Abstract
Hippocampal synaptic structure and function exhibit marked variations during the estrus cycle of female rats. Estradiol activates the mitogen-activated protein (MAP) kinase pathway in numerous cell types, and MAP kinase has been shown to play a critical role in the mechanisms underlying synaptic plasticity. Here, we report that endogenous estrogen produces a tonic phosphorylation/activation of extracellular signal-regulated kinase 2 (ERK2)/MAP kinase throughout the female rat brain and an increase in tyrosine phosphorylation of NR2 subunits of N-methyl-d-aspartate (NMDA) receptors. Moreover, cyclic changes in estrogen levels during the estrus cycle of female rats are associated with corresponding changes in the levels of activation of ERK2, the state of tyrosine phosphorylation of NR2 subunits of NMDA receptors, and the magnitude of long-term potentiation in hippocampus. Thus, cyclic changes in female sexual hormones result in marked variations in the state of activation of a major cellular signaling pathway critical for learning and memory and in a cellular model of learning and memory.
Estrogens have profound effects on hippocampal structure and physiology (1–4) and on hippocampal-dependent learning and memory (5, 6). In particular, estrogens have been shown to increase the density of dendritic spines on CA1 pyramidal neurons (7). In hippocampal slices, 17β-estradiol increases electrophysiological responses elicited by activation of both α-amino-3-hydroxy-5-methylisoxazole propionic acid and N-methyl-d-aspartate (NMDA) receptors and the magnitude of long-term potentiation (LTP) in field CA1 (8, 9). At the cellular level, numerous laboratories have shown that, in addition to direct genomic effects, 17β-estradiol activates the extracellular regulated kinase/mitogen-activated protein (ERK/MAP) kinase pathway (10–12), an effect associated with the neurotrophic/neuroprotective actions of estrogen (13, 14). We recently reported that estrogen-mediated activation of the ERK/MAP kinase pathway in hippocampus was involved in the rapid effects of estrogen on NMDA receptors and LTP through tyrosine phosphorylation of NR2 subunits of NMDA receptors (15).
The ERK/MAP kinase pathway is a central cellular signaling pathway linking numerous extracellular signals to membrane receptors, transcription factors, and gene regulation (16) and is critically involved in synaptic plasticity, learning, and memory (17–20). Pharmacological manipulations directed at blocking this pathway have consistently produced impairment in synaptic plasticity, learning, and memory, and this pathway is activated with LTP-inducing tetanus or in different learning paradigms. The present study analyzed whether endogenous estrogen regulates the state of activation of the ERK/MAP kinase pathway and whether cyclic variations in estrogen levels during the female estrus cycle suffice to modify this pathway in the brain. Moreover, we determined the state of tyrosine phosphorylation of NMDA-receptor subunits, as well as the magnitude of LTP in field CA1 of hippocampal slices obtained from adult female rats with low (diestrus) or high (proestrus) endogenous estrogen levels. Our results indicate that cyclic changes in female sexual hormones are associated with marked variations in the state of activation of a major cellular signaling pathway in the brain and in a cellular model of learning and memory.
Materials and Methods
Ovariectomy.
A total of 11 sexually naive, Sprague–Dawley female rats (Harlan Breeders, Indianapolis) were used. Ovariectomy (OVX) was performed at 9 weeks, and 17β-estradiol (n = 5) or placebo (n = 6) implantation was done at 12 weeks. Animals were killed by decapitation at 14 weeks; brains were rapidly removed and kept at −70°C until use. To verify the efficacy of the procedures, uterine tissues were weighted at the time of killing with the following results: OVX + 17β-estradiol = 0.35 ± 0.03 g, OVX + placebo = 0.08 ± 0.01 g. The 17β-estradiol 60-day release pellets (0.72 mg per pellet) and placebo pellets were purchased from Innovative Research of America.
Tissue Preparation and Western Blotting.
