Abstract
Small cell carcinoma of the prostate (SCCP) is a rare and the most aggressive variant of prostate cancer. There is no effective cure or treatment for SCCP. Therefore, there is an urgent need for new therapy to improve the prognosis of patients with SCCP. DUSP1 is a dual specific phosphatase with an increasingly recognized in tumor biology. Altered expression of DUSP1 induced changes in the expression of genes involved in various biological pathways, including cell-cell signaling and angiogenesis. To understand more about the role of DUSP1 in SCCP, we evaluated the biological function and associated regulatory mechanism of DUSP1. In this study, DUSP1 was significantly down-regulated in human SCCP compared with the non-carcinoma tissues (P < 0.05). Overexpression of DUSP1 was found to suppress MAPK signaling and cell proliferation in PC-3 cells. Additionally, silencing of DUSP1 enhanced MAPK signaling and PC-3 cell proliferation. Moreover, it was observed that DUSP1 blocked the phosphorylation of p38 MAPK induced by anisomycin. Taken together, this investigation suggests that DUSP1 is involved in the progression of SCCP and may provide a new therapeutic target for SCCP treatment.
Keywords: DUSP1, MAPK signaling, Cell proliferation, Small cell carcinoma, Prostate
1. Introduction
Small cell carcinoma of the prostate (SCCP) is an uncommon type of prostate cancer and a high grade malignant neoplasm with neuroendocrine differentiation (Furtado et al., 2011). The morphologic features of small cell including small tumor cells with minimal cytoplasm, nuclear molding, fine chromatin pattern and so on. As other cancers, aberrant gene regulation features significantly in the SCCP.
Dual specificity phosphatase (DUSP) family belongs to phosphatase that can act on tyrosine or serine/tyrosine residues. DUSP2 is able to inactive ErK2 (Zhang et al., 2005). DUSP1 is a mitogen and stress-inducible dual specificity protein phosphatase. It has been reported that DUSP1/MKP1 can regulate MAPKs activity with an important role in tumor biology (Moncho-Amor et al., 2011). In addition, DUSP1 level has been found to be decreased in lung squamous carcinoma with cancer progression (Wang et al., 2011). However, the role of DUSP1 is still incompletely understood in the SCCP.
Mitogen-activated protein kinase (MAPK) signaling pathway is a well-known signaling that is evolutionarily conserved kinase modules. It links extracellular signals to the machinery that controls fundamental cellular processes including growth, proliferation, apoptosis, and so on (Dhillon et al., 2007). The mammalian family of MAPKs includes extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal kinase (JNK) and p38. The core components identified for each of the three major MAPK pathways consist of a MAPK kinase kinase (MAP3K), a MAPK kinase (MAP2K), and a MAPK (Kim and Choi, 2010). P38 MAPK signaling pathway functions in a cell context-specific and cell type specific manner to affect cell proliferation, migration and survival (Wagner and Nebreda, 2009). Dysregulation of p38 MAPK signaling is associated with the development, progression and short survival of different cancer types including breast, bladder, liver, and so on (Dhillon et al., 2007, Koul et al., 2013). However, it is still unclear whether p38 MAPK signaling plays important roles in the SCCP. In this study, we found DUSP1 level was reduced in human prostatic tumors. In addition, DUSP1 overexpression was found to repress MAPK signaling and cell proliferation in PC-3 cells. Loss of DUSP1 could reverse the effect of DUSP1 overexpression on PC-3 cells. Moreover, we demonstrated that the phosphorylation of p38 MAPK could be blocked by DUSP1. Our studies investigated the role of DUSP1 in the SCCP, and DUSP1 may be an unexplored target of a novel therapy for the SCCP.
2. Materials and methods
2.1. Cell culture and Materials
Recently, PC-3 cell line has shown specific association of those cases of the SCCP based on the molecular characterization (Furtado et al., 2011). In addition, the PC-3 cell line is also considered to be one of the classical human prostate cancer cell lines for study human prostate cancer (Alimirah et al., 2006). ATCC human prostate cancer cell line PC-3 was cultured as suggested. Briefly, PC-3 cells were maintained in ATCC formulated F12K Medium containing 10% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma-Aldrich, St Louis, MO) in a humidified atmosphere of 5% CO2 and 95% air at 37°C.
