used to assess gene expression.
Immunohistochemical and immunofluorescence staining in human lung.
Deidentified formalin-fixed paraffin-embedded lung tissue from IPF subjects and normal controls were obtained from the LTRC. Briefly, for immunohistochemical staining, sections were deparaffinized with xylene and rehydrated using a graded ethanol series. Heat-induced antigen retrieval was performed in 10 mM citric scid (pH 6.0). Slides were blocked for 60 min at room temperature using 2.5% horse serum in PBS. Primary antibodies for C3 (sc-28294; Santa Cruz Biotechnology, Dallas, TX), C3d (C3d29 antibody as described previously; Ref. 34), and C4d (12-5000; American Research Products, Waltham, MA) were diluted in blocking solution (1:15,000, 1:4,000, and 1:500, respectively). Slides were applied to sections for overnight incubation at 4°C. Secondary staining was done (ImmPRESS Reagent Kit; Vector Laboratories, Burlingame, CA) at room temperature for 60 min. For immunofluorescence staining, slides were blocked for 60 min at room temperature using 10% normal goat serum, 1% BSA, 0.1% Tween 20 in PBS. Primary antibodies for C3 and MUC5B (sc-20119; Santa Cruz Biotechnology) were diluted in 5% normal goat serum and 1% BSA (1:20 and 1:100, respectively). Slides were applied to sections for overnight incubation at 4°C. After being washed, slides were incubated with goat anti-mouse secondary antibody Alexa 488 (1:200 diluted; Abcam, Cambridge, MA) and goat anti-rabbit secondary antibody Alexa 594 (1:200 diluted; Abcam) at room temperature for 60 min. The slides were imaged with AXIO Observer A1 inverted microscope (Zeiss, Thornwood, NY).
Mice.
Wild-type (WT) C57BL/6 mice were obtained from the Jackson Laboratory. Homozygous C3-deficient mice (C3−/−) were provided by Dr. Banda and had been backcrossed to the C57BL/6 background for >20 generations. C3−/− mice were genotyped by PCR using the following primers: forward 5′-ATCTTGAGTGCACCAAGCC-3′; reverse for C3 WT 5′-GGTTGCAGCAGTCTATGAAGG-3′; and reverse for C3 mutant 5′-GCCAGAGGCCACTTGTGTAG-3′. Muc5B-deficient mice (Muc5B−/−) were described previously (9).
All WT mice and C3−/− mice were male to eliminate the potential effect of sex. Mice were between 8 and 12 wk old at the initiation of the experiments. All animals were kept in a barrier animal facility with a climate-controlled environment providing 12-h light-dark cycles at the University of Colorado. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee at University of Colorado [Protocol No. B95914(05)1E].
Repeat bleomycin model in mice.
Bleomycin was prepared by mixing bleomycin product (APP Pharmaceuticals, Schaumburg, IL) with sterile normal saline (HOSPIRA, Lake Forest, IL). Bleomycin was administered intratracheally at three time points: 2.5 U/kg (day 0) and 1.5 U/kg (days 14 and 28). For this procedure, mice were anesthetized with inhaled isoflurane by inhalation. The mice were killed at day 49.
Bronchoalveolar lavage in mice.
Bronchoalveolar lavage (BAL) was performed as the follows. Briefly, three 0.5-ml lavages of sterile PBS were performed using a 20-gauge blunt tipped needle inserted into the trachea via cannula. Samples were centrifuged at 1,200 g for 10 min. Cells were resuspended using 1 ml of sterile PBS. Cell counts were performed by manual counting under light microscopy using a hemocytometer. Two-hundred microliters of sample were loaded onto slides using Cytospin 4 (Thermo Fisher Scientific). These slides were stained using the PROTOCOL Hema3 manual staining system (Thermo Fisher Scientific), and at least 200 cells were counted to obtain differential cell counts.
Hydroxyproline assay.
The right lung was harvested, homogenized, and suspended in 550 μl of sterile PBS. Two-hundred microliters of 12 M HCl (Sigma-Aldrich, St. Louis, MO) were then added, and the samples were heated overnight at 100°C. Five microliters of the resulting acid hydrolysate were transferred to a 96-well plate containing 5 μl of citric acetate buffer (5% citric acid, 7.24% sodium acetate, 3.4% sodium hydroxide, and 1.2% glacial acetic acid, pH 6.0). One-hundred microliters of chloramine T solution [141 mg of chloramine T (Sigma-Aldrich), 1 ml of H2O, 1 ml of 1-propanol (Sigma-Aldrich), and 8 ml of citric acetate buffer] were added to this, and the mixture was incubated for 20 min at room temperature. One-hundred microliters of Ehrlich's solution [2.5 g 4-dimethylaminobenzaldehyde (Sigma-Aldrich), 9.3 ml n-propanol (Sigma-Aldrich), and 3.9 ml 70% perchloric acid (GFS Chemicals, Powell, OH)] were added to this, and the mixture was incubated for 20 min at 65°C before the absorbance (optical density of 550 nm) of the solution was measured.
Dot blot for MUC5B.
The right lung of each mouse was homogenized and treated with SDS (Thermo Fisher Scientific) and proteinase inhibitor cocktail (Sigma-Aldrich). Five micrograms of protein were incubated in 3 M urea and 50 mM DTT. The samples were blotted to PVDF membrane using a Minifold I Dot-Blot system (GE Healthcare, Pittsburgh, PA). The membrane was incubated with rabbit anti-mouse polyclonal MUC5B antibody (1:5,000) as described previously (38). The membrane was subsequently incubated with a donkey anti-rabbit secondary antibody (LI-COR, Lincoln, NE) at room temperature for 1 h and scanned on an Odyssey CLx (LI-COR). Images were analyzed with Image Studio Lite (Li-Cor).
ELISA.
C3a levels in mouse BAL were measured using C3a Mouse Elisa Kit (Quidel, San Diego, CA) as per manufacturer’s protocol.
Immunohistochemical and immunofluorescence staining in mouse lung.
For immunohistochemical staining, primary antibody of MUC5B described previously (38), C3 antibody (MP Biomedicals), and C4 antibody (Hycult Biotech, Plymouth Meeting, PA) were diluted to 1:1,000, 1:20,000, and 1:20. Slides were applied to sections for overnight incubation at 4°C. After being washed, secondary staining was done (ImmPRESS Reagent Kit; Vector Laboratories) at room temperature for 60 min. For immunofluorescence staining, primary antibody of C3 (MP Biomedicals) and MUC5B described previously (38) was diluted (1:200 and 1:200, respectively). Slides were applied to sections for overnight incubation at 4°C. After being washed, slides were incubated with donkey anti-goat secondary antibody Alexa 488 (1:200 diluted; Abcam) and donkey anti-rabbit secondary antibody Alexa 594 (1:200 diluted; Abcam) at room temperature for 60 min. The slides were imaged with AXIO Observer A1 inverted microscope (Zeiss).
Cell culture.
A549 cells were grown in Ham’s F-12 media (Corning) supplemented with 10% heat-inactivated FBS, 1% pennicilline/streptomycin at 37°C and 5% CO2. A549 cells were incubated with C3a (Millipore, Burlington, MA) at different concentrations for 8 h. RNA was extracted by RNeasy Mini Kit and analyzed Muc5b gene expression by quantitative PCR.
