Abstract
Leptin is a pleiotropic hormone produced by white adipose tissue that regulates appetite and many physiological functions, including the immune response to infection. Genetic leptin deficiency in humans and mice impairs host defenses against respiratory tract infections. Since leptin deficiency is associated with obesity and other metabolic abnormalities, we generated mice that lack the leptin receptor (LepRb) in cells of the myeloid linage (LysM-LepRb-KO) to evaluate its impact in lean metabolically normal mice in a murine model of pneumococcal pneumonia. We observed higher lung and spleen bacterial burdens in LysM-LepRb-KO mice following an intratracheal challenge with Streptococcus pneumoniae. Although numbers of leukocytes recovered from bronchoalveolar lavage fluid did not differ between groups, we did observe higher levels of pulmonary IL-13 and TNFα in LysM-LepRb-KO mice 48 h post infection. Phagocytosis and killing of ingested S. pneumoniae were also impaired in alveolar macrophages (AMs) from LysM-LepRb-KO mice in vitro and were associated with reduced LTB4 and enhanced PGE2 synthesis in vitro. Pretreatment of AMs with LTB4 and the cyclooxygenase inhibitor, indomethacin, restored phagocytosis but not bacterial killing in vitro. These results confirm our previous observations in leptin-deficient (ob/ob) and fasted mice and demonstrate that decreased leptin action, as opposed to metabolic irregularities associated with obesity or starvation, is responsible for the defective host defense against pneumococcal pneumonia. They also provide novel targets for therapeutic intervention in humans with bacterial pneumonia.
Keywords: bacterial pneumonia, host defense, leptin receptor, lung, Streptococcus pneumoniae
INTRODUCTION
Pneumococcal pneumonia is the most common cause of community-acquired pneumonia, resulting in more than 400,000 annual hospitalizations in the United States (22). Despite the availability of effective 13-valent pneumococcal conjugate vaccines that provide protection against the most common strains of this bacterium, the incidence of pneumococcal pneumonia is rising (50). The individuals most vulnerable to pneumococcal pneumonia are the elderly, smokers, children under the age of 5 yr, and patients with diabetes and chronic heart, lung, liver, and kidney diseases (13). Estimated United States health care costs for this disease totaled $3.4 billion in 2004 and are expected to rise as the population ages (22). In addition, the increase in antimicrobial-resistant strains of Streptococcus pneumoniae has complicated treatment, highlighting the need to identify novel targets for therapeutic intervention (11, 16).
Leptin is a hormone produced by adipocytes that regulates many physiological functions, including appetite, metabolic rate, and immune function (17). In general, leptin levels correlate with white adipose tissue mass, with the highest levels found in obesity and the lowest levels in humans and animals with very low levels of adipose tissue and/or acute caloric deficits (1). Additionally, leptin also plays an important role in regulating the innate immune response (18). We have previously reported that leptin levels increase in blood, lung homogenates, and bronchoalveolar lavage fluid (BALF) in mice during Klebsiella and pneumococcal pneumonia, and this contributes to enhanced leukocyte phagocytosis and bactericidal functions (19, 31, 32). These effects are mediated by the long form of the leptin receptor (LepRb), which activates downstream signaling events mediated by the JAK-STAT, ERK, and phosphatidyl inositol 3-kinase pathways (9, 15). The LepRb is expressed in most cells of the immune system, including alveolar macrophages (AMs) (36). Db/db mice, which lack a functional LepRb, resulting in obesity and diabetes, have been useful in identifying physiological functions of the leptin receptor (10). However, this model of leptin receptor deficiency is complicated by endocrine and other systemic abnormalities observed in these animals (6).
We have previously used leptin receptor mutant mice (s/s and l/l) to identify which LepRb-mediated intracellular signaling events contribute to the host response to bacterial pneumonia (35, 36). Using the s/s mouse, which cannot signal through the LepRb→-STAT3 pathway and is obese, we observed that leptin-mediated ERK activation enhances AM leukotriene (LT) synthesis, AM phagocytosis, and bacterial killing, resulting in protection against pneumococcal pneumonia in vivo (36). We have also observed that interference with LepRb-mediated ERK activation (in l/l mice) impairs AM phagocytosis and bacterial killing (35). These responses occurred via enhanced PGE2 synthesis and subsequent elevation of cAMP (35). In this report, by deleting this receptor from cells of the myeloid lineage, we examined the impact of LepRb deficiency in the host response to pneumococcal pneumonia in a lean mouse with normal glucose homeostasis.
MATERIALS AND METHODS
Animals.
LysM-Cre C57BL6 mice were purchased from the Jackson Laboratories, housed in the University of Michigan Unit for Laboratory Animal Medicine (Ann Arbor, MI), and intercrossed for two subsequent generations with mice harboring loxP sites flanking the LepRb. We used this approach to generate age- and gender-matched male and female (LysM-Cre; Leprflox/flox) and control (LysM-Cre Lepr+/+) littermates, which were used in experiments at 8 to 14 wk of age. The genotype of the LysM-Cre Leprflox/flox (LysM-LepRb-KO) mice was confirmed by quantitative real-time PCR (qPCR), using tail snips for each mouse and primers that flank both LeprΔ (226 bp) and Leprflox (643 bp): Forward 5′-AATGAAAAAGTTGTTTTGGGACGA and Reverse 5′-CAGGCTTCAGAACATGAACACAACAAC, as previously described (43). We confirmed deletion of Lepr by comparing the gene maker for the excised Lepr in tail snips and AMs. Animals were treated according to National Institutes of Health guidelines for the Use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals.
Preparation of S. pneumoniae and infection.
S. pneumoniae (ATCC 6303, Type 3) (American Type Culture Collection, Manassas, VA) was grown in Todd-Hewett Broth with 0.5% yeast extract (Difco, Detroit, MI) to mid-log phase, washed 2 times by centrifugation at 13,000 revolutions/min for 3 min, and the pellet was resuspended in PBS. S. pneumoniae was then serially diluted, and mice were infected with 5 × 104 colony-forming units (CFUs) through a 26-guage needle, introduced into the trachea of mice aestheticized with ketamine and xylazine, as previously described (36). To confirm the dose of the inoculum, bacteria were serially diluted and plated on soy-based blood agar (Difco) for CFU determinations.
Determination of lung and spleen bacterial loads, cytokines, LTB4, and PGE2.
