Abstract
The human endometrial carcinoma is one of the most common female malignancies, and there is an urgent requirement to explore new therapeutic strategies. There is accumulating evidence that microRNAs (miRNAs) can serve as potential diagnostic and prognostic biomarkers for various types of cancer, but the significance of miR-582-5p still remains largely unknown in the endometrial carcinoma. The aims of this study were to understand and identify the influence of miR-582-5p on the proliferation and apoptosis of human endometrial carcinoma and its relevant mechanism. First, quantitative real-time PCR (qRT-PCR) was used to detect miR-582-5p and AKT3 expression in human tissue samples and cells. Then, CyQuant assay and 2D colony assay were employed to evaluate cell proliferation. Western blotting was used to determine protein expression. Subsequently, the luciferase reporter assay was used to identify the target of miR-582-5p. Finally, Annexin V assay was used to detect cell apoptosis. We found that miR-582-5p expression was significantly decreased in human endometrial carcinoma tissues, and miR-582-5p upregulation in human endometrial carcinoma cells inhibit cell proliferation and promote apoptosis. Moreover, AKT3 was validated as a target of miR-582-5p and AKT3 expression was inversely correlated with miR-582-5p expression. Besides, AKT3 upregulation efficiently abrogates the effect of miR-582-5p on the cells. These results demonstrated that miR-582-5p regulates cell proliferation and apoptosis in human endometrial carcinoma via AKT3. Thus, miR-582-5p represents a potential therapeutic target in human endometrial carcinoma meriting further investigation.
Keywords: miR-582-5p, AKT3, Proliferation, Apoptosis, Endometrial carcinoma
1. Introduction
The human endometrium is a highly dynamic tissue and undergoes complex dynamic changes under the control hormones (Yin et al., 2015). Endometrial cancer has become the most common gynecological malignancy and the fourth most common diagnosed malignancy among women. Recently, despite a great improvement in endometrial cancer treatment and diagnosis, advanced stages of the disease are still difficult to manage (Bansal et al., 2009). The incidence of endometrial cancer increases, and the lack of powerful treatment strategies calls for the need of developing novel and effective therapeutic strategies for this malignancy. MicroRNAs (miRNAs) have become as one of the novel class of diagnostic biomarkers in human cancers (Koturbash et al., 2011, Shah and Calin, 2014). MiRNAs are small non-coding RNAs that negatively regulate gene expression. Over the last decade, extensive research has demonstrated the importance of miRNAs in cancer biology as powerful regulators of cellular processes in cancer initiation, progression and metastasis (Croce, 2009, Shah and Calin, 2014). Many miRNAs have been identified to be involved in the cancer development, such as miR17, miR-34 and miR-145 (Maet al., 2012, Wuet al., 2012, Pagliucaet al., 2013). MiR-582-5p has been shown to be associated with various biological progression of cancer cells. For example, miR-582-5p has been reported to inhibit tumor proliferation in bladder cancer (Uchino et al., 2013). MiR-582-5p overexpression was also found to inhibit cell proliferation, cell cycle progression and invasion in human colorectal carcinoma (Zhang et al., 2015a, Zhang et al., 2015b). However, its biological role and underlying molecular mechanism are still poor understand in human endometrial carcinoma.
AKT belongs to the most hyperactivated signaling pathways in human cancer to regulate various cellular functions including cell growth, proliferation, invasion, and survival (Altomare and Testa, 2005, Gonzalez and McGraw, 2009, Khan et al., 2013). AKT1, AKT2, and AKT3 share roughly 80% overall sequence identity (Cheng et al., 2008). Among these isoforms, AKT2, and possibly AKT3, are important for disease progression and maintenance, but not AKT1 (Michiue et al., 2009, Mureet al., 2010). Akt3 has been also shown to contribute to the regulation of cell proliferation, migration, and invasion in many cancers such as thyroid cancer (Lin et al., 2017). However, the significance of AKT3 is still not clear in the miRNA regulatory network of human endometrial carcinoma.
In this study, the biological role of miR-582-5p in human endometrial carcinoma were explored. Additionally, we obtained evidence indicating miR-582-5p regulate the cell proliferation and apoptosis via AKT3.