2.2. Quantitative real-time PCR (qRT-PCR)
Total RNA of human tissues or cells were isolated by Trizol reagent (Invitrogen, Carlsbad, CA) as the manufacturer’s suggestion. After removal of DNA by DNA-free DNase (Ambion, Austin, TX), residual RNAs were reverse transcribed into cDNA with M-MLV reverse transcriptase (BRL, Gaithersburg, MD). The Taqman qRT-PCR was used for DUSP1 gene expression analysis. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for comparative analyses of gene expression. All specific taqman probes were purchased from Applied Biosystems, (Foster City, CA). All Taqman qRT- PCR studies were performed in triplicate on an ABI 7500 system (Applied Biosystems) and the data were presented as mean ±SE. The amount of each miRNA relative to GAPDH was calculated as previously described (Yin et al., 2015).
2.3. DUSP1 overexpression and knockdown
PC-3 cells were engineered to overexpress DUSP1 by lentiviral system. The lentiviral control vector (Con-OE) contains a non-relevant sequence as a negative control. All the lentiviruses were purchased from Applied Biological Materials Inc (Richmond, BC). The overexpression was performed according to the manufacturer's instructions. For the DUSP1 silencing lentiviral system, short hairpin RNA (shRNA) of DUSP1 lentiviral vector and corresponded lentiviral control vector (Con-KD) as a negative control were purchased System Biosciences (Mountain View, CA). Transfections shRNA lentiviral particles were generated as previously describe (Zhang et al., 2015).
2.4. Western blotting
The 4–20% gradient SDS-polyacrylamide gel (Invitrogen) was used to separate the protein lysis from PC-3 cells. After then, the gel was electrophoretically transferred onto a PVDF membrane (GE) and the membranes were incubated with anti-DUSP1 (Abcam, Cambridge, MA), anti-pp38 (New England Biolabs, Hitchin, Hertfordshire, United Kingdom), anti-p38 (New England Biolabs) primary antibody or GAPDH (Abcam, Cambridge, MA) overnight. After washing with TBST for 5 min, the Horseradish peroxidase (HRP)-conjugated secondary Abs (Bio-Rad, Hercules, CA) were added the next day. The proteins were visualized by ECL chemiluminescence and exposed to the X-ray film. All the studies were performed in triplicate. Anisomycin (Sigma) was used to induce the p38 MAPK signaling at the indicated concentration.
2.5. Cell proliferation assay
Of 5000 cells were seeded in triplicate in each well of the 96-well tissue culture plates. At indicated time points, a CyQuant assay (Thermo Fisher Scientific, Boston, MA) was performed as the suggestion of manufacture to monitor the growth of the cells. Briefly, the CyQuant solution was prepared immediately before use. 100ul of CyQuant solution was added to the wells after removal of media and incubated in the dark for 45min at room temperature. The plate was read at excitation at 497nm and emission at 520 nm. All studies were performed in 3 independent cell preparation and the data was presented as mean ± SE.
2.6. Dual-luciferase assay
The luciferase reporter assay was performed to detect the MAPK signaling pathway activity as previous description (Zhang et al., 2015). Briefly, cells were seeded in 96-well plate at a density of 2 × 104 cells per well. PC-3 cells overexpressed with DUSP1 or Con-OE transfected with MAPK pathway-luciferase-reporter construct (SABiosciences, Frederick, MD) with lipofectamine 2000. After culture 48h, the cells were harvested for the Dual Luciferase Reporter Assay (Promega). MAPK signaling pathway was also detected in PC-3 cells silenced with DUSP1 or Con-KD by the dual-luciferase reporter assay. Sh-Con was used as a negative vector control.
2.7. Statistics
GRAPHPAD PRISM (version 6.07 for windows, Graphpad Software, La Jolla, CA) was used for statistical analyses. Data are expressed as mean ± SE (n = 3 independent cell preparation). Unpaired student t-test was performed to ascertain statistical significance between the two groups. P (probability) less than 0.05 was concerned as significant difference.
3. Results
3.1. DUSP1 mRNA decreased in the tumor tissues
Previous studies showed that DUSP1 was down-regulated in many types of human cancers (Persson et al., 2011, Newie et al., 2014, Schmitt et al., 2015), suggesting a possible significance of DUSP1 in cancer development and/or progression. In this present study, we first examined DUSP1 mRNA level in prostatic tumors and normal tissues. 18 prostatic tumors and 14 noncancerous normal tissues were used to determine DUSP1 expression by qRT-PCR assay. All samples were collected in accordance the ethical guidelines of the First Hospital of Shijiazhuang. GAPDH was used as the endogenous control. We found that DUSP1 mRNA levels in human prostatic tumors were significantly down-regulated compared to the non-cancerous normal tissues (P < 0.05) (Fig. 1). It was indicated that down-regulation of DUSP1 may be involved in the progression of SCCP.
