Abstract
Public interest in complementary and alternative medicine has been increased worldwide, due to its wide applications in cancer prevention and treatment. Cordycepin is one of the most common and crucial types of complementary and alternative medicine. Cordycepin (3′-deoxyadenosine), a derivative of adenosine, was first isolated from medicine drug Cordyceps militaris. Cordycepin has been widely used as one compound for antitumor, which has been found to exert antiangiogenic, anti-metastatic, and antiproliferative effects, as well as inducing apoptosis. However, the mechanism of its anti-tumor activity is not well known. This review will clarify anti-tumor mechanisms of Cordycepin, which regulate signaling pathways related with tumor growth and metastasis. Cordycepin inhibit tumor growth via upregulating tumor apoptosis, inducing cell cycle arrest and targeting cancer stem cells (CSCs). Cordycepin regulates tumor microenvironment via suppressing tumor metastasis-related pathways. Thus, Cordycepins may be one of important supplement or substitute medicine drug for cancer treatment.
Keywords: Cordycepin, Anti-tumor, Anti-metastasis
1. Introduction
The number of people suffering from cancer in the world will continue to grow steadily, according to the latest report about the trend of global cancer released by the World Health Organization (WHO). It is estimated that the number of newly-increased cancer patients will reach 19 million or even more by 2025. Cancer is the second killer of human death in the world (Anderson and Flanigan, 2015). Cancer is caused by an imbalance between the progression of cell cycle and programmed cell death (Apoptosis) (Lowe et al., 2004). Therefore, the majority of anticancer medical drugs exert their anti-proliferative activity through cell cycle arrest and induction of apoptosis (Bai et al., 2017, Evan and Vousden, 2001). The cytotoxic nucleoside analogues were the first chemotherapeutic agents used for the therapy of cancer. The some researched cytotoxic nucleoside analogues are isolated from Cordyceps militaris (Tian et al., 2015).
Cordycepin was first found from the fermented broth of the medicinal mushroom Cordyceps militaris, which is the fungus that grows parasitically on lepidopteron larvae and insect pupae (Cunningham et al., 1950). The genus Cordyceps is well-known in traditional Chinese medicine and exhibits a variety of clinical health effects including immunomodulatory, anticancer, antioxidant, anti-inflammatory and anti-microbial activities (Tuli et al., 2014, Yue et al., 2013). Recently, more and more studies have demonstrated Cordycepin, as one bioactive compound of Cordyceps militaris, have abroad roles of anti-tumor (Hsu et al., 2017, Hwang et al., 2017a, Wang et al., 2017a, Wang et al., 2017b, Zeng et al., 2017). However, little is known about the active ingredients as well as the mechanism underlying these roles. The review summarizes the anti-tumor mechanism of Cordycepin.
2. Main active components of Cordyceps militaris
2.1. Chemical features of cordycepin
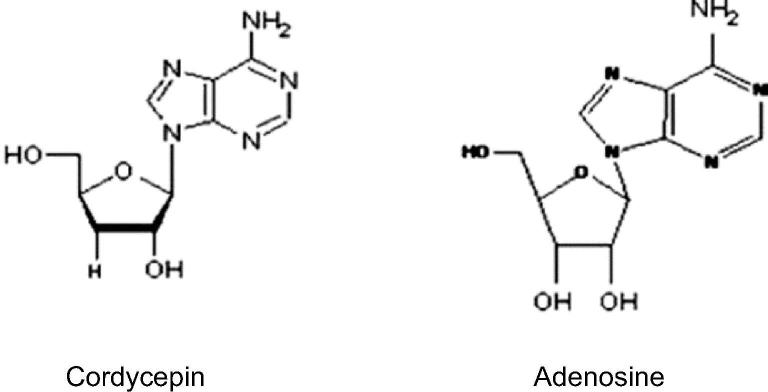
The structure of Cordycepin is very much similar with cellular nucleoside, adenosine and acts like a nucleoside analogue (Li et al., 2015). The structure of cordycepin comprises a purine (adenine) nucleoside molecule attached to one ribose sugar moiety. The chemical synthesis of cordycepin is completed mainly through the replacement of the OH group at the 3′-position in the ribofuranosyl moiety with H, generating a deoxy analogue of adenosine (Fig. 1) (Tuli et al., 2013).
Fig. 1.
The difference of chemical structures between Cordycepin and adenosine.