Brain tissues were homogenized in 20% (wt/vol) sucrose in PBS with 3.0 mM EGTA. Aliquots of homogenates (30–40 μg of protein per lane) were diluted with equal volumes of 2× sample buffer [2% SDS/50 mM Tris⋅HCl, pH 6.8/10% (vol/vol) 2-mercaptoethanol/10% glycerol (vol/vol)/0.1% bromophenol blue]. After heating samples at 90- to 100°C for 5 min, proteins were subjected to SDS/PAGE (21) and transferred onto nitrocellulose membranes (22). Anti-NR2 and anti-phosphotyrosine antibodies were obtained from Chemicon and Upstate Biotechnology (Lake Placid, NY), respectively. Anti-p38 (activated/diphosphorylated p38) and anti-MAP kinase antibodies (nonphosphorylated ERK and activated/diphosphorylated ERK-1 and -2) were purchased from Sigma. Immunoblots were scanned and the digitized images were quantitatively analyzed by densitometry with the imagequant program providing peak areas and apparent molecular weights. For immunoprecipitation, samples were first centrifuged at 14,000 × g for 15–20 min. The supernatant was discarded, the pellet resuspended in 100 μM EGTA in distilled water, and centrifuged again as above. The pellet was resuspended in Tris-acetate (100 mM, pH 7.4) containing 100 μM EGTA and centrifuged again. The supernatant was discarded, and this last step was repeated at least once. The final pellet was resuspended in the Tris-acetate buffer and frozen at −70°C until used. Membranes fractions (≈ 300 μg of protein) were lysed in PBS containing protease inhibitors (0.1 mM PMSF/1 μg/ml pepstatin A/1 μg/ml leupeptin) and 2 mM EDTA. Membranes then were sonicated and centrifuged at 4°C for 10 min at 100,000 × g. Membrane fractions were resuspended in lysis buffer containing 1% SDS and heated at 95°C for 5 min, followed by dilution in 4 volumes of cold lysis buffer containing 2% (vol/vol) Triton X-100. Insoluble materials were removed by centrifugation at 100,000 × g for 10 min. Protein A-agarose (Upstate Biotechnology)/anti-NR2A/B antibody mixtures (3 μg of each) were added to the supernatant and incubated with gentle agitation overnight at 4°C. Immunoreactive complexes were recovered by centrifugation at 14,000 × g for 5 sec. The pellets were washed first with lysis buffer [containing 2% (vol/vol) Triton X-100] then with PBS. Proteins were finally eluted in 2× sample buffer and incubated at 95°C for 5 min. Immunoprecipitated proteins were subjected to SDS/8% PAGE and to immunoblotting as above.
Estrus-Cycle Testing and Hippocampal Slice Preparation.
Adult female Sprague–Dawley rats (Harlan Breeders) were monitored by means of photomicrographs of vaginal smears obtained for at least 10 consecutive days before experimentation. Brain tissues from rats in either proestrus or diestrus were obtained on the morning in which these conditions were first observed. Trunk blood was collected and analyzed for hormonal content through RIA. Serum-estrogen levels were significantly higher (P < 0.05) in the proestrus group (mean = 52.6 pg/ml) compared with the diestrus group (mean = 31.9 pg/ml).
Hippocampal slices (400 μm thick) from rats in either proestrus or diestrus were prepared on the morning in which these conditions were first observed, and transferred to an interface-recording chamber (31.5°C) where the slices were perfused with oxygenated artificial cerebrospinal fluid, as described (9). Extracellular field-excitatory postsynaptic potential recordings (fEPSPs) were obtained from the apical dendritic layer of the CA1 region by using glass microelectrodes filled with 2 M NaCl. fEPSPs were recorded by applying single-test pulses (0.05 Hz, 100 μs) at voltage intensities sufficient to evoke 50% of the maximal fEPSP. LTP was induced by a brief period of high-frequency stimulation (hfs; five trains of 20 pulses at 100 Hz) after a total of 40 min of pre-hfs stimulation. Subsequent fEPSPs were monitored for 30 min post-hfs induction at the pre-hfs stimulation rate and intensity. Electrophysiological recordings were collected, digitized, and stored in a computer using axobasic software.