Fig. 1.
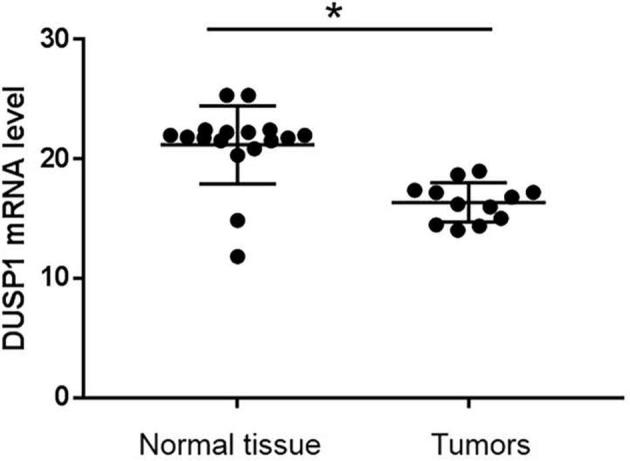
DUSP1 mRNA level in the prostatic tumors and non-cancerous tissues. *p < 0.05 vs Normal tissue.
3.2. DUSP1 regulates human prostate cancer cell proliferation
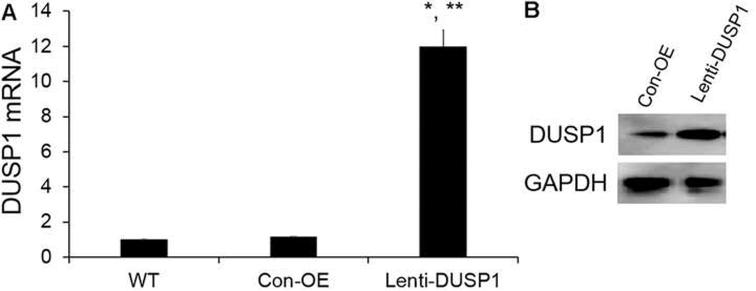
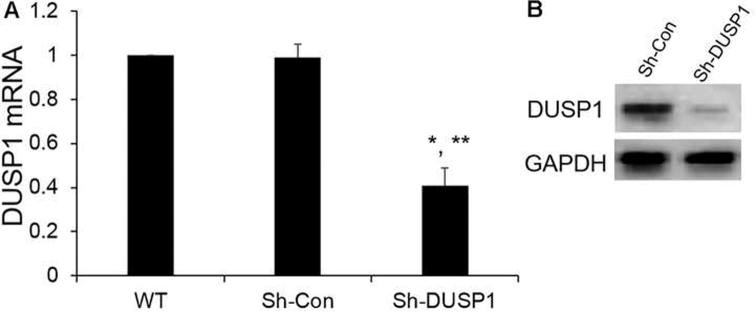
We next determined the effects of DUSP1 on human prostate cancer cell line in vitro. We first determined DUSP1 expression levels after infecting PC-3 cells with MOI = 10 of DUSP1 lentivirus or Con-OE lentivirus. As shown in Fig. 2, the DUSP1 mRNA and protein expression levels were significantly enhanced after infection compared with Con-OE and wide type (WT) cell groups. The cell proliferation was monitored by CyQuant assay. We observed that DUSP1 overexpression significantly suppressed PC-3 cell proliferation (Fig. 3). To further confirm DUSP1 effect on PC-3 cell proliferation, we next silenced DUSP1 by DUSP1 shRNA lentivirus. As shown in Fig. 4, DUSP1 shRNA significantly reduced the expression of DUSP1 protein expression in PC-3 cells. In addition, DUSP1 shRNA reversed the effects on PC-3 cell proliferation caused by DUSP1 overexpression (Fig. 5).
Fig. 2.
DUSP1 expression was increased after PC-3 cells overexpressed with DUSP1 by lentiviral system. (A) DUSP1 mRNA expression level was determined by qRT-PCR; (B) DUSP1 protein expression were determined by WB. WT: PC-3 cells without infection. GAPDH was used as an internal control. Results were mean ± SE (n = 3). *P < 0.05 vs WT; **P < 0.05 vs Con-OE.
Fig. 3.