2.2. Function of cordycepin
Cordycepin has many biological and pharmacological actions in immunological, hepatic, renal, cardiovascular systems as well as an anti-cancer agent. Those functions are related to its structure (Tuli et al., 2014). During the process of RNA synthesis (transcription), some enzymes are not able to distinguish between an adenosine and Cordycepin which leads to incorporation of Cordycepin to induce premature termination of transcription (Chen et al., 2008, Holbein et al., 2009). In addition, The IC50 (the concentration at which 50% inhibition of cell growth was achieved) of cordycepin in human gallbladder cancer cell lines NOZ and GBC-SD cells at 48 h was approximately 19.2 μg/mL and 398.1 μg/mL, respectively (Wang et al., 2014). For human lung cancer cell lines. IC50 of cordycepin was 60 μg/ml (Hwang et al., 2017b). The function of Cordycepin treatment in tumor is dependent on tumor types and concentration (Cho and Kang, 2018, Fong et al., 2018).
The high dosage of Cordycepin can block mTOR (mammalian target of rapamycin) signaling pathway (Wong et al., 2010). The name mTOR has been derived from the drug rapamycin, because the drug inhibits mTOR activity. Some of mTOR inhibitors have been tested as anti-cancer drugs, since they suppress cancer through mTOR signaling pathway (Bjornsti and Houghton, 2004, Sabatini, 2006). The study found that Cordycepin can activate AMPK which blocks the activity of mTORC1/mTORC2 complex. The inactivated complex cannot activate AKT 1 kinase fully, which suppress mTOR signal transduction inhibiting translation, and further cell proliferation and growth (Wong et al., 2010). Those functions of Cordycepin elucidate their structure–function relationship, and further explain the anti-tumor roles of the compound. Cordycepin had been shown to regulate AMPK/mTORC1 signaling pathway to down-regulate multiple drug resistant to HIF-1α in GBC-SD gallbladder cancer cells (Wu et al., 2014). The anti-tumor roles and mechanisms of Cordycepin are descripted detail below.
3. Cordycepin inhibits tumor growth
Many complementary and alternative medicine are developed in applications of cancer prevention and therapy due to chemotherapy resistance and metastasis (Wong et al., 2017). Traditional Chinese medicine is one treatment for complementary and alternative therapy (Wong et al., 2015, Ye et al., 2018). Cordycepin is an active compound and has been used in cancer treatment in past studies.
3.1. Induction of tumor apoptosis
Cordycepin can induce cancer cell apoptosis in caspase-dependent pathways. Apoptosis of Human liver cancer (HepG2) cells were induced by the activation of caspase, interaction between Fas and FADD, and modulation of the protein levels of Bid and tBid (Shao et al., 2016). Cordycepin also decreased human bladder carcinoma cells (T24 cells) survival, which was regulated by the activation of A3 adenosine receptor and the subsequent inactivation of Akt pathways, leading to the increases in cleaved Caspase-3 and apoptosis (Cao et al., 2017). In addition, Cordycepin reduced cell viability, inhibited cell proliferation, and enhanced lactate dehydrogenase release and reactive oxygen species (ROS) accumulation of human breast cancer cell (MCF-7 and MDA-MB-231 cells) through up-regulating the activation of pro-apoptotic proteins, such as caspase-3, 8, 9 and suppressing the expression of the anti-apoptotic protein, B-cell lymphoma 2 (Bcl-2) (Wang et al., 2016).
Cordycepin induced the apoptosis of human renal cancer cells by triggering the MKK7-JNK signaling pathway through inhibition of anti-apoptotic protein cellular caspase 8 (FLICE)-like inhibitory protein (c-FLIP) expression and the consequent activation of the Bax/caspase-3/PARP-mediated pathway (Hwang et al., 2017a). In human Non-Small Cell Lung Cancer (NSCLC), Cordycepin-induced apoptosis was also associated with down-regulation of protein c-FLIP, which inhibited the activity of caspase-8. Cordycepin inhibited cell growth by inducing apoptosis and autophagy. The cordycepin-stimulated autophagy were mediated by suppressing mTOR signaling pathway in lung cancer cells. In addition, suppression of autophagy could also elevate the protein level of c-FLIP which indicated cordycepin-triggered autophagy promoted the degradation of c-FLIP. Therefore, Cordycepin induced apoptosis through autophagy-mediated downregulation of c-FLIP in human NSCLC cells. In addition, cordycepin also inhibits the ERK/Slug signaling pathway through the activation of GSK3β which, upregulates Bax to result in apoptosis of lung cancer cells (Hwang et al., 2017b). Taken together, cordycepin may serve as one promising therapeutic compound, which acts on multiple molecular targets in lung cancer treatment (Yu et al., 2017).