Statistics.
All statistical analyses were computed in Prism software (v. 5) (GraphPad, San Diego, CA) and Stata/IC 12 (StataCorp, College Station, TX). Differences among multiple groups were assessed using a Kruskal-Wallis test with a Holm-Bonferroni post hoc test. Differences between two groups were assessed using a Mann-Whitney test. Linear regression analyses were used to explore the relationship between C3 expression and the rs35705950 genotype controlling for age, diagnosis, smoking history, sex, and forced vital capacity. ΔCT values for C3 were regressed on the continuous variables for age and forced vital capacity as well as categorical variables for sex and diagnosis (control vs. IPF) and rs35705950 genotypes. Data are presented as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
C3 expression in human lung tissue.
Baseline characteristics of the study population are shown in Table 1. IPF subjects were older and had a higher percentage of male sex. More were former smokers, and there was a higher average pack-year smoking history than control subjects. As expected, IPF subjects also had a higher percentage of the GT or TT genotype for the MUC5B promoter variant rs35705950 than controls. The odds ratio for disease among subjects who were heterozygous and homozygous for the minor allele of rs35705950 was 2.76 [95% confidence interval (CI) 1.85–4.18; P < 0.0001].
Table 1.
Clinical characteristics
| Control (n = 175) | IPF (n = 300) | P Value | |
|---|---|---|---|
| Age (95% CI) | 55.4 (52.9–57.9) | 62.9 (61.9–63.8) | <0.0001 |
| Males, % | 89 (50.9) | 200 (66.7) | 0.001 |
| Smoking historycurrent/former/never, % | 41/73/61 (23/42/35) | 18/187/95 (6/62/32) | <0.0001 |
| Pack-years (95% CI) | 17.0 (13.7–20.4) | 25.8 (22.1–29.5) | 0.0018 |
| %Predicted FVC (95% CI) | 95.9% (92.4–99.4) | 68.6% (66.3–70.9) | <0.0001 |
| %Predicted DLco (95% CI) | 85.1% (80.7–89.5) | 47.4% (45.2–49.7) | <0.0001 |
| MUC5B genotype GG/GT/TT, % | 128/45/2 (73/26/1) | 151/126/23 (50/42/8) | <0.0001 |
Clinical characteristics of the lung tissue subjects used for expression and genotyping analysis. Statistical tests performed using two-tailed t-tests for means and for the proportions where appropriate. The percent predicted forced vital capacity (FVC) was available on control (n = 87) and idiopathic pulmonary fibrosis (IPF) (n = 285). The percent predicted diffusing capacity of the lung for carbon monoxide (DLco) was available on control (n = 78) and IPF (n = 259). CI, confidence interval.
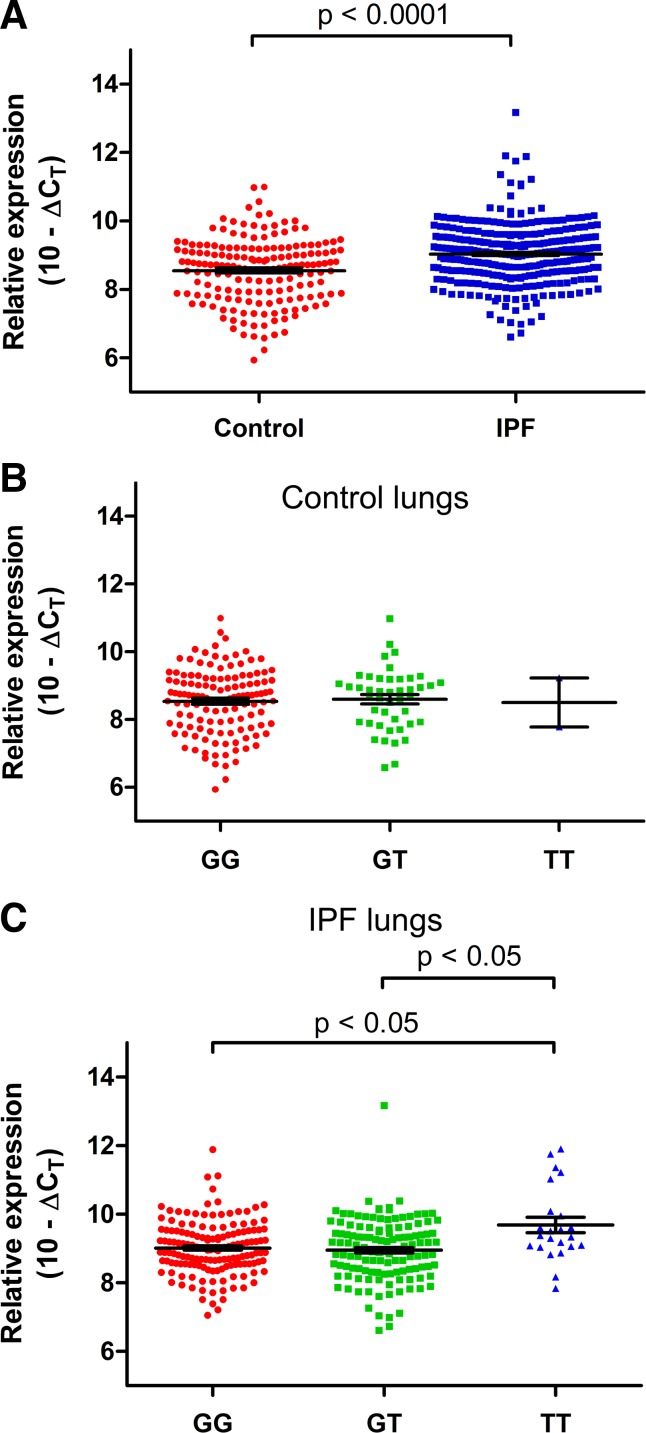
C3 gene expression was significantly higher in IPF lung compared with control lung (P < 0.0001; 1.40-fold increase; 95% CI 1.31–1.50; Fig. 1A). C3 expression remained significantly higher in IPF lungs compared with controls after controlling for age, sex, and smoking history (P < 0.0001). C3 expression in the lung was unaffected by age in the IPF subjects (r = −0.004; P = 0.50), but C3 expression decreased with increasing age in control subjects (r = −0.009; P = 0.05).
Fig. 1.
Relative complement component 3 (C3) expression in human lung tissues from 175 controls and 300 idiopathic pulmonary fibrosis (IPF) cases by quantitative PCR. A: IPF lung tissue had significantly higher C3 expression than control lung. Relative C3 expression in human lung tissue compared with genotypes at rs35705950 from 175 controls and 300 IPF cases by quantitative PCR. B: in control lungs, there is no significant difference in C3 expression between genotypes. C: in IPF lung tissue, the TT genotype had significantly higher C3 expression than GG or GT. Statistical tests were performed on Δcycle threshold (ΔCT) values (C3 CT values normalized to housekeeping gene GAPDH), and relative expression is visually depicted as (10 – ΔCT) on this graph. Lines indicate mean values with bars denoting ± SE.
C3 expression and MUC5B promoter variant (rs35705950).