2. Material and methods
2.1. Tissue collection
The human endometrial carcinoma and normal endometrial tissue samples for use in research were obtained according to the operational and ethical guidelines of Luoyang central hospital affiliated to Zhengzhou university. All patients are female. Tissues were collected and frozen in liquid nitrogen immediately after mincing on ice and then stored at −80 °C until analyze.
2.2. Cell culture
The endometrial carcinoma cell line ECC1 was cultured in the DMEM/F12 medium (Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (FBS), penicillin and streptomycin (100 U/mL and 100 μg/mL). Cells were then maintained at 37 °C in the humidified chamber with 5% CO2.
2.3. mi-582-5p mimic transfection
The cells were seeded in to 12-well plate at a density of 1 X 105 per well. The has-miR-582-5p and its negative control (miR-NC) were commercially available at Genepharma Co. (Shanghai, China). The cells were transfected with 25 nmol/L of miR-582-5p or miR-NC by RNAiMAX (life technologies) according to the manufacturer’s instructions. The effect of miR-582-5p mimic transfection was determined by quantitative real time-PCR.
2.4. Quantitative real time-PCR (qRT-PCR)
The total RNA was extracted from human tissues or cells by mirVanaTM miRNA isolation kit (Thermo Fisher Scientific, Waltham, MA) following the manufacturer’s protocol. The quantitative real time-PCR (qRT-PCR) was used to determine the mRNA and miRNA expression. For specific gene expression determination, the complement DNA (cDNA) was generated by RNA-to-cDNA kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The gene specific probes were purchased from Applied Biosystems (Foster City, CA). GAPDH was used as an internal reference gene.
The Applied Biosystems TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems) was used to generate the cDNA template of miR-582-5p. The U6 snRNA was used as an internal control. U6 cDNA was prepared by the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems).
2.5. Cell proliferation assay
The Cyquant assay was employed to monitor the cell viability (Thermo Fisher Scientific, Waltham, MA). The untransfected cells and transfected cells were plated into a 96-well plate at a density of 5000 cells per well. The plate was frozen at indicated time for the 1, 2, 3, 4 and 5 days incubation. Subsequently, the fresh prepared 100 μl CyQuant solution was added to the well to read with excitation at 485 nm and emission at 530 nm.
2.6. 2D colony formation assay
The colony formation assay is an in vitro assay to detect the ability of a single cell to grow into a colony. The suspended ECC1 cells were seeded in the 6-well plate (BD Biosciences, Bedford, MA) at a density of 1000 cells per well. The colonies were fixed with 80% ethanol and stained with crystal violet (0.5% w/v) (Millipore, Temecula, CA).
2.7. 3′-UTR luciferase assay
The ECC1 cells were seeded in the 6-well plate at the density of 0.2 M cells per well. After being incubated for 24 h, the cells were co-transfected with 20 nM miR-582-5p or miR-NC and 1 μg AKT3 3′-UTR luciferase reporter construct (GeneCopoeia, Rockville, MD) by lipofectamine 2000 (Invitrogen). Cells were lysed 48 h after transfection, and the renilla and firefly luciferase activity was detected by the Premega dual luciferase assay kit. Data was expressed as the ratio of firefly luciferase activity/renilla luciferase activity. The control luciferase reporter construct (Ctrl) was also available from GeneCopoeia.
2.8. AKT3 overexpression
To overexpress AKT3 in vitro, we used AKT3 vector construct (OriGene Technologies, Inc., Rockville, MD) and followed the manufacturer’s instructions. The negative control vector (VC) was also commercially available at OriGene Technologies.
2.9. Apoptosis assay
The Annexin V Fluorescein Isothiocyanate kit (BD Pharmingen, San Diego, CA) was used to detect cell apoptosis. The cells were collected after 48hrs transfection with miRNA mimic or gene construct. After then, cells were suspended in 100 ml binding buffer (1 × 106 cells/ml) and incubated with Annexin V-FITC and propidium iodide for 15 min. The apoptosis was detected on a BD FACSCalibur™ system (Becton-Dickinson, Franklin Lakes, NJ, USA). miR-NC or VC were used as the negative controls for miRNA or gene, respectively.