Cordycepin also induces cancer cell apoptosis in caspase-independent pathways. Cordycepin decreased cell mitosis and EGFR signaling in one murine oral tumor mouse model. In accordance, the treatment distinctly reduced the levels of ki-67 and EGFR signaling molecules to induce cancer cell apoptosis (Hsu et al., 2017). For human lung adenocarcinoma, Cordycepin induced cancer cell apoptosis by caveolin-1-upregulated JNK/Foxo3a signaling pathway, and significantly decreased tumor volume in nude mice (Joo et al., 2017). Cordycepin also increase ROS levels and induce apoptosis in MA-10 mouse Leydig tumor cells but not cause cell death of primary mouse Leydig cells on moderate concentration through down-regulating the p38 MAPK and PI3K/AKT signaling pathways (Pan et al., 2015) (Table 1).
Table 1.
Inhibition roles of Cordycepin on tumor growth.
| Tumor types (cell lines) | Mechanism of anti-tumor | Molecular targets | References |
|---|---|---|---|
| Human gastric cancer (SGC-7901) | Induction of apoptosis | PI3K/AKT↑ | (Nasser et al., 2017) |
| Human non-small cell lung cancer cells | Induction of apoptosis/autophagy | c-FLIPL↓ | (Yu et al., 2017) |
| Human liver cancer (HepG2) | Induction of apoptosis | Caspase-8, Fas, FADD↑ | (Shao et al., 2016) |
| Human renal cancer cells (TK-10) | Induction of apoptosis | MKK7, JNK↑ | (Hwang et al., 2017a) |
| Human uterine cervical cancer cell (ME180 and HeLa cell) | Induction of G2/M arrest | Cyclin A2↓ | (Seong da et al., 2016) |
| Human Leukemia cells (NB-4 and U937 cells) | Induction of apoptosis/cell cycle arrest | Cyclin A2, cyclin E, and CDK2↓ p53↑ | (Liao et al., 2015) |
| Human Bladder cancer (T-24) | Induction of apoptosis | A3 adenosine receptors↑ | (Cao et al., 2017) |
| Human bladder cancer cell (5637 and T-24 cells) | G2/M cell cycle arrest | Phosphorylation of c-Jun | (Lee et al., 2009) |
| Human breast cancer cells (MCF-7 and MDA-MB-231) | Induction of apoptosis | Caspase-3,8,9↑, BCL-2↓ | (Wang et al., 2016) |
| Murine oral cancer (4NAOC-1) | Induction of apoptosis, decrease cell mitosis and EGFR signaling | Caspase-3↑ EGFR, IL-17RA↓ | (Hsu et al., 2017) |
| Murine Leydig tumor cell (MA-10) | Induction of apoptosis | p38 MAPK↑ | (Hsu et al., 2017) |
3.2. Cell cycle arrest
Cordycepin incorporates mitochondrial-mediated apoptosis in gastric cancer cell (SGC‑7901 cells) with regulating mitochondrial extrinsic pathways by inhibition of A3 adenosine receptor (A3AR) and drive activation of death receptor DR3, which promote the activation of PI3K/Akt protein expression as well as collapse of mitochondrial membrane potential (MMP). Phosphorylation of PI3K/Akt and DNA damage by cordycepin induced the production of ROS and regulated SGC‑7901 cell cycle cessation at S phase (Nasser et al., 2017). Cordycepin also increased radio-sensitivity in human uterine cervical cancer cells, such as ME180 and HeLa cells, and induced the increased number of those tumor cells in the G2/M phase, which is related to the induction of p53-mediated apoptosis and modulation of the expression of cell cycle checkpoint molecules (Seong da et al., 2016). The increased expression of p53 by Cordycepin treatment promoted the release of cytochrome c from mitochondria to the cytosol, to further activate caspase-9 and promote the apoptosis of leukemia cells (NB-4 and U937 cells) (Liao et al., 2015). In addition, cordycepin inhibits the expression of cyclin A2, cyclin E, and CDK2, which leads to the accumulation of those leukemia cells in S-phase through the activation of Chk2-Cdc25A pathway (Liao et al., 2015).
Recently, Lee at al found that Cordycepin causes p21WAF1-mediated G2/M cell cycle arrest by upregulating c-Jun N-terminal kinase activation in human bladder cancer cells. They blocked JNK function using JNK-specific inhibitor and small interfering RNA of JNK to rescue cordycepin-dependent p21WAF1 expression and decrease of cell cycle proteins (Lee et al., 2009). These results suggest that cordycepin could be an effective treatment for bladder cancer.