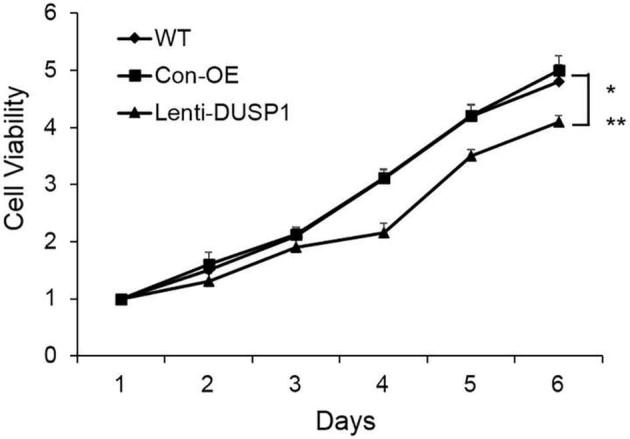
Effect of DUSP1 overexpression on PC-3 cell growth. PC-3 cell growth was significantly decreased from day 3 after DUSP1 overexpression. Data was mean ± SE (n = 3). *P < 0.05 vs WT; **P < 0.05 vs Con-OE.
Fig. 4.
DUSP1 expression were decreased after PC-3 cells infected DUSP1 silencing shRNA by lentiviral system. (A) DUSP1 mRNA expression level was determined by qRT-PCR; (B) DUSP1 protein expression was determined by WB. WT: PC-3 cells without infection. GAPDH was used as an internal control. Results were mean ± SE (n = 3). *P < 0.05 vs WT; **P < 0.05 vs Sh-Con.
Fig. 5.
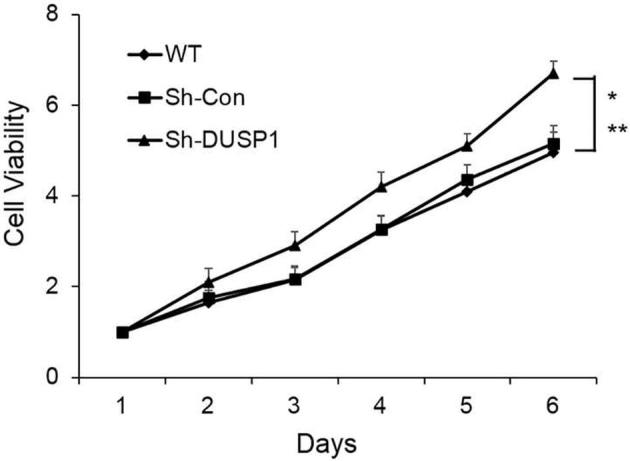
Effect of DUSP1 silencing on PC-3 cell growth. PC-3 cell growth was significantly increased from day 2 after DUSP1 silencing. Data was mean ± SE (n = 3). *P < 0.05 vs WT; **P < 0.05 vs Sh-Con.
3.3. DUSP1 regulates MAPK signaling pathway in vitro
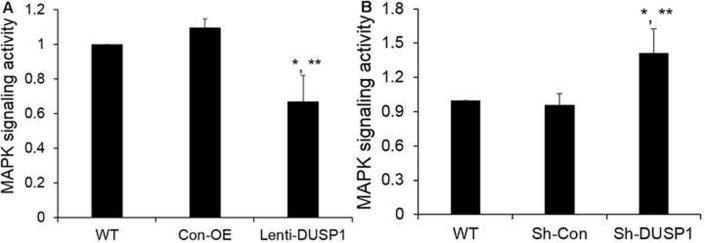
We further determined the effect of DUSP1 on MAPK signaling pathway since DUSP1 is one of the important regulators of the cascades of MAPK signaling pathway. Using a simple MAPK dual-luciferase reporter assay, we investigated that DUSP1 overexpression reduced the activity of MAPK signaling compared with Con-OE (Fig. 6A). DUSP1 silencing reversed the effect of DUSP1 overexpression on MAPK signaling activity in the PC-3 cells (Fig. 6B). The luciferase data suggested that DUSP1 regulated the activity of MAPK signaling pathway in the PC-3 cells.
Fig. 6.
Effect of DUSP1 on MAPK signaling pathway. (A) PC-3 cells overexpressing DUSP1 were transfected with a MAPK luciferase-reporter construct. (B) PC-3 cells with DUSP1 knockdown were transfected with MAPK luciferase-reporter construct. MAPK activity was normalized to Renilla luciferase activity. Results were mean ± SE (n = 3). *P < 0.05 vs WT; **P < 0.05 vs Con-OE or Sh-Con.
