Abstract
T-cell function in female animal models of hypertension is poorly understood since most research is conducted in males. Our findings in Dahl-salt-sensitive and Dahl salt-resistant rats support prior research showing sex-specific T-cell effects in the pathophysiology of hypertension. Further studies are needed to inform clinical studies in both sexes and to provide clues into immune mechanisms underlying susceptibility and resilience to developing hypertension and associated disease.
Introduction
The role of the immune system in the development of hypertension has been investigated for over five decades. Early transplant experiments were the first to demonstrate a direct link between the immune system and blood pressure. In 1982, Ba et al. (1) reported that blood pressure was reduced in the male spontaneously hypertensive rat (SHR) after receiving male organ transplants from thymus, lymph nodes, or spleen from a normotensive Wistar Kyoto (WKY) rat or after thymus grafts from Fischer F344 rats and C57BL/6 mice. The finding that blood pressure in the SHR was reduced after transplants from lymphocytic beds but not from the liver provided evidence that the immune system modulates blood pressure. Later studies in rats using the immune-suppressive agent mycophenolate mofetil (MMF) demonstrated that inhibiting lymphocyte proliferation attenuated the development of salt-sensitive (3, 4, 14, 20, 21) and spontaneous (29) hypertension. These immunosuppressive agents, however, are not specific to T cells since many other immune cell types are affected. The involvement of T cells in blood pressure regulation gained broad acceptance after Guzik et al. (9) showed male mice lacking the recombinant activating gene (Rag1−/−) exhibited blunted pressor responses to angiotensin II (Ang II), whereas adoptive transfer of T but not B cells from male wild-type (WT) mice restored pressor sensitivity to Ang II.
Sex-Specific T-Cell Modulation of Blood Pressure
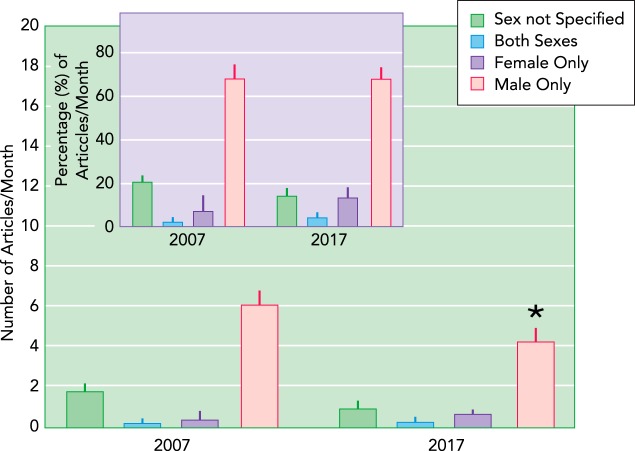
The majority of basic science research in hypertension has been conducted in male models of disease. This fact is illustrated by an analysis of studies published in the high-impact journal Hypertension. Between January and July 2007, 62 articles involving animal studies were published in this journal. Of these, 21% did not specify the sex of the animal used in the research. Ten years later, there has been a slight improvement in the number of articles specifying the sex of the animal, and the number of studies that were solely conducted in males was reduced by 30% (P < 0.02, two-way ANOVA); however, no increase was apparent in the percent of animal studies conducted in females (FIGURE 1), probably because fewer articles involving animal studies were published in 2017 compared with 2007.
FIGURE 1.
Sex of animals published in the journal Hypertension
Shown are the number of animal studies published per month ± SE and percentage of articles published per month ± SE (inset) between January and July in the years 2007 and 2017 in the journal Hypertension. Animal studies are reported based on the use of both sexes and those that reported data solely in one sex (male or female). Articles that did not specify the sex of the animals studied are also reported. *Significant difference vs. 2007 [P < 0.05; two-way ANOVA (time, sex studied) followed by Sidak’s multiple comparison test].