3.3. Resistance of cancer stem cell
Cancer stem cells (CSCs) are a limitless cell source for the initiation and maintenance of cancer cells. CSCs can generate cancer cells through the stem cell processes of self-renewal and differentiation into multiple tumor cell types (Batlle and Clevers, 2017, Visvader, 2011). Thus, The intrinsic resistance of CSCs to conventional therapy is regarded as a potential therapeutic target of cancer (Reya et al., 2001). Activation of the Wnt/β-catenin pathway is required for the survival and development of CSCs, such as leukemia stem cells (LSCs) (Nusse and Clevers, 2017). Therefore, targeting β-catenin is considered a therapeutic strategy for the treatment of leukemia. cordycepin can block the effect of β-catenin in leukemia cells by regulating GSK-3β to inhibit the growth of LSCs (Ko et al., 2013). CSCs escape chemotherapy and lead to chemo-resistance due to the induction of TGF-β. Cordycepin efficiently inhibited cell viability, the percentage of ovarian cancer stem cells, and the levels of matrix metalloproteinases (MMPs) in TGF-beta-induced SKOV-3 ovarian cancer cells. Thus, cordycepin acted as a complementary agent for ovarian cancer therapy that against chemoresistance (Wang et al., 2017c).
4. Regulation of cordycepin on tumor microenvironment
4.1. Inhibition of migration and invasion of tumor cell
Cordycepin inhibited the migration and invasion of human oral squamous cell carcinoma (OSCC) cell through upregulating E-cadherin and downregulating N-cadherin protein expression, implying the inhibition of Cordycepin on epithelial-mesenchymal transition (EMT) (Yu et al., 2017). In addition, Cordycepin have been shown to suppress the migration of the human glioblastoma cell lines U87MG and LN229 in transwell and wound healing assays in vitro, since Cordycepin decreased protein expression of integrin α1, focal adhesion kinase (FAK), p-FAK, paxillin and p-paxillin. The lysosomal inhibitor NH4Cl can block the ability of cordycepin to inhibit focal adhesion protein expression and glioma cell migration. The protein phosphatase inhibitors Calyculin A and okadaic acid also blocked the cordycepin-mediated reduction in p-Akt, p-FAK and further suppress tumor cell line migration. Hematoxylin and eosin staining of mouse xenografts demonstrated that brain tumor sizes were reduced after Cordycepin treatment in vivo. Thus, cordycepin inhibited the migration and invasion of human glioblastoma cells by affecting lysosomal degradation and protein phosphatase activation (Hueng et al., 2017). These data are in consistent with the finding that cordycepin inhibits the migration and invasion of LNCaP cells (human prostate carcinoma cells). Cordycepin significantly downregulated the activity of tight junctions and suppressed the expression and activity of MMP-2 and MMP-9, which regulated tumor metastasis. These anti-metastatic roles were mediated by inactivation of the phosphoinositide 3-kinase (PI3K)/Akt pathway in LNCaP cells (Jeong et al., 2012).
4.2. Blockage of tumor metastasis
Anti-metastatic activities of cordycepin were demonstrated in mouse models where cordycepin inhibited B16 mouse melanoma liver metastasis in vivo (Kubo et al., 2010). The potential roles of cordycepin in melanoma cell metastasis and the underlying molecular mechanisms were addressed further. Zhang et al. found that cordycepin could suppress melanoma invasion via MMPs and metastasis via actomyosin machinery through LXR/RXR activation-dependent upregulation of miR-33b. Cordycepin also suppressed the expressions of HMGA2, Twist1 and ZEB1 through miR-33b. The up-regulation of miR-33b by cordycepin inhibited melanoma metastasis in vivo (Zhang et al., 2015). In another in vivo mouse melanoma studies, Yoshikawa at al demonstrated that adenosine-5′-diphosphate (ADP)-induced platelet aggregations accelerated lung metastasis on mouse melanoma. Cordycepin treatment reduced the number of metastatic lung nodules through blocking ADP-induced platelet aggregations (Yoshikawa et al., 2009). Those data indicated that cordycepin inhibit melanoma metastasis through different anti-metastatic mechanisms.
Cordycepin can suppress mitochondrial fusion-induced EMT in ovarian carcinoma cells through inhibiting estrogen-related receptor (ERR)-α, which is a co-transcription factor for gene expressions associated with mitochondrial fusion Thus, cordycepin suppresses metastasis and migration of ovarian carcinoma cells via inhibiting mitochondrial activity (Wang et al., 2017b). In addition, Cordycepin also blocked EMT through regulating TGF-β (Wang et al., 2017c).