A separate group of animals was used for biochemical analysis of MAP kinase activation and NMDA-receptor subunit phosphorylation, as described above.
Results
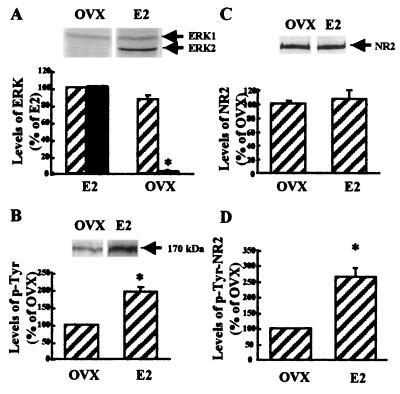
We first examined the state of phosphorylation of ERK/MAP kinases in whole brains of ovariectomized female rats with or without estrogen implants. This experimental approach provides for controlled states of low or high endogenous circulating estrogen levels. The results were quite remarkable, as levels of phosphorylated (active) ERK2 were lowered by more than 95% by OVX without estrogen (E2) implants (Fig. 1A). Similar results were obtained for different brain regions (hippocampus, hypothalamus, piriform and frontal cortex; data not shown). In contrast, no difference was observed for total ERK/MAP kinase levels (phosphorylated plus unphosphorylated) under these conditions.
Figure 1.
Effects of ovariectomy on ERK- and NR2-subunit tyrosine phosphorylation in female rat brains. Brain tissues from ovariectomized female rats without (OVX) or with (E2) estrogen implants were homogenized in Tris-acetate buffer (100 mM, pH 7.4) containing 0.1 mM EGTA and processed for Western blotting. Aliquots of homogenates (50–60 μg protein per lane) were subjected to SDS/10% PAGE. Proteins were transferred onto nitrocellulose membranes. (A) Membranes were processed with monoclonal antibodies against active/phosphorylated ERK. (Upper) Representative blot. (Lower) Blots were quantified, and results for ERK1 (hatched bars) and ERK2 (black bars) were expressed as percentages of the values found in samples from female rats with estrogen implants (means ± SEM of 6–8 animals). *, P < 0.01. (B) Membranes were processed with anti-phosphotyrosine antibodies that stain a band with an apparent Mr of 170 kDa, corresponding to NR2 subunits of NMDA receptors (Upper). (Lower) Blots were quantified and results were expressed as percentages of the values found in samples from female rats with estrogen implants (means ± SEM of 6–8 animals). *, P < 0.05. (C) Membranes were processed with anti-NR2A/B antibodies (Upper). (Lower) Blots were quantified, and results were expressed as percentages of the values found in samples from female rats with estrogen implants (means ± SEM of 6–8 animals). (D) Brain tissues were homogenized and NR2A/B receptors were immunoprecipitated with anti-NR2A/B antibodies. Immunoprecipitated proteins were subjected to SDS/PAGE, and Western blotting was performed with anti-phosphotyrosine antibodies (0.5–2 μg/ml). The bands migrating at 170 kDa were quantified, and results were expressed as percentages of the values found in samples from female rats with estrogen implants (means ± SEM of 6–8 animals). *, P < 0.01.
As we previously found that estrogen treatment of hippocampal slices resulted in tyrosine phosphorylation of NR2 subunits of NMDA receptors (15), we determined the state of tyrosine phosphorylation of NR2 subunits in brain after OVX with or without E2 implants. Brain extracts were first immunoblotted with anti-phosphotyrosine or anti-NR2A/B antibodies (Fig. 1 B and C). In both cases, the antibodies recognized a band with an apparent Mr of 170 kDa, corresponding to NR2 subunits of NMDA receptors. Although no differences in the levels of NR2 subunits were found in the brains of ovariectomized females with or without estrogen implants, E2 treatment increased the levels of the phosphorylated 170-kDa band by 100%. To extend these results, brain extracts first were immunoprecipitated with anti-NR2 antibodies before estimating the amounts of tyrosine phosphorylated NR2 subunits with Western blots stained with anti-phosphotyrosine antibodies (Fig. 1D). Levels of tyrosine phosphorylation of NR2 subunits of NMDA receptors were 100% higher in ovariectomized females receiving estrogen implants.