Mice were euthanized by CO2 asphyxiation 24 and 48 h after infection; the lungs and spleen were dissected, homogenized in PBS, serially diluted, and plated on blood agar plates to determine bacterial loads. Cytokines [CXCL2 (MIP-2), IL-6, IL-10, IL-13, IFNγ, and TNF-α] were assayed in whole lung homogenates by the University of Michigan Immunology Core using commercially available enzyme immunoassay kits (EMD Biosciences and R&D Duoset, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Quantification of lung leukocytes in bronchoalveolar lavage fluid and peripheral blood.
In a separate group of mice, euthanized by CO2 asphyxiation at 24 and 48 h after infection, we removed the lungs and performed bronchoalveolar lavage using 2 ml of lavage buffer (HEPES-buffered saline solution), as previously described (42). Briefly, AMs were adhered to 96-well polystyrene cell culture plates in RPMI 1640 (Life Technologies, Invitrogen, Carlsbad, CA) at a concentration of 1 × 105 cells per well for 1 h. Leukocytes were enumerated using a hemocytometer, spun onto glass slides using a cytocentrifuge, and stained with a modified Wright-Geimsa stain (American Scientific Products, McGraw Park, IL). Total and differential counts were determined as previously described (31).
Isolation and culture of alveolar macrophages.
In a separate group of mice that were not infected, AMs were recovered from mice by BALF and were cultured overnight in RPMI with 10% fetal calf serum and penicillin-streptomycin. On the following day, the cell culture media was replaced with fresh RPMI alone or with media with 1 × 108 CFUs of heat-killed S. pneumoniae for 4 h at 37°C in a 5% CO2 incubator. The cell culture media was recovered and stored at −70°C and assayed using commercially available enzyme-linked immunosorbent assays for lipid mediators, LTB4, and PGE2 (Cayman Chemical, Ann Arbor, MI), according to the manufacturer’s instructions.
Recovery and identification of lung leukocytes from lung homogenates for flow cytometry.
In another separate group of mice infected with S. pneumoniae, animals were euthanized 24 and 48 h post infection. Lungs were perfused with 10 ml of cold Dulbecco's PBS (DPBS) medium (GIBCO, ThermoFisher, Carlsbad, CA), excised, and collected in 20 ml of cold DPBS medium with 30 μg/ml of DNase (Sigma-Aldrich, St. Louis, MO). Lung lobes were blended in a Waring blender at low speed for 30 s, and the entire lung homogenate was centrifuged. The resuspended homogenate was filtered through a 40-μm cell strainer (BD Biosciences, San Jose, CA), and recovered cells were subsequently washed 3 times by centrifugation before staining.
Flow cytometry.
Cells were suspended in 2 ml of DPBS staining buffer with 2% (v/v) fetal bovine serum for staining. Cells were first incubated with 10 μg/ml of TruStain fcX anti-CD16/32 block (BioLegend, San Diego, CA) for 10 min at 4°C. Cell samples were then equally divided, and 100 μl antibody cocktails were added using 0.5 μl of antibodies per 100 μl of staining buffer, using either the lymphoid marker panel [CD103-PE, B220-PE Texas Red (BD PharMingen, San Jose, CA), CD4-Pacific Blue, CD8-APC-Cy7, CD3e-PerCP-Cy5.5, CD44-AF700, NK1.1-AF647, CD62L-AF488, CD25-PE-Cy7, and CD69-PE-Cy5 (BioLegend)] or the DC marker panel [CD103-PE, B220-PE Texas Red (BD PharMingen), CD4-Pacific Blue, CD8-APC-Cy7, F4/80-PerCP-Cy5.5, CD45.2 PE-Cy7, CD11c-PE-Cy5, and CD11b-AF488 (BioLegend)]. Preparations were incubated for 30 min at 4°C and were washed with 2 ml of staining buffer. A FACScan LSRII 12-color flow cytometer (BD Biosciences) and FlowJo software version 7.5.5 (Tree Star Inc., Ashland, OR) were used for data acquisition and analysis as previously described (38).
Fluorometric assay for AM phagocytosis of S. pneumoniae.
AM phagocytosis of S. pneumoniae was assessed using a previously published protocol for determining the ingestion of fluorescent, fluorescein isothiocyanate (FITC)-labeled heat-killed S. pneumoniae (42). Murine AMs were obtained from the BALF of age-matched mice, and 1.25 × 105 AMs/well were adhered to 384-well tissue culture plates with opaque sides and optically clear bottoms (Costar, Corning Life Sciences, Lowell, MA). AMs were cultured overnight with RPMI 1640 with 5% penicillin-streptomycin and 10% fetal calf serum (Invitrogen). On the following day, the media was replaced, and AMs were pretreated with RPMI media alone, indomethacin (10 µM), LTB4 (1 nM), or indomethacin and LTB4 (Cayman Chemical) for 30 min. S. pneumoniae was suspended in RMPI 1640 and opsonized with 10% normal rat serum (Invitrogen, ThermoFisher Scientific) by incubating this suspension on a rotating platform for 30 min at 37°C. To allow phagocytosis, AMs were cultured with FITC-labeled heat-killed S. pneumoniae for 60 min. Trypan blue (250 μg/ml) (Harleco, EMD Millipore, Billerica, MA) was added for 1 min to quench the fluorescence of extracellular bacteria, then fluorescence was determined using a SPECTRAMax GEMINI EM fluorometer 485ex/535em (Molecular Devices, Sunnyvale, CA). The phagocytic index was calculated as previously described in relative fluorescence units (5).
Tetrazolium dye reduction assay of S. pneumoniae killing.
The ability of murine AMs to kill ingested S. pneumoniae was quantified using a tetrazolium reduction assay as previously described (41, 43, 47). In this assay, 2 × 105 AMs were plated in duplicate 96-well tissue culture dishes. On the following day, S. pneumoniae was opsonized with 5% normal rat serum as previously reported (35). AMs were cultured with media alone, LTB4 (1 nM), indomethacin (10 μM), or LTB4 and indomethacin 20 min before the addition of bacteria and for the duration of the killing assay. AMs were infected with 1 × 107 CFUs of S. pneumoniae for a multiplicity of infection of 50:1 and cultured for 1 h to allow phagocytosis to occur. The plates were washed 3 times with Hanks’ buffered salt solution. One plate (phagocytosis plate) was cooled to 4°C and the other plate (killing plate) was incubated at 37°C for an additional 90 min and cooled to 4°C. AMs in both plates were then lysed with 5% saponin, and methylthiazolyldiphenyl-tetrazolium bromide (MTT) (5 mg/ml) was added to all wells and incubated for 90 min at 37°C. The intensity of the absorbance at 595 nm (A595) was directly proportional to the number of ingested bacteria within the macrophages. Results were expressed as percentage of ingested bacteria that were killed, where % killing = 100% × (A595 phagocytosis plate − A595 killing plate/A595 phagocytosis plate). Preliminary experiments compared this colorimetric assay with a conventional CFU-based (serial dilution) assay, and similar results were obtained (data not shown).