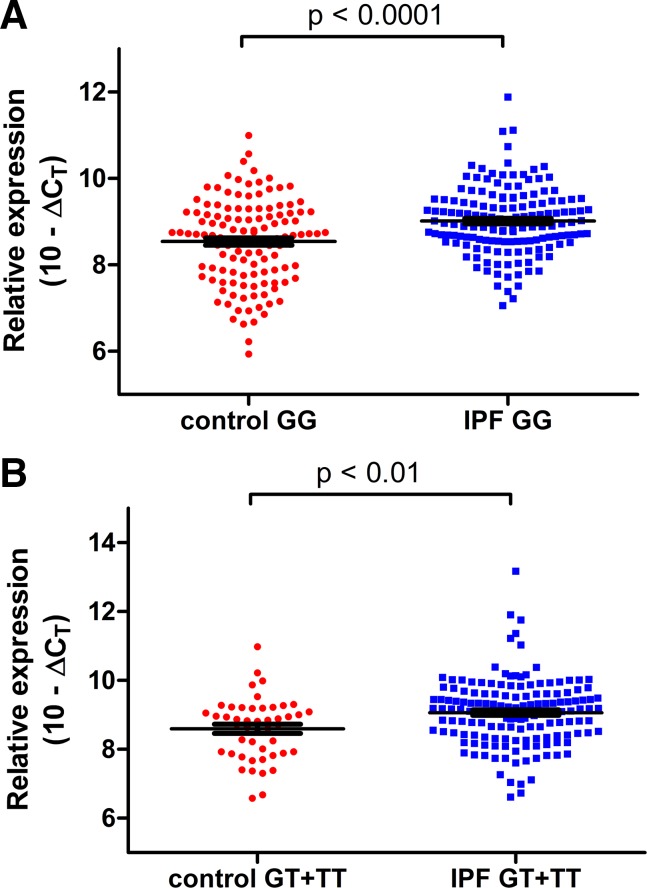
In unaffected control lung tissue, there was no significant difference in C3 expression based on rs35705950 genotypes (P = 0.95) (Fig. 1B). In contrast, in IPF lung tissue, C3 expression was significantly higher in subjects who were homozygous for the MUC5B promoter risk allele (TT) compared with either GT or GG subjects (Fig. 1C). Among subjects with IPF, there was a 1.59-fold increase (95% CI 1.15–2.20; P < 0.05) in C3 expression in TT subjects compared with GG and a 1.66-fold increase (95% CI 1.20–2.30; P < 0.05) in C3 expression in TT subjects compared with GT. In IPF lung tissue, the TT variant of the MUC5B promoter remained significantly associated with increased C3 expression (P = 0.03) after controlling for age, sex, smoking history, and forced vital capacity (Table 2). Comparing the relationship of the C3 expression based on the MUC5B promoter genotypes in the control and IPF, we found there was a 1.39-fold increase (95% CI 1.28–1.52; P < 0.0001) in C3 expression in IPF subjects with GG compared with control subjects with GG and a 1.38-fold increase (95% CI 1.26–1.54; P < 0.01) in IPF subjects with T allele compared with control subjects with T allele (Fig. 2). Interestingly, among subjects with IPF, C3 and MUC5B gene expression was inversely related for those with the GT (r = −0.25; P = 0.006) and TT (r = −0.41; P = 0.05) genotypes but not the GG genotype (r = −0.02; P = 0.85).
Table 2.
Linear regression model for C3 expression
| Coefficients | SE | P Value | 95% CI | |
|---|---|---|---|---|
| Control lungs | ||||
| Age | −0.009 | 0.004 | 0.045 | (−0.017, −0.0002) |
| Sex | 0.088 | 0.143 | 0.540 | (−0.194, 0.370) |
| Smoking history | 0.018 | 0.094 | 0.186 | (−0.169, 0.204) |
| MUC5B genotype | 0.056 | 0.150 | 0.372 | (−0.241, 0.353) |
| IPF lungs | ||||
| Age | −0.006 | 0.007 | 0.366 | (−0.019, 0.007) |
| Sex | −0.157 | 0.114 | 0.171 | (−0.381, 0.068) |
| Smoking history | −0.097 | 0.097 | 0.321 | (−0.288, 0.095) |
| FVC | −0.001 | 0.003 | 0.850 | (−0.006, 0.005) |
| MUC5B genotype | 0.216 | 0.083 | 0.033 | (0.051, 0.380) |
Linear regression model for complement component 3 (C3) expression [10 − Δcycle threshold (ΔCT)] after controlling for age, sex, smoking history, and MUC5B genotype. IPF, idiopathic pulmonary fibrosis; CI, confidence interval; FVC, forced vital capacity.
Fig. 2.
The relationship of the complement component 3 (C3) expression based on the MUC5B promoter rs35705950 genotypes in the control and idiopathic pulmonary fibrosis (IPF) lung tissues by quantitative PCR. A: IPF lung tissue with GG had significantly higher C3 expression than control lung with GG. B: IPF lung tissue with T allele had significantly higher C3 expression than control lung with T allele. Statistical tests were performed on Δcycle threshold (ΔCT) values (C3 ΔCT values normalized to housekeeping gene GAPDH), and relative expression is visually depicted as (10 – ΔCT) on this graph. Lines indicate mean values with bars denoting ± SE.
C3 expression and IPF phenotypes.
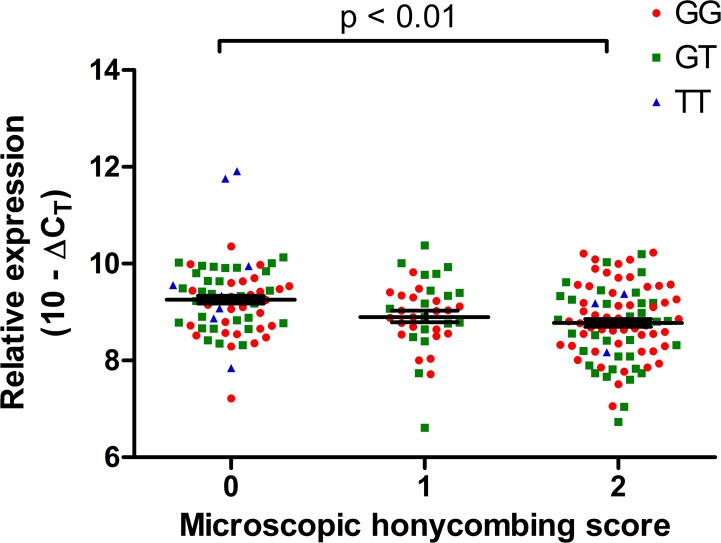
We compared radiographic honeycombing on high-resolution computed tomography between subjects of upper and lower quartile of C3 expression in IPF lung tissue and found no significant difference of lobe-specific radiographic honeycombing scores (P = 0.76; Table 3). However, the histologic presence of microscopic honeycombing in IPF was significantly associated with low C3 expression and the absence of microscopic honeycombing was similarly associated with high C3 expression (P = 0.008; Table 4), consistent with the inverse relationship between C3 and MUC5B gene expression. Comparing C3 expression in IPF lung to microscopic honeycombing scores, C3 expression was significantly higher in lung tissue without microscopic honeycombing than in lung tissue with microscopic honeycombing [1.40-fold increase (95% CI 1.23–1.59); P < 0.01; Fig. 3]. In contrast, fibroblastic foci were not associated with C3 expression in the IPF lung (P = 0.24), similar to what we had observed previously between MUC5B expression and fibroblastic foci (36).