2.10. Western blot analysis
The total protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto nitrocellulose membranes (Millipore). After blocked with 5% nonfat milk, the membranes were then incubated with the anti-AKT3 antibody (Abcam, Cambridge, MA) or anti-GAPDH antibody (Abcam), which was used as the internal control. Then, goat anti-mouse or anti-rabbit horseradish peroxidase secondary antibody (Sigma, St. Louis, MO) was used for further incubation. The enhanced chemiluminescence reagents was introduced to detect the protein complex (Thermo Fisher Scientific).
2.11. Statistical analysis
Results were expressed as the means ± SE from 3 independent measurements. Data from 2 treatments were analyzed using the Student's t-test. One-way analysis of variance (ANOVA) test, followed by Tukey’s multiple comparison for multiple groups were used to compare the significance of differences. P < 0.05 were considered as statistically significant differences. The analyses were performed by the SPSS 16.0 version (SPSS, Chicago, IL).
3. Results
3.1. miR-582-5p is downregulated in endometrial carcinoma
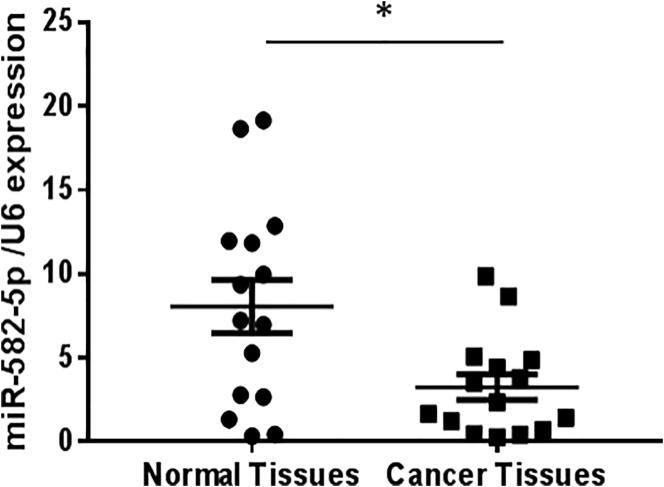
We first analyzed the expression patterns of miR-582-5p in human endometrial carcinoma and normal endometrial tissues. As shown in Fig. 1, lower expression of miR-582-5p was found in the endometrial carcinoma compared to their normal counterparts. In this study, we used 15 pairs of endometrial carcinoma and the adjacent normal endometrial tissues.
Fig. 1.
miR-582-5p expression in human endometrial carcinoma tissues. Taqman qRT-PCR was used to determine miR-582-5p expression in 15 cases of cancer tissues and paired non tumor tissues. U6 was used as an internal reference. *P < 0.05 vs non tumor tissues.
3.2. Upregulation of miR-582-5p inhibits cellular proliferation
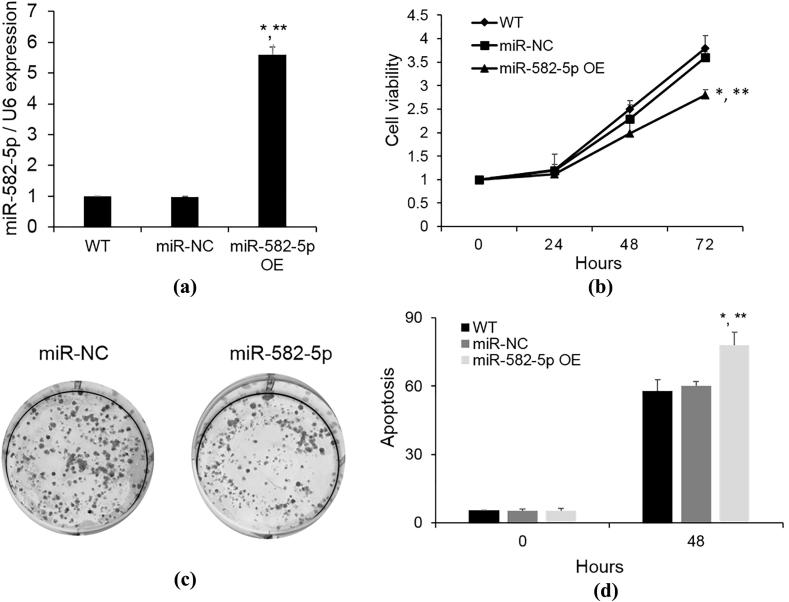
To further investigate the biological role of miR-582-5p in the endometrial carcinoma, we next transfected the ECC1 cells with miR-582-5p mimics or miR-NC as a negative control. MiR-582-5p expression was dramatically increased after miR-528-5p transfection (Fig. 2A). The Cyquant assay demonstrated that miR-528-5p strongly repressed ECC1 cell proliferation on a time dependent manner (Fig. 2B). Furthermore, the 2D colony formation assay also revealed that miR-528-5p upregulation markedly inhibit ECC1 cell growth (Fig. 2C). Our results suggested that miR-582-5p could significantly inhibit the proliferation of endometrial carcinoma cells.