Assessment of AM nitric oxide and reactive oxygen intermediates.
AMs were adhered to 96-well plates at a concentration of 1.25 × 105 cells/well and were cultured for 24 h with DMEM (high glucose; 4.5 g/l) supplemented with 1% sodium pyruvate (Invitrogen) containing 10% FCS and penicillin-streptomycin, with or without 10 µg/ml lipoteichoic acid from Staphylococcus aureus (Sigma-Aldrich) and 100 ng/ml IFN-γ (R&D systems). We determined nitric oxide (NO) production by measuring stable nitrite concentrations using a modified Griess reaction according to the manufacturer’s instructions (Cayman Chemical).
Reactive oxygen intermediate (ROI) production was assessed in AMs adhered to 384-well plates at 1.25 × 105 cells/well. AMs were cultured overnight in RPMI 1640 containing 10% FCS with 1% penicillin/streptomycin/amphotericin B (Invitrogen). On the next day, the medium was replaced with PBS containing 10 μM 2′,7′-dichlorodihydrofluorescein diacetate, and the cells were cultured for 1 h. The medium was then replaced with warmed Hanks’ buffered salt solution with calcium and magnesium, and the cells were stimulated with heat-killed S. pneumoniae using a multiplicity of infection of 50:1. We assessed ROI production 120 min after stimulation by measuring fluorescence using a Spectramax Gemini XS fluorometer (Molecular Devices) with excitation/emission setting at 493/522 nm.
Analysis of inducible nitric oxide synthase and cyclooxygenase gene expression.
RNA was isolated from AMs cultured with lipoteichoic acid and IFNγ, as mentioned above, using Trizol according to the manufacturer’s protocol (Invitrogen). To quantify expression of inducible NO synthase (iNOS), we performed qPCR as previously described (12), normalizing mRNA levels to GAPDH. Primers for murine iNOS included Forward 5′-CCCTCCTGATCTTGTGTTGGA-3′ and Reverse 5′-CAACCCGAGCTCCTGGAA-3′. Primers for murine cyclooxygenase-2 included: Forward 5′-TGACCCCCAAGGCTCAAAT-3′ and Reverse 5′-GAACCCAGGTCCTCGCTTATG-3′. Primers for murine GAPDH included Forward 5′-TGCACCACCAACTGCTTAG-3′ and Reverse 5′-GGATGCAGGGATGATGTTC-3′.
Statistical analyses.
Statistical analyses were conducted using Prism 6.0 software (GraphPad Software, La Jolla, CA). Where appropriate, mean values were compared using a paired Student t-test or a one-way analysis of variance (ANOVA) followed by Bonferroni correction. Differences were considered significant if P < 0.05. Unless otherwise stated, all experiments were performed on at least three separate occasions. Data are presented as means ± SE, unless noted otherwise.
RESULTS
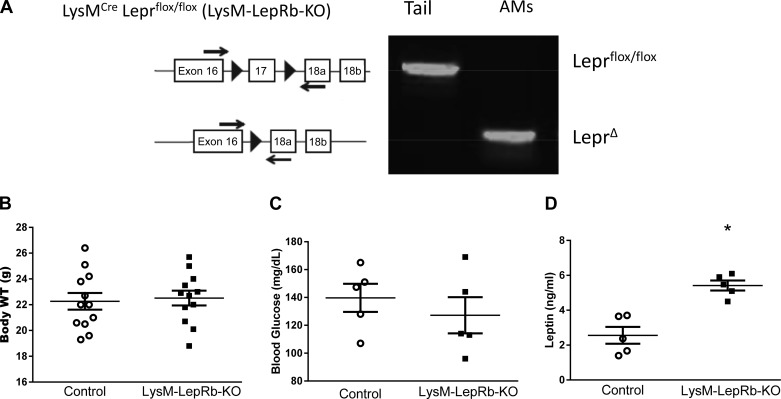
Previously, we used two different approaches to assess the impact of leptin deficiency on host responses against pneumococcal pneumonia: elicited by fasting of wild-type mice as well as ob/ob mice (which are genetically deficient in leptin because of spontaneous deletion of the leptin gene) (32). In this study, we used the Cre-Lox approach to specifically eliminate the LepRb in cells of the myeloid lineage to determine if the lack of signaling initiated by this receptor impacts the innate immune response against S. pneumoniae. PCR genotyping confirmed the excision of exon 17 from AMs but not tail tissue (Fig. 1A). As expected, body weights and blood glucose levels of LysM-LepRb-KO mice were not statistically different from those of control mice, a finding consistent with the ability of leptin to control adiposity via the central nervous system (Fig. 1, B and C). However, we did observe modest but statistically higher levels of serum leptin in LysM-LepRb-KO mice (Fig. 1D). These results suggest that LepRb deletion in myeloid cells did not affect feeding behavior and glucose homeostasis but modestly impacted circulating leptin.
Fig. 1.
Characterization of LysM-LepRb-KO mice. A: genetic deletion of LepRb from AMs by LysM-Cre is specific for cells of the myeloid lineage. B: body weights. C and D: blood glucose and serum leptin levels in control (open circles) and LysM-LepRb-KO (closed squares) mice at 8 wk of age. *P < 0.05 vs. control using the Student’s t-test. Lines indicate means ± SE. AM, alveolar macrophage; LepRb, long form of the leptin receptor; WT, weight.