The findings of Guzik et al. (9) have been reproduced by several laboratories, including ours (12, 19); however, we also found that adoptive transfer of female T cells into the male Rag1/– mouse did not restore pressor sensitivity to Ang II in the host (12). Furthermore, adoptive transfer of male T cells from WT mice into the female Rag1−/−−/− host did not increase the pressor sensitivity to Ang II (19). The two major T-cell populations, CD4 and CD8, have been investigated as possible contributors to the pathophysiology of hypertension in rodents (24, 30, 33, 35). Our studies show that adoptive transfer of either male CD4 or CD8 cells restored the pressor response to Ang II in male Rag1−/− mice; however, these effects were sex-specific, since adoptive transfer of either CD4 or CD8 cells from female WT mice did not restore the pressor response to Ang II in male Rag1−/− mice (24).
Recently, studies from our laboratory as well as others showed the pressor dose response to Ang II in the male Rag1−/− mouse purchased from Jackson Laboratories between 2015 and 2016 was identical to their WT litter mates (11). These findings suggest mutations have occurred in this mouse line that mask the role of T cells in the pathophysiology of Ang II-induced hypertension. This conclusion is supported by our previous work showing that resistance to Ang II-induced hypertension in the male Rag1−/− mouse was dependent on the dose of Ang II (12). At low doses (200–490 ng·kg–1·min–1), the magnitude of Ang II-induced increases in mean arterial pressure (MAP) was less in the male Rag1−/− mouse compared with male WT littermates, whereas at a higher dose (1,000 ng·kg–1·min–1), no differences were observed in the time course of Ang II-induced hypertension between Rag 1−/− mice and their WT littermates.
Similar to the findings in male Rag1−/− mice (9, 12, 24), the Mattson laboratory demonstrated that male DSS rats bred in Milwaukee (DSS/Mcwi), and in which the Rag1 gene was deleted (DSS-Rag1em1Mcwi; DSSRag1−/−), have lower MAP responses when switched to a high sodium diet compared with WT DSS/Mcwi (15). Moreover, this group found that dietary sodium-induced increases in MAP were also attenuated in male DSS/Mcwi rats expressing non-functional T cells due to a deletion in the CD3 zeta chain (CD247−/−) (23). These studies suggest that T cells contribute to the pathophysiology of salt-sensitive hypertension in male DSS/Mcwi rats; however, they also found that the resistance to developing salt-sensitive hypertension was time-dependent; the MAP in male DSSRag1−/− rats continued to rise over 3 wk on the high sodium diet, and the MAP reached ~155 mmHg by the end of the study period (15). These observations indicate that both T-cell-dependent and T-cell-independent mechanisms contribute to the development of hypertension in the male DSS/Mcwi rat. Thus future studies need to determine the contribution of T-cell-dependent and T-cell-independent mechanisms in the pathogenesis of hypertension. This knowledge would guide prioritization of drug targets for intervention and clinical development.
CD4/CD8 Ratio as a Clinical Biomarker
There recently has been increasing interest in the CD4/CD8 ratio as a potential biomarker of hypertension and associated disease. Recent clinical studies reported that an increase in the CD4/CD8 ratio in peripheral blood from men and women was associated with essential hypertension (16) and an increased risk of coronary artery disease in men and women (8). In addition, Youn et al. (35) reported that both CD4 and CD8 T-cell infiltration was significantly higher in the tubulointerstitium of patients with hypertensive nephrosclerosis compared with healthy participants. Based on reported T-cell values, the CD4/CD8 ratio in individuals with hypertension was higher than observed in healthy subjects [kidney (CD4/CD8): control, 1.15 ± 0.0 vs. hypertension, 1.32 ± 0.03; P < 0.0001 by t-test; n = 7–9]. Likewise, a clinical study found that patients with pulmonary arterial hypertension (PAH) had a twofold higher CD4/CD8 ratio in the circulation compared with healthy participants (31).