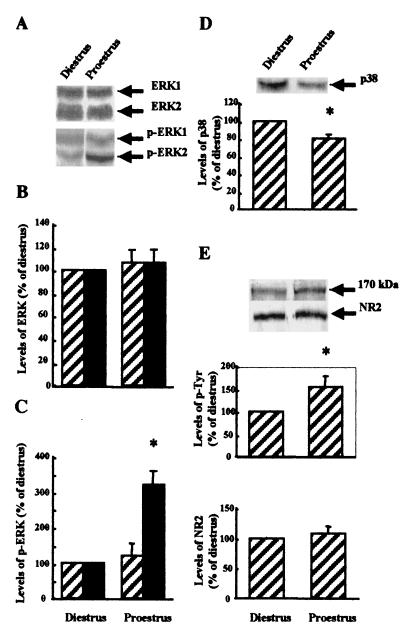
Although the previous results strongly suggested that endogenous estrogen produces a tonic activation of the ERK/MAP kinase pathway, we determined the state of activation of this pathway during the phases of the estrus cycle associated with low (diestrus) and high (proestrus) levels of circulating estrogen. Brain samples at proestrus exhibited a 3-fold increase in the state of phosphorylation/activation of ERK2 kinase with only small changes in the state of phosphorylation of ERK1 kinase as compared with diestrus (Fig. 2A–C). Moreover, overall levels of either ERK1 or ERK2 were not different between the two states. Interestingly, the state of phosphorylation of another kinase belonging to this family of kinases, p38, was decreased in proestrus as compared with diestrus (Fig. 2D). We also compared the levels of tyrosine phosphorylation of NR2 subunits of NMDA receptors between the two states (Fig. 2E). Levels of tyrosine phosphorylation of NR2 subunits were increased by 50% at proestrus above diestrus.
Figure 2.
Changes in ERK-, p38-, and NR2-subunit tyrosine phosphorylation in brain of female rats in proestrus and diestrus. Brain tissues from female rats in diestrus or proestrus were homogenized in Tris-acetate buffer (100 mM, pH 7.4) containing 0.1 mM EGTA and processed for Western blotting. (A) Membranes were processed with monoclonal antibodies against total (top half) or phosphorylated ERK (bottom half). (B and C) Blots similar to those shown in A were quantified, and results for ERK1 (hatched bars) and ERK2 (black bars) were expressed as percentages of the values found in samples from females in diestrus (means ± SEM of 6–8 animals). *, P < 0.01. (B, total ERK; C, phospho-ERK). (D) Membranes were processed with antibodies against activated/phosphorylated p38 (Upper). (Lower) blots were quantified, and results were expressed as percentages of the values found in samples from females in diestrus (means ± SEM of 6–8 animals). *, P < 0.05. (E) Membranes were processed with anti-phosphotyrosine antibodies (Upper) or anti-NR2A/B antibodies (Lower). Blots were quantified, and results were expressed as percentages of the values found in samples from females in diestrus (means ± SEM of 6–8 animals). *, P < 0.01.