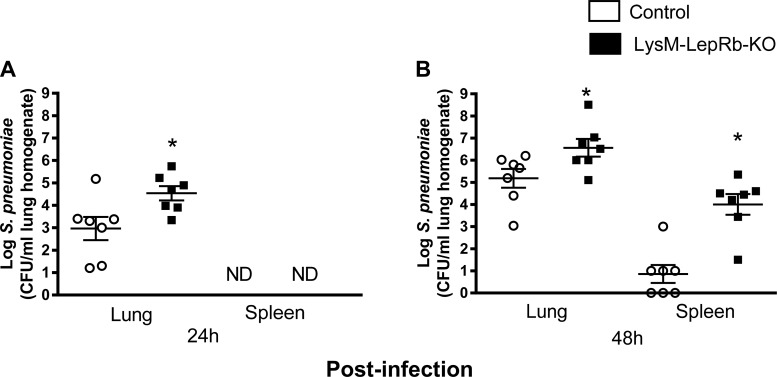
To determine if LepRb deletion impairs the innate immune response against bacterial infection, we assessed pulmonary clearance of S. pneumoniae. Following an intratracheal infection with S. pneumoniae, bacterial burdens were two log units higher in the lungs of LysM-LepR-KO mice compared with control mice 24 h post infection (Fig. 2A). At this time point, bacteria were not detected in any spleens. Forty-eight hours after infection, pulmonary and spleen bacterial loads were ~1.5 and 2.5 log units higher, respectively, in LysM-LepRb-KO mice compared with the controls (Fig. 2B). These results imply that targeted deletion of LepRb from myeloid lineage cells impairs lung clearance of bacteria.
Fig. 2.
Increased lung and spleen bacterial loads in LysM-LepRb-KO mice following intratracheal S. pneumoniae infection. Control (open circles) and LysM-LepRb-KO (closed squares) mice were infected with 5 × 104 CFUs of S. pneumoniae, and bacterial burdens were quantified in lungs and spleens harvested 24 h (A) and 48 h (B) post infection. *P < 0.05 compared with control using the Student’s t-test. Lines indicate means ± SE from 2 separate experiments. CFU, colony-forming unit; ND, not detected.
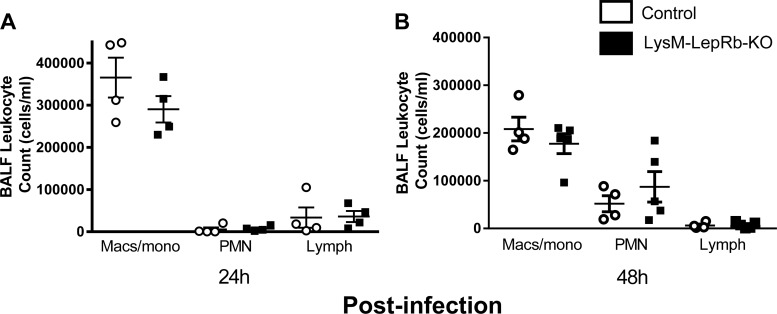
Recruitment of leukocytes to the alveolar focus of infection is critical for pulmonary clearance during bacterial pneumonia. We previously reported reduced numbers of neutrophils in BALF and peripheral blood following S. pneumoniae challenge in mice depleted of leptin by fasting (32). By contrast, leukocyte counts in BALF following infection with S. pneumoniae did not differ significantly between control mice and LysM-LepRb-KO mice in macrophage/monocytes, neutrophils, or lymphocytes at 24 h and 48 h post infection (Fig. 3, A and B). We also examined leukocyte subsets in lung homogenates by flow cytometry 48 h after infection and observed no differences between wild-type and LysM- LepRb-KO animals in numbers of AMs, exudative macrophages, dendritic cells, neutrophils, or lymphocytes (data not shown). In addition, we did not observe differences in peripheral blood leukocyte counts (data not shown).
Fig. 3.
Leukocyte counts in BALF from control and LysM-LepRb-KO mice following intratracheal S. pneumoniae infection. Control (open circles) and LysM-LepRb-KO (closed squares) mice were infected with 5 × 104 CFUs of S. pneumoniae, and leukocytes were recovered from BALF 24 h (A) and 48 h (B) post infection. Lines indicate means ± SE from 2 separate experiments. BALF, bronchoalveolar lavage fluid; CFU, colony-forming unit; PMN, polymorphonuclear neutrophils.
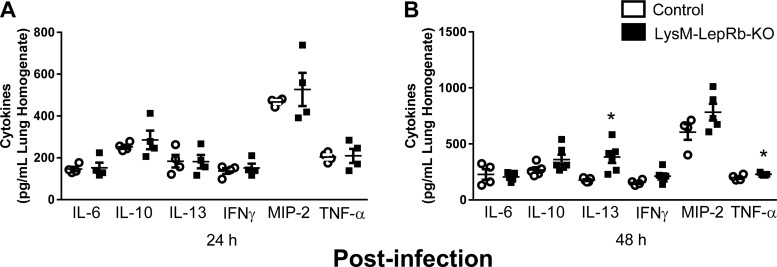
Next, we examined lung production of inflammatory innate immune-relevant cytokines (IL-6, IL-10, IL-13, IFN-γ, MIP-2, and TNF-α) following infection. Although there were no differences between control mice and LysM-LepRb-KO mice in any of these cytokines in lung homogenates 24 h post infection (Fig. 4A), we detected higher levels of IL-13 and TNF-α 48 h post infection in LysM-LepRb-KO mice (Fig. 4, A and B). These results suggest that the lack of LepRb signaling in cells of the myeloid lineage had a selective effect on IL-13 and TNF-α production, but not on other cytokines measured, during pneumococcal pneumonia in LysM-LepRb-KO mice.
Fig. 4.
Elevated IL-13 and TNF-α in lung homogenates from control and LysM-LepRb-KO mice 48 h following intratracheal S. pneumoniae infection. Lung homogenates were prepared from control (open circles) and LysM-LepRb-KO (closed circles) mice 24 h (A) and 48 h (B) post infection with 5 × 104 CFUs of S.pneumoniae. Cytokine concentrations in lung homogenates were measured using ELISA. *P < 0.05 compared with control to LysM-LepRb-KO mice using the Student’s t-test. Lines indicate means ± SE from 2 separate experiments. CFU, colony-forming unit.