Fig. 2.
Effect of miR-582-5p overexpressing on proliferation and apoptosis of the ECC1 cells. (A) ECC1 cells were overexpressed miR-582-5p; (B) cell proliferation was evaluated by Cyquant assay. Data are expressed as fold change (mean ± SE) compared with 0 h. (C) 2D colony formation assay was used to evaluate cell growth; (D) after overexpression of miR-582-5p for 48 hours, Annexin V assay was used to detect the cell apoptosis. *P < 0.05 vs WT, **P < 0.05 vs miR-NC.
3.3. Overexpression of miR-582-5p enhances cell apoptosis
Annexin V assay was employed to determine the effect of miR-582-5p on cell apoptosis. As shown in Fig. 2D, miR-582-5p overexpression significantly increased the apoptosis rate of ECC1 cells compared with the miR-NC and non-transfected cells (p < 0.05). This result suggested that miR-582-5p may serve as a pro-apoptosis factor in the endometrial carcinoma cells.
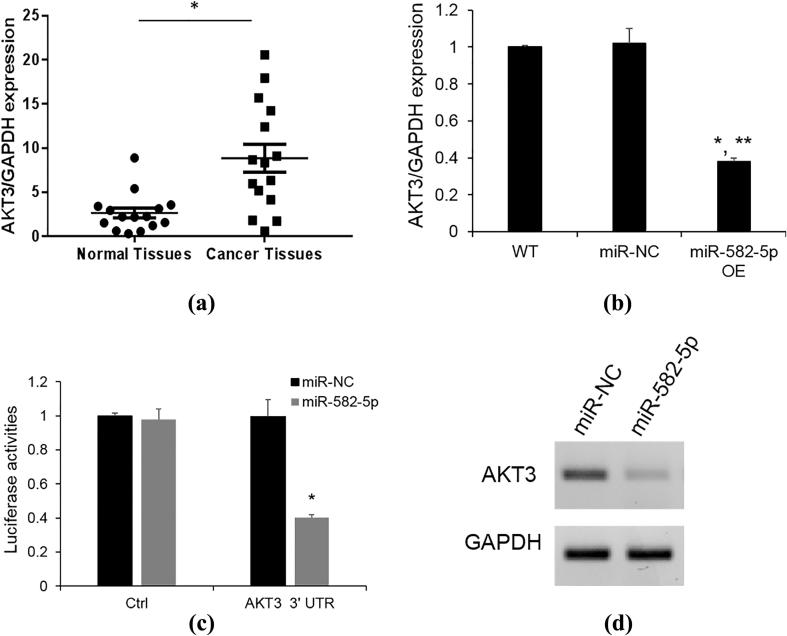
3.4. miR-582-5p targets the 3′-UTR of AKT3
Based on previous studies (Zhang et al., 2015a, Zhang et al., 2015a), we hypothesized that there is a significant correlation between miR-582-5p and AKT3. As expected, AKT3 was found to be upregulated in the endometrial carcinoma than their normal counterparts (Fig. 3A). To verify the regulatory capacity of miR-582-5p for AKT3, we used 3′-UTR luciferase reporter assays in the ECC1 cells by co-transfecting the miR-582-5p or miR-NC with the AKT3 3′-UTR reporter construct.
Fig. 3.