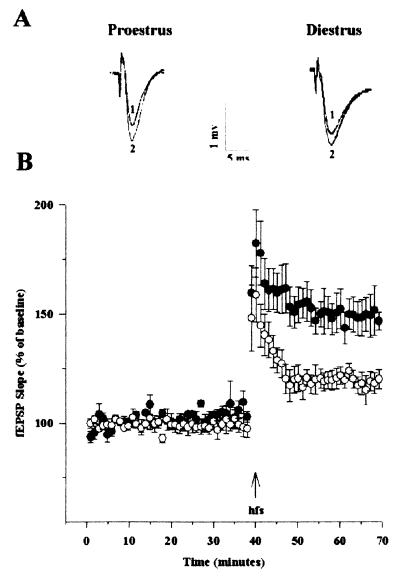
After our observations that estrogen increased the magnitude of LTP in field CA1 of hippocampal slices from male rats (9, 15), it was of interest to evaluate the magnitude of LTP in slices from females in proestrus or diestrus conditions (Fig. 3). Extracellular fEPSPs were recorded in CA1 for 40 min before administering hfs to the Schaffer collateral pathway. Hfs was immediately followed by posttetanic potentiation (PTP) that decayed to reach a stable potentiation, which was recorded for 30 min. No difference in the magnitude of PTP was observed between slices prepared from females in proestrus as compared with diestrus. On the other hand, a significant difference was observed in the magnitude of LTP between the two groups, with slices prepared from females in proestrus exhibiting greater LTP than slices from females in diestrus (56 ± 9% vs. 25 ± 6%, means ± SEM of six experiments; P < 0.05).
Figure 3.
Changes in LTP in field CA1 of hippocampal slices from female rats in proestrus and diestrus. Hippocampal slices from female rats in proestrus or diestrus were prepared as described (9). fEPSP amplitude and slope values were obtained for each slice and averaged across slices to produce one average for before and after the train of hfs. fEPSP amplitudes and slopes were normalized for the 10-min pre-hfs period for each slice. Separate ANOVAs and planned two-tailed t tests for the pre-hfs and post-hfs periods were used to evaluate the effects of estrus cycle on fEPSP slope and amplitude. (A) Representative waveforms from female rats in proestrus and diestrus for pre-hfs (1) and post-hfs (2) periods. (B) Means ± SEM of fEPSP slopes recorded in slices from female rats in proestrus (black circles; n = 6) and diestrus (white circles; n = 5).
Discussion
Our results clearly establish that endogenous estrogen is a major regulator of the important intracellular signaling cascade consisting of ERK/MAP kinase pathway throughout the brain. First, ovariectomy, a procedure that reduces circulating estrogen levels by more than 90%, resulted in an almost complete elimination of the activated/phosphorylated state of ERK2. This effect was completely reversed by restoring normal levels of estrogen with estrogen pellet implantation. Second, cyclic changes in estrogen levels occurring during the estrus cycle in female rats also were associated with changes in the state of activation/phosphorylation of ERK2. This effect was relatively specific for ERK2, as only minimal changes for ERK1 and a decrease in p38 phosphorylation were observed. Changes in activation/phosphorylation of ERK2 were associated with parallel changes in the state of tyrosine phosphorylation of NR2 subunits of NMDA receptors. This result was not unexpected, as it was shown previously that various tyrosine kinases, including Src and MAP kinase, can phosphorylate NR2 subunits (15, 23). Finally, changes in estrogen levels during the estrus cycle also were associated with changes in the magnitude of LTP in field CA1 of hippocampal slices. This finding fits well with our previous results showing that estrogen, by activating the MAP kinase pathway and by increasing the state of tyrosine phosphorylation of NR2 subunits of NMDA receptors, produced an increase in the magnitude of LTP in hippocampal slices (15). It is also consistent with reports indicating facilitation of LTP induction by estrogen in ovariectomized female rats (24), increased LTP in the afternoon of proestrus of female rats (25), and increased LTP magnitude in proestrus as compared with diestrus in CA1 of adult female rats (26). Therefore, we propose that circulating levels of estrogen regulate the phosphorylation/activation state of ERK2. In turn, this regulation results in the tyrosine phosphorylation of NR2 subunits of NMDA receptors, an effect known to increase NMDA-receptor function (27). These changes in NMDA-receptor function would be well suited to account for changes in the magnitude of LTP observed with changes in circulating estrogen levels.