3.4. DUSP1 regulated the phosphorylation of p38
P38 MAPK phosphorylation was found to be regulated by DUSP1 in airway smooth muscle (ASM) cells (Manetsch et al., 2012, Prabhala and Ammit, 2015). DUSP1 has also been reported to inactivate p38 MAPK in the innate immunity (Arthur and Ley, 2013). Our next step was to determine whether DUSP1 had a similar function on p38 MAPK in the PC-3 cells. To address this, we compared the impact of DUSP1 overexpression on p38 MAPK phosphorylation in PC-3 cells treated with anisomycin, which was the specific p38 MAPK activator. As expected, anisomycin strongly activated p38, but DUSP1 overexpression repressed p38 MAPK phosphorylation induced by anisomycin (Fig. 7).
Fig. 7.
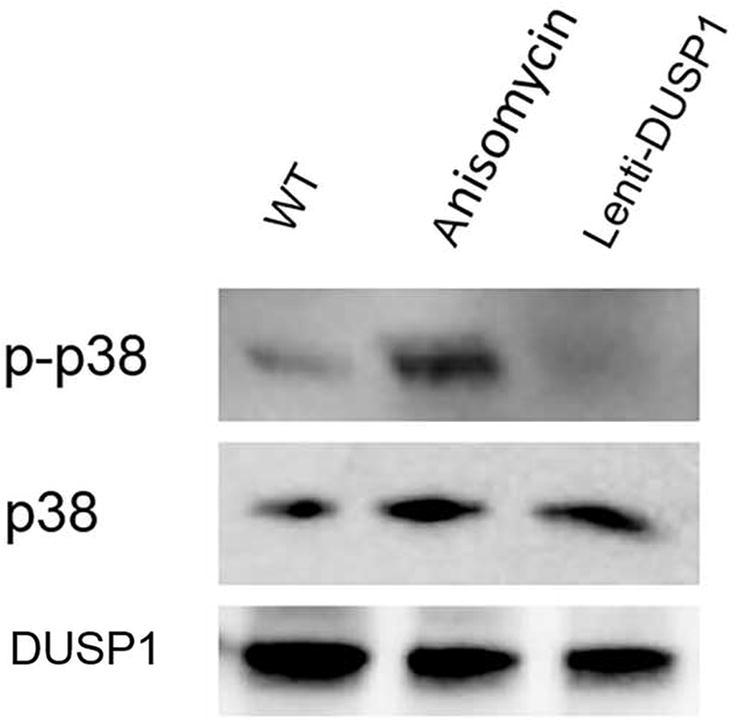
Effects of anisomycin and DUSP1 overexpression on the level of p-p38 and p38. Western blot was used to analyze p-p38 and p38 proteins in the PC-3 cells. GAPDH was used as the internal control. The data shown was from one representative experiment of 3 independent experiments.
4. Discussion
SCCP is accounting for less than 1% of all prostate cancers. A wide variety of human tumors contain abnormal expression of DUSP1 and abnormal MAPK signaling associated with various processes such as cell proliferation, differentiation and metabolism. Down-regulation of DUSP1 has been reported in cancer. Currently, DUSP1 has become an attractive regulator gene. However, the roles of DUSP1 in the progression of human SCCP is still largely unknown. In this study, we observed that DUSP1 mRNA was down-regulated in human prostatic tumors compared with normal tissues. It has been reported that DUSP1 was silencing in primary oral squamous cell carcinoma (Khor et al., 2013). And, MAPK signaling depended on increased DUSP1 expression (Kao et al., 2013). Additionally, DUSP1 expression was found to inversely correlate with NF-κB activity and expression in prostate cancer and promotes apoptosis through a p38 MAPK dependent mechanism (Gil-Araujo et al., 2014). In this present study, we also found that DUSP1 overexpression suppressed MAPK signaling. In addition, DUSP1 overexpression remarkably suppressed PC-3 cell proliferation. DUSP1 silencing reversed the effects of DUSP1 overexpression on PC-3 cells. DUSP1 was also found to regulate the phosphorylation of p38 MAPK in the PC-3 cells. These results suggested that DUSP1 was involved in the progression of the SCCP through p38 MAPK signaling pathway. Our data was derived from a small number of patients. The present study generates hypotheses and further validation in larger datasets is necessary.