The CD4/CD8 ratio in PBMC and around pulmonary vessels has also been investigated in PAH, which is a complex, progressive condition arising from a variety of etiologies. Certain pathophysiological features overlap between PAH and persistent systemic hypertension, including structural alterations in the vascular wall and increased stiffening and thickening of the (pulmonary) arteries (27). A clinical study performed on idiopathic PAH (iPAH) and connective tissue disease-associated PAH reported that the CD4/CD8 ratio in PBMC was a predictor of survival using a ratio threshold of 3.6; ratios ≥ 3.6 were associated with 20% transplant free survival after 3 yr, whereas ratios below 3.6 were associated with 60% survival (5). Interestingly, 87% of the patient population was female. Another study performed on human explanted lung tissues from subjects (60% female patients) with iPAH demonstrated the CD4/CD8 ratio was almost twofold elevated in the hypertensive iPAH patients compared with normotensive donors (26).
Sex Differences in the CD4/CD8 Ratio
To gain further insights into the potential use of the CD4/CD8 ratio as a biomarker of hypertensive disease, we performed a systematic literature search and identified 20 unique records reporting both CD4 and CD8 frequencies in rat models of hypertension. We focused on rats because the majority of physiological research into mechanisms of hypertension has been conducted on rats. Twelve of these 20 reports were relevant to our search; the remaining articles either did not study hypertension or did not report the effect of CD4 or CD8 T-cell numbers and/or frequencies. We calculated the ratios for articles that reported values for CD4 and CD8 T cells and expressed the ratios as means ± SE. It should be noted, however, that experimental details regarding gating and validation of antibodies in rats is missing in many papers citing flow cytometry. Therefore, our interpretation of this published data highlighted with an asterisk has the caveat that we assumed appropriate controls were used for the flow cytometry. Unfortunately, this may not be the case.
Tipton et al. (28) reported male SHRs have ~33 mmHg higher MAP and a 2.2-fold higher renal CD4/CD8 ratio [kidney (CD4/CD8): female SHR, 0.8 ± 0.02 vs. male SHR, 1.8 ± 0.07; P < 0.03 by t-test; n = 11–13] than age-matched female SHRs. This study demonstrates that sex differences exist in the CD4/CD8 ratio. Furthermore, the higher ratio along with the higher blood pressure in male SHRs compared with female SHRs supports the idea that the CD4/CD8 ratio positively correlates with blood pressure.
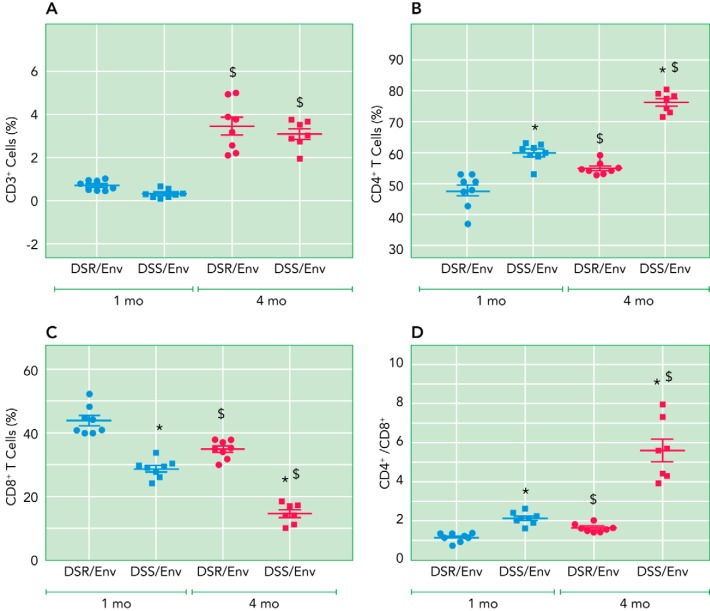
Our laboratory investigated T-cell profiles in the kidney of female Dahl hypertensive salt-sensitive and normotensive salt-resistant rats purchased from Envigo (2015–2016) (DSS/Env & DSR/Env). We found that frequencies of CD3 T cells did not differ between DSS/Env and DSR/Env rats at either 1 or 4 mo of age, but both rat strains had more T cells at 4 mo compared with 1 mo (FIGURE 2A). The frequency of CD4 T cells in DSS/Env was higher than DSR/Env rats at both 1 and 4 mo (FIGURE 2B), whereas the frequency of CD8 in DSS/Env was lower than DSR/Env rats at both time points (FIGURE 2C). As a result, the CD4/CD8 ratio was higher in DSS/Env compared with DSR/Env rats at both ages (FIGURE 2E). Although the ratio increased over the 3-mo period in both strains, the ratio rose more dramatically in the DSS/Env strain [ΔCD4/CD8 (4–1 mo): DSR/Env, 0.5 ± 0.15 vs. DSS/Env, 3.5 ± 0.7; P < 0.001 by t-test; n = 7–8] (FIGURE 2D). At 1 mo, both DSS/Env and DSR/Env rats were normotensive (17). Thus the striking increase in the CD4/CD8 ratio was associated with the marked increase in MAP observed in the 4-mo DSS/Env female rat (17).