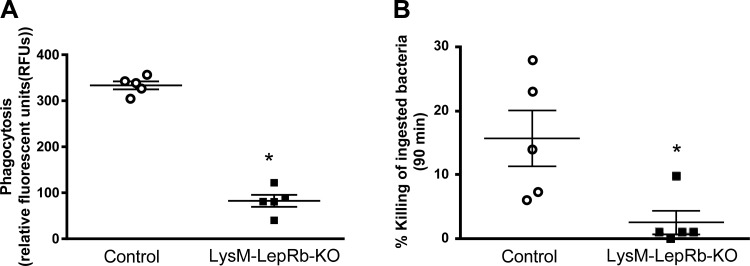
Phagocytosis and killing of ingested S. pneumoniae were also reduced in AMs from LysM-LepRb-KO as compared with control mice (Fig. 5). These results agree with and extend our previous reports demonstrating diminished AM antibacterial host defense in mice with leptin deficiency and a role for leptin receptor signaling in this protective response (21, 31, 32).
Fig. 5.
Impaired phagocytosis and killing of S. pneumoniae in AMs from LysM-LepRb-KO mice. Phagocytosis (A) and intracellular killing (B) were assessed in AMs from wild-type (open circles) and LysM-LepRb-KO (closed squares) mice. Lines indicate means ± SE with at least 5 replicates per experiment. *P < 0.05 compared with control by Student’s t-test. AM, alveolar macrophage.
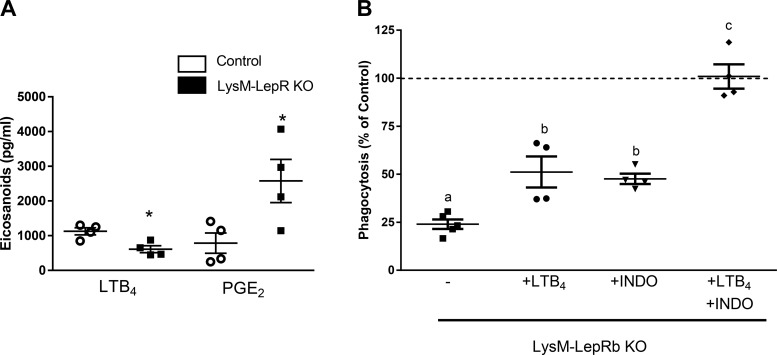
LTB4 synthesis is reduced and PGE2 synthesis is increased in AMs from leptin receptor signaling mutant mice (35). We therefore assessed AM synthesis of these lipid mediators, which regulate leukocyte bactericidal functions (3, 34, 45, 46). Following stimulation with S. pneumoniae, LTB4 synthesis was reduced, whereas PGE2 production was enhanced in AMs from LysM-LepR-KO mice as compared with control mice (Fig. 6A). Because LTB4 potently stimulates leukocyte antibacterial functions, we tested whether adding LTB4 to AMs from LysM-LepRb-KO mice could restore phagocytosis (34, 45). Although we did observe a slight increase in phagocytosis, this enhancement did not reach statistical significance (Fig. 6B). Similarly, phagocytosis was not restored by inhibiting PGE2 synthesis using the cyclooxygenase inhibitor, indomethacin. However, phagocytosis was restored when AMs from LysM-LepR-KO mice were treated with both LTB4 and indomethacin. In contrast, pretreating AMs from LysM-LepRb-KO mice with LTB4, indomethacin alone, or in combination, did not restore killing (data not shown). We did not observe differences in cyclooxygenase-2 mRNA, the enzyme responsible for PGE2 synthesis, using qPCR (data not shown). These data suggest that combined effects of diminished LTB4 and increased PGE2 synthesis contributed to the impairment in phagocytosis but not killing in AMs from LysM-LepR-KO mice.
Fig. 6.
Reduced LTB4 and increased PGE2 in AMs from LysM-LepRb-KO mice and exogenous LTB4 and indomethacin restores phagocytosis of S. pneumoniae in AMs from LysM-LepRb-KO mice. A: AMs were stimulated with 1 × 108 CFUs of heat-killed S. pneumoniae overnight, and LTB4 and PGE2 were measured in cell culture media. B: phagocytosis of S. pneumoniae was assessed in AMs from control (dashed line) or LysM-LepRb-KO mice treated with media alone (closed squares), LTB4 (10 nM) (solid circles), INDO (10 µM) (closed triangles), or LTB4 (10 nM) and INDO (10 µM) (closed diamonds). Data are expressed as a percentage of the control. Lines indicate means ± SE with 4–5 replicates per experiment. *P < 0.05 compared with control by Student’s t-test or by ANOVA with different letters indicating differences between groups. AM, alveolar macrophage; CFU, colony-forming unit; INDO, indomethacin.
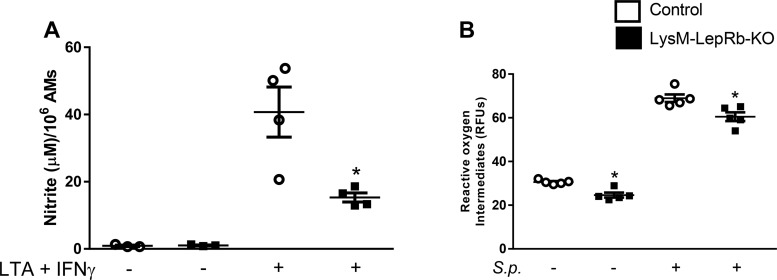
Finally, we assessed NO and ROI synthesis because these mediators have been shown to contribute to AM killing of S. pneumoniae in vitro (12, 25). Previously, we reported lower levels of ROI and nonsignificantly lower levels of NO in AMs from leptin receptor signaling mutant (l/l) mice (35). AMs from LysM-LepRb-KO mice showed a substantial reduction in nitrite production, indicating impaired NO synthesis and a modest deficit in ROI synthesis (Fig. 7). There were no differences in iNOS mRNA expression as assayed by qPCR (data not shown). These results provide a partial mechanistic explanation for the reduced bactericidal capacity of AMs from LysM-LepRb-KO mice.
Fig. 7.
Reduced nitric oxide and ROI synthesis in AMs from LyzM-LepRb-KO mice. A: AMs stimulated for 24 h with LTA (10 g/ml) and IFN-γ (100 ng/ml) for nitrite production. B: AMs stimulated for 24 h with heat-killed S. pneumoniae for ROI synthesis. Lines indicate means ± SE with 4–5 replicates per experiment. *P < 0.05 compared with control by Student’s t-test. AM, alveolar macrophage; LTA, lipoteichoic acid; RFU, relative fluorescent unit.