AKT3 is a direct target of miR-582-5p. (A) AKT3 expression level was detected by Taqman qRT-PCR. *P < 0.05 vs normal tissues; (B) AKT3 mRNA level was determined by Taqman qRT-PCR after miR-582-5p overexpression; (C) 3′UTR luciferase assay. The ECC1 cells were co-transfected with AKT3 3′ UTR or negative control construct (Ctrl) reporter with miR-NC or mir-582-5p. Luciferase assays were performed after 48hrs. *p < 0.05 vs miR-NC; (D) AKT3 protein level was analyzed by western blotting after overexpression of miR-582-5p for 48 h.
Overexpression of miR-582-5p showed a significant reduction of luciferase activity in comparison to the miR-NC (Fig. 3B).
We next determined whether miR-582-5p overexpression affect AKT3 expression in the ECC1 cells. As expected, miR-582-5p significantly decreased both mRNA and protein expression of AKT3 (Fig. C, D). These results validated that miR-582-5p directly inhibit the expression of AKT3.
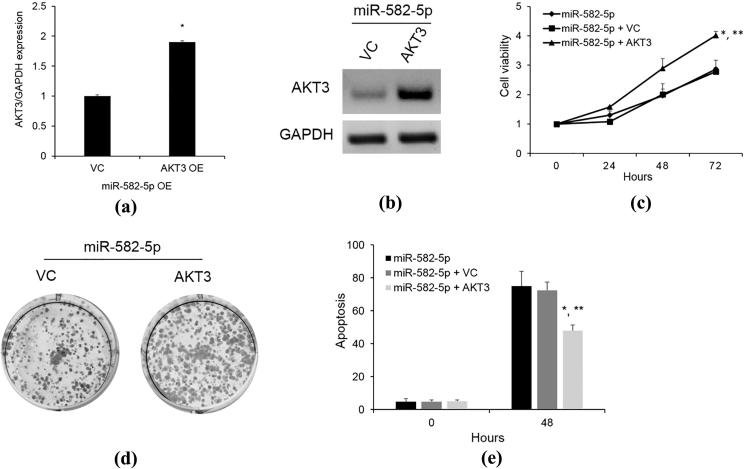
3.5. AKT3 overexpression obligates the effect of miR-582-5p on cells
To further validate whether AKT3 is a functional target of miR-528-5p in the ECC1 cells, we overexpressed AKT3 in the ECC1 cells which were transfected with miR-582-5p mimic. As shown in Fig. 4A, B, AKT3 overexpression rescued the inhibited expression of AKT3 by miR- 582-5p upregulation. As expected, AKT3 overexpression obligates the inhibitory effect of miR- 582-5p on cell proliferation (Fig. 4C, D). The cell apoptosis induced by miR-582-5p overexpression was also decreased after AKT3 upregulation (Fig. 4E). These data collectively indicate that miR-582-5p regulated ECC1 cell growth and apoptosis through AKT3.
Fig. 4.
AKT3 upregulation obligated the effect of miR-582-5p on cells. (A) AKT3 mRNA level after ATK3 upregulation in the ECC1 cells overexpressing miR-582-5p was detected by Taqman qRT-PCR. *P < 0.05 vs VC; (B) AKT3 protein level was determined by western blotting after AKT3 upregulation in ECC1 cells overexpressing miR-582-5p; (C) cell proliferation was analyzed with Cyquant assay. ECC1 cells were transfected with ATK3 construct to overexpress AKT3 after miR-582-5p upregulated in ECC1 cells. Luciferase assays were performed after 48 h; (D) Cell growth after AKT3 overexpression was evaluated by 2D colony formation assay; (E) AKT3 effect on cell apoptosis was detected by Annexin V assay. *P < 0.05 vs miR-582-5p; P < 0.05 vs miR-582-5p + VC.