FIGURE 2.
Renal T-cell profile of 1- and 4-mo female DSS/Env and DSR/Env rats
Shown are frequencies of CD3 (A), CD4 (B), or CD8 (C) T cells; and CD4/CD8 ratios (D) in kidneys of 1- and 4-mo DSS/Env and DSR/Env female rats. The data are expressed as means ± SE (n = 7–8/ group). Significant difference (two-way ANOVA followed by Sidak’s multiple comparison test): *P < 0.05 vs. DSR/Env same age; $P < 0.05 1 mo, same strain.
A recent study in male DSS/Mcwi rats demonstrated that increased renal perfusion pressure, induced by a high sodium diet, drove infiltration of CD4 and CD8 T cells into the kidney (6). Reduced renal perfusion pressure did not, however, alter the CD4/CD8 ratio in the kidney. Furthermore, preventing the increase or lowering the renal perfusion pressure in the kidney did not change the CD4/CD8 ratio either. Thus, unlike our findings in female DSS/Env rat kidneys, the CD4/CD8 ratio in male DSS/Mcwi rats did not increase with higher blood pressures.
One explanation for these observational differences could be the biological variable sex; we studied female DSS rats, whereas Milwaukee studied male DSS rats. In this regard, Zhang et al. also showed that the CD4/CD8 ratio in splenocytes from male SHR was no different than the ratio in normotensive WKY rats at 4 mo of age (37). No differences were detected in the CD4/CD8 ratio in the kidney of SHR compared with WKY when the SHRs were still normotensive (22).
In addition to sex differences, genetic differences between the DSS strain purchased from Envigo and the DSS strain bred in Milwaukee could contribute to differences in T-cell function. Environmental factors including diet could also account for these observed differences between the female DSS/Env and male DSS/Mcwi rat. Clearly, more studies are needed to elaborate the relationship between blood pressure and the CD4/CD8 ratio to determine what factors impact this ratio and the function of CD4 and CD8 cells in the circulation, lymphocytic tissues, and end organs damaged by hypertension. Future investigations into these possible factors would provide insight into the mechanisms of how a high CD4/CD8 ratio could be a cause or a consequence of hypertension and under what conditions it could be used as a biomarker of disease pathogenesis.
Changes in the CD4/CD8 Ratio in Response to Antihypertensive Treatment
Antihypertensive treatments are both positively and negatively correlated with changes in the CD4/CD8 ratio. Tipton et al. (28) reported the combination of a thiazide (hydrochlorothiazide) and vasodilator (reserpine) for 6 wk (HR6) attenuated the development of hypertension in the female SHR, and this antihypertensive treatment also prevented the age-associated increase in the CD4/CD8 ratio; the HR6-treated females had a 23% lower renal CD4/CD8 ratio as well as 30 mmHg lower systolic blood pressure compared with the untreated female SHRs. In contrast, after hypertension was already established in the female SHR, 2 wk of treatment with this drug combination (HR2) reduced MAP, but these conditions did not alter the infiltration of CD4 and CD8 T cells in the kidney.