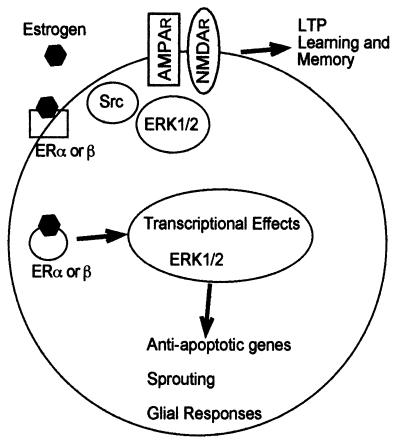
The MAP kinase pathway occupies a central place in the regulation of synaptic plasticity (refs. 17 and 18; Fig. 4). Pharmacological manipulations directed at blocking this pathway have consistently produced impairment in synaptic plasticity, learning, and memory, and this pathway is activated with LTP-inducing tetanus or in different learning paradigms (28–31). Our results indicate that circulating estrogen produces a tonic activation of this pathway throughout the brain. This effect could therefore account for the various effects of estrogen on synaptic plasticity reported in various brain structures. In particular, activation of the MAP kinase pathway by estrogen could well be responsible for the effects of estrogen on NMDA receptor-mediated responses and on synaptic connectivity in field CA1 of hippocampus (4, 5).
Figure 4.
Schematic representation of the cellular effects of estrogen in the brain. Estrogen produces both the regulation of genomic events through its binding to traditional hormonal receptors, the translocation of the estrogen-receptor complexes to the nucleus, and the activation of a major intracellular cascade. The former pathway is expected to account for the slow effects of estrogen on cells and tissues, including its neuroprotective/neurotrophic effects via the activation of antiapoptotic genes. In addition, estrogen activates the intracellular cascade consisting of the ERK/MAP kinase pathway. This pathway is well suited to regulate both additional genomic events after translocation of activated ERK into the nucleus and also membrane receptors [such as the α-amino-3-hydroxy-5-methylisoxazole propionic acid (AMPA) or NMDA receptors], ion channels, and intracellular enzymes. This pathway is expected to account for the rapid effects of estrogen on cells and tissues. In particular, we postulate that tyrosine phosphorylation of NMDA receptors results in the increased function of the receptors and facilitation of LTP formation. This pathway also could be involved in the synaptic remodeling taking place in hippocampus and other brain structures during the estrus cycle.
Estrogen affects hippocampal-dependent learning and memory (5, 32). It has beneficial effects on working memory in humans (33) and in spatial memory (34) or delayed matching-to-place (35) in rats. Our results not only provide a cellular explanation for these effects but also indicate that fluctuations in endogenous levels of estrogen have profound influences on mechanisms that play central roles in these processes. Studies related to changes in learning and memory processes during the estrus cycle in females have provided variable results. In hippocampal-dependent tasks, Warren and Juraska (36) found worse learning in proestrus, Berry et al. (37) reported no difference for various phases of the cycle, whereas Frick and Berger-Sweeney (38) reported increased learning performance in proestrus. Interestingly, in a hippocampal-independent task, Shors et al. (39) found substantially enhanced learning performance in proestrus. Inconsistent findings have been reported in humans (40, 41). In any event, our results raise some challenging questions regarding the functional significance of linking cellular processes critical for cognitive processes with signals critical for reproductive functions.
Acknowledgments
The authors wish to thank Dr. X. Bi (University of California, Irvine) for helpful comments on the manuscript. This research was supported by National Institute on Aging Grant AG14751.