Taken together, DUSP1 expression reduced in prostatic tumors and DUSP1 can regulate PC-3 cell proliferation through p38 MAPK signaling pathway. It indicates that appropriate regulation of DUSP1 has the potential to be used for the therapy of SCCP.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alimirah F., Chen J., Basrawala Z., Xin H., Choubey D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: implications for the androgen receptor functions and regulation. FEBS Lett. 2006;580:2294–2300. doi: 10.1016/j.febslet.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Arthur J.S., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Furtado P., Lima M.V., Nogueira C., Franco M., Tavora F. Review of small cell carcinomas of the prostate. Prostate Cancer. 2011;2011:543272. doi: 10.1155/2011/543272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Araujo B., Toledo Lobo M.V., Gutierrez-Salmeron M., Gutierrez-Pitalua J., Ropero S., Angulo J.C., Chiloeches A., Lasa M. Dual specificity phosphatase 1 expression inversely correlates with NF-kappaB activity and expression in prostate cancer and promotes apoptosis through a p38 MAPK dependent mechanism. Mol Oncol. 2014;8:27–38. doi: 10.1016/j.molonc.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T.C., Wu C.H., Yen G.C. Glycyrrhizic acid and 18beta-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomedicine. 2013;20:295–302. doi: 10.1016/j.phymed.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Khor G.H., Froemming G.R., Zain R.B. DNA methylation profiling revealed promoter hypermethylation-induced silencing of p16, DDAH2 and DUSP1 in primary oral squamous cell carcinoma. Int. J. Med. Sci. 2013;10:1727–1739. doi: 10.7150/ijms.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E.K., Choi E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Koul H.K., Pal M., Koul S. Role of p38 MAP kinase signal transduction in solid tumors. Genes Cancer. 2013;4:342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetsch M., Che W., Seidel P., Chen Y., Ammit A.J. MKP-1: a negative feedback effector that represses MAPK-mediated pro-inflammatory signaling pathways and cytokine secretion in human airway smooth muscle cells. Cell. Signal. 2012;24:907–913. doi: 10.1016/j.cellsig.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Moncho-Amor V., Ibanez de Caceres I., Bandres E. DUSP1/MKP1 promotes angiogenesis, invasion and metastasis in non-small-cell lung cancer. Oncogene. 2011;30:668–678. doi: 10.1038/onc.2010.449. [DOI] [PubMed] [Google Scholar]
- Newie I., Sokilde R., Persson H. The HER2-encoded miR-4728-3p regulates ESR1 through a non-canonical internal seed interaction. PLoS One. 2014;9:e97200. doi: 10.1371/journal.pone.0097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Kvist A., Rego N. Identification of new microRNAs in paired normal and tumor breast tissue suggests a dual role for the ERBB2/Her2 gene. Cancer Res. 2011;71:78–86. doi: 10.1158/0008-5472.CAN-10-1869. [DOI] [PubMed] [Google Scholar]
- Prabhala P., Ammit A.J. Tristetraprolin and its role in regulation of airway inflammation. Mol. Pharmacol. 2015;87:629–638. doi: 10.1124/mol.114.095984. [DOI] [PubMed] [Google Scholar]
- Schmitt D.C., Madeira da Silva L., Zhang W. ErbB2-intronic microRNA-4728: a novel tumor suppressor and antagonist of oncogenic MAPK signaling. Cell Death Dis. 2015;6:e1742. doi: 10.1038/cddis.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E.F., Nebreda A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang M., Qian Y.Y., Ding Z.Y., Lv J.H., Shen H.H. Imbalanced expression of mitogen-activated protein kinase phosphatase-1 and phosphorylated extracellular signal-regulated kinases in lung squamous cell carcinoma. J. Zhejiang Univ. Sci. B. 2011;12:828–834. doi: 10.1631/jzus.B1100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q., Fischer L., Noethling C. In vitro-assessment of putative antiprogestin activities of phytochemicals and synthetic UV absorbers in human endometrial Ishikawa cells. Gynecol. Endocrinol. 2015;31:578. doi: 10.3109/09513590.2015.1047448. [DOI] [PubMed] [Google Scholar]
- Zhang L., Huang C., Guo Y. MicroRNA-26b Modulates the NF-kappaB Pathway in Alveolar Macrophages by Regulating PTEN. J. Immunol. 2015;195:5404–5414. doi: 10.4049/jimmunol.1402933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Muller M., Chen C.H., Zeng L., Farooq A., Zhou M.M. New insights into the catalytic activation of the MAPK phosphatase PAC-1 induced by its substrate MAPK ERK2 binding. J. Mol. Biol. 2005;354:777–788. doi: 10.1016/j.jmb.2005.10.006. [DOI] [PubMed] [Google Scholar]