Studies with the immune-suppressive agent MMF demonstrated a negative correlation between the CD4/CD8 ratio and blood pressure. MMF administration reduced MAP in both male and female SHR and reduced the frequency of renal as well as circulating CD4 and CD8 T cells; however, MMF-treated females (2.1 ± 0.1) had more than a twofold higher CD4/CD8 ratio (P < 0.0001) compared with the untreated female SHR. MMF treatment did not alter the CD4/CD8 ratio in male SHR kidneys (29).
In the induced model of hemolysis-elevated liver enzymes-low platelet (HELLP) syndrome, a model of preeclampsia, increases in MAP were associated with increases in the renal CD4/CD8 ratio in the females due to increased renal infiltration of CD4 but not CD8 cells (32). Increases in the renal CD4/CD8 ratio were also observed after chronic infusion of soluble fms-like tyrosine kinase 1 and soluble endoglin in HELLP rats (37). Although the ratio of CD4/CD8 T cells was higher in the kidney, the ratio in PBMC and the spleen was indistinguishable between the normotensive and hypertensive animals. This suggests the association of the CD4/CD8 with hypertension is tissue-specific.
Orencia administration (T-cell co-stimulation blocker) reduced MAP in female HELLP rats and lowered CD4 and CD8 T cells in the circulation. Even though the treated group’s MAP was 23 mmHg lower than untreated HELLP rats, the CD4/CD8 ratio was significantly higher than the untreated group [PBMC (CD4/CD8): HELLP, 1.3 ± 0.1 vs. HELLP+Orencia, 2.2 ± 0.4; P < 0.03 by t-test; n = 5–6] (2). Similarly, in a male rat model of experimental autoimmune myocarditis, the olmesartan treatment group had lower blood pressure (systolic and diastolic) and fewer CD4 and CD8 T cells infiltrating the myocardium, yet the CD4/CD8 T-cell ratio was higher in the myocardium compared with untreated rats with myocarditis (36). These findings show two experimental conditions of hypertension in which the changes in blood pressure negatively correlate with changes in the CD4/CD8 ratio.
Experiments in male rat models of PAH suggest that treatment with pyrrolidine dithiocarbamate or dexamethasone lowers right ventricular systolic pressure under conditions in which the CD4/CD8 ratio around lung vessels remains elevated (7, 34). Taking all these reports into consideration, it appears that the association between CD4/CD8 T-cell ratio and hypertension is complex. Several factors such as sex, tissue origin, treatment, and methods employed can influence this relationship. Further studies are thus needed in both sexes to determine the correlation between changes in blood pressure and whether these changes correlate with a threshold CD4/CD8 T-cell ratio or magnitude change in the ratio in various models of hypertension. These studies would then provide clues into the mechanisms by which CD4 and CD8 T cells modulate blood pressure or the reverse, i.e., modulated by changes in blood pressure. Further research could also establish normal ranges of CD4 and CD8 cells in the circulation, lymphocytic tissues, and end organs as a function of blood pressure, sex, and the model of hypertension. Future studies should also investigate the mechanisms of why pretreatment with hydrochlorothiazide and reserpine attenuated hypertension and lowered the CD4/CD8 ratio in females, whereas this phenomenon was not observed after treatment with immunosuppressants (MMF, orencia) or the Ang II receptor antagonist (olmesartan).