Abbreviations
- NMDA
N-methyl-d-aspartate
- LTP
long-term potentiation
- ERK
extracellular regulated kinase
- MAP
mitogen-activated protein
- OVX
Ovariectomy
- fEPSPs
field-excitatory postsynaptic potential recording
- hfs
high-frequency stimulation
References
- 1.Woolley C S, McEwen B S. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy D D, Cole N B, Greenberger V, Segal M. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanapat P, Hastings N B, Reeves A J, Gould E. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolley C S, Weiland N G, McEwen B S, Schwartzkroin P A. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen B, Akama K, Alves S, Brake W G, Bulloch K, Lee S, Li C, Yuen G, Milner T A. Proc Natl Acad Sci USA. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wise P M, Dubal D B, Wilson M E, Rau S W, Liu Y. Front Neuroendocrinol. 2001;22:33–66. doi: 10.1006/frne.2000.0207. [DOI] [PubMed] [Google Scholar]
- 7.Woolley C S. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 8.Gu Q, Moss R L. J Physiol. 1998;506:745–754. doi: 10.1111/j.1469-7793.1998.745bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foy M R, Xu J, Xie X, Brinton R D, Thompson R F, Berger T W. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 10.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 11.Singer C A, Figueroa-Masot X A, Batchelor R H, Dorsa D M. J Neurosci. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh M, Setalo G, Jr, Guan X, Frail D E, Toran-Allerand C D. J Neurosci. 2000;20:1694–1700. doi: 10.1523/JNEUROSCI.20-05-01694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toran-Allerand C D, Singh M, Setalo G J. Front Neuroendocrinol. 1999;20:97–121. doi: 10.1006/frne.1999.0177. [DOI] [PubMed] [Google Scholar]
- 14.Lee S J, McEwen B S. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- 15.Bi R, Broutman G, Foy M, Thompson R F, Baudry M. Proc Natl Acad Sci USA. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. . (First Published March 21, 2000; 10.1073/pnas.060034497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cobb M R, Goldsmith E J. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 17.Mazzucchelli C, Brambilla R. Cell Mol Life Sci. 2000;57:604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweatt J D. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 19.Coogan A N, O'Leary D M, O'Connor J J. J Neurophys. 1999;81:103–110. doi: 10.1152/jn.1999.81.1.103. [DOI] [PubMed] [Google Scholar]
- 20.Schafe G E, Atkins C M, Swank M W, Bauer E P, Sweatt J D, LeDoux J E. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu X M, Salter M W. Proc Natl Acad Sci USA. 1999;96:7697–7704. doi: 10.1073/pnas.96.14.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cordoba Montoya D A, Carrer H F. Brain Res. 1997;778:430–438. doi: 10.1016/s0006-8993(97)01206-7. [DOI] [PubMed] [Google Scholar]
- 25.Warren S G, Humphreys A G, Juraska J M, Greenough W T. Brain Res. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- 26.Good M, Day M, Muir J L. Europ J Neurosci. 1999;11:4476–4480. doi: 10.1046/j.1460-9568.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu X-M, Askalan R, Keil G J, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 28.Brambilla R, Gnesutta N, Minichiello L, White G, Roylance A J, Herron C E, Ramsey M, Wolfer D P, Cestari V, Rossi-Arnaud C, et al. Nature (London) 1997;290:281–286. doi: 10.1038/36849. [DOI] [PubMed] [Google Scholar]
- 29.Berman D E, Hazvi S, Rosenblum K, Seger R, Dudai Y. J Neurosci. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blum S, Moore A N, Adams F, Dash P K. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selcher J C, Atkins C M, Trzaskos J M, Paylor R, Sweatt J D. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinton R D. Learn Mem. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- 33.Duff S J, Hampson E. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- 34.Luine V N, Richards S T, Wu V Y, Beck K D. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- 35.Sandstrom N J, Williams C L. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- 36.Warren S G, Juraska J M. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- 37.Berry B, McMahan R, Gallagher M. Behav Neurosci. 1997;111:267–274. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- 38.Frick K M, Berger-Sweeney J. Behav Neurosci. 2001;115:229–237. doi: 10.1037/0735-7044.115.1.229. [DOI] [PubMed] [Google Scholar]
- 39.Shors T J, Beylin A V, Wood G E, Gould E. Behav Brain Res. 2000;110:39–52. doi: 10.1016/s0166-4328(99)00183-7. [DOI] [PubMed] [Google Scholar]
- 40.Phillips S M, Sherwin B B. Psychoneuroendocrinology. 1992;17:497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- 41.Sherwin B B. Novartis Found Symp. 2000;230:188–196. doi: 10.1002/0470870818.ch14. [DOI] [PubMed] [Google Scholar]