4. Discussion
The value of miRNAs in cancer cell proliferation, angiogenesis and apoptosis has been explored. Accumulated evidence shows that miRNAs play important regulatory roles through targeting oncogenes, tumor suppressors or specific genes (Di Leva et al., 2014). Many miRNAs have been reported to be aberrantly expressed in the endometrial carcinoma (Snowdonet al., 2011, Schirmeret al., 2014, Liuet al., 2016). Among those miRNAs, miR-582-5p has been reported to contribute to the development and progression of several human cancers such as bladder cancer and colorectal carcinoma (Uchinoet al., 2013, Zhang et al., 2015a, Zhang et al., 2015a). MiR-34a has been shown as a suppressor of L1CAM in endometrial carcinoma (Schirmer et al., 2014). MiR-372 inhibited the endometrial carcinoma development through regulating Ras homolog gene family member C (RhoC) (Liu et al., 2016). In addition, miR-505 served as a tumor suppressor in endometrial cancer by targeting TGF-α (Chen et al., 2016). However, the exact function and its molecular mechanism of miR-582-5p in endometrial carcinoma is still largely unknown. In this study, we found miR-582-5p was remarkably decreased in the endometrial carcinoma tissues. Upregulation of miR-582-5p negatively regulated ECC1 cell proliferation but enhanced the cell apoptosis.
AKT3 plays critical roles in regulating cancer progression. Recently, AKT3 was validated as one of the direct targets of miR-582-5p in regulating the progression of hepatocellular carcinoma (HCC) (Zhang et al., 2015a, Zhang et al., 2015a). Downregulation of AKT3 has been shown to increase migration and metastasis in triple negative breast cancer cells by upregulating S100A4 (Grottke et al., 2016). AKT3 has also been confirmed to mediate resistance to apoptosis in B-RAF-targeted melanoma cells (Shao et al., 2010). Our results, AKT3 has been observed to be reversely correlated with miR-582-5p. Furthermore, we also verified AKT3 is a direct target of miR-582-5p in the endometrial carcinoma. Moreover, AKT3 is also involved in regulation of cell proliferation and apoptosis in ECC1 cells.
In summary, miR-582-5p was demonstrated to be downregulated in the human endometrial carcinoma tissues. Overexpression of miR-582-5p repressed ECC1 cell proliferation and increased cell apoptosis. Our results also validate miR-582-5p directly targeting AKT3. Consequently, upregulation of AKT3 abrogates the effect of miR-582-5p on the ECC1 cells. Collectively, these data proved that miR-582-5p regulate cell proliferation and apoptosis through AKT3. Additionally, our research provided some hints for a profound learning of miR-582-5p.
Footnotes
Peer review under responsibility of King Saud University.
References
- Altomare D.A., Testa J.R. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- Bansal N., Yendluri V., Wenham R.M. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- Chen S., Sun K.X., Liu B.L., Zong Z.H., Zhao Y. MicroRNA-505 functions as a tumor suppressor in endometrial cancer by targeting TGF-alpha. Mol. Cancer. 2016;15:11. doi: 10.1186/s12943-016-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G.Z., Park S., Shu S., He L., Kong W., Zhang W., Yuan Z., Wang L.H., Cheng J.Q. Advances of AKT pathway in human oncogenesis and as a target for anticancer drug discovery. Curr. Cancer Drug Targets. 2008;8:2–6. [PubMed] [Google Scholar]
- Croce C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leva G., Garofalo M., Croce C.M. MicroRNAs in cancer. Annu. Rev. Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E., McGraw T.E. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8:2502–2508. doi: 10.4161/cc.8.16.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottke A., Ewald F., Lange T., Norz D., Herzberger C., Bach J., Grabinski N., Graser L., Hoppner F., Nashan B., Schumacher U., Jucker M. Downregulation of AKT3 increases migration and metastasis in triple negative breast cancer cells by upregulating S100A4. PLoS One. 2016;11:e0146370. doi: 10.1371/journal.pone.0146370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K.H., Yap T.A., Yan L., Cunningham D. Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin. J. Cancer. 2013;32:253–265. doi: 10.5732/cjc.013.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I., Zemp F.J., Pogribny I., Kovalchuk O. Small molecules with big effects: the role of the microRNAome in cancer and carcinogenesis. Mutat. Res. 2011;722:94–105. doi: 10.1016/j.mrgentox.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Michiue H., Eguchi A., Scadeng M., Dowdy S.F. Induction of in vivo synthetic lethal RNAi responses to treat glioblastoma. Cancer Biol. Ther. 2009;8:2306–2313. doi: 10.4161/cbt.8.23.10271. [DOI] [PubMed] [Google Scholar]