In summary, our studies on female DSS/Env and DSR/Env rats support the potential use of CD4/CD8 ratios as a clinical biomarker of hypertensive disease. We found the CD4/CD8 ratio was twofold higher in DSS/Env compared with DSR/Env female rats under normotensive conditions at 1 mo, and this difference in the ratio between the normotensive and hypertensive strain increased to sevenfold at 4 mo when the DSS/Env animals were hypertensive. These studies also support the prior literature demonstrating sex-specific T-cell effects in the pathophysiology of hypertension. A systematic literature review of studies on T cells conducted in rats revealed contradicting observations with respect to the CD4/CD8 ratio and its correlation with blood pressure and response to antihypertensive agents. These findings suggest subpopulations of CD4 and CD8 T cells play distinct roles depending on the sex, strain, model of hypertension, and antihypertensive treatment. Examining the literature also suggests T cells are modulators of hypertension rather than triggers since hypertension can exist in the absence of functional T cells. This observation could inform drug target prioritization in the development of new antihypertensives.
In conclusion, further studies in male and female animal models of hypertensive disease are needed to fully understand the impact of biological sex on T-cell polarization and activity, and the extent to which T cells play a role (either as a cause or consequence) in the pathogenesis of hypertension and associated end-organ injury in both sexes. Unlocking the mysteries of the understudied female rat should greatly advance this goal.
Methods
Literature Reviews
All papers published in the journal Hypertension in 2007 and 2017 between the months of January and July were reviewed, as previously described (25). Articles that reported data in animals were stratified based on the sex of the animals studied. In addition to reviewing the methods, figure legends, and table legends for each article, the entire article was electronically searched for the terms male, man, men, female, woman, women, sex, and gender. Data were collected and analyzed for trends (TM).
A systematic literature search on Ovid MEDLINE was conducted using keywords including T cell(s) OR T cell(s) OR T lymphocyte(s) AND hypertension OR hypertensive OR blood pressure OR arterial pressure AND rat(s) AND CD4(+) AND CD8(+). We found 20 reports. Twelve of these 20 reports were relevant to our search; the remaining articles either did not study hypertension or did not report the effect of CD4 or CD8 T-cell numbers and/or frequencies.
Animals
Female DSS and DSR (rapp) inbred rats were purchased between November 2015 and December 2016 from Envigo (Indianapolis, IN) (DSS/Env & DSR/Env). At Envigo, animals were maintained on a low sodium diet (0.1% sodium) (no. 7034; Teklad, Madison, WI) after weaning, and at Georgetown University they were maintained on a purified phytoestrogen-free low sodium diet (0.128% NaCl) (no. 120597; Teklad) (10). Food and water were provided ad libitum. All methods were approved by the Georgetown University Animal Care and Use Committee.
Flow Cytometry of Kidney Cells
Enzyme digestion of right kidney poles was followed by percoll centrifugation, as described previously (13, 18). One million cells were stained for 20 min on ice in the dark with antibodies for surface markers including CD3 (T-cells marker; REA223; 130-102-677; Miltenyi Biotec, Auburn, CA), CD4 (helper T-cell marker; W3/25; 201518; Biolegend, San Diego, CA), CD8 (cytotoxic T-cell marker; OX-8; 25-0084-82; eBioscience, San Diego, CA). All antibodies were titrated to optimize multicolor flow cytometric staining.
OneComp eBeads Compensation Beads (no. 01-1111-42; Thermo Fisher Scientific, Waltham, MA, then eBioscience) were used for positive and negative staining controls and to avoid spillover signals from fluorochromes. Dead cells were excluded by using the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (L34966; Thermo Fisher Scientific). Prepared samples were run on an eight-color flow cytometer (LSRFortessa; BD Biosciences, San Jose, CA) by the Lombardi Comprehensive Cancer Center Flow Cytometry and Cell Sorting Shared Resource (Georgetown University), and data were collected using FACSDIVA software (BD Biosciences, San Jose, CA). Gating and flow cytometry analysis were performed in a blinded manner (AVP) on FlowJo software (FLOWJO, Ashland, OR).