Solid tumors grow fast if they induce the development of new blood vessels, a process known as tumor angiogenesis, which is the main process of tumor growth and metastasis (Carmeliet and Jain, 2000, Carmeliet and Jain, 2011). Angiogenesis was assessed using a tube formation assay (Yang et al., 2008). Anti-angiogenic drugs have been broadly used for clinical studies to suppress the growth and metastasis of tumors (Ferrara and Adamis, 2016). Cordycepin inhibited tube formation (total length of tubular structure) of human umbilical vein endothelial cell line (HUVEC) and the migration of those cells. Cordycepin also efficiently suppressed the invasion and migration of hepatocellular carcinoma cell line (HepG2) (Lu et al., 2014) (Table 2).
Table 2.
Inhibition roles of Cordycepin on tumor migration and metastasis.
| Tumor types (cell lines) | Anti-metastatic mechanism | Molecular targets | References |
|---|---|---|---|
| Human oral squamous cell carcinoma | Inhibition of epithelial-mesenchymal transition (EMT) | E-cadherin, N-cadherin↓ | (Su et al., 2017) |
| Human hepatocellular carcinoma (HepG2) | Anti-metastatic and anti-angiogenic | (Lu et al., 2014) | |
| Human glioblastoma cell (U87MG and LN229) | Inhibition of tumor cell motility | Lysosomal degradation, protein phosphatase activation↑ | (Hueng et al., 2017) |
| Human prostate carcinoma (LNCaP) | Inhibition of migration and invasion of tumor | AKT↓ | (Jeong et al., 2012) |
| Human ovarian carcinoma (OVCAR-3) | EMT | Mitochondrial activity↓, Estrogen-related receptor α↓ | (Wang et al., 2017b) |
| Human melanoma | Inhibition of invasion and metastasis | miR-33b↓, HMGA2, Twist1, ZEB1↑ | (Zhang et al., 2015) |
4.3. Disruption between cancer cells and mesenchymal stromal cells (MSCs)
Mesenchymal stromal cells (MSCs), as the main cell type of tumor microenvironment, promote tumor growth and metastasis, and stromal cells support tumor progression and resistance to chemotherapy (Ridge et al., 2017, Wan et al., 2013). Thus, targeting the niche-based microenvironment may be one new approach for cancer therapy (Singh et al., 2018). Cordycepin reduces the numbers of CD34+CD38-cells in leukemia such as U937 and K562, and induces Dkk1 expression to disrupt the association of both leukemia and MSCs. Cordycepin also suppressed cell attachment of leukemia with MSCs and downregulates N-cadherin in leukemia and VCAM-1 in MSCs (Liang et al., 2017). Therefore, the results indicated the potential of cordycepin as a multitarget drug in anti-metastatic therapy.
5. Conclusion and future prospect
Numerous studies have shown that Cordycepin as one valuable compound, can inhibit many malignant tumors through different pathways. Since Cordycepin-induced death of cancer cells are performed via multi-target pathways, it is difficult to some extent for cancer cells to develop drug resistance. Moreover, another advantage of Cordycepin is that the small side effect is shown when inhibiting the growth and progression of cancer cells. Therefore, Cordycepin may be considered as one wonderful drug candidate for cancer treatment.
Acknowledgements
This work was supported by Jilin province science and technology development program (20160201001YY); Jilin province science and technology development program (20170520043JH); Foundation of Jilin Educational Committee (JJKH20170726KJ); the Intramural Research Program at the NCI, NIH; the National Natural Science Foundation of China (Grant No. 81572868); Natural Science Foundation of Shangdong (Grant No. ZR2014ZM023).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Peng Yu, Email: yupengcczy@163.com.
Peng Qu, Email: peng.qu@nih.gov.