DISCUSSION
In this study, we examined gene-targeted mice lacking LepRb in cells of the myeloid linage (LysM-LepRb-KO mice) and observed defective pulmonary clearance of S. pneumoniae in vivo and impaired phagocytosis and killing of ingested pneumococci in vitro. The latter findings were associated with reduced LTB4 and enhanced PGE2 synthesis and phagocytosis that could be significantly reversed by the combination of exogenous LTB4 and indomethacin. These results provide the most definitive evidence to date that leptin receptor signaling contributes to innate immune defenses in a cell-autonomous fashion, thus implicating this pathway as a potential therapeutic target.
These results extend our previous findings and those of others who demonstrated that db/db mice, the most common animal model of LepRb deficiency, exhibit impaired host defense against bacterial infections (23, 48). Because of a spontaneous mutation in the db/db gene, which encodes LepRb, these mice lack LepRb signaling in the hypothalamus, become hyperphagic, and develop obesity (44, 47). However, owing to the global nature of the defect in db/db mice, it is difficult to distinguish the impact of LepRb deficiency on innate immune cells from the confounding effects of obesity, diabetes, or some other endocrine abnormality. To circumvent these limitations, we generated LysM-LepRb-KO mice, allowing us to examine the effects of LepRb deficiency targeted specifically to myeloid lineage cells on the innate immune response against S. pneumoniae. Unlike previously published reports, in which leptin or LepRb deficiency in mice causes diabetes, obesity, glucocorticoid excess, and other metabolic abnormalities, the LysM-LepRb-KO mice exhibit normal metabolic function. Hence, they provide an uncomplicated model to study the role of LepRb signaling in myeloid cells known to play a critical role in host defense (29, 31, 49). Using this approach, we demonstrate for the first time that myeloid-targeted LepRb deletion, in the absence of endocrine abnormalities, impairs pulmonary host defense against pneumococcal pneumonia.
AMs play an important role in host defense of the alveolar surface, as their removal before bacterial infection of the lung results in increased pulmonary bacterial burdens, alveolar epithelial injury, a more profound inflammatory response, and reduced survival (8, 26, 27). Our finding of impaired phagocytosis of S. pneumoniae in AMs from LysM-LepRb-KO mice agrees with and extends similar in vitro findings in macrophages from ob/ob and db/db mice (20, 31). These defects were partially due to reduced LTB4 synthesis in AMs from LysM-LepRb-KO mice, a lipid mediator that is produced only in cells of the myeloid lineage and is essential for host defense against S. pneumoniae (33). LTB4 enhances AM phagocytosis by activating phosphatidyl inositol 3-kinase-mediated actin polymerization and by reducing intracellular cAMP-mediated activation of exchange protein directly activated by cAMP (4, 28, 33, 40). We previously demonstrated that LTB4 synthesis is impaired in AMs from mice rendered leptin deficient by genetic means and by fasting, and the administration of exogenous leptin in vivo or in vitro can restore LTB4 synthesis by enhancing phospholipase A2-gamma expression (30, 31). The lack of differences in leukocyte recruitment to the lungs in LysM-LepRb-KO mice despite reduced AM LTB4 production is consistent with previous reports demonstrating impaired pulmonary bacterial clearance in 5-lipoxygenase knockout mice and ob/ob mice during bacterial pneumonia (6, 32).
Elevated production of PGE2 by AMs from LysM-LepRb-KO mice following stimulation in vitro is important because this potent lipid mediator suppresses AM phagocytosis, bacterial killing, and ROI production via intracellular signaling events initiated by the EP2 receptor (2, 3, 46), predominantly increased cAMP. This result was unsurprising, as we have previously reported that l/l mice, a transgenic strain in which LepRb-mediated signaling via ERK1/2 pathway is attenuated, also produced excess levels of PGE2 because of increased expression of microsomal prostaglandin E synthase-1. The current finding that the defective phagocytosis observed in AMs from LysM-LepRb-KO mice could be reconstituted by the addition of both exogenous LTB4 and indomethacin, but neither alone supports our previous data showing that reduced production of LTs or enhanced PGE2 synthesis impairs host defense against pneumococcal pneumonia (3, 35, 46).
AMs from LysM-LepRb-KO mice showed defective bactericidal function associated with reduced synthesis of ROI and NO, which contribute to killing of S. pneumoniae (12, 33). The reduced LTB4 and enhanced PGE2 production in AMs from LysM-LepRb-KO mice likely contributed to this defect. LTB4 activates nicotine adenine dinucleotide phosphatase oxidase to enhance bactericidal function in AMs and to enhance ROI synthesis in AMs (33, 45). In contrast, PGE2 inhibits nicotine adenine dinucleotide phosphatase oxidase activation and can suppress AM ROI synthesis (46). However, unlike phagocytosis, AM bactericidal activity could not be restored with exogenous LTB4 and the cyclooxygenase inhibitor indomethacin (data not shown). Hence, LepRb deficiency likely impairs other mechanisms that contribute to AM bactericidal function, a topic for future investigation.
We previously reported that exogenous leptin restores circulating and intrapulmonary polymorphonuclear neutrophils (PMNs) in fasted mice during bacterial pneumonia (32). In the current study, we did not observe differences between wild-type and LysM-LepRb-KO in PMN counts in either peripheral blood at baseline or in BALF following infection. This suggests that leptin affects PMN counts indirectly. For example, fasting increases while leptin decreases glucocorticoids known to induce apoptosis in PMNs (32). LysM is expressed in PMNs, and it is acknowledged that ablation of LepRb in these cells may have impacted pulmonary host defense, a subject for future investigation.
The increased levels of pulmonary IL-13 48 h after S. pneumoniae challenge of LysM-LepRb-KO may also contribute to defective bacterial clearance. IL-13, a Th2 cytokine best known for its role as an essential mediator of atopic asthma, is produced primarily by Th2 cells, innate lymphoid 2 cells, and macrophages (37, 39). Importantly, however, IL-13 can also diminish macrophage expression of NO synthase-2 by reducing both mRNA and protein levels (7). Interestingly, db/db mice produce higher levels of IL-13 in the airways and an increased number of pulmonary innate lymphoid 2 cells IL13+ cells following ozone exposure (24, 37). It is possible that the elevated IL-13 observed in LysM-LepRb-KO mice may be responsible for the reduced production of NO.