Table 3.
The association C3 expression with HRCT findings
| HRCT: Lobe-Specific Honeycombing Score | High C3 | Low C3 | P Value |
|---|---|---|---|
| 0: Normal/none | 10 | 19 | |
| 1: Mild (1–25%) | 4 | 7 | |
| 2: Moderate (26–50%) | 2 | 3 | |
| 3: Marked (51–75%) | 1 | 1 | |
| 4: Severe (>75%) | 1 | 0 | Fisher’s exact test (0 vs. 1–4), P = 0.762 |
The comparison of high-resolution computed topography scan (HRCT) between cases in upper and lower quartile of complement component 3 (C3) expression (High C3 and Low C3, respectively). Extent of honeycombing in the lobe that mRNA was extracted from were determined by HRCT as previously described (36).
Table 4.
The association C3 expression with histological findings
| Score | High C3 | Low C3 | P Value |
|---|---|---|---|
| Microscopic honeycombing score | |||
| 0 (absent) | 23 | 9 | |
| 1 (rare) | 7 | 8 | |
| 2 (present) | 18 | 31 | χ2-test, P = 0.008 |
| Fibroblastic foci score | |||
| 0 (absent) | 9 | 8 | |
| 1 (rare) | 13 | 7 | |
| 2 (present) | 21 | 28 | χ2-test, P = 0.24 |
The comparison of pathological findings between cases upper and lower quartile of complement component 3 (C3) expression (High C3 and Low C3, respectively).
Fig. 3.
The comparison of complement component 3 (C3) expression related to microscopic honeycombing scores. Regarding microscopic honeycombing scores, 0, absent; 1, rare; 2, present. The C3 expression was significantly higher for 0 than 1 or 2 score. Statistical tests wwere performed on Δcycle threshold (ΔCT) values (C3 Ct values normalized to housekeeping gene GAPDH), and relative expression is visually depicted as (10 – ΔCT) on this graph. The data of GG, GT, and TT subjects are plotted in red, green, and blue colors, respectively. Lines indicate mean values with bars denoting ± SE.
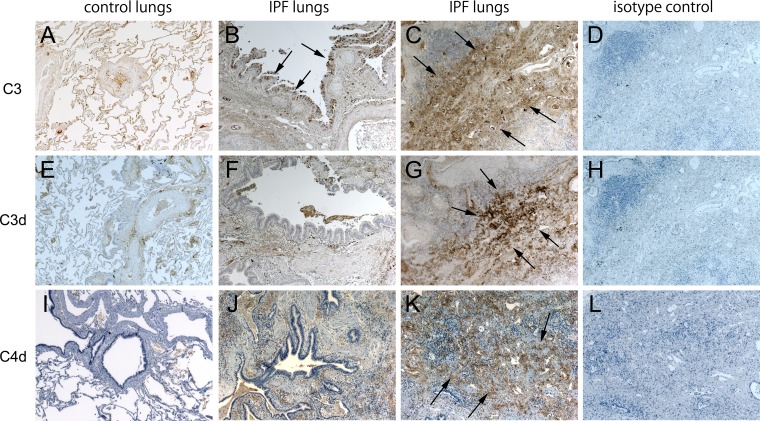
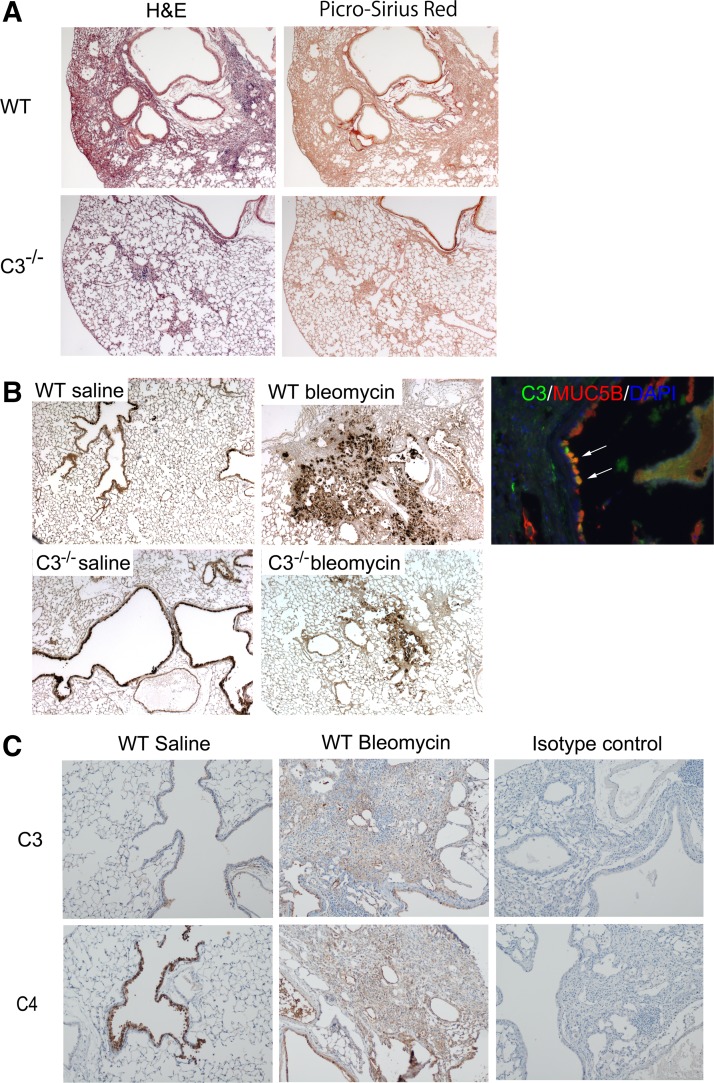
Immunohistochemical staining for C3 in control lung tissue indicates that C3 was expressed in airway epithelial cells, alveolar macrophages, and vascular walls (Fig. 4A). In IPF lung tissue, C3 was expressed not only in airway epithelial cells and vascular walls but also in the interstitial lesions (Fig. 4, B and C). C3 was not present in fibroblastic foci. To ensure that tissue C3 deposition was due to complement activation and not simply due to leakage of plasma C3 into the tissues, we also stained lungs using an antibody specific for the C3d activation fragment. Additionally, to investigate which complement activation pathway is involved, C4d staining was also performed. In IPF lung tissue, C3d and C4d were expressed in the vascular walls and interstitial lesions but not in airway epithelial cells and fibroblastic foci (Fig. 4, F, G, J, and K). Double immunofluorescence staining for C3 and MUC5B in IPF lung tissue demonstrated that there were C3 and MUC5B double-positive cells in airway epithelia (Fig. 5A, C, and D). In honeycomb cysts, the epithelial lining cells and intraluminal material expressed for MUC5B but not C3 (Fig. 5E).