Statistical Analyses
Prism 7.0 (GraphPad Software, La Jolla, CA) was used to analyze all data. Unpaired parametric t-test was used to analyze differences between groups. Two-way ANOVA followed by Sidak’s multiple comparisons analysis was used as post hoc text to analyze differences in the effects of age between rat groups and to analyze the effect of year of publication on the sex of the animal model used in research. All data are expressed as scatter plots showing means ± SE, unless otherwise described. The significance threshold was defined as 0.05.
Acknowledgments
This work was supported by National Institutes of Health Grants TL1-TR-001431, UL1-TR-001409, and R01-HL-119380.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.V.P. and K.S. conceived and designed research; A.V.P. and T.M. performed experiments; A.V.P. and T.M. analyzed data; A.V.P., T.M., and K.S. interpreted results of experiments; A.V.P. and T.M. prepared figures; A.V.P. and K.S. drafted manuscript; A.V.P. and K.S. edited and revised manuscript; A.V.P., T.M., and K.S. approved final version of manuscript.
References
- 1.Ba D, Takeichi N, Kodama T, Kobayashi H. Restoration of T cell depression and suppression of blood pressure in spontaneously hypertensive rats (SHR) by thymus grafts or thymus extracts. J Immunol 128: 1211–1216, 1982. [PubMed] [Google Scholar]
- 2.Bean C, Spencer SK, Bowles T, Kyle PB, Williams JM, Gibbens J, Wallace K. Inhibition of T-cell activation attenuates hypertension, TNFα, IL-17, and blood-brain barrier permeability in pregnant rats with angiogenic imbalance. Am J Reprod Immunol 76: 272–279, 2016. doi: 10.1111/aji.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boesen EI, Williams DL, Pollock JS, Pollock DM. Immunosuppression with mycophenolate mofetil attenuates the development of hypertension and albuminuria in deoxycorticosterone acetate-salt hypertensive rats. Clin Exp Pharmacol Physiol 37: 1016–1022, 2010. doi: 10.1111/j.1440-1681.2010.05428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 298: R1136–R1142, 2010. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards AL, Gunningham SP, Clare GC, Hayman MW, Smith M, Frampton CM, Robinson BA, Troughton RW, Beckert LE. Professional killer cell deficiencies and decreased survival in pulmonary arterial hypertension. Respirology 18: 1271–1277, 2013. doi: 10.1111/resp.12152. [DOI] [PubMed] [Google Scholar]
- 6.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension 70: 543–551, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas D, Alhussaini AA, Kraskauskas D, Kraskauskiene V, Cool CD, Nicolls MR, Natarajan R, Farkas L. Nuclear factor κB inhibition reduces lung vascular lumen obliteration in severe pulmonary hypertension in rats. Am J Respir Cell Mol Biol 51: 413–425, 2014. doi: 10.1165/rcmb.2013-0355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao P, Rong HH, Lu T, Tang G, Si LY, Lederer JA, Xiong W. The CD4/CD8 ratio is associated with coronary artery disease (CAD) in elderly Chinese patients. Int Immunopharmacol 42: 39–43, 2017. doi: 10.1016/j.intimp.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension 44: 405–409, 2004. doi: 10.1161/01.HYP.0000142893.08655.96. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Pai AV, West CA, Wu X, Speth RC, Sandberg K. Loss of Resistance to Angiotensin II-Induced Hypertension in the Jackson Laboratory Recombination-Activating Gene Null Mouse on the C57BL/6J Background. Hypertension 69: 1121–1127, 2017. doi: 10.1161/HYPERTENSIONAHA.117.09063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, Sandberg K. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 64: 573–582, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martina MN, Bandapalle S, Rabb H, Hamad AR. Isolation of double negative αβ T cells from the kidney. J Vis Exp 87: 51192, 2014. doi: 10.3791/51192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension 48: 149–156, 2006. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 15.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013. doi: 10.1152/ajpregu.00304.