References
- Anderson B.O., Flanigan J. Novel methods for measuring global cancer burden: implications for global cancer control. JAMA Oncol. 2015;1:425–427. doi: 10.1001/jamaoncol.2015.1426. [DOI] [PubMed] [Google Scholar]
- Bai J., Li Y., Zhang G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017;14:348–362. doi: 10.20892/j.issn.2095-3941.2017.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E., Clevers H. Cancer stem cells revisited. Nat. Med. 2017;23:1124–1134. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- Bjornsti M.A., Houghton P.J. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Cao H.L., Liu Z.J., Chang Z. Cordycepin induces apoptosis in human bladder cancer cells via activation of A3 adenosine receptors. Tumour Biol. 2017;39 doi: 10.1177/1010428317706915. 1010428317706915. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Jain R.K. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.S., Stellrecht C.M., Gandhi V. RNA-directed agent, cordycepin, induces cell death in multiple myeloma cells. Br. J. Haematol. 2008;140 doi: 10.1111/j.1365-2141.2007.06955.x. 682 391. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Kang I.C. The inhibitory effect of Cordycepin on the proliferation of cisplatin-resistant A549 lung cancer cells. Biochem. Biophys. Res. Commun. 2018;498:431–436. doi: 10.1016/j.bbrc.2018.02.188. [DOI] [PubMed] [Google Scholar]
- Cunningham K.G., Manson W., Spring F.S., Hutchinson S.A. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) Link. Nature. 1950;166:949. doi: 10.1038/166949a0. [DOI] [PubMed] [Google Scholar]
- Evan G.I., Vousden K.H. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Adamis A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- Fong P., Ao C.N., Tou K.I., Huang K.M., Cheong C.C., Meng L.R. Experimental and in silico analysis of cordycepin and its derivatives as endometrial cancer treatment. Oncol. Res. 2018 doi: 10.3727/096504018X15235274183790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbein S., Wengi A., Decourty L., Freimoser F.M., Jacquier A., Dichtl B. Cordycepin interferes with 3′ end formation in yeast independently of its potential to terminate RNA chain elongation. RNA. 2009;15:837–849. doi: 10.1261/rna.1458909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P.Y., Lin Y.H., Yeh E.L., Lo H.C., Hsu T.H., Su C.C. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget. 2017;8:93712–93728. doi: 10.18632/oncotarget.21477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hueng D.Y., Hsieh C.H., Cheng Y.C., Tsai W.C., Chen Y. Cordycepin inhibits migration of human glioblastoma cells by affecting lysosomal degradation and protein phosphatase activation. J. Nutr. Biochem. 2017;41:109–116. doi: 10.1016/j.jnutbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Hwang I.H., Oh S.Y., Jang H.J., Jo E., Joo J.C., Lee K.B., Yoo H.S., Lee M.Y., Park S.J., Jang I.S. Cordycepin promotes apoptosis in renal carcinoma cells by activating the MKK7-JNK signaling pathway through inhibition of c-FLIPL expression. PLoS One. 2017;12:e0186489. doi: 10.1371/journal.pone.0186489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.H., Park S.J., Ko W.G., Kang S.M., Lee D.B., Bang J., Park B.J., Wee C.B., Kim D.J., Jang I.S. Cordycepin induces human lung cancer cell apoptosis by inhibiting nitric oxide mediated ERK/Slug signaling pathway. Am. J. Cancer Res. 2017;7:417–432. [PMC free article] [PubMed] [Google Scholar]
- Jeong J.W., Jin C.Y., Park C., Han M.H., Kim G.Y., Moon S.K., Kim C.G., Jeong Y.K., Kim W.J., Lee J.D. Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int. J. Oncol. 2012;40:1697–1704. doi: 10.3892/ijo.2012.1332. [DOI] [PubMed] [Google Scholar]
- Joo J.C., Hwang J.H., Jo E., Kim Y.R., Kim D.J., Lee K.B., Park S.J., Jang I.S. Cordycepin induces apoptosis by caveolin-1-mediated JNK regulation of Foxo3a in human lung adenocarcinoma. Oncotarget. 2017;8:12211–12224. doi: 10.18632/oncotarget.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko B.S., Lu Y.J., Yao W.L., Liu T.A., Tzean S.S., Shen T.L., Liou J.Y. Cordycepin regulates GSK-3beta/beta-catenin signaling in human leukemia cells. PLoS One. 2013;8:e76320. doi: 10.1371/journal.pone.0076320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo E., Yoshikawa N., Kunitomo M., Kagota S., Shinozuka K., Nakamura K. Inhibitory effect of Cordyceps sinensis on experimental hepatic metastasis of melanoma by suppressing tumor cell invasion. Anticancer Res. 2010;30:3429–3433. [PubMed] [Google Scholar]