Fig. 4.
Immunohistochemistry of complement component 3 (C3), C3d, and C4d. Normal lungs are stained by C3, C3d, and C4d (A, E, and I). Idiopathic pulmonary fibrosis (IPF) lungs are stained by C3 (B and C), C3d (F and G), and C4d (J and K) (arrows). Isotype controls for C3, C3d, and C4d are shown (D, H, and L). Airway epithelial cells (B, F, and J) and interstitial lesions (C, G, and K) are shown. In IPF lung tissue C3 was expressed in airway epithelial cells, vascular walls, and interstitial lesions (B and C). C3d and C4d were expressed in the vascular walls and interstitial lesions but not in airway epithelial cells (F, G, J, and K). Brown color, DAB; blue color, hematoxylin. Magnification: ×4.
Fig. 5.
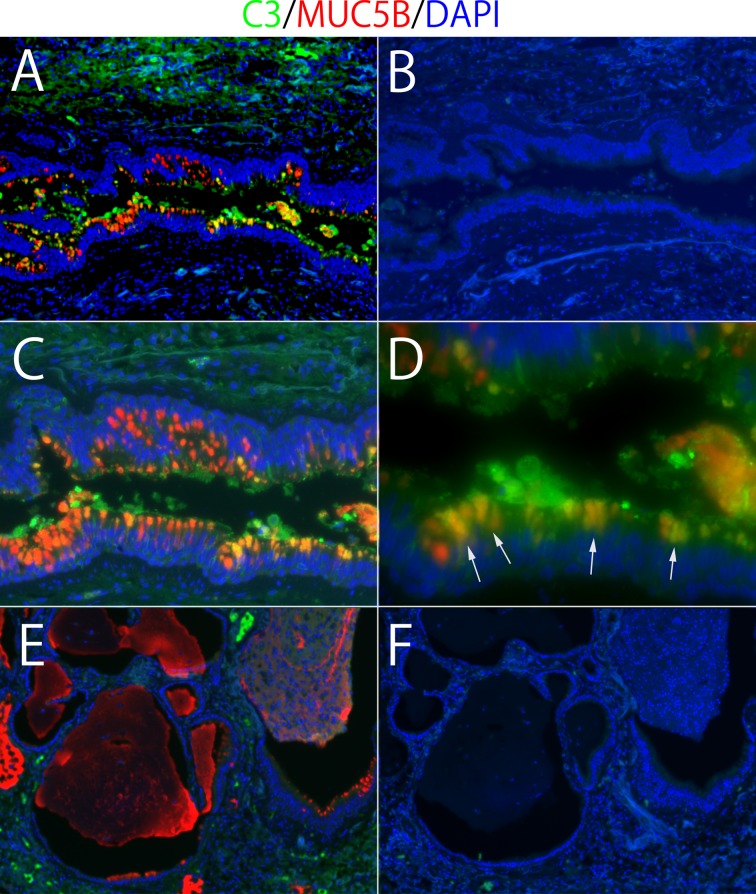
Immunofluorescence double staining of complement component 3 (C3) and MUC5B in human lung tissue. Double positive cells (arrows) denote airway epithelial cells (A, C, and D). There is no C3 expression in honeycomb cysts (E). B and F are isotype controls for A and E, respectively. Magnifications: A, B, E, and F: ×10; C: ×20; D: ×40. Fluorescent colors: green, C3 staining; red, MUC5B staining: yellow, overlay; blue, DAPI.
C3−/− mice demonstrate reduced bleomycin-induced lung injury.
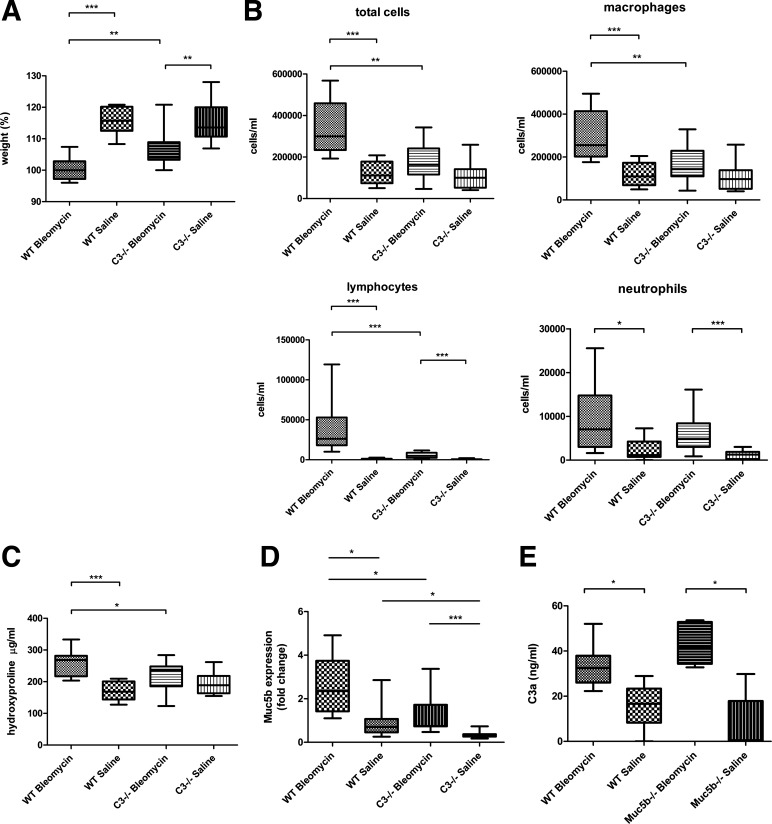
The body weight increased significantly more in C3−/− mice than treated WT mice with bleomycin (Fig. 6A). In BAL fluid, total cell and lymphocyte counts were significantly higher in WT mice than C3−/− mice treated with bleomycin (P < 0.001; Fig. 6B). Mouse lung hydroxyproline levels were significantly higher in WT mice than C3−/− mice treated with bleomycin (P < 0.05; Fig. 6C). Histologically, lung fibrosis in C3−/− mice was diminished compared with WT treated with bleomycin (Fig. 7A). To investigate complement activation, mouse lungs were stained with antibodies to C3 and C4. C3 and C4 were stained similarly in airway epithelial cells and injured regions (Fig. 7C).
Fig. 6.
The phenotype of complement component 3-deficient (C3−/−) mice compared with wild-type (WT) mice in the repeat bleomycin model. A: body weight increased more significantly in C3−/− mice with bleomycin treatment compared with WT mice with bleomycin treatment. B: in bronchoalveolar lavage (BAL) profile, total cells, macrophages, and lymphocytes were significantly lower in C3−/− mice with bleomycin treatment than WT mice with bleomycin treatment. C: the hydroxyprolin concentration was significantly lower in C3−/− mice with bleomycin treatment than WT mice with bleomycin treatment. D: Muc5b expression in BAL was significantly lower in C3−/− mice compared with WT mice treated with bleomycin. E: there was no significant difference of C3a in BAL between WT mice and Muc5b−/− mice treated with bleomycin. Values are presented as maximum, upper quartile, mean, lower quartile, and minimum. Each group: n = 9–16. *P < 0.05; **P < 0.01; ***P < 0.001.