In summary, although deleting LepRb from myeloid cells in mice results in normal body weight and glucose homeostasis, these mice exhibit defective pulmonary host defense against pneumococcal pneumonia in vivo, which was associated with impaired phagocytosis and killing of S. pneumoniae in AMs in vitro. Whether or not leptin receptor deficiency in humans is associated with greater susceptibility to bacterial pneumonia has not been reported, but there is evidence of greater susceptibility to infection in humans with congenital leptin deficiency (41). In addition, leptin receptor mutations in humans are associated with greater susceptibility to amoeba infections of the colon (14). Future research should examine potential links between leptin receptor abnormalities and infections of the respiratory tract in humans.
GRANTS
This research was supported by National Institutes of Health Grants RO1-HL-077417 (to P. Mancuso) and R01-AI-083334 (to J. Weinberg) and the Department of Veterans Affairs via Merit Review Awards I01 BX001389 (to C. Freeman) and I01 CX000911 (to J. Curtis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
P.M., J.L.C., M.P.-G., J.B.W., and M.G.M. conceived and designed research; P.M., C.M.F., J.B.W., and M.G.M. performed experiments; P.M., C.M.F., and M.G.M. analyzed data; P.M., J.L.C., C.M.F., M.P.-G., J.B.W., and M.G.M. interpreted results of experiments; P.M. prepared figures; P.M. drafted manuscript; P.M., J.L.C., C.M.F., M.P.-G., J.B.W., and M.G.M. edited and revised manuscript; P.M., J.L.C., C.M.F., M.P.-G., J.B.W., and M.G.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Deepti Goel, Edmund O’Brien, Molly MacDonald, and Lindsay Ward for technical assistance.
REFERENCES
- 1.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 2.Aronoff DM, Bergin IL, Lewis C, Goel D, O’Brien E, Peters-Golden M, Mancuso P. E-prostanoid 2 receptor signaling suppresses lung innate immunity against Streptococcus pneumoniae. Prostaglandins Other Lipid Mediat 98: 23–30, 2012. doi: 10.1016/j.prostaglandins.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 173: 559–565, 2004. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 4.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174: 595–599, 2005. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 5.Aronoff DM, Lewis C, Serezani CH, Eaton KA, Goel D, Phipps JC, Peters-Golden M, Mancuso P. E-prostanoid 3 receptor deletion improves pulmonary host defense and protects mice from death in severe Streptococcus pneumoniae infection. J Immunol 183: 2642–2649, 2009. doi: 10.4049/jimmunol.0900129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev 10: 131–145, 2014. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogdan C, Thüring H, Dlaska M, Röllinghoff M, Weiss G. Mechanism of suppression of macrophage nitric oxide release by IL-13: influence of the macrophage population. J Immunol 159: 4506–4513, 1997. [PubMed] [Google Scholar]
- 8.Broug-Holub E, Toews GB, van Iwaarden JF, Strieter RM, Kunkel SL, Paine R III, Standiford TJ. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun 65: 1139–1146, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruno A, Conus S, Schmid I, Simon HU. Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J Immunol 174: 8090–8096, 2005. doi: 10.4049/jimmunol.174.12.8090. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84: 491–495, 1996. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 11.Cilloniz C, Albert RK, Liapikou A, Gabarrus A, Rangel E, Bello S, Marco F, Mensa J, Torres A. The effect of macrolide resistance on the presentation and outcome of patients hospitalized for Streptococcus pneumoniae pneumonia. Am J Respir Crit Care Med 191: 1265–1272, 2015. doi: 10.1164/rccm.201502-0212OC. [DOI] [PubMed] [Google Scholar]
- 12.Dolan JM, Weinberg JB, O’Brien E, Abashian A, Procario MC, Aronoff DM, Crofford LJ, Peters-Golden M, Ward L, Mancuso P. Increased lethality and defective pulmonary clearance of Streptococcus pneumoniae in microsomal prostaglandin E synthase-1-knockout mice. Am J Physiol Lung Cell Mol Physiol 310: L1111–L1120, 2016. doi: 10.1152/ajplung.00220.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect 20, Suppl 5: 45–51, 2014. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 14.Duggal P, Guo X, Haque R, Peterson KM, Ricklefs S, Mondal D, Alam F, Noor Z, Verkerke HP, Marie C, Leduc CA, Chua SC Jr, Myers MG Jr, Leibel RL, Houpt E, Gilchrist CA, Sher A, Porcella SF, Petri WA Jr. A mutation in the leptin receptor is associated with Entamoeba histolytica infection in children. J Clin Invest 121: 1191–1198, 2011. doi: 10.1172/JCI45294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyckerman S, Broekaert D, Verhee A, Vandekerckhove J, Tavernier J. Identification of the Y985 and Y1077 motifs as SOCS3 recruitment sites in the murine leptin receptor. FEBS Lett 486: 33–37, 2000. doi: 10.1016/S0014-5793(00)02205-5. [DOI] [PubMed] [Google Scholar]
- 16.Feldman C, Anderson R. Epidemiology, virulence factors and management of the pneumococcus. F1000 Res 5: 2320, 2016. doi: 10.12688/f1000research.9283.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernández-Riejos P, Najib S, Santos-Alvarez J, Martín-Romero C, Pérez-Pérez A, González-Yanes C, Sánchez-Margalet V. Role of leptin in the activation of immune cells. Mediators Inflamm 2010: 568343, 2010. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gainsford T, Willson TA, Metcalf D, Handman E, McFarlane C, Ng A, Nicola NA, Alexander WS, Hilton DJ. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci USA 93: 14564–14568, 1996. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest 97: 2152–2157, 1996. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hellmann J, Zhang MJ, Tang Y, Rane M, Bhatnagar A, Spite M. Increased saturated fatty acids in obesity alter resolution of inflammation in part by stimulating prostaglandin production. J Immunol 191: 1383–1392, 2013. doi: 10.4049/jimmunol.1203369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol 150: 332–339, 2007. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine 29: 3398–3412, 2011. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 23.Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes 54: 182–189, 2005. doi: 10.2337/diabetes.54.1.182. [DOI] [PubMed] [Google Scholar]