- Lee S.J., Kim S.K., Choi W.S., Kim W.J., Moon S.K. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-Jun N-terminal kinase activation in human bladder cancer cells. Arch. Biochem. Biophys. 2009;490:103–109. doi: 10.1016/j.abb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Li G., Nakagome I., Hirono S., Itoh T., Fujiwara R. Inhibition of adenosine deaminase (ADA)-mediated metabolism of cordycepin by natural substances. Pharmacol. Res. Perspect. 2015;3:e00121. doi: 10.1002/prp2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S.M., Lu Y.J., Ko B.S., Jan Y.J., Shyue S.K., Yet S.F., Liou J.Y. Cordycepin disrupts leukemia association with mesenchymal stromal cells and eliminates leukemia stem cell activity. Sci. Rep. 2017;7:43930. doi: 10.1038/srep43930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Ling J., Zhang G., Liu F., Tao S., Han Z., Chen S., Chen Z., Le H. Cordycepin induces cell cycle arrest and apoptosis by inducing DNA damage and up-regulation of p53 in Leukemia cells. Cell Cycle. 2015;14:761–771. doi: 10.1080/15384101.2014.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S.W., Cepero E., Evan G. Intrinsic tumour suppression. Nature. 2004;432:307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- Lu H., Li X., Zhang J., Shi H., Zhu X., He X. Effects of cordycepin on HepG2 and EA.hy926 cells: potential antiproliferative, antimetastatic and anti-angiogenic effects on hepatocellular carcinoma. Oncol. Lett. 2014;7:1556–1562. doi: 10.3892/ol.2014.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser M.I., Masood M., Wei W., Li X., Zhou Y., Liu B., Li J., Li X. Cordycepin induces apoptosis in SGC7901 cells through mitochondrial extrinsic phosphorylation of PI3K/Akt by generating ROS. Int. J. Oncol. 2017;50:911–919. doi: 10.3892/ijo.2017.3862. [DOI] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Pan B.S., Wang Y.K., Lai M.S., Mu Y.F., Huang B.M. Cordycepin induced MA-10 mouse Leydig tumor cell apoptosis by regulating p38 MAPKs and PI3K/AKT signaling pathways. Sci. Rep. 2015;5:13372. doi: 10.1038/srep13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T., Morrison S.J., Clarke M.F., Weissman I.L. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ridge S.M., Sullivan F.J., Glynn S.A. Mesenchymal stem cells: key players in cancer progression. Mol. Cancer. 2017;16:31. doi: 10.1186/s12943-017-0597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D.M. mTOR and cancer: insights into a complex relationship. Nat. Rev. Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- Seong da B., Hong S., Muthusami S., Kim W.D., Yu J.R., Park W.Y. Cordycepin increases radiosensitivity in cervical cancer cells by overriding or prolonging radiation-induced G2/M arrest. Eur. J. Pharmacol. 2016;771:77–83. doi: 10.1016/j.ejphar.2015.12.022. [DOI] [PubMed] [Google Scholar]
- Shao L.W., Huang L.H., Yan S., Jin J.D., Ren S.Y. Cordycepin induces apoptosis in human liver cancer HepG2 cells through extrinsic and intrinsic signaling pathways. Oncol. Lett. 2016;12:995–1000. doi: 10.3892/ol.2016.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Yelle N., Venugopal C., Singh S.K. EMT: mechanisms and therapeutic implications. Pharmacol. Ther. 2018;182:80–94. doi: 10.1016/j.pharmthera.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Su N.W., Wu S.H., Chi C.W., Liu C.J., Tsai T.H., Chen Y.J. Metronomic cordycepin therapy prolongs survival of oral cancer-bearing mice and inhibits epithelial-mesenchymal transition. Molecules. 2017;22 doi: 10.3390/molecules22040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Li Y., Shen Y., Li Q., Wang Q., Feng L. Apoptosis and inhibition of proliferation of cancer cells induced by cordycepin. Oncol. Lett. 2015;10:595–599. doi: 10.3892/ol.2015.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli H.S., Sandhu S.S., Sharma A.K. Pharmacological and therapeutic potential of Cordyceps with special reference to Cordycepin. 3. Biotech. 2014;4:1–12. doi: 10.1007/s13205-013-0121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuli H.S., Sharma A.K., Sandhu S.S., Kashyap D. Cordycepin: a bioactive metabolite with therapeutic potential. Life Sci. 2013;93:863–869. doi: 10.1016/j.lfs.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Visvader J.E. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Wan L., Pantel K., Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat. Med. 2013;19:1450–1464. doi: 10.1038/nm.3391. [DOI] [PubMed] [Google Scholar]