Fig. 7.
A: the histology of mouse lungs in the repeat bleomycin mice model after staining with hematoxylin and eosin (H&E) and picro-sirius red staining (×4). B: immunostaining for MUC5B in bleomycin-induced lung injury. MUC5B is expressed in airway epithelial cells and interstitial lesions. Muc5b expression in lung tissue of complement component 3-deficient (C3−/−) mice treated with bleomycin is diminished compared with WT treated with bleomycin. There are double positive cells as arrows (C3 and MUC5B). C: C3 and C4 staining in mice lungs. C3 and C4 are expressed in airway epithelial cells and injured interstitial lesions.
C3−/− mice with bleomycin treatment reduced Muc5b expression.
C3−/− mice with bleomycin had less Muc5b expression in BAL significantly compared with WT mice with bleomycin (P < 0.05; Fig. 6D). On the other hand, there was no significant difference of C3a levels in BAL between WT and Muc5b−/− mice with bleomycin (Fig. 6E). Immunohistochemically, Muc5b was expressed in airway epithelial cells and interstitial lesions in both WT mice and C3-deficient mice (Fig. 7B). Muc5b was expressed more in lung tissue of WT mice treated with bleomycin compared with WT mice treated with saline. A similar pattern was observed in C3-deficient mice. Muc5b expression in lung tissue of C3−/− mice treated with bleomycin was also diminished compared with WT treated with bleomycin. Double positive staining for C3 and MUC5B was observed in airway epithelial cells (Fig. 7B).
C3a increased Muc5b gene expression in vitro.
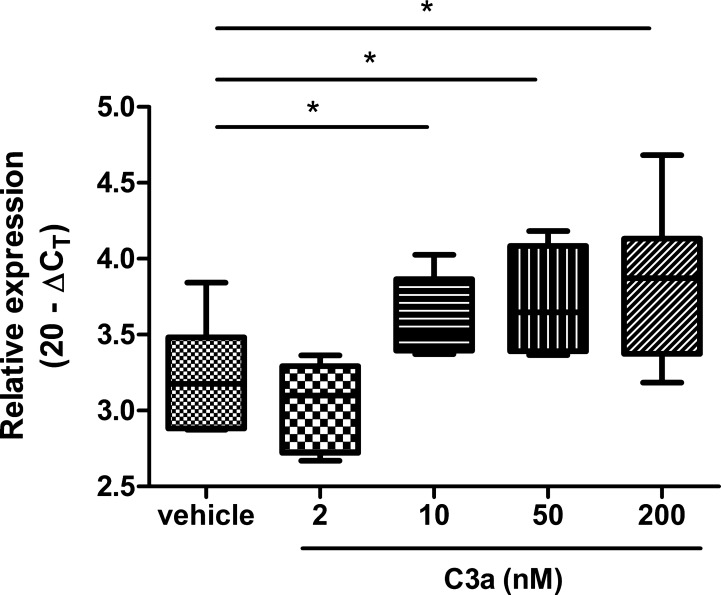
To verify the relationship between C3 and Muc5b, we exposed A549 cells to different concentrations of C3a. Muc5b gene expression increased in a dose-dependent manner (Fig. 8).
Fig. 8.
The association between C3a and Muc5b in A549 cells. Muc5b gene expression increased 8 h after exposure of C3a in a dose-dependent manner. The experiment was performed twice with the same result. Values are presented as maximum, upper quartile, mean, lower quartile, and minimum. ΔCT, Δcycle threshold. *P < 0.05.
DISCUSSION
Our findings indicate that C3 expression is increased in IPF compared with normal lungs and that being homozygous for the MUC5B promoter variant rs35705950 is associated with increased C3 expression in subjects with IPF but not controls. Furthermore, C3−/− mice demonstrate reduced bleomycin-induced lung injury compared with WT mice. This suggests that in IPF the gain-of-function MUC5B promoter variant is associated with complement activation, suggesting that excessive activation of complement may be involved in the pathogenesis of MUC5B associated IPF.
A recent genome-wide association study of IPF subjects identified genes linked to host defense, cell-cell adhesion, and DNA repair (6). We hypothesize that defects in these mechanisms contribute to the development of IPF. Fibroproliferation is a complex tissue response that is, in part, due to an essential component of host defense in respond to pathogens. Excessive or reduced concentrations of MUC5B may impair lung host defense locally and lead to recurrent injury (5). Lung injury, either via environmental exposures, endogenous defects in critical homeostatic mechanisms, or subtle defects in host defense, may, over years, lead to pulmonary fibrosis. Fibroproliferative responses were studied as a component of the innate and adaptive immune response to certain pathogens and autoimmunity (12, 20, 35). The complement system plays a key role in both innate and adaptive immune responses and therefore affects host defense. As we have shown here, C3 expression is related to MUC5B promoter variant in the lung. Although the association between impaired host defense and fibrotic lung disease remains unclear, our findings suggests that complement activation may be secondary to a primary defect in host defense.
The presence of C3d and C4d, cleaved products of C3 and C4, implies that complement activation occurs within the fibrotic lesion. Given the presence of C4d staining in the IPF lung and C4 staining in the mouse lung injured by bleomycin, the classical pathway and lectin pathway of complement activation may be involved in IPF. A previous study reported that classical pathway complement activation was operative in IPF, and fragment Ba, a marker of alternative pathway, indicates disease severity (19). To clarify which complement activation pathway is involved in IPF is important not only for understanding of the pathogenesis of IPF but also for identifying appropriate approaches to treatment through inhibition of components of the complement cascade.
Here, C3-deficient mice were protected from bleomycin-induced lung injury. This suggests that C3 may play an important role in bleomycin-induced lung injury in mice. This result supports our finding in humans in whom C3 expression was higher in IPF compared with control lungs. Several studies have also demonstrated a relationship between complement activation and pulmonary fibrosis using a mouse model. With the use of C5-deficient mice, we demonstrated that C5, a downstream product of C3, promoted fibrosis and that blocking C5 attenuated fibrosis (1). Thrombin can generate C5a even in C3-deficient mice (10). However, our results that the C3-deficient mouse is resistant to bleomycin-induced fibrosis suggest that complement activation induced by bleomycin may be derived from the complement cascade rather than the coagulation pathway. Consistent with a role for complement, blockade of the C3a and the C5a receptor arrested the progression of fibrosis (7). In addition, the epithelial injury in IPF can be enhanced by transforming growth factor-β1, which promotes loss of complement inhibitory proteins and leads to excessive complement activation (8). According to our results, the inhibition of complement activation may emerge as an option for the treatment of pulmonary fibrosis. Further studies are indicated to clarify the mechanism of C3 in the pathogenesis.
The number of infiltrating macrophages and lymphocytes was significantly lower in C3−/− mice treated with bleomycin compared with WT mice, indicating that complement activation may be important in bleomycin-induced lung inflammation. Complement activation generates several chemotactic molecules, such as C5a, and may promote trafficking of these cells to the injured lung (2). The infiltrating macrophages and lymphocytes, in turn, might be important mediators of fibrosis. Our in vitro studies also indicate that complement activation directly induces lung epithelial cells to produce Muc5b and this may be another mediator of pulmonary fibrosis.