- 24.Johnston RA, Theman TA, Lu FL, Terry RD, Williams ES, Shore SA. Diet-induced obesity causes innate airway hyperresponsiveness to methacholine and enhances ozone-induced pulmonary inflammation. J Appl Physiol (1985) 104: 1727–1735, 2008. doi: 10.1152/japplphysiol.00075.2008. [DOI] [PubMed] [Google Scholar]
- 25.Kerr AR, Wei XQ, Andrew PW, Mitchell TJ. Nitric oxide exerts distinct effects in local and systemic infections with Streptococcus pneumoniae. Microb Pathog 36: 303–310, 2004. doi: 10.1016/j.micpath.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Knapp S, Leemans JC, Florquin S, Branger J, Maris NA, Pater J, van Rooijen N, van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am J Respir Crit Care Med 167: 171–179, 2003. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 27.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun 66: 3164–3169, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SP, Serezani CH, Medeiros AI, Ballinger MN, Peters-Golden M. Crosstalk between prostaglandin E2 and leukotriene B4 regulates phagocytosis in alveolar macrophages via combinatorial effects on cyclic AMP. J Immunol 182: 530–537, 2009. doi: 10.4049/jimmunol.182.1.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemos MP, Rhee KY, McKinney JD. Expression of the leptin receptor outside of bone marrow-derived cells regulates tuberculosis control and lung macrophage MHC expression. J Immunol 187: 3776–3784, 2011. doi: 10.4049/jimmunol.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2γ) protein expression. Am J Physiol Lung Cell Mol Physiol 287: L497–L502, 2004. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 31.Mancuso P, Gottschalk A, Phare SM, Peters-Golden M, Lukacs NW, Huffnagle GB. Leptin-deficient mice exhibit impaired host defense in Gram-negative pneumonia. J Immunol 168: 4018–4024, 2002. doi: 10.4049/jimmunol.168.8.4018. [DOI] [PubMed] [Google Scholar]
- 32.Mancuso P, Huffnagle GB, Olszewski MA, Phipps J, Peters-Golden M. Leptin corrects host defense defects after acute starvation in murine pneumococcal pneumonia. Am J Respir Crit Care Med 173: 212–218, 2006. doi: 10.1164/rccm.200506-909OC. [DOI] [PubMed] [Google Scholar]
- 33.Mancuso P, Lewis C, Serezani CH, Goel D, Peters-Golden M. Intrapulmonary administration of leukotriene B4 enhances pulmonary host defense against pneumococcal pneumonia. Infect Immun 78: 2264–2271, 2010. doi: 10.1128/IAI.01323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancuso P, Standiford TJ, Marshall T, Peters-Golden M. 5-Lipoxygenase reaction products modulate alveolar macrophage phagocytosis of Klebsiella pneumoniae. Infect Immun 66: 5140–5146, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancuso P, Myers MG Jr, Goel D, Serezani CH, O’Brien E, Goldberg J, Aronoff DM, Peters-Golden M. Ablation of leptin receptor-mediated ERK activation impairs host defense against Gram-negative pneumonia. J Immunol 189: 867–875, 2012. doi: 10.4049/jimmunol.1200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mancuso P, Peters-Golden M, Goel D, Goldberg J, Brock TG, Greenwald-Yarnell M, Myers MG Jr. Disruption of leptin receptor-STAT3 signaling enhances leukotriene production and pulmonary host defense against pneumococcal pneumonia. J Immunol 186: 1081–1090, 2011. doi: 10.4049/jimmunol.1001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathews JA, Krishnamoorthy N, Kasahara DI, Cho Y, Wurmbrand AP, Ribeiro L, Smith D, Umetsu D, Levy BD, Shore SA. IL-33 drives augmented responses to ozone in obese mice. Environ Health Perspect 125: 246–253, 2017. doi: 10.1289/EHP272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCubbrey AL, Sonstein J, Ames TM, Freeman CM, Curtis JL. Glucocorticoids relieve collectin-driven suppression of apoptotic cell uptake in murine alveolar macrophages through downregulation of SIRPα. J Immunol 189: 112–119, 2012. doi: 10.4049/jimmunol.1200984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210: 535–549, 2013. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morato-Marques M, Campos MR, Kane S, Rangel AP, Lewis C, Ballinger MN, Kim SH, Peters-Golden M, Jancar S, Serezani CH. Leukotrienes target F-actin/cofilin-1 to enhance alveolar macrophage anti-fungal activity. J Biol Chem 286: 28902–28913, 2011. doi: 10.1074/jbc.M111.235309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 84: 3686–3695, 1999. doi: 10.1210/jcem.84.10.5999. [DOI] [PubMed] [Google Scholar]
- 42.Phipps JC, Aronoff DM, Curtis JL, Goel D, O’Brien E, Mancuso P. Cigarette smoke exposure impairs pulmonary bacterial clearance and alveolar macrophage complement-mediated phagocytosis of Streptococcus pneumoniae. Infect Immun 78: 1214–1220, 2010. doi: 10.1128/IAI.00963-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajala MW, Patterson CM, Opp JS, Foltin SK, Young VB, Myers MG Jr. Leptin acts independently of food intake to modulate gut microbial composition in male mice. Endocrinology 155: 748–757, 2014. doi: 10.1210/en.2013-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ring LE, Zeltser LM. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J Clin Invest 120: 2931–2941, 2010. doi: 10.1172/JCI41985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood 106: 1067–1075, 2005. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol 37: 562–570, 2007. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, Muir C, Sanker S, Moriarty A, Moore KJ, Smutko JS, Mays GG, Wool EA, Monroe CA, Tepper RI. Identification and expression cloning of a leptin receptor, OB-R. Cell 83: 1263–1271, 1995. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 48.Ubags ND, Burg E, Antkowiak M, Wallace AM, Dilli E, Bement J, Wargo MJ, Poynter ME, Wouters EF, Suratt BT. A comparative study of lung host defense in murine obesity models. Insights into neutrophil function. Am J Respir Cell Mol Biol 55: 188–200, 2016. doi: 10.1165/rcmb.2016-0042OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, Ventrone S, Zabeau L, Tavernier J, Poynter ME, Parsons PE, Dixon AE, Wargo MJ, Littenberg B, Wouters EF, Suratt BT. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight 1: e82101, 2016. doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wroe PC, Finkelstein JA, Ray GT, Linder JA, Johnson KM, Rifas-Shiman S, Moore MR, Huang SS. Aging population and future burden of pneumococcal pneumonia in the United States. J Infect Dis 205: 1589–1592, 2012. doi: 10.1093/infdis/jis240. [DOI] [PubMed] [Google Scholar]