- Lin Y., Cheng K., Wang T., Xie Q., Chen M., Chen Q., Wen Q. miR-217 inhibits proliferation, migration, and invasion via targeting AKT3 in thyroid cancer. Biomed. Pharmacother. 2017;95:1718–1724. doi: 10.1016/j.biopha.2017.09.074. [DOI] [PubMed] [Google Scholar]
- Liu B.L., Sun K.X., Zong Z.H., Chen S., Zhao Y. MicroRNA-372 inhibits endometrial carcinoma development by targeting the expression of the Ras homolog gene family member C (RhoC) Oncotarget. 2016;7:6649–6664. doi: 10.18632/oncotarget.6544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang P., Wang F., Zhang H., Yang Y., Shi C., Xia Y., Peng J., Liu W., Yang Z., Qin H. Elevated oncofoetal miR-17-5p expression regulates colorectal cancer progression by repressing its target gene P130. Nat. Commun. 2012;3:1291. doi: 10.1038/ncomms2276. [DOI] [PubMed] [Google Scholar]
- Mure H., Matsuzaki K., Kitazato K.T., Mizobuchi Y., Kuwayama K., Kageji T., Nagahiro S. Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Oncol. 2010;12:221–232. doi: 10.1093/neuonc/nop026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliuca A., Valvo C., Fabrizi E., di Martino S., Biffoni M., Runci D., Forte S., De Maria R., Ricci-Vitiani L. Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene. 2013;32:4806–4813. doi: 10.1038/onc.2012.495. [DOI] [PubMed] [Google Scholar]
- Schirmer U., Doberstein K., Rupp A.K., Bretz N.P., Wuttig D., Kiefel H., Breunig C., Fiegl H., Muller-Holzner E., Zeillinger R., Schuster E., Zeimet A.G., Sultmann H., Altevogt P. Role of miR-34a as a suppressor of L1CAM in endometrial carcinoma. Oncotarget. 2014;5:462–472. doi: 10.18632/oncotarget.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M.Y., Calin G.A. MicroRNAs as therapeutic targets in human cancers. Wiley Interdiscip Rev. RNA. 2014;5:537–548. doi: 10.1002/wrna.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Aplin A.E. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670–6681. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon J., Zhang X., Childs T., Tron V.A., Feilotter H. The microRNA-200 family is upregulated in endometrial carcinoma. PLoS One. 2011;6:e22828. doi: 10.1371/journal.pone.0022828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino K., Takeshita F., Takahashi R.U., Kosaka N., Fujiwara K., Naruoka H., Sonoke S., Yano J., Sasaki H., Nozawa S., Yoshiike M., Kitajima K., Chikaraishi T., Ochiya T. Therapeutic effects of microRNA-582-5p and -3p on the inhibition of bladder cancer progression. Mol. Ther. 2013;21:610–619. doi: 10.1038/mt.2012.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Wu G., Lv L., Ren Y.F., Zhang X.J., Xue Y.F., Li G., Lu X., Sun Z., Tang K.F. MicroRNA-34a inhibits migration and invasion of colon cancer cells via targeting to Fra-1. Carcinogenesis. 2012;33:519–528. doi: 10.1093/carcin/bgr304. [DOI] [PubMed] [Google Scholar]
- Yin Q., Fischer L., Noethling C., Schaefer W.R. In vitro-assessment of putative antiprogestin activities of phytochemicals and synthetic UV absorbers in human endometrial Ishikawa cells. Gynecol. Endocrinol. 2015;31:578–581. doi: 10.3109/09513590.2015.1047448. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Y., Yang J., Li S., Chen J. Upregulation of miR-582-5p inhibits cell proliferation, cell cycle progression and invasion by targeting Rab27a in human colorectal carcinoma. Cancer Gene Ther. 2015;22:475–480. doi: 10.1038/cgt.2015.44. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Huang W., Ran Y., Xiong Y., Zhong Z., Fan X., Wang Z., Ye Q. miR-582-5p inhibits proliferation of hepatocellular carcinoma by targeting CDK1 and AKT3. Tumour. Biol. 2015;36:8309–8316. doi: 10.1007/s13277-015-3582-0. [DOI] [PubMed] [Google Scholar]