Muc5b protein expression in the mouse lung increased in mice treated with bleomycin. While Muc5b was expressed in airway epithelial cells, it was much more prevalent in the distal airway and injured alveoli following bleomycin exposure (Fig. 7B). Additionally, Muc5b expression in BAL and lung tissue of C3−/− mice treated with bleomycin was also diminished compared with WT treated with bleomycin (Figs. 6D and 7B). This suggests that C3 may play a role in the response to bleomycin through Muc5b production in the injured lung. There was no significant difference of C3a levels in BAL between WT mice and Muc5b−/− mice treated with bleomycin (Fig. 6E). C3a levels 1 wk after bleomycin administration provided the same result (data not shown), indicating that Muc5b may be downstream reaction of complement activation in this mouse model. We also demonstrated the direct relationship that C3a increased Muc5b expression in lung epithelial cells. However, further studies are needed to clarify the mechanistic relationship between C3 and MUC5B.
The presence of microscopic honeycombing, a phenotype of end-stage of fibrosis, was related to low C3 expression in the lungs. While honeycomb cysts are lined by cells producing MUC5B and filled with MUC5B containing mucus (28), we have not found that the MUC5B promoter variant is associated with more microscopic honeycombing. Therefore, C3 expression may be influenced by the concentration of MUC5B, which is determined by the MUC5B promoter variant. Another reason for the relationship between low C3 expression and honeycomb cysts may be changes of the cell composition. Type II alveolar epithelial cells, airway epithelial cells, alveolar macrophages, and fibroblasts can also synthesize C3 (3, 25, 30). Therefore, the cell composition, especially in the remodeled lung, may have a profound effect on the local levels of C3 transcription. Additionally, inflammatory stage may change in the honeycombing lungs. It has been suggested that patients with IPF have active inflammatory cell infiltration early in the disease but subsequently have decreasing inflammation as the disease progresses (29).
In conclusion, C3 expression was increased in IPF compared with control lungs. Additionally, especially high levels of tissue C3 mRNA were observed in IPF subjects who were homozygous for the MUC5B promoter variant. C3-deficient mice had attenuated bleomycin-induced pulmonary fibrosis. These findings suggest that C3, a central component of complement cascade related to innate immunity and inflammation, as well as additional complement activation products, likely contributes to the pathogenesis of IPF in humans.
GRANTS
Support for this study was provided by National Institutes of Health Grants UH2-HL-123442, R01-HL-097163, R01-HL-130140, R01-AR-51749, R01-DK-076690, and R21/R33- HL-120770 and Veterans Affairs Medical Center Grant 1I01BX001534.
DISCLOSURES
J. M. Thurman receives royalties from Alexion Pharmaceuticals, Inc. No other conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.O., M.I.S., N.K.B., J.M.T., I.V.Y., and D.A.S. conceived and designed research; T.O. performed experiments; T.O., S.K.M., A.L.S., N.K.B., and V.M.H. analyzed data; T.O., S.K.M., C.E.H., L.A.H., A.D.W., A.L.S., K.K.B., D.A.L., G.P.C., S.D.G., C.D.C., M.I.S., N.K.B., J.M.T., I.V.Y., V.M.H., and D.A.S. interpreted results of experiments; T.O. prepared figures; T.O. drafted manuscript; T.O., M.I.S., N.K.B., J.M.T., I.V.Y., V.M.H., and D.A.S. edited and revised manuscript; T.O., S.K.M., C.E.H., L.A.H., A.D.W., A.L.S., K.K.B., D.A.L., G.P.C., S.D.G., C.D.C., M.I.S., N.K.B., J.M.T., I.V.Y., V.M.H., and D.A.S. approved final version of manuscript.
REFERENCES
- 1.Addis-Lieser E, Köhl J, Chiaramonte MG. Opposing regulatory roles of complement factor 5 in the development of bleomycin-induced pulmonary fibrosis. J Immunol 175: 1894–1902, 2005. doi: 10.4049/jimmunol.175.3.1894. [DOI] [PubMed] [Google Scholar]
- 2.Al-Rayahi IA, Sanyi RH. The overlapping roles of antimicrobial peptides and complement in recruitment and activation of tumor-associated inflammatory cells. Front Immunol 6: 2, 2015. doi: 10.3389/fimmu.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole FS, Matthews WJ Jr, Rossing TH, Gash DJ, Lichtenberg NA, Pennington JE. Complement biosynthesis by human bronchoalveolar macrophages. Clin Immunol Immunopathol 27: 153–159, 1983. doi: 10.1016/0090-1229(83)90065-X. [DOI] [PubMed] [Google Scholar]
- 4.Dreisin RB, Schwarz MI, Theofilopoulos AN, Stanford RE. Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med 298: 353–357, 1978. doi: 10.1056/NEJM197802162980701. [DOI] [PubMed] [Google Scholar]
- 5.Evans CM, Fingerlin TE, Schwarz MI, Lynch D, Kurche J, Warg L, Yang IV, Schwartz DA. Idiopathic pulmonary fibrosis: a genetic disease that involves mucociliary dysfunction of the peripheral airways. Physiol Rev 96: 1567–1591, 2016. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, Loyd JE, Cosgrove GP, Lynch D, Groshong S, Collard HR, Wolters PJ, Bradford WZ, Kossen K, Seiwert SD, du Bois RM, Garcia CK, Devine MS, Gudmundsson G, Isaksson HJ, Kaminski N, Zhang Y, Gibson KF, Lancaster LH, Cogan JD, Mason WR, Maher TM, Molyneaux PL, Wells AU, Moffatt MF, Selman M, Pardo A, Kim DS, Crapo JD, Make BJ, Regan EA, Walek DS, Daniel JJ, Kamatani Y, Zelenika D, Smith K, McKean D, Pedersen BS, Talbert J, Kidd RN, Markin CR, Beckman KB, Lathrop M, Schwarz MI, Schwartz DA. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet 45: 613–620, 2013. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu H, Fisher AJ, Mickler EA, Duerson F 3rd, Cummings OW, Peters-Golden M, Twigg HL 3rd, Woodruff TM, Wilkes DS, Vittal R. Contribution of the anaphylatoxin receptors, C3aR and C5aR, to the pathogenesis of pulmonary fibrosis. FASEB J 30: 2336–2350, 2016. doi: 10.1096/fj.201500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu H, Mickler EA, Cummings OW, Sandusky GE, Weber DJ, Gracon A, Woodruff T, Wilkes DS, Vittal R. Crosstalk between TGF-β1 and complement activation augments epithelial injury in pulmonary fibrosis. FASEB J 28: 4223–4234, 2014. doi: 10.1096/fj.13-247650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hancock LA, Hennessy CE, Dobrinskikh E, Estrella A, Yang IV, Evans C, Schwartz DA. The concentration of muc5b is directly related to the severity of bleomycin-induced lung injury in mice. Am J Respir Crit Care Med 195: A2381, 2017. [Google Scholar]
- 10.Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med 12: 682–687, 2006. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 11.Jansen HM, Schutte AJ, Elema JD, Giessen MV, Reig RP, Leeuwen MA, Sluiter HJ, The TH. Local immune complexes and inflammatory response in patients with chronic interstitial pulmonary disorders associated with collagen vascular diseases. Clin Exp Immunol 56: 311–320, 1984. [PMC free article] [PubMed] [Google Scholar]