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ni X, Wang A, Zhang L, Shan LY, Zhang HC, Li L, Si JQ, Luo J, Li XZ, Ma KT. Up-regulation of gap junction in peripheral blood T lymphocytes contributes to the inflammatory response in essential hypertension. PLoS One 12: e0184773, 2017. doi: 10.1371/journal.pone.0184773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai AV, West CA, Souza A, Kadam PS, Pollner EJ, West DA, Ji H, Wu X, Sandberg K. Abstract P285: Hydralazine attenuates the development of hypertension in the female Dahl salt-sensitive rat in a T cell-independent manner. Hypertension 70, Suppl 1: AP285, 2017. [Google Scholar]
- 18.Pindjakova J, Hanley SA, Duffy MM, Sutton CE, Weidhofer GA, Miller MN, Nath KA, Mills KH, Ceredig R, Griffin MD. Interleukin-1 accounts for intrarenal Th17 cell activation during ureteral obstruction. Kidney Int 81: 379–390, 2012. doi: 10.1038/ki.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 64: 384–390, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quiroz Y, Pons H, Gordon KL, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ, Rodríguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol 281: F38–F47, 2001. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001. doi: 10.1046/j.1523-1755.2001.00737.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Iturbe B, Quiroz Y, Ferrebuz A, Parra G, Vaziri ND. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am J Nephrol 24: 587–594, 2004. doi: 10.1159/000082313. [DOI] [PubMed] [Google Scholar]
- 23.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program . CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol 294: 95–101, 2015. doi: 10.1016/j.cellimm.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandberg K, Pai AV, Maddox T. Sex and rigor: the TGF-β blood pressure affair. Am J Physiol Renal Physiol 313: F1087–F1088, 2017. doi: 10.1152/ajprenal.00381.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 186: 897–908, 2012. doi: 10.1164/rccm.201202-0335OC. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 91: 297–309, 2013. doi: 10.1007/s00109-013-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 64: 557–564, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol 303: R359–R367, 2012. doi: 10.1152/ajpregu.00246.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration 75: 272–280, 2008. doi: 10.1159/000111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace K, Morris R, Kyle PB, Cornelius D, Darby M, Scott J, Moseley J, Chatman K, Lamarca B. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertens Pregnancy 33: 41–54, 2014. doi: 10.3109/10641955.2013.835820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallace K, Richards S, Dhillon P, Weimer A, Edholm ES, Bengten E, Wilson M, Martin JN Jr, LaMarca B. CD4+ T-helper cells stimulated in response to placental ischemia mediate hypertension during pregnancy. Hypertension 57: 949–955, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Wang YL, Chen XY, Li YT, Hao W, Jin YP, Han B. Dexamethasone attenuates development of monocrotaline-induced pulmonary arterial hypertension. Mol Biol Rep 38: 3277–3284, 2011. doi: 10.1007/s11033-010-0390-x. [DOI] [PubMed] [Google Scholar]
- 35.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, Yoo OJ, Shin EC, Park S. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 62: 126–133, 2013. doi: 10.1161/HYPERTENSIONAHA.113.00689. [DOI] [PubMed] [Google Scholar]
- 36.Yuan Z, Nimata M, Okabe TA, Shioji K, Hasegawa K, Kita T, Kishimoto C. Olmesartan, a novel AT1 antagonist, suppresses cytotoxic myocardial injury in autoimmune heart failure. Am J Physiol Heart Circ Physiol 289: H1147–H1152, 2005. doi: 10.1152/ajpheart.00078.2005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang HC, Zhang ZS, Zhang L, Wang A, Zhu H, Li L, Si JQ, Li XZ, Ma KT. Connexin 43 in splenic lymphocytes is involved in the regulation of CD4+CD25+ T lymphocyte proliferation and cytokine production in hypertensive inflammation. Int J Mol Med 41: 13–24, 2018. doi: 10.3892/ijmm.2017.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]