- Wang C., Mao Z.P., Wang L., Zhang F.H., Wu G.H., Wang D.Y., Shi J.L. Cordycepin inhibits cell growth and induces apoptosis in human cholangiocarcinoma. Neoplasma. 2017;64:834–839. doi: 10.4149/neo_2017_604. [DOI] [PubMed] [Google Scholar]
- Wang C.W., Hsu W.H., Tai C.J. Antimetastatic effects of cordycepin mediated by the inhibition of mitochondrial activity and estrogen-related receptor alpha in human ovarian carcinoma cells. Oncotarget. 2017;8:3049–3058. doi: 10.18632/oncotarget.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.W., Lee B.H., Tai C.J. The inhibition of cordycepin on cancer stemness in TGF-beta induced chemo-resistant ovarian cancer cell. Oncotarget. 2017;8:111912–111921. doi: 10.18632/oncotarget.22951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Zhang Y., Lu J., Wang Y., Wang J., Meng Q., Lee R.J., Wang D., Teng L. Cordycepin, a natural antineoplastic agent, induces apoptosis of breast cancer cells via caspase-dependent pathways. Nat. Prod. Commun. 2016;11:63–68. [PubMed] [Google Scholar]
- Wang X.A., Xiang S.S., Li H.F., Wu X.S., Li M.L., Shu Y.J., Zhang F., Cao Y., Ye Y.Y., Bao R.F. Cordycepin induces S phase arrest and apoptosis in human gallbladder cancer cells. Molecules. 2014;19:11350–11365. doi: 10.3390/molecules190811350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A.S., Che C.M., Leung K.W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat. Prod. Rep. 2015;32:256–272. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]
- Wong, J.H., Cho Wing Sze, S., Ng, T.B., Chi Fai Cheung, R., Tam, C., Zhang, K.Y., Dan, X., Chan, Y.S., Chi Shing Cho, W., Cheuk Wing Ng, C., et al., 2017. Apoptosis and anti-cancer drug discovery: the power of medicinal fungi and plants. Curr. Med. Chem. [DOI] [PubMed]
- Wong Y.Y., Moon A., Duffin R., Barthet-Barateig A., Meijer H.A., Clemens M.J., de Moor C.H. Cordycepin inhibits protein synthesis and cell adhesion through effects on signal transduction. J. Biol. Chem. 2010;285:2610–2621. doi: 10.1074/jbc.M109.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.D., Hu Z.M., Shang M.J., Zhao D.J., Zhang C.W., Hong D.F., Huang D.S. Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1alpha through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. Int. J. Mol. Sci. 2014;15:12778–12790. doi: 10.3390/ijms150712778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Huang J., Ren X., Gorska A.E., Chytil A., Aakre M., Carbone D.P., Matrisian L.M., Richmond A., Lin P.C. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J., Zhang R., Wu F., Zhai L., Wang K., Xiao M., Xie T., Sui X. Non-apoptotic cell death in malignant tumor cells and natural compounds. Cancer Lett. 2018;420:210–227. doi: 10.1016/j.canlet.2018.01.061. [DOI] [PubMed] [Google Scholar]
- Yoshikawa N., Kunitomo M., Kagota S., Shinozuka K., Nakamura K. Inhibitory effect of cordycepin on hematogenic metastasis of B16–F1 mouse melanoma cells accelerated by adenosine-5′-diphosphate. Anticancer Res. 2009;29:3857–3860. [PubMed] [Google Scholar]
- Yu X., Ling J., Liu X., Guo S., Lin Y., Liu X., Su L. Cordycepin induces autophagy-mediated c-FLIPL degradation and leads to apoptosis in human non-small cell lung cancer cells. Oncotarget. 2017;8:6691–6699. doi: 10.18632/oncotarget.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue K., Ye M., Zhou Z., Sun W., Lin X. The genus Cordyceps: a chemical and pharmacological review. J. Pharm. Pharmacol. 2013;65:474–493. doi: 10.1111/j.2042-7158.2012.01601.x. [DOI] [PubMed] [Google Scholar]
- Zeng Y., Lian S., Li D., Lin X., Chen B., Wei H., Yang T. Anti-hepatocarcinoma effect of cordycepin against NDEA-induced hepatocellular carcinomas via the PI3K/Akt/mTOR and Nrf2/HO-1/NF-kappaB pathway in mice. Biomed. Pharmacother. 2017;95:1868–1875. doi: 10.1016/j.biopha.2017.09.069. [DOI] [PubMed] [Google Scholar]
- Zhang P., Huang C., Fu C., Tian Y., Hu Y., Wang B., Strasner A., Song Y., Song E. Cordycepin (3′-deoxyadenosine) suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. Oncotarget. 2015;6:9834–9853. doi: 10.18632/oncotarget.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]