- 12.Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, Bon J, Soejima M, Levesque MC, Lindell KO, Gibson KF, Kaminski N, Banga G, Oddis CV, Pilewski JM, Sciurba FC, Donahoe M, Zhang Y, Duncan SR. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med 187: 768–775, 2013. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerr AR, Paterson GK, Riboldi-Tunnicliffe A, Mitchell TJ. Innate immune defense against pneumococcal pneumonia requires pulmonary complement component C3. Infect Immun 73: 4245–4252, 2005. doi: 10.1128/IAI.73.7.4245-4252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 15.Köhl J, Gessner JE. On the role of complement and Fc gamma-receptors in the Arthus reaction. Mol Immunol 36: 893–903, 1999. doi: 10.1016/S0161-5890(99)00111-X. [DOI] [PubMed] [Google Scholar]
- 16.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clin Epidemiol 5: 483–492, 2013. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marc MM, Korosec P, Kosnik M, Kern I, Flezar M, Suskovic S, Sorli J. Complement factors c3a, c4a, and c5a in chronic obstructive pulmonary disease and asthma. Am J Respir Cell Mol Biol 31: 216–219, 2004. doi: 10.1165/rcmb.2003-0394OC. [DOI] [PubMed] [Google Scholar]
- 18.Mathai SK, Pedersen BS, Smith K, Russell P, Schwarz MI, Brown KK, Steele MP, Loyd JE, Crapo JD, Silverman EK, Nickerson D, Fingerlin TE, Yang IV, Schwartz DA. Desmoplakin variants are associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 193: 1151–1160, 2016. doi: 10.1164/rccm.201509-1863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meliconi R, Senaldi G, Sturani C, Galavotti V, Facchini A, Gasbarrini G, Vergani D. Complement activation products in idiopathic pulmonary fibrosis: relevance of fragment Ba to disease severity. Clin Immunol Immunopathol 57: 64–73, 1990. doi: 10.1016/0090-1229(90)90022-I. [DOI] [PubMed] [Google Scholar]
- 20.Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, Murphy E, Johnston SL, Schwartz DA, Wells AU, Cookson WO, Maher TM, Moffatt MF. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 190: 906–913, 2014. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandya PH, Wilkes DS. Complement system in lung disease. Am J Respir Cell Mol Biol 51: 467–473, 2014. doi: 10.1165/rcmb.2013-0485TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phan SH, Thrall RS. Inhibition of bleomycin-induced pulmonary fibrosis by cobra venom factor. Am J Pathol 107: 25–28, 1982. [PMC free article] [PubMed] [Google Scholar]
- 23.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richeldi L, Cottin V, Flaherty KR, Kolb M, Inoue Y, Raghu G, Taniguchi H, Hansell DM, Nicholson AG, Le Maulf F, Stowasser S, Collard HR. Design of the INPULSIS™ trials: two phase 3 trials of nintedanib in patients with idiopathic pulmonary fibrosis. Respir Med 108: 1023–1030, 2014. doi: 10.1016/j.rmed.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Rothman BL, Merrow M, Bamba M, Kennedy T, Kreutzer DL. Biosynthesis of the third and fifth complement components by isolated human lung cells. Am Rev Respir Dis 139: 212–220, 1989. doi: 10.1164/ajrccm/139.1.212. [DOI] [PubMed] [Google Scholar]
- 26.Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, Alexander SN, Bellinghausen LK, Song AS, Petrova YM, Tuvim MJ, Adachi R, Romo I, Bordt AS, Bowden MG, Sisson JH, Woodruff PG, Thornton DJ, Rousseau K, De la Garza MM, Moghaddam SJ, Karmouty-Quintana H, Blackburn MR, Drouin SM, Davis CW, Terrell KA, Grubb BR, O’Neal WK, Flores SC, Cota-Gomez A, Lozupone CA, Donnelly JM, Watson AM, Hennessy CE, Keith RC, Yang IV, Barthel L, Henson PM, Janssen WJ, Schwartz DA, Boucher RC, Dickey BF, Evans CM. Muc5b is required for airway defence. Nature 505: 412–416, 2014. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 28.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, Evans CM, Garantziotis S, Adler KB, Dickey BF, du Bois RM, Yang IV, Herron A, Kervitsky D, Talbert JL, Markin C, Park J, Crews AL, Slifer SH, Auerbach S, Roy MG, Lin J, Hennessy CE, Schwarz MI, Schwartz DA. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med 364: 1503–1512, 2011. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest 128, Suppl 1: 526S–532S, 2005. doi: 10.1378/chest.128.5_suppl_1.526S. [DOI] [PubMed] [Google Scholar]
- 30.Strunk RC, Eidlen DM, Mason RJ. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J Clin Invest 81: 1419–1426, 1988. doi: 10.1172/JCI113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki H, Lasbury ME, Fan L, Vittal R, Mickler EA, Benson HL, Shilling R, Wu Q, Weber DJ, Wagner SR, Lasaro M, Devore D, Wang Y, Sandusky GE, Lipking K, Pandya P, Reynolds J, Love R, Wozniak T, Gu H, Brown KM, Wilkes DS. Role of complement activation in obliterative bronchiolitis post-lung transplantation. J Immunol 191: 4431–4439, 2013. doi: 10.4049/jimmunol.1202242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeda K, Thurman JM, Tomlinson S, Okamoto M, Shiraishi Y, Ferreira VP, Cortes C, Pangburn MK, Holers VM, Gelfand EW. The critical role of complement alternative pathway regulator factor H in allergen-induced airway hyperresponsiveness and inflammation. J Immunol 188: 661–667, 2012. doi: 10.4049/jimmunol.1101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Z, Lu B, Hatch E, Sacks SH, Sheerin NS. C3a mediates epithelial-to-mesenchymal transition in proteinuric nephropathy. J Am Soc Nephrol 20: 593–603, 2009. doi: 10.1681/ASN.2008040434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM. Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest 123: 2218–2230, 2013. doi: 10.1172/JCI65861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vannella KM, Luckhardt TR, Wilke CA, van Dyk LF, Toews GB, Moore BB. Latent herpesvirus infection augments experimental pulmonary fibrosis. Am J Respir Crit Care Med 181: 465–477, 2010. doi: 10.1164/rccm.200905-0798OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang IV, Coldren CD, Leach SM, Seibold MA, Murphy E, Lin J, Rosen R, Neidermyer AJ, McKean DF, Groshong SD, Cool C, Cosgrove GP, Lynch DA, Brown KK, Schwarz MI, Fingerlin TE, Schwartz DA. Expression of cilium-associated genes defines novel molecular subtypes of idiopathic pulmonary fibrosis. Thorax 68: 1114–1121, 2013. doi: 10.1136/thoraxjnl-2012-202943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Köhl J. A complex role for complement in allergic asthma. Expert Rev Clin Immunol 6: 269–277, 2010. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, Dickey BF, Davis CW. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol 586: 1977–1992